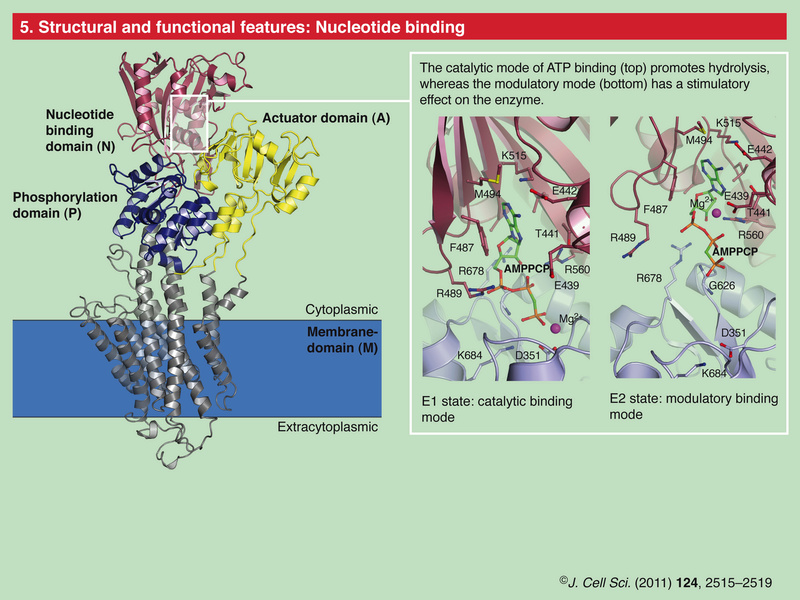
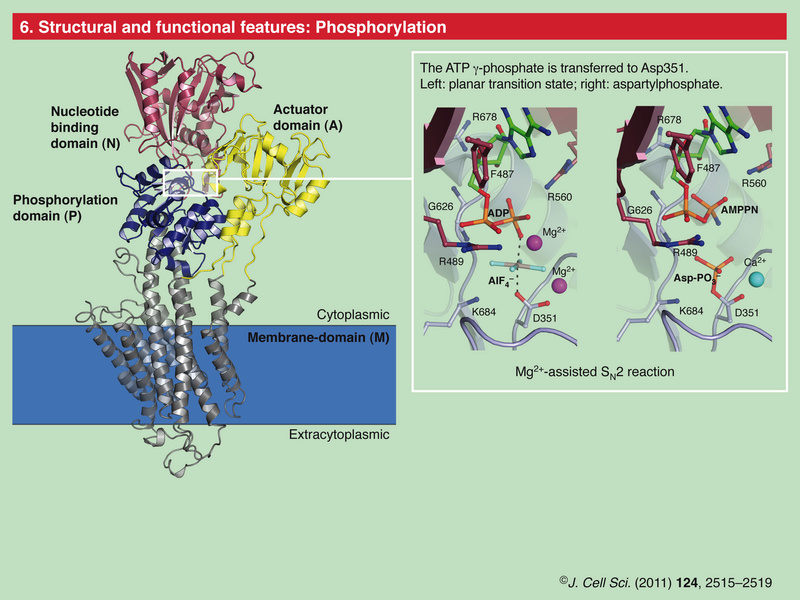
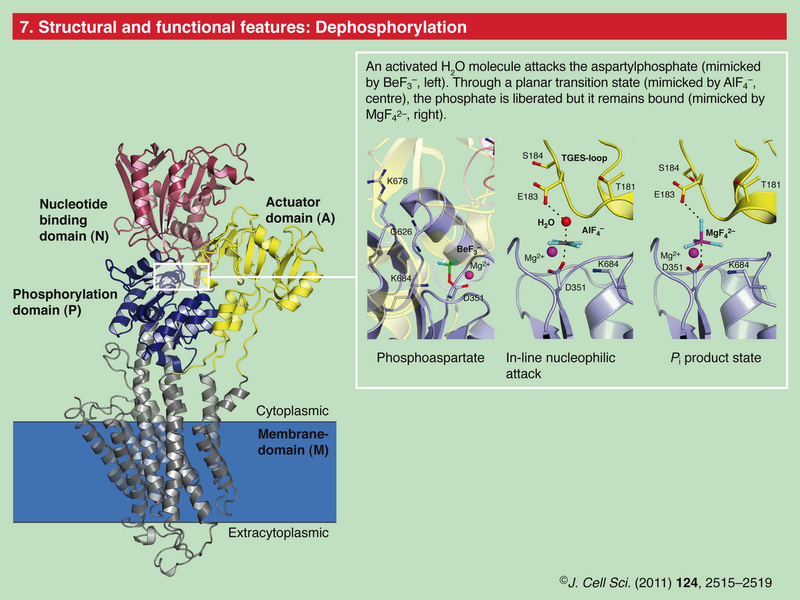
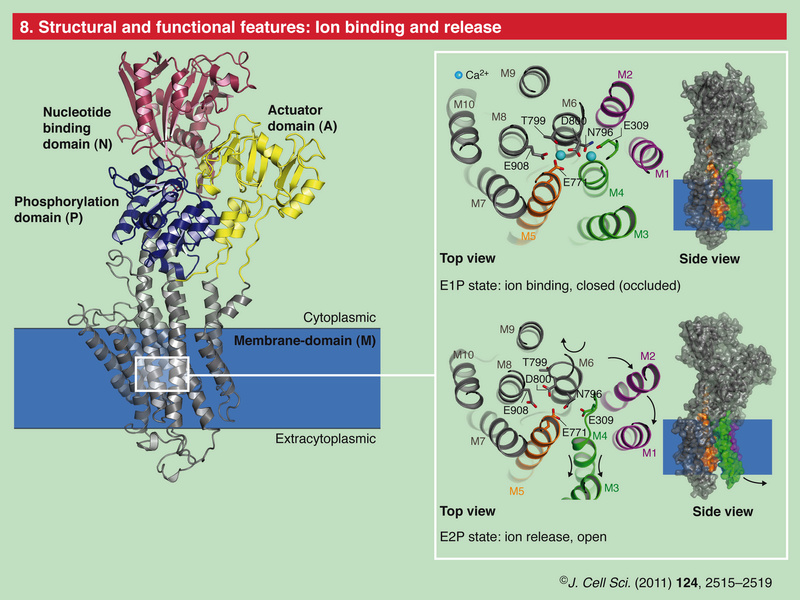
Role of Calcium Ions in the Cell and Bacterial Calcium Metabolism 1
http://reasonandscience.heavenforum.org/t2448-role-of-calcium-ions-in-the-cell-and-bacterial-calcium-metabolism
The make of a power gradient is always a engineering achievement, and a lot of knowledge, planning and intelligence is required for setup. Hydroelectric dams are highly complex, and always the result of years of planning by the most skilled, educated and knowledged engineers of large companies. As for many human inventions, the engineering solutions discovered by man are employd in nature at least since life began in a far more elaborated and sophisticated way.In view of the importance of Ca2+ as a universal intracellular regulator, it is surprising how little of it is mentioned and debated when it comes to elucidate how life began.
Why Calcium? How Calcium became the best communicator.To adapt to changing environments, cells must signal, and signaling requires messengers whose concentration varies with time. Filling this role, calcium ions (Ca2+) and phosphate ions have come to rule cell signaling. 19 An oversupply of certain elements, even essential ones, may subject an organism to stress. Accordingly, cellular systems need counteractive measures to overcome the threat imposed by their habitat. In order to illustrate the kind of defense strategies adopted by living cells for managing hazardous compounds, the case of Ca2+ is presented. This element is an especially sensitive indicator because the cell has to maintain a critical level of it at all times and any changes, up or down, will severely impair the cell and eventually kill it. 18 The critical chemical step at the start of life was not to make lots of DNA or RNA, but must have been to get rid of Ca2+. 