Proteins are large, complex molecules that are essential for the structure, function, and regulation of cells and organisms. They are polymers composed of smaller units called amino acids, which are linked together by peptide bonds. Polymers are large molecules composed of repeating subunits called monomers. These monomers are connected through covalent bonds to form a long chain-like structure. The process of combining monomers to form polymers is known as polymerization. Proteins are one of the fundamental building blocks of life and play diverse roles in various biological processes. Proteins exhibit a wide range of structures and functions, determined by their unique amino acid sequences. The sequence of amino acids in a protein is encoded in the genetic material, such as DNA or RNA, and is transcribed and translated into a specific protein molecule during cellular processes.
In biology, transcription and translation are two fundamental processes involved in gene expression, where the information encoded in DNA is converted into functional proteins. Transcription is the process by which the genetic information stored in DNA is copied or transcribed into a complementary RNA molecule. It takes place in the nucleus of eukaryotic cells or the cytoplasm of prokaryotic cells. The steps involved in transcription are as follows: RNA polymerase, along with other proteins called transcription factors, binds to a specific region on the DNA called the promoter. This marks the beginning of the gene to be transcribed. RNA polymerase moves along the DNA template strand, synthesizing a complementary RNA molecule. The RNA is assembled based on the sequence of the DNA template, with adenine (A) pairing with uracil (U), cytosine (C) with guanine (G), and thymine (T) with adenine (A). The termination signal on the DNA is reached, causing RNA polymerase to detach from the DNA template and release the newly synthesized RNA molecule. After transcription, the resulting RNA molecule, called messenger RNA (mRNA), undergoes processing in eukaryotes, including the removal of introns (non-coding regions) and addition of a protective cap and a poly-A tail. The processed mRNA then exits the nucleus and moves into the cytoplasm for translation.
Translation is the process by which the genetic information carried by mRNA is decoded and used to assemble a specific sequence of amino acids, forming a protein. It occurs in the ribosomes, which are cellular structures found in the cytoplasm. The steps involved in translation are as follows: The mRNA binds to the ribosome, and the process begins at a specific start codon (usually AUG). Transfer RNA (tRNA) molecules carrying amino acids recognize and bind to the codons on the mRNA through complementary base pairing. The ribosome moves along the mRNA molecule, reading each codon and matching it with the corresponding tRNA carrying the appropriate amino acid. The amino acids are joined together by peptide bonds to form a growing polypeptide chain. When a stop codon is reached on the mRNA, the translation process is terminated. The newly synthesized polypeptide chain is released from the ribosome. After translation, the polypeptide may undergo additional modifications, such as folding into a specific three-dimensional structure, association with other polypeptide chains, or chemical modifications, to become a functional protein.
Many proteins act as enzymes, catalyzing biochemical reactions and facilitating the conversion of substrates into products. Enzymes play a crucial role in metabolism, facilitating chemical reactions necessary for energy production, nutrient breakdown, and the synthesis of cellular components. Proteins provide structural support to cells and tissues. They form the backbone of cellular structures, such as the cytoskeleton, and contribute to the structural integrity of tissues like skin, muscles, and bones. Proteins like collagen and keratin are particularly important in providing strength and elasticity to various tissues. Certain proteins act as carriers and transport molecules within cells or across cell membranes. For example, hemoglobin transports oxygen in red blood cells, and membrane transport proteins facilitate the movement of ions, nutrients, and other molecules across cellular membranes. Some proteins also serve as storage molecules, storing substances like iron or nutrients for later use. Proteins play a crucial role in cellular signaling and communication. Receptor proteins, located on the cell membrane, recognize and bind to specific signaling molecules, initiating cellular responses. Other proteins involved in signal transduction pathways relay and amplify signals, leading to various physiological responses within the cell. Proteins are integral components of the immune system and play a vital role in defending the body against pathogens. Antibodies, produced by specialized immune cells, recognize and neutralize foreign substances, such as bacteria or viruses. Other proteins, like cytokines, regulate immune responses and facilitate communication between immune cells. Proteins are involved in regulating gene expression and controlling cellular processes. Transcription factors bind to specific DNA sequences and control the transcription of genes, thereby influencing the production of proteins. Protein kinases and phosphatases regulate the activity of other proteins by adding or removing phosphate groups, respectively. Some proteins, such as actin and myosin, are responsible for muscle contraction and cellular movement. They interact to generate force and enable the movement of cells, organelles, and other structures within the cell.
Proteins are remarkable molecular entities with a wide range of essential functions in living organisms. They are intricately designed and specifically crafted to carry out specific tasks necessary for the functioning and survival of life.
Proteins as catalysts
The remarkable efficiency and specificity of enzymes are critical for the functioning of biological networks. Without enzymes, many essential reactions would occur too slowly or not at all, hindering the production of crucial biomolecules like pyrimidine ribonucleotides. The need for catalysis in multiple reactions within the network highlights the interdependence and complexity of biochemical systems. From an intelligent design (ID) perspective, the catalytic properties of proteins, including enzymes, provide compelling evidence for the involvement of an intelligent agent in the design and creation of life. Proteins exhibit remarkable specificity and efficiency as catalysts, allowing them to accelerate chemical reactions in living systems. The specific arrangement of amino acids within a protein's structure enables it to bind to specific substrates and facilitate the conversion of reactants into products with high precision. The active sites of enzymes, for example, are specifically shaped to accommodate particular substrates, leading to efficient and selective catalysis. The functional effectiveness of protein catalysts depends on their precise arrangement and sequence of amino acids. The probability of a random sequence of amino acids spontaneously folding into a functional protein with catalytic properties is extremely low. The vast number of possible amino acid sequences makes the chance formation of a functional protein through undirected processes highly improbable. Moreover, the emergence of a single functional protein is not sufficient for the development of complex metabolic networks. The successful functioning of these networks relies on the coordinated action of multiple proteins working together in a synergistic manner. This interdependence and complexity of protein interactions further suggest the involvement of intelligent agency in the design and assembly of these systems. Intelligent design proposes that the intricate and purposeful arrangement of proteins as catalysts in living organisms reflects the work of an intelligent agent. The complexity, specificity, and efficiency of protein catalysts go beyond what can be reasonably attributed to undirected natural processes. The fine-tuning and functional integration of proteins in metabolic pathways and cellular processes strongly suggest intelligent design as the best explanation for their existence and functionality.
