Antifreeze proteins: By evolution, or design?
Cells have developed antifreeze proteins in response to severe cold. Failure to have done this in time would, of course, have stopped them in their tracks permanently. The antifreeze molecules lower the freezing point of water contained within the cell, disrupting the freezing process by limiting the growth of water-hungry ice crystals. Various kinds are shown above (illus: David S. Goodsell)
When water begins to freeze, many small crystals form, but then a few small crystals dominate and grow larger and larger, stealing water molecules from the surrounding small crystals. Antifreeze proteins counteract this recrystallization effect. They bind to the surface of the small ice crystals and slow or prevent the growth into larger dangerous crystals.
Antifreeze proteins lower the freezing point of water by a few degrees, but surprisingly, they don’t change the melting point. This process of depressing the freezing point while not affecting the melting point is termed thermal hysteresis. The most effective antifreeze proteins are made by insects, which lower the freezing point by about 6 degrees. However, antifreeze proteins, even the ones from plants and bacteria that have smaller effects on freezing point, are useful in another way. They are placed outside cells where they control the size of ice crystals and prevent catastrophic ice crystal formation when the temperature drops below the (lowered) freezing point.
https://web.archive.org/web/20130503211721/http://iaincarstairs.wordpress.com/2013/03/25/as-smart-as-molecules/
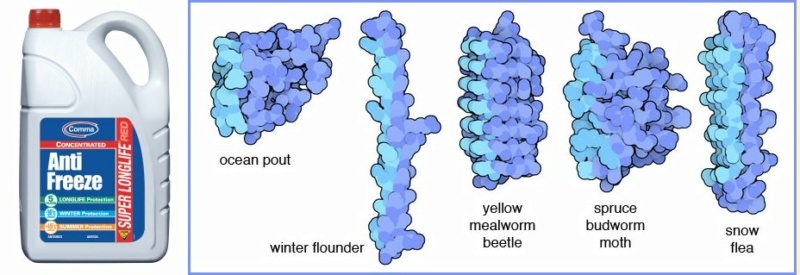
Cells have developed antifreeze proteins in response to severe cold. Failure to have done this in time would, of course, have stopped them in their tracks permanently. The antifreeze molecules lower the freezing point of water contained within the cell, disrupting the freezing process by limiting the growth of water-hungry ice crystals. Various kinds are shown above (illus: David S. Goodsell)
When water begins to freeze, many small crystals form, but then a few small crystals dominate and grow larger and larger, stealing water molecules from the surrounding small crystals. Antifreeze proteins counteract this recrystallization effect. They bind to the surface of the small ice crystals and slow or prevent the growth into larger dangerous crystals.
Antifreeze proteins lower the freezing point of water by a few degrees, but surprisingly, they don’t change the melting point. This process of depressing the freezing point while not affecting the melting point is termed thermal hysteresis. The most effective antifreeze proteins are made by insects, which lower the freezing point by about 6 degrees. However, antifreeze proteins, even the ones from plants and bacteria that have smaller effects on freezing point, are useful in another way. They are placed outside cells where they control the size of ice crystals and prevent catastrophic ice crystal formation when the temperature drops below the (lowered) freezing point.
https://web.archive.org/web/20130503211721/http://iaincarstairs.wordpress.com/2013/03/25/as-smart-as-molecules/


