https://reasonandscience.catsboard.com/t1438-hexameric-helicases-some-of-the-most-complex-machines-on-earth
The enzymes which couple chemical energy to unwind the DNA duplex are commonly referred to as helicases. Related motors also work as chromatin remodelers, which restructure chromosome organization and thereby enabling or restricting access to DNA.
The proteins that drive DNA replication—the force behind cellular growth and reproduction—are some of the most complex machines on Earth.
DNA helicases are essential during DNA replication because they separate double-stranded DNA into single strands allowing each strand to be copied.
The helicase rotational speed of up to 10,000 rotations per minute !!!!! How astonishing and marvelous.
Helicase must have emerged before life began, since its essential for DNA replication
"The genesis of the DNA-unwinding machinery is wonderfully complex and surprising," said study coauthor Huilin Li, a biologist at Brookhaven Lab and Stony Brook University. "Seeing this helicase enzyme prepare to surround and unwind the DNA at the molecular level helps us understand the most fundamental process of life and how that process might go wrong. Errors in copying DNA are found in certain cancers, and this work could one day help develop new treatment methods that stall or break dangerous runaway machinery."
Before a cell divides
What is the driving force to make the cell divide ? Why at all did dividing start ?
it has to replicate its DNA so that the daughter cell receives a copy of the genome. The DNA helix consists of two complementary DNA strands.
Why at all did a complementary strand arise ?
Therefore, each of the two strands serves as a template for the construction of the other strand. Under normal conditions the DNA is packed into a compact structure called chromatin. To be able to replicate, the cell has to unfold and unwind the DNA,
What is the reason that it started to unwind ?
and also has to separate the two strands from each other. The cell has a complex machinery to perform these tasks.
How did this machinery arise ? What was the force behind it ? What is the origin of the information to make this machinery ?
When it is time to replicate, special initiator proteins attach to the DNA at regions called replication origins.
For what reasos did these special initiator proteins arise ? Where came the information come from to encode these proteins ?
These regions are characterised by a weak bond between the two DNA strands.
How did this bond arise ?
There are around 10,000 replication origins on the DNA in a cell; this arrangement increases the rate of replication tremendously. The initiator proteins pry the two strands apart
How did these proteins become able to pry the two strands apart ?
and a small gap is created at the replication origin. Once the strands are separated another group of proteins, that carry out the DNA replication, attaches and go to work.
How did these other group of proteins start to be able to attach at the right position, and go to work ?
This group of proteins includes helicase, which serves as an unzipper by breaking the bonds between the two DNA strands.
The hexameric helicases are ubiquitous proteins (related enzymes are found on both sides of the bacterial-archaean divide) involved in unwinding double-stranded DNA and RNA.
this is a highly organized, sophisticated and orchestrated movement, like a robot, executing a specific task:
In order to be able to unwind the dna strand, the initial opening of the double helix (at the origin of replication) is performed by an initiator protein.
How did chance, physical necessity, or natural selection " know " an initiator protein would be needed, and how it would have to be, and where employed ?
The helicase rotational speed of up to 10,000 rotations per minute !!!!! How astonishing and marvelous .
This unzipping takes place in both directions from the replication origins, creating a replication bubble. The replication is therefore said to be bi-directional.
How did they start and " know " the need to be bidirectional ?
Once the two strands are separated a small piece of RNA, called an RNA primer , is attached to the DNA by an enzyme called DNA primase. 4
Primases synthesize short RNA strands on single-stranded DNA templates, thereby generating the hybrid duplexes required for the initiation of synthesis by DNA polymerases. 5
These primers are the beginnings of all new DNA chains since the enzyme responsible for the copying of the DNA, DNA polymerase, can not start from scratch. It is a self-correcting enzyme and copies the DNA template with remarkable fidelity.
How did it " learn " to self-correct itself?
The DNA polymerase can only read in the 3' to 5' direction. This gives rise to some trouble since the two strands of the DNA are antiparallel. On the upper strand which runs from 3' to 5', nucleotide polymerisation can take place continuously without any problems. This strand is called the leading strand. But how does the polymerase copy the other strand then when it runs in the opposite direction, from 5' to 3'? On this so called lagging strand the polymerase produces short DNA fragments, called okazaki fragments, by using a backstitching technique.
How and why did it begin the backstitiching technique ?
These lagging strand fragments are primed by short RNA primers and are subsequently erased and replaced by DNA.
In order for DNA replication machinery to gain access to the genetic code, the two strands of the double helix must first be “unwound.” In some cases, because DNA is tightly packaged in chromatin, these protein–DNA complexes need to be restructured to expose the DNA region of interest. The enzymes which couple chemical energy to unwind the DNA duplex are commonly referred to as helicases. Related motors also work as chromatin remodelers, which restructure chromosome organization and thereby enabling or restricting access to DNA.