Structural Support
The complexity and precision of protein structures suggest their creation by intelligent planning and foresight. Proteins like collagen, with their fibrous structure and ability to form strong connective tissues, demonstrate a level of sophistication that is far beyond what could be attributed to chance or undirected processes alone. The specific arrangement of amino acids within proteins is critical for their structural stability. Proteins often possess modular domains that allow for specific interactions and assembly into larger structures. The intricate interplay between different proteins in extracellular matrices, such as the coordination of collagen fibers with other components like elastin or proteoglycans, further emphasizes their remarkable sophistication. The diversity of protein structures and functions across different organisms also raises questions about the origin of this complexity. ( The Protein Data Bank (PDB), which is a widely used resource for structural information on proteins, contains structures for more than 180,000 unique proteins. That is, IMHO, just a fraction of the diversity known to exist. The human body alone is estimated to have millions of protein species )
Transport and Signaling
Proteins are multifunctional, able to transport molecules and ions across cell membranes, as well as operate as signaling molecules. Proteins that act as channels and transporters in cell membranes play a crucial role in maintaining the internal environment of cells. They selectively allow specific molecules and ions to pass through the membrane, controlling the movement of substances in and out of cells. The selective permeability of cell membranes is essential for life and had to be present when life began. Cell membranes act as barriers that separate the internal environment of the cell from the external environment, allowing for the regulation of molecular movement in and out of the cell. This selective permeability is crucial for maintaining homeostasis and facilitating essential cellular processes. The ability of cell membranes to selectively allow specific molecules and ions to pass through is vital for several reasons: The selective permeability of cell membranes allows the entry of nutrients while preventing the passage of unwanted or harmful substances. Cells produce metabolic waste products that need to be eliminated from the cell. The selective permeability of the membrane enables the removal of waste materials while retaining necessary molecules inside the cell. Cell membranes help maintain the proper balance of ions and molecules inside the cell, creating an optimal internal environment for cellular processes to occur. This selective permeability is crucial for maintaining the appropriate concentrations of ions such as potassium, sodium, and calcium, which are involved in various cellular functions. Cell membranes contain receptors that can recognize specific signaling molecules, such as hormones or neurotransmitters. These receptors allow the cell to respond to external signals and initiate appropriate cellular responses. Precise regulation is vital for cellular homeostasis and proper functioning. The specificity and selectivity of these transport proteins, ensuring that only certain substances are allowed to cross the membrane, indicate a sophisticated design strategy aimed at maintaining cellular integrity and functionality.
The ability of proteins to act as signaling molecules further emphasizes their role in coordinating physiological processes. Signaling proteins transmit information within cells and between cells, enabling cells to communicate and respond to external stimuli. They are involved in processes such as cell growth, differentiation, immune responses, and neuronal signaling. Signaling proteins often possess specific binding sites that allow them to interact with other molecules, triggering a cascade of events that ultimately leads to a cellular response. The complexity and specificity of these signaling pathways suggest a deliberate design to ensure accurate and efficient communication within biological systems.
The instantiation of communication and the establishment of communication channels typically involve deliberate acts of intelligent setup. Communication is a process by which information is exchanged between individuals or systems, allowing for the transmission of thoughts, ideas, data, or instructions. While some forms of communication in nature may occur instinctively or through simple signaling mechanisms, the deliberate setup of communication channels requires intelligent intervention. Here are a few reasons why: The setup of communication channels involves the design and organization of systems that facilitate the exchange of information. This requires intelligence to plan and implement the necessary infrastructure, protocols, and mechanisms for communication to occur effectively. For example, in human communication, the development of language, the creation of written symbols, or the design of technological communication networks all require deliberate intelligent actions. Communication channels are typically established with a specific purpose or goal in mind. Intelligent agents consciously design and set up these channels to achieve desired outcomes. Whether it is to share information, coordinate actions, convey emotions, or express complex concepts, communication channels are intentionally created to serve a particular objective.
Communication channels often need to be adaptable and capable of handling complex information. Intelligent beings can design communication systems that allow for flexibility, scalability, and the encoding of nuanced meanings. They can create intricate systems such as language with grammar, syntax, and semantics to convey rich and diverse information. The deliberate act of setting up such sophisticated communication channels requires intelligence.
Intelligent agents can establish feedback mechanisms within communication channels to facilitate learning and improvement. They can assess the effectiveness of the communication process, make adjustments, and learn from previous interactions. This ability to adapt and optimize communication channels based on feedback is a characteristic of intelligent systems. While natural systems, such as some animal communication or chemical signaling in cells, may involve innate or instinctive communication mechanisms, the deliberate setup of communication channels with specific objectives and complex features typically requires intelligent intervention. Human communication, in particular, exemplifies the intentional design and implementation of sophisticated communication systems.
Non-intelligent mechanisms are incapable of instantiating communication channels that are irreducible and interdependent for several reasons: Communication channels often require a deliberate design and purposeful organization to facilitate effective information exchange. Non-intelligent mechanisms lack the capability to plan, design, and purposefully set up communication channels to achieve specific objectives. They operate based on predefined rules or physical interactions without the ability to adapt or optimize the communication process. Communication channels often need to be flexible and capable of handling complex information. They should be able to transmit and interpret diverse messages, adapt to changing contexts, and support nuanced meanings. Non-intelligent mechanisms, typically driven by simple physical or chemical processes, lack the capacity to handle the complexity and variability required for sophisticated communication. Communication often involves the use of symbols or representations that convey meaning. Symbols are abstract entities that represent objects, ideas, or concepts, and their interpretation requires shared understanding. Non-intelligent mechanisms generally do not possess the ability to create or interpret symbolic representations, limiting their capacity for meaningful communication. Effective communication often involves feedback mechanisms that allow for learning, adjustment, and improvement. Intelligent systems can assess the effectiveness of communication, make adjustments based on feedback, and learn from previous interactions. Non-intelligent mechanisms typically lack the ability to analyze feedback, adapt their communication processes, or learn from experience. Communication channels are often established with specific intentions and goals in mind. Intelligent agents can set up communication systems with purpose and direct them toward achieving desired outcomes. Non-intelligent mechanisms do not possess intentions or goals and operate based on deterministic or stochastic processes without purposeful direction. Communication is highly context-dependent, requiring an understanding of the situation.