3 Calcium is a divalent cation (Ca2+), ubiquitous in natural waters. It has a variable degree of hydration of 6 to 8 water molecules that can be exchanged very rapidly. This makes calcium the fastest binding agent of all the available divalent ions in the environment. Mg2+ reacts 103 times slower.
That shows that calcium regulation had to be full setup right from the start, when life began.
The book Intracellular Calcium, page 581 confirms this notion :
Our knowledge of the biological chemistry of Ca2+ argues strongly that Ca2+ must also have played a key part in the biochemistry of the first, primeval cells. Given the prevalence of calcium in the Earth’s crust, these primeval cells will have been surrounded by at least tens of micromolar free Ca2+, if not millimolar. This is high enough to mess up many reactions inside contemporary cells. At millimolar concentrations, Ca2+ competes with Mg2+, binds to DNA and RNA, and clogs it up. Ca2+ binds to nucleotides, so they do not work properly. And crucially Ca2+, at microto millimolar concentrations, precipitates carbonate, phosphate and sulphate. So if a primeval cell was to work, it had to get rid of Ca2+, lowering it at least to submillimolar levels, if not submicromolar.
In fact, without control of intracellular Ca2+, I have argued life would never have got off the ground or even been able to get going in the ocean! Control of intracellular Ca2+ had to be a crucial step in allowing the original cells to survive and replicate, evenbefore RNA or DNA synthesis could begin in earnest.There are three ways this can be done across a semipermeable membrane:
1. A membrane potential, positive inside, instead of the negative one in all contemporary cells, so that Ca2+ is at its equilibrium potential.