The proteins that drive DNA replication—the force behind cellular growth and reproduction—are some of the most complex machines on Earth. 5 The multistep replication process involves hundreds of atomic-scale moving parts that rapidly interact and transform. Mapping that dense molecular machinery is one of the most promising and challenging frontiers in medicine and biology. "The genesis of the DNA-unwinding machinery is wonderfully complex and surprising," said study coauthor Huilin Li, a biologist at Brookhaven Lab and Stony Brook University. "Seeing this helicase enzyme prepare to surround and unwind the DNA at the molecular level helps us understand the most fundamental process of life and how that process might go wrong. But DNA replication is a bi-directional process with two helicases moving in opposite directions. The key question, then, was how does a second helicase core get recruited and loaded onto the DNA in the opposite orientation of the first?
The first DNA helicase, Escherichia coli, was purified and characterized in 1976. As more helicases were identified and reported in the literature, helicase “signature motifs” were identified. These highly conserved amino acid domains are involved in the binding and hydrolysis of nucleoside triphosphate (NTP), the energy source required to separate the stable double-stranded DNA (dsDNA). It is estimated that approximately 1% of the prokaryotic and eukaryotic genomes encode for proteins containing helicase signature motifs. In order for a helicase to unwind DNA processively, it must also be able to move along the DNA filament (i.e., to translocate) and couple this directional motion along the DNA lattice to strand separation activity.
Processive DNA unwinding requires a helicase to undergo a series of repeated “steps” along the DNA lattice until the duplex is fully unwound. Each step involves a number of processes such as
NTP binding,
hydrolysis,
phosphate release,
base pair melting or capturing of the spontaneously melted bases, and
translocation
increasing evidence demonstrates that some helicases also possess rewinding activity—in other words, they can anneal two complementary single-stranded nucleic acids. All five members of the human RecQ helicase family, helicase PIF1, mitochondrial helicase TWINKLE, and helicase/nuclease Dna2 have been shown to possess strand-annealing activity. 4 The number of helicases expressed in higher organisms is strikingly high, with approximately 1% of the genes in many eukaryotic genomes apparently encoding RNA or DNA helicases. Helicases are involved in virtually all aspects of nucleic acid metabolism, including replication, repair, recombination, transcription, chromosome segregation, and telomere maintenance. The mechanism of this novel strand annealing activity and its biological consequences remain largely unknown
Helicase unwinds the DNA1
Helicases are enzymes that bind and may even remodel nucleic acid or nucleic acid protein complexes. There are DNA and RNA helicases. DNA helicases are essential during DNA replication because they separate double-stranded DNA into single strands allowing each strand to be copied. During DNA replication, DNA helicases unwind DNA at positions called origins where synthesis will be initiated. DNA helicase continues to unwind the DNA forming a structure called the replication fork, which is named for the forked appearance of the two strands of DNA as they are unzipped apart. The process of breaking the hydrogen bonds between the nucleotide base pairs in double-stranded DNA requires energy. To break the bonds, helicases use the energy stored in a molecule called ATP, which serves as the energy currency of cells. DNA helicases also function in other cellular processes where double-stranded DNA must be separated, including DNA repair and transcription. RNA helicases are involved in shaping the form of RNA molecules, during all processes involving RNA, such as transcription, splicing, and translation.
The hexameric helicases are ubiquitous proteins (related enzymes are found on both sides of the bacterial-archaean divide) involved in unwinding double-stranded DNA and RNA.
this is a highly organized, sophisticated and orchestrated movement, like a robot, executing a specific task:
In order to be able to unwind the dna strand, the initial opening of the double helix (at the origin of replication) is performed by an initiator protein.
How did chance, physical necessity, or natural selection " know " an initiator protein would be needed, and how it would have to be, and where employed ?
The helicase rotational speed of up to 10,000 rotations per minute !!!!! How astonishing and marvelous.
Helicase must have emerged before life began, since its essential for DNA replication
"The genesis of the DNA-unwinding machinery is wonderfully complex and surprising," said study coauthor Huilin Li, a biologist at Brookhaven Lab and Stony Brook University. "Seeing this helicase enzyme prepare to surround and unwind the DNA at the molecular level helps us understand the most fundamental process of life and how that process might go wrong. Errors in copying DNA are found in certain cancers, and this work could one day help develop new treatment methods that stall or break dangerous runaway machinery."
How different helicase families with a conserved catalytic ‘helicase core’ evolved to function on varied RNA and DNA substrates by diverse mechanisms remains unclear. 2 Before DNA and RNA can perform their essential tasks in cells, enzymes called helicases must separate the interacting strands. A large group of helicases, known as superfamily 1 and 2, are involved in virtually all aspects of the control of RNA and DNA structure. Helicases use the energy released from breaking down molecules called nucleotides to pull apart the bonds that hold DNA and RNA strands together.