The interdependence and integration of transport proteins and signaling molecules in cellular processes raise questions about their origin and functionality. The intricate coordination of various proteins and their ability to work together harmoniously point to an intelligent setup. The specific structures, functions, and interactions of these proteins imply a purposeful design aimed at achieving optimal cellular processes and responses. The remarkable diversity and conservation of transport proteins and signaling molecules across different organisms indicate the existence of a common blueprint or design principles underlying these systems. This suggests the involvement of an intelligent designer who implemented similar functional strategies across various life forms. The specificity, selectivity, and coordination of proteins in facilitating the movement of substances across cell membranes and transmitting signals within and between cells point to the work of an intelligent agent who carefully designed these systems to ensure the proper functioning and communication of living organisms. The complexity and interdependence of these proteins provide compelling evidence for intelligent design as the best explanation for their origin and functionality.
Immune Defense
Proteins play a vital role in the immune system's defense mechanisms against pathogens and foreign invaders. Antibodies, which are specialized proteins produced by immune cells, exhibit remarkable specificity in binding to antigens found on the surface of these invaders. This precise recognition enables antibodies to mark the pathogens for destruction by other immune cells, effectively neutralizing the threat. The ability of antibodies to selectively bind to antigens is a remarkable example of molecular recognition and targeting. The specific structure of antibodies, with their variable regions that can adapt to different antigens, suggests a purposefully designed arrangement to enable a diverse range of pathogens to be recognized and targeted effectively.
Antibodies have specific binding sites, often referred to as protein pockets or antigen-binding sites, within their variable regions. These pockets are responsible for recognizing and binding to antigens, which are specific molecular structures found on pathogens. The design of these pockets involves a complex arrangement of amino acid residues that create a complementary shape to the target antigen. This precise pocket design allows antibodies to selectively bind to specific antigens with high affinity. The probability of randomly generating such a precise protein pocket design is astronomically low, making unguided random events an improbable mechanism for its creation.
The binding between an antibody and its target antigen relies on a complementary fit between their structures. The protein pocket of the antibody must match the shape, charge distribution, and other molecular features of the antigen. This complementary fit ensures specific and stable binding interactions. Achieving such precise complementarity by random events alone is highly unlikely due to the vast number of possible protein sequences and conformations. Antibodies have to perform their functions with a high degree of specificity and efficiency. They need to recognize and neutralize a wide variety of pathogens while avoiding unnecessary interactions with self-molecules. This necessitates a purposeful design that ensures proper antigen recognition and discrimination. Random events are unlikely to produce the level of specificity and discrimination required for antibodies to fulfill their protective role effectively. The structure and function of antibodies are conserved across different species, suggesting that the design is not a result of random events. Antibodies have undergone millions of years of evolutionary refinement to achieve their current effectiveness. The evolution of complex structures, such as antibodies, involves gradual modifications and selection of favorable variants over time. Unguided random events alone are unlikely to account for the precise arrangement and functionality observed in antibodies.
Additionally, proteins contribute to immune surveillance by participating in the recognition and elimination of abnormal cells, such as cancerous cells. Certain proteins act as checkpoints to ensure the identification and elimination of cells displaying abnormal behavior or presenting foreign molecules. These proteins help maintain the integrity and health of the organism by removing potentially harmful cells from the body. The ability of proteins to recognize specific targets, mount immune responses, and coordinate the intricate defense mechanisms required to protect the body from pathogens strongly suggests an intelligent setup. When considering the origin of such intricate and precisely coordinated systems, the concept of intelligent design provides a compelling explanation. The intricate interplay between proteins, their specific functions, and their coordinated responses to external threats suggests that these systems were purposefully designed by an intelligent agent with the capability to anticipate and address the challenges faced by living organisms. While acknowledging the scientific principles and mechanisms involved, the complexity and purposeful nature of these systems strongly support the idea that an intelligent designer played a pivotal role in the origin and functionality of proteins and the immune system as a whole.
Regulation and Control
Proteins serve as essential regulators and controllers of cellular processes, exhibiting a finely tuned and orchestrated functionality. One significant role they play is acting as molecular switches, governing the activation or inhibition of specific biochemical pathways and gene expression. Transcription factors, a class of proteins, exemplify this function by binding to specific DNA sequences and influencing the transcription of genes. Transcription factors are key players in crucial biological processes, including development, cellular differentiation, and the response to environmental signals. Their ability to precisely bind to DNA and initiate or suppress gene expression provides a means of regulating the intricate molecular events that guide the formation and function of cells and tissues. Proteins involved in regulation and control exhibit an intricate interplay of structure, function, and interaction, allowing for the fine-tuning and coordination of cellular activities. The intricate nature of transcription factors and their ability to modulate gene expression governs the development and functioning of organisms. Transcription factors and gene regulation were crucial to the emergence of life for several reasons: Gene regulation allows organisms to control when and to what extent specific genes are expressed. This regulation is essential for the proper development and functioning of cells and organisms. By selectively activating or inhibiting gene expression, transcription factors play a vital role in ensuring that genes are expressed in the appropriate cells, at the right time, and in response to specific signals. This regulation is critical for response to environmental cues. Gene regulation mediated by transcription factors enables cells to respond to changes in their environment. It allows them to adjust their gene expression patterns and cellular activities in response to external cues such as nutrients, stressors, or signaling molecules. This adaptability enhances the cells ability to survive, reproduce, and thrive in different environmental conditions. Gene regulation ensures the proper balance and coordination of cellular activities. Transcription factors help maintain homeostasis by fine-tuning the expression of genes involved in metabolic pathways, signaling cascades, and other essential cellular processes. By modulating gene expression, they ensure that cells respond appropriately to internal and external signals, maintain proper cell function, and prevent abnormalities or dysfunction.