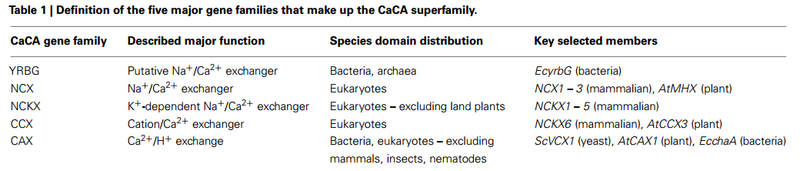
2. A Ca2+ exchanger, a Ca2+ anion symport (e.g. phosphate) or a Ca2+/cation antiport, (e.g. Na+ or H+).
3. A Ca2+ pump.
Most discussions about evolution start with RNA versus DNA worlds and the origins of the organelles in eukaryotes. Those discussing the evolution of the Ca2+ signalling system usually focus on the need for a Ca2+ pump, then Ca2+ channels and then Ca2+ target sites such as the EF-hand . But, it is much more likely that the primeval cells found a way of getting rid of intracellular Ca2+ without the need for a pump. The only way that this can be done is to generate a membrane potential, positive inside instead of the negative potential of contemporary cells.
For 3800 million years it has been essential for all cells, whether they were animal, plant or microbe, whether they were Bacteria, Archaea or Eukaryota, to maintain a free Ca2+ in the soluble part of the cell, the cytosol, very low, in the micromolar to submicromolar range. Without this low intracellular free Ca2+, particularly in the presence of millimolar Ca2+ outside the cell, damaging inorganic and organic Ca2+ precipitates would form, and the essential biochemical processes of energy metabolism, DNA, RNA, protein, lipid and carbohydrate synthesis and degradation could not take place.
Membrane PotentialAll contemporary cells have a membrane potential, negative inside. So, without a Ca2+ pump, Ca2+ would concentrate inside. At equilibrium, a cell with a large, negative membrane potential would have a cytosolic free Ca2+ concentration as high as tens of millimolar or even 1M! Hence contemporary cells have to have a Ca2+ pump, the Ca2+-MgATPase and/or the Na+/Ca2+ exchanger, in order to prevent Ca2+ concentrating inside the cell. Pumps on internal organelles can remove Ca2+ from the cytosol, but without an efflux mechanism on the plasma membrane, Ca2+ would eventually reach its electrochemical equilibrium.
Most bacterial and eukaryotic cells have a cytosolic K+ at least as high as 150mM, with a cytosolic Na+ concentration of around 5–10mM, 1mM Mg2+ and submicromolar free Ca2+. The K+/Na+ gradient across the plasma membrane is maintained by the sodium pump, which exchanges three Na+ out for two K+ in. Thus, this pump is slightly electrogenic, contributing 5–10mV to the membrane potential, as shown by the small drop in membrane potential when the Na+ pump inhibitor ouabain is present. However, the bulk of the resting membrane potential in contemporary eukaryotic cells is due to the selective permeability of the plasma membrane to K+ ( Potassium ) compared with Na+( Sodium ), the membrane potential being close to the equilibrium potential for K+, typically –70mV in muscle, –60 to –90mV in nerve and –30mV in liver, all negative inside. But the primeval cell would not have evolved this far.
So, in the absence of pumps, how could a cell get rid of Ca2+? The answer must be that it had a membrane potential, positive inside, maintained by a
Donnan potential . This can be generated by a gradient of permeant anions, where there is higher concentration inside than out. This will occur if there is a higher concentration of impermeant anions outside the cell than inside. Alternatively, a positive membrane potential inside could be generated by cations if there is a higher concentration of impermeant cations inside than outside, causing the permeant cations to be higher outside than in.
After much speculation, at the end, the authors confess: These arguments emphasise the importance of relating the electrophysiology to the biochemistry of Ca2+, if we are to understand how the Ca2+ signalling system evolved and how it works in cells today. Once a ‘Ca2+ pressure’ had been established, and proteins started to be produced in earnest, the scene was set for the Ca2+ signalling proteins to appear. First would have been the Ca2+ pumps and exchangers, taking over from the Donnan potential, allowing a membrane potential, negative inside, to develop. Only when there were fully active plasma membrane pumps and exchangers was it possible to maintain the cytosolic free Ca2+ low in the presence of high concentrations outside a cell with a negative membrane potential. These early molecular processes for Ca2+ may have taken hundreds or even a billion years to develop effectively. By the time of Cambrian explosion, some 590 million years ago, the majority of Ca2+-regulated cellular events must have been in place – nerve action potentials and nerve terminal secretion, all forms ofmuscle contraction, vesicular secretion, invertebrate vision, gamete fusion, plant regulation, defence mechanism, and cell death by apoptosis.