Rotary Firing in Ring-Shaped Protein Explains Unidirectionality 3
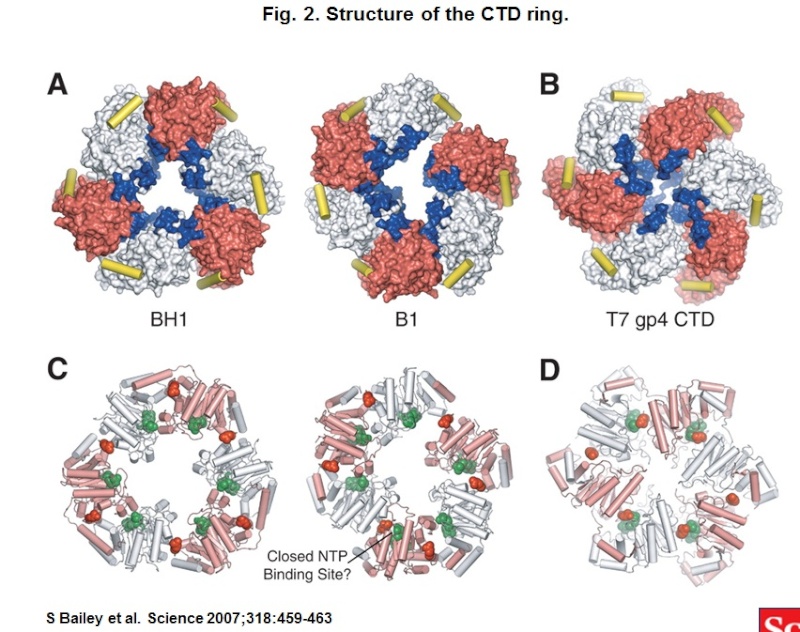
Hexameric motor proteins represent a complex class of molecular machines that variously push and pull on biological molecules using adenosine triphosphate (ATP) as chemical fuel. A specialized class of ring-shaped motor proteins, hexameric helicases, can unwind DNA strands and perform large-scale manipulations of single-stranded nucleic acids in processes such as DNA replication, DNA repair, and gene expression. To understand how certain hexameric helicases walk with directional polarity along single-stranded nucleic acids, Berkeley researchers used x-ray crystallography at the ALS to solve the structure of a hexameric helicase, the Rho transcription termination factor (from E. coli), bound to both ATP mimics and an RNA substrate. The results showed that Rho functions like a rotary engine: as the motor spins, it pulls RNA strands through it's interior. Interestingly, the rotary firing order of the motor is biased so that the Rho protein can walk in only one direction along the RNA chain.
Rings Running in Reverse
The Rho factor is a ring-shaped motor protein made up of six subunits (or, in analogy to combustion engines, six "cylinders"). Such motor proteins (also known as hexameric helicases) are found in all organisms and are involved in unwinding and moving DNA and RNA strands (nucleic acids) around the cell. There are two subfamilies of hexameric helicases: AAA+ and RecA. Rho belongs to the RecA family, which is most common in bacteria. AAA+ motors are predominantly found in eukaryotes, including humans, as well as some human pathogens, such as the papillomavirus. Although these motors evolved from a common ancestor, they have distinct properties, most notably the predisposition to walk along nucleic acid tracks in opposite directions.
To understand how such a biological mechanism works and perhaps eventually develop a therapeutic drug that will gum up the works and stop the motor from doing its job, it helps to know how the protein is constructed. Thomsen et al. are the first group to determine the crystal structure of a RecA-class hexameric helicase in a translocation state bound to both its nucleic-acid track and a molecule that mimics the role of its chemical energy source, ATP. In doing so, they fortuitously caught an atomic-level snapshot of the motor in the act of tracking along an RNA chain. Their analysis showed that the proteins from different subfamilies move in opposite directions by reversing the rotational firing order of the ATP sites—essentially by reversing gears as opposed to turning around.
Inspection of the model reveals that Rho binds to RNA in a helical conformation, using a "spiral staircase" arrangement of RNA binding loops that project from five of the protein subunits to interact with the RNA's sugar–phosphate backbone. The sixth subunit does not significantly contact the RNA and lies midway between the top and bottom steps of the staircase. The positional relationships between the six Rho subunits generate four distinct classes of ATP binding sites that together represent a complete ATP turnover cycle. The organization of these sites around the ring indicates that ATP is consumed by a sequential mechanism akin to a rotary or radial engine. The cycle of ATP binding, hydrolysis, and release is carried out as each subunit passes through the six conformational states observed in the structure, creating a rotary wave motion within the RNA binding loops to power nucleic acid translocation in the proper direction.
1. http://phys.org/news/2014-10-genesis-enzyme-dna-helix-cell.html
2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4383044/
3. http://www.als.lbl.gov/als/science/sci_archive/206hexhelicase.html
4. https://www.hindawi.com/journals/jna/2012/140601/
5. https://www.bnl.gov/newsroom/news.php?a=111672
More readings:
Cryo-EM structure of a helicase loading intermediate containing ORC–Cdc6–Cdt1–MCM2-7 bound to DNA
http://www.nature.com.secure.sci-hub.cc/nsmb/journal/v20/n8/full/nsmb.2629.html
Last edited by Admin on Wed Jul 18, 2018 1:20 pm; edited 13 times in total