The gene regulatory network is essential for life. It plays a fundamental role in the development, functioning, and maintenance of living organisms. The gene regulatory network refers to the complex system of interactions between genes, transcription factors, and other regulatory molecules that control gene expression. Here are some reasons why the gene regulatory network is crucial for life: The gene regulatory network is essential for maintaining cellular homeostasis by regulating the expression of genes involved in metabolic processes, signaling pathways, and cellular functions. It allows cells to respond to changing conditions, adapt to internal and external cues, and maintain a stable internal environment. The gene regulatory network enables organisms to respond and adapt to changes in their environment. It allows for the activation or suppression of genes in response to various signals, such as stressors, nutrients, or signaling molecules. This responsiveness helps organisms survive and thrive in different environmental conditions. Dysregulation of the gene regulatory network can lead to various diseases and disorders. Problems in gene expression control can result in abnormal cell growth, developmental abnormalities, or malfunctioning cellular processes. : The gene regulatory network plays a role in evolutionary processes by providing mechanisms for genetic variation and adaptation. Changes in the regulatory interactions within the network can lead to the emergence of new traits and the exploration of novel evolutionary pathways. It allows for the diversification and adaptation of species over time.
Transcription factors and gene regulation were crucial to the emergence and functionality of life for several reasons: Gene regulation allows organisms to control when and to what extent specific genes are expressed. This regulation is essential for the proper development and functioning of cells and organisms. By selectively activating or inhibiting gene expression, transcription factors play a vital role in ensuring that genes are expressed at the right time, and in response to specific signals. This regulation is critical to respond to environmental cues. Gene regulation mediated by transcription factors enables organisms to respond to changes in their environment. It allows them to adjust their gene expression patterns and cellular activities in response to external cues such as nutrients, stressors, or signaling molecules. This adaptability enhances an organism's ability to survive, reproduce, and thrive in different environmental conditions. The specific binding of transcription factors to DNA sequences, coupled with their capacity to influence gene expression in response to internal and external cues allows organisms to adapt and respond to their environment. The ability of these proteins to coordinate and modulate cellular processes with remarkable specificity and efficiency implies the work of an intelligent agent capable of designing and implementing such sophisticated systems. The underlying purpose, precision, and adaptability of these systems strongly support the notion that an intelligent agent played a pivotal role in the origin and functionality of proteins involved in regulating and controlling cellular processes. Transcription factors work in a highly coordinated manner with other molecules involved in gene expression regulation. They interact with DNA sequences in a specific and sequence-dependent manner, binding to promoter or enhancer regions of genes. This binding, in turn, influences the recruitment of other proteins and the transcriptional machinery to initiate or suppress gene expression. The interplay between transcription factors, DNA sequences, and other regulatory molecules demonstrates a finely tuned and interdependent system. Transcription factors exhibit remarkable specificity in recognizing and binding to their target DNA sequences. They possess unique structural features, such as DNA-binding domains, that enable them to recognize specific nucleotide sequences and form stable complexes. This specificity ensures that transcription factors selectively bind to their target genes, avoiding random interactions and ensuring precise control over gene expression. The highly specific and selective nature of transcription factor-DNA interactions suggests a purposeful design to achieve regulatory precision. Transcription factors often possess complex protein architectures that contribute to their functionality. They can contain multiple domains with distinct functions, including DNA-binding domains, activation or repression domains, and protein-protein interaction domains. These domains allow transcription factors to interact with various molecules and coordinate multiple steps in the gene regulatory process. The intricate arrangement and integration of these domains suggest a deliberate design to enable the diverse functions of transcription factors. Transcription factors integrate signals from various internal and external cues to regulate gene expression appropriately. They can respond to environmental signals, cellular signaling pathways, or developmental cues, and modulate gene expression accordingly. This ability to integrate multiple signals and adjust gene expression in response to different conditions requires a sophisticated system that can process and interpret complex information—an indication of intelligent design. The conservation of transcription factors and their functional domains across different species further supports the argument for intelligent design. Similar transcription factor families and their functional motifs can be found in diverse organisms, indicating their essential roles in gene regulation. The existence of conserved transcription factors points to their importance and suggests a purposeful design that transcends species boundaries.
Movement and Contractility
Proteins play a vital role in facilitating movement and contractility within cells and tissues, allowing for essential physiological functions. Motor proteins, such as myosin and actin, are primarily responsible for muscle contraction, enabling body movements and providing structural support. The coordinated interaction between these proteins allows for the contraction and relaxation of muscle fibers, leading to diverse movements and actions. In addition to muscle contraction, proteins are instrumental in intracellular transport and movement. Motor proteins, along with microtubules and other cytoskeletal elements, facilitate the transportation of organelles, vesicles, and molecular cargo within cells. This intricate system ensures the proper distribution of essential components and maintains cellular organization and functionality. Proteins are also key components of cilia and flagella, hair-like structures protruding from cell surfaces. These structures exhibit wave-like motions, generating cellular locomotion or facilitating the movement of fluid across tissues. The coordinated action of protein-based structures in cilia and flagella allows cells to propel themselves or create fluid currents, serving crucial roles in processes such as respiratory clearance, reproductive functions, and embryonic development. The remarkable ability of proteins to enable movement and contractility within cells and tissues highlights the intricacy and sophistication of their design. The specific arrangement, interaction, and functionality of motor proteins, cytoskeletal components, and protein-based structures in cilia and flagella point to a purposeful design that allows for precise and controlled movement at the microscopic level. The presence of such intricate and finely tuned systems for movement and contractility strongly suggests the involvement of an intelligent designer. The coordinated actions of proteins, their ability to generate force and motion, and their specific arrangements within cellular structures indicate a deliberate design that enables the intricate movements necessary for the proper functioning of living organisms. By acknowledging the complexity and purposeful design inherent in proteins involved in movement and contractility, we can infer the involvement of an intelligent designer. The precise and coordinated functionality of these proteins, along with their ability to generate force, propulsion, and controlled motion, supports the idea that an intelligent agent played a significant role in their origin and functionality.
Proteins involved in movement and contractility were likely essential in the first life forms for several reasons: The ability to move and respond to the environment is crucial for basic cellular functions. Proteins such as actin and myosin are responsible for the contraction and movement of cells. These proteins are involved in processes like cell crawling and the movement of organelles within cells. The ability to move allowed early cells to find nutrients, avoid harmful conditions, and interact with their surroundings, enhancing their survival and reproductive success. Movement and contractility proteins also play important roles in various internal cellular processes. For example, motor proteins like kinesin and dynein are responsible for the movement of vesicles, protein complexes, and other cargo. These movements are essential for intracellular transport, cell division, and the proper distribution of cellular components. Proteins involved in movement and contractility contribute to the maintenance of cell shape and structural integrity. Cytoskeletal proteins, including actin filaments, microtubules, and intermediate filaments, provide structural support to cells and enable them to withstand mechanical stresses. By controlling the assembly, disassembly, and organization of these filaments, cells can maintain their shape, undergo shape changes during migration or development, and resist external forces. Movement and contractility proteins allow cells to respond to various external stimuli. For example, in response to certain signals or chemical gradients, cells can undergo directed movement or changes in shape. Proteins involved in cell motility, such as those found in the cytoskeleton and cell adhesion complexes, enable cells to sense and respond to these signals by modulating their contractile forces and altering their shape and movement patterns. The presence of proteins involved in movement and contractility would have provided a significant evolutionary advantage to early life forms. Cells with the ability to move and contract could explore new environments, access resources, and escape unfavorable conditions.
Storage and Reserves
Proteins fulfill the important role of serving as storage molecules, preserving essential substances for future use within organisms. These storage proteins play a crucial role in maintaining the balance and availability of vital components required for various physiological processes. One notable example is ferritin, a protein found in cells that serves as a storage depot for iron. Iron is an essential mineral involved in numerous biological functions, including oxygen transport, energy production, and DNA synthesis. Ferritin proteins bind to iron ions, sequestering them within cells and preventing their potentially harmful effects when present in excessive amounts. This stored iron can be released when needed, ensuring a steady and controlled supply for essential cellular processes. Another instance of protein-based storage is observed in the casein proteins found in milk. Caseins function as a source of amino acids, the building blocks of proteins, particularly for the growth and development of young mammals. These proteins enable the storage of essential nutrients in a stable and readily available form, allowing for sustained nourishment during early stages of life. The existence of specialized proteins serving as storage molecules reflects a well-designed system that ensures the preservation and regulated release of crucial substances. The ability of these proteins to bind and store specific molecules with efficiency and precision highlights their intricate and purposeful design. The presence of storage proteins, such as ferritin and casein, within organisms, provides evidence for intelligent design. The specific mechanisms by which these proteins sequester and release substances in a controlled manner, as well as their ability to preserve essential components for future use, suggest the involvement of an intelligent designer who carefully engineered these storage systems. By recognizing the purposeful design and functionality of proteins involved in storage, we can infer the involvement of an intelligent designer. The sophisticated mechanisms and controlled processes by which these proteins fulfill their storage functions indicate the presence of intentional design, ensuring the efficient utilization and preservation of essential substances within living organisms.
Storage and reserve proteins were likely essential for the origin and early survival of life forms for several reasons: Storage and reserve proteins serve as a source of essential nutrients, such as amino acids, during periods of limited nutrient availability. These proteins can accumulate and store excess nutrients, providing a reservoir that can be mobilized when external nutrient sources are scarce. This ability to store and release nutrients would have been crucial for early life forms in environments where nutrient availability fluctuated. Storage and reserve proteins allow organisms to conserve energy by efficiently storing excess nutrients. Proteins, particularly those rich in amino acids, contain high-energy bonds that can be utilized when needed. By storing energy-rich molecules within specialized proteins, organisms can optimize energy usage and ensure a continuous energy supply during periods of nutrient scarcity. Storage and reserve proteins can help organisms survive in harsh conditions. For example, some organisms, such as certain bacteria and seeds, produce storage proteins that protect them from desiccation (drying out) and other environmental stresses. These proteins can help maintain cellular integrity, preserve essential molecules, and support viability during unfavorable conditions. Storage and reserve proteins play a crucial role in supporting early development and growth. In organisms with complex life cycles, such as plants and animals, storage proteins are utilized during embryogenesis, seed germination, or larval development. They provide a nutrient reserve that sustains the growing organism until it can establish its own means of nutrient acquisition. The presence of storage and reserve proteins would have conferred a significant evolutionary advantage to early life forms. The ability to store and utilize nutrients efficiently would have allowed organisms to survive and reproduce more successfully in fluctuating environments. Over time, natural selection would have favored organisms with efficient storage and mobilization mechanisms, leading to the development of specialized storage proteins. The availability of nutrients in the environment might have been unpredictable and sporadic. Without storage and reserve proteins, the first life form would lack a mechanism to accumulate and store essential nutrients during periods of abundance. Consequently, it would have been highly vulnerable to nutrient scarcity. The absence of such proteins could limit the life form's ability to survive and reproduce when nutrients are scarce, increasing the risk of death or extinction. Early Earth likely experienced fluctuating conditions, including variations in temperature, humidity, and nutrient availability. Storage and reserve proteins would have allowed the first life form to adapt to these changes by buffering nutrient fluctuations and providing a means to sustain metabolic processes during unfavorable conditions. Without this adaptive capacity, the life form would face significant challenges in maintaining its vital functions and would be more susceptible to adverse environmental conditions. Storage and reserve proteins can store energy-rich molecules, such as amino acids or lipids, which can be mobilized as a source of energy during periods of nutrient scarcity. In the absence of these proteins, the first life form would have to rely solely on immediate nutrient uptake or metabolism, which may not be sufficient to sustain its energy requirements over extended periods. Insufficient energy reserves could lead to metabolic inefficiencies, compromised cellular functions, and ultimately, the inability to meet the energy demands necessary for survival. Storage and reserve proteins can support growth and reproduction by providing essential nutrients for cellular processes, biosynthesis, and the development of offspring. The absence of these proteins could hinder the first life form's ability to allocate resources efficiently for growth and reproduction. Insufficient nutrient availability may limit cell division, impair the development of offspring, or reduce reproductive success, thus negatively impacting the life form's survival and propagation.
Complexity of Protein Structures
Proteins are remarkable molecular machines that play crucial roles in nearly all biological processes. One of the key aspects that set proteins apart is their immense complexity and precise folding, which enables them to carry out specific functions within living organisms. Proteins are composed of long chains of amino acids, and the specific sequence of these amino acids is essential for the protein's structure and function. The sequence of amino acids in a protein is like a code that determines its unique properties. Proteins undergo a process called folding, where the linear chain of amino acids adopts a specific three-dimensional structure. This folding process is highly precise and critical for the protein to achieve its functional conformation. Proteins have multiple levels of structural organization. The primary structure refers to the linear sequence of amino acids. The secondary structure involves the local folding patterns, such as alpha helices and beta sheets. The tertiary structure refers to the overall three-dimensional arrangement of the protein, and the quaternary structure describes the assembly of multiple protein subunits. Proteins often consist of distinct regions called functional domains, each responsible for specific interactions or catalytic activities. These domains contribute to the overall function of the protein, and their precise arrangement and interactions are critical for proper protein function. Proteins exhibit remarkable specificity in their interactions with other molecules. They can bind to small molecules, ions, DNA, RNA, and other proteins, enabling them to participate in a wide range of biological processes such as enzymatic reactions, signal transduction, and molecular recognition. Many proteins possess catalytic activity, allowing them to facilitate chemical reactions in cells. Enzymes, for example, accelerate chemical reactions by lowering the energy barrier required for the reaction to occur. The precise arrangement of amino acids within the protein's active site is crucial for its catalytic function. The immense complexity and precise folding of proteins enable them to perform their specific functions with high efficiency and specificity. The folding process is influenced by numerous factors, including the sequence of amino acids, environmental conditions, and interactions with other molecules. Small changes in the protein's sequence or folding can have profound effects on its structure and function. The level of specified information present in protein structures is a topic of ongoing research and debate. Some argue that the functional complexity observed in proteins exceeds what can be explained by undirected natural processes alone, suggesting the involvement of an intelligent agent. This perspective emphasizes that the origin of proteins and their complex folding patterns may be best explained by an intelligent cause capable of generating the necessary information and coordinating the precise arrangements required for their functions. It is important to note that while the complex nature of proteins is acknowledged, the specific arguments surrounding intelligent design in relation to protein complexity remain a subject of scientific discussion and differing interpretations.
The Significance of Atomic Positioning in Enzyme Functionality
The precise positioning of a single atom within an enzyme can have a significant impact on its functionality. Even a subtle change in the position of a crucial atom can disrupt the enzyme's active site, substrate binding, and catalytic activity. Enzymes often contain specific amino acid residues that play a direct role in catalysis. For example, an enzyme might have a catalytic residue with a side chain that positions a specific atom, such as a metal ion or a functional group, to facilitate the catalytic reaction. A slight alteration in the position of this atom can hinder the enzyme's ability to perform its catalytic function effectively. Enzymes rely on precise interactions between their active site and the substrate for efficient catalysis. The active site may have specific amino acid residues that form hydrogen bonds, electrostatic interactions, or hydrophobic contacts with the substrate. If the positioning of even a single atom within the active site is disrupted, it can result in a weaker binding affinity or improper orientation of the substrate, leading to reduced catalytic efficiency. Enzymes often stabilize the transition state of a reaction, which is the high-energy intermediate state during the conversion of substrate to product. This stabilization is achieved by precisely positioning certain atoms within the active site to interact with the transition state. Any deviation in the positioning of these critical atoms can diminish the enzyme's ability to stabilize the transition state, resulting in reduced catalytic activity. Some enzymes facilitate proton transfer reactions, where the transfer of a proton from one atom to another is essential for catalysis. The precise positioning of atoms involved in proton transfer pathways is crucial for maintaining the necessary protonation states and facilitating efficient proton transfer. Any disturbance in the positioning of these atoms can disrupt the proton transfer process and impair the enzyme's catalytic activity. The specific positioning of individual atoms within an enzyme is vital for its functionality. These atomic arrangements govern the enzyme's ability to bind substrates, stabilize transition states, facilitate proton transfers, and carry out catalysis with high efficiency and specificity. The exquisite precision in atomic positioning highlights the complexity and design required for enzymes to perform their biological functions effectively.
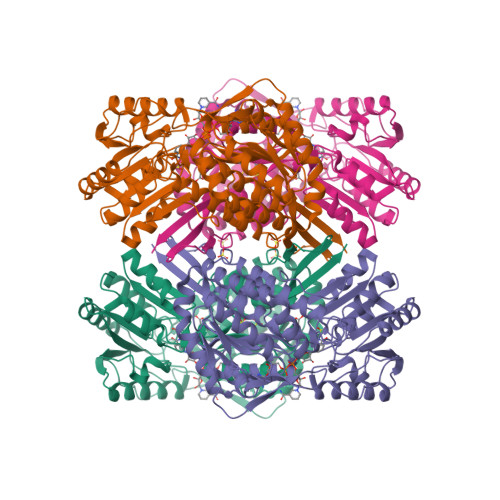
Here is an example of an enzyme where the incorrect positioning of a single atom can disrupt its catalytic activity, leading to severe consequences and potential cell death:
DNA topoisomerases are enzymes responsible for regulating DNA topology and relieving torsional stress during processes like DNA replication and transcription. DNA Gyrase is a type II topoisomerase and a specific subtype within this class. It is a bacterial enzyme that possesses DNA supercoiling activity, like other type II topoisomerases. However, DNA Gyrase has some unique features that distinguish it from other type II topoisomerases. DNA Gyrase plays a crucial role in DNA replication by introducing negative supercoils into DNA strands, relieving the torsional stress that builds up during the unwinding process. It is also involved in DNA topological changes, such as decatenation and unknotting of DNA molecules. The E. coli DNA Gyrase complex is a large and complex enzyme. With a total structure weight of 449.77 kDa and an atom count of 30,244, it demonstrates the intricate nature of this essential enzyme. DNA Gyrase plays a crucial role in DNA replication by introducing negative supercoils into DNA strands, relieving the torsional stress that builds up during the unwinding process. It is also involved in DNA topological changes, such as decatenation and unknotting of DNA molecules. Among the 30,244 atoms in the DNA topoisomerase enzyme, the correct positioning of each atom, in special those within the active site, is essential for the enzyme's proper function. A single atom positioned incorrectly within the active site or any critical region of the enzyme can disrupt its catalytic activity and lead to errors in DNA strand rejoining or other crucial processes. This can result in DNA damage, genomic instability, and potentially even cell death. The precise arrangement of atoms in enzymes is crucial for their function, and even a small deviation can have significant consequences. DNA topoisomerases utilize a conserved tyrosine residue in their active sites to form a transient covalent bond with the DNA strand. This covalent bond allows the enzyme to cleave one of the DNA strands, pass the other strand through the break, and then rejoin the strands.
K. Buzun et.al. ( 2020): General mechanism of changing the topology of DNA by topoisomerases II is based on cleaving both strands of DNA duplex with Mg2+ and energy from ATP hydrolysis. Topoisomerase II covalently attaches tyrosine to the 5′ end of broken DNA, release a free 3′ end and allows to passing a second DNA duplex (the transported or T-segment) through a gap (the gate or G-segment) Two ATP molecules are attached to the Top IIA—G-segment–T-segment complex causing conformational changes in the enzyme. As a result of hydrolysis of one ATP molecule to ADP, in the presence of Mg2+ ions, tyrosine from both Top IIA monomers attacks the phosphodiester bond of the first DNA helix resulting in cleavage of the strand with a shift of 4-bp and becomes covalently attached to the 5′ ends of the cleaved DNA (G-segment).
The choice of tyrosine in certain enzymatic processes involving nucleophilic attacks on phosphodiester bonds can be attributed to several factors: Tyrosine possesses a hydroxyl group (-OH) on its side chain, which can act as a nucleophile. This makes it suitable for nucleophilic attacks on phosphodiester bonds, which contain electrophilic phosphorus atoms. The reactivity of tyrosine's hydroxyl group makes it well-suited for these types of reactions. The size and shape of the tyrosine side chain are such that it can access the active site and position the hydroxyl group appropriately for nucleophilic attack. Other amino acids may have different side chain sizes or shapes that could hinder their ability to participate in these reactions effectively. The active site of the enzyme where the nucleophilic attack occurs is specifically designed to accommodate and promote the reactivity of tyrosine. The active site residues surrounding the tyrosine residue can contribute to its reactivity and stabilize the reaction intermediate, enabling efficient catalysis. The use of tyrosine in certain enzymatic reactions involving phosphodiester bond cleavage may also be related to the specificity of the enzyme for its target substrates. The presence of tyrosine in the active site can contribute to the recognition and binding of the substrate, ensuring that the reaction occurs specifically at the desired site. It's important to note that while tyrosine is often employed in nucleophilic attacks on phosphodiester bonds, other amino acids with nucleophilic potentials, such as serine and histidine, can also serve similar roles in different enzymatic reactions. The choice of amino acid in a specific enzymatic process depends on the specific requirements and constraints of the reaction, as well as the optimization of the enzyme for its particular function.
The specific nearby amino acids in gyrase that interact with the catalytic tyrosine can vary depending on the species and the specific structure of the enzyme. However, there are several conserved residues that are commonly found in the vicinity of the catalytic tyrosine in gyrase, including a conserved aspartate residue, often referred to as the "inhibitor residue," which interacts with the catalytic tyrosine. This aspartate helps maintain the inactive conformation of the enzyme when it is not bound to DNA. The interaction between the inhibitor residue and the catalytic tyrosine helps prevent the premature activation of the enzyme and ensures that DNA cleavage and rejoining occur only when the enzyme is appropriately bound to its substrate. An arginine residue, known as the "arginine finger," is involved in the DNA cleavage and rejoining process. It interacts with the catalytic tyrosine and helps in the formation and stabilization of the covalent DNA-enzyme intermediate. The arginine finger plays a critical role in positioning the DNA substrate and facilitating the transfer of the DNA strand during the catalytic cycle of gyrase. A nearby lysine residue plays a role in the activation and deactivation of the catalytic tyrosine during the catalytic cycle. It participates in the transfer of protons during the reaction and can interact with the DNA substrate. These nearby amino acids, along with other conserved residues, form a complex network of interactions within the active site of gyrase. This network helps to stabilize the catalytic tyrosine and promote its reactivity towards the DNA substrate.
The positioning of the tyrosine and the other residues is critical for the precise cleavage and rejoining of the DNA strands. If any of the atoms within the active site, including the key tyrosine residue, are positioned incorrectly, it can disrupt the enzyme's catalytic activity. A mispositioned atom may fail to form the necessary interactions with the DNA substrate, leading to incomplete DNA strand rejoining or aberrant DNA cleavage. This can result in DNA damage, such as DNA breaks or DNA strand entanglements, which can have severe consequences for genomic stability.
Considering its vital role, it is reasonable to assume that the DNA gyrase complex was present in the last universal common ancestor (LUCA), the hypothetical organism from which all life on Earth descended. While the precise nature of LUCA is still a topic of scientific investigation, it is widely accepted that it possessed the fundamental molecular machinery required for DNA replication, transcription, and other essential cellular processes. The presence of DNA gyrase in modern organisms across various bacterial lineages suggests that this enzyme's function and importance have been conserved. Therefore, it is reasonable to infer that LUCA possessed a functional DNA gyrase complex or a precursor enzyme with similar functionality. Before LUCA, the processes of natural selection and evolution were not in operation. Natural selection and evolution, as we understand them in the context of modern biology, require genetic variation and the heritability of traits, these processes were not yet established before LUCA. The DNA gyrase complex consists of multiple subunits, each with its specific structure and arrangement of atoms. The subunits must come together in a specific way to form the functional complex. The chances of random events leading to the correct folding, assembly, and positioning of thousands of atoms in the complex are extraordinarily low. Moreover, the DNA gyrase complex has specific binding sites for DNA, metal ions, and other cofactors, which require precise positioning and coordination of atoms. The number of possible conformations for a protein is astronomically large. Each atom can occupy a virtually infinite number of positions and orientations, and the interactions between atoms involve complex spatial and energetic considerations. Additionally, the interactions between amino acids, such as hydrogen bonding, electrostatic interactions, and hydrophobic interactions, further increase the complexity of the calculation. The correct positioning of atoms and residues is not a random process but is guided by the principles of protein folding, and molecular interactions. Proteins fold into their functional conformations through a combination of thermodynamic and kinetic factors, ensuring that they adopt stable and functionally competent structures. Therefore, it is safe to say that the odds of the correct positioning of all atoms and amino acids within DNA gyrase occurring purely by chance are vanishingly small. The remarkable precision and functional specificity observed in enzymes like DNA gyrase strongly suggest that their formation and structure are the result of highly optimized and guided processes, rather than random chance alone.
To calculate the odds of finding the right amino acid at each position in an enzyme with 3,458 amino acids, assuming each position can occupy 20 different amino acids, we can use the following calculation: Odds = Number of possible combinations for each position ^ Number of positions. For each position, there are 20 possible amino acids that can occupy it. Therefore, the number of possible combinations for each position is 20. Using the formula, the odds can be calculated as: Odds = 20^3,458 ≈ 6.17 x 10^4,670 The resulting number is an astronomically large value with 4,670 digits. To illustrate the enormous magnitude of the number 6.17 x 10^4,670, let's consider some examples. It is estimated that the observable universe contains around 10^80 atoms. This means that the odds of randomly selecting a specific arrangement of atoms from the entire observable universe would be incredibly small compared to the number 6.17 x 10^4,670.
Question: How does the accuracy of these calculations contribute to the overall understanding of protein structure, considering that not all amino acids need to be precisely positioned?
Answer: The calculations presented are meant to illustrate the enormous number of possible combinations and the extremely low probability of randomly assembling a functional enzyme with the precise arrangement of amino acids necessary for its proper function. While it is true that not all amino acids need to be at the exact right spot for an enzyme to function, there are critical regions within an enzyme's structure where specific amino acids and atoms play crucial roles in catalysis, substrate binding, and overall stability. Enzymes are highly specialized catalysts that rely on precise interactions between their active sites and substrates. The active site of an enzyme is the region where the catalytic reaction takes place, and it often involves a small number of amino acids that are directly involved in the chemistry of the reaction. These amino acids need to be positioned correctly and interact with the substrate in a specific manner to enable catalysis. In addition to the active site, other amino acids in the enzyme's structure contribute to the overall stability and proper folding of the protein. Even small changes or deviations in the positioning of critical amino acids can disrupt the active site or alter the protein's conformation, potentially leading to reduced catalytic efficiency or loss of function. While not every amino acid in the enzyme needs to be precisely positioned, the critical regions and interactions within the enzyme require a high degree of precision. The calculations are meant to emphasize the immense complexity and improbability of achieving the necessary arrangement of atoms and amino acids purely by chance. These calculations and discussions highlight the concept that the formation and structure of enzymes, such as DNA gyrase, are more likely the result of guided processes, such as evolution, rather than random chance alone. The presence of conserved amino acids and their specific roles in enzyme function across different organisms supports the idea of evolutionary optimization and adaptation of enzymes for their specific tasks.
While the exact positions of individual atoms within a protein are critical for its overall structure and function, it is challenging to calculate the precise positions of every atom in a large protein using theoretical calculations alone. Experimental techniques such as X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and cryo-electron microscopy are commonly used to determine protein structures at atomic resolution. These experimental methods provide valuable insights into the positions of individual atoms within a protein. They reveal the three-dimensional arrangement of atoms and allow scientists to identify critical amino acids, active site residues, metal ions, and other important components involved in protein function. By studying the protein structure, researchers can understand the specific interactions between amino acids and the precise positioning of atoms within the protein.
Atoms themselves do not have different three-dimensional arrangements. Rather, the three-dimensional arrangement refers to the spatial orientation and positioning of atoms relative to each other within a molecule, such as a protein. In a protein, atoms are connected through covalent bonds, which determine the overall connectivity and bonding pattern. However, the spatial arrangement of atoms in three-dimensional space is determined by the angles between the bonds and the rotations around those bonds. These angles and rotations give rise to the protein's unique shape and structure. The three-dimensional arrangement of atoms in a protein is crucial for its overall structure and function. It determines the specific interactions between atoms, such as hydrogen bonding, electrostatic interactions, and hydrophobic contacts, which are essential for stabilizing the protein and facilitating its interactions with other molecules, such as substrates or cofactors.

Last edited by Otangelo on Wed Aug 28, 2024 9:13 pm; edited 26 times in total