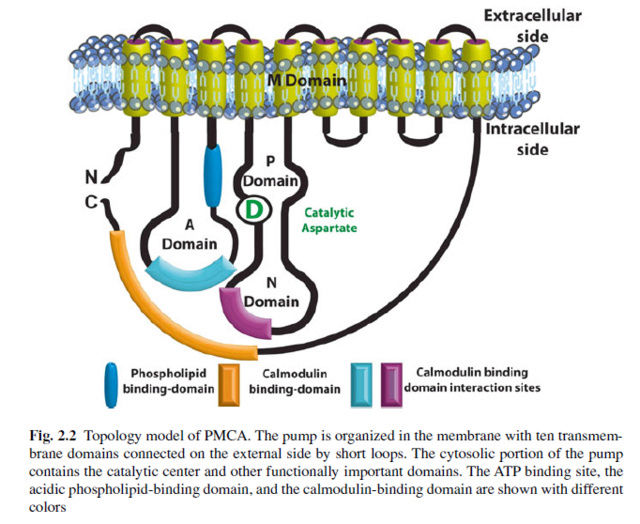
As so often, there is a hudge gap between the unsatisfying just so speculation of how the Ca2+ emerged, and the extant solution and its complexity. If the Donnal potential was sufficient, why did cells evolve highly sophisticated, diversified Ca2+ pumps, extremely complex as exposed below, where Calcium ATPases require Calmodulin in a interdependent manner for proper function ? As so often, the authors have no answer by detailled chronological narrative of the events that triggered the production of highly complex and regulation requiring membrane pumps. The pseudo-scientific assertions are remarkable. Calcium signaling is one of the most extensively employed signal transduction mechanisms in life. 10 Calcium ions (Ca2+) serve as a universal signal to
modulate almost every aspect of cellular function in all cells. Cells in the three domains all have a number of universalities, including intracellular Ca2+. 3 Calcium carries messages to virtually all important functions of cells. Ca2+ signaling pathway plays a key second messenger role in regulating many cellular processes in virtually all types of animal cells including fertilization, contraction, exocytosis, transcription, apoptosis, and learning and memory. 11 Most likely its unique coordination chemistry has been a decisive factor as it makes its binding by complex molecules particularly easy, even in the presence of large excesses of other cations, e.g. magnesium. Its free concentration within cells can thus be maintained at the very low levels demanded by the signaling function. A large cadre of proteins exist to bind or transport calcium. They all contribute to buffer it within cells, but a number of them also decode its message for the benefit of the target. The most important of these "calcium sensors" are the EF-hand proteins.
In bacteria, Ca2+ is implicated in a wide variety of cellular processes, including the cell cycle and cell division. 17 The growing number of proteins containing various Ca2+-binding motifs supports the importance of Ca2+, which controls various protein functions by affecting protein stability, enzymatic activity or signal transduction. Calcium is an ambivalent messenger:
while essential to the correct functioning of cell processes if not carefully controlled spatially and temporally within cells it generates variously severe cell dysfunctions, and even cell death. A prolonged high level of intracellular free Ca2+ irreversibly damages mitochondria and can cause chromatin condensation, precipitation of phosphate and protein and activation of degradative enzymes such as proteases, nucleases and phospholipases 18
The molecular mechanisms of the multiple effects of Ca2+ range from Ca2+ playing a role as a structural component stabilizing lipopolyscharide layer and cell wall to Ca2+ playing a role in structural stability of proteins or protein complexes ] to Ca2+ effecting transcription of two component systems, regulatory proteins, andproteinphosphorylation, whichmayhave eittherpositive or negative regulatory outcomes . When a cell is exposed to external Ca2+, in addition to Ca2+ uptake and its possible effect on the production of other intracellular messengers (ex, cAMP), it may be recognized by two component regulatory systems whose primary role is adjusting bacterial physiology to environmental conditions.
Had the control not have to be setup right from the beginning ? Given the central role of intracellular calcium signaling in the living world, a better understanding of the constitution of this calcium-signaling toolkit, and the proteins that comprise it, is crucial to our global understanding of what was required for cells to emerge. 8 These scientific studies highlight the high conservation of the calcium toolkit from prokaryotes to metazoa and the increasing complexity of the proteins that make it up. The necessity of exporting Ca2+ from cells is a direct consequence of the ambivalent nature of the Ca2+ signal.14 Ca2+ is essential to cells: it presides over the origin of new life at fertilization and assists cells when their vital cycle has come to an end. Between origin and end, however, Ca2+ guides cells in most of what they must do to fulfil the tasks assigned to them. The balance of Ca2+ between cells and the outside ambient must be regulated with outmost precision: any escape that would somehow alter the balance by letting internal Ca2+ increase over the optimal level spells doom for cells.
Controlled environment is the essence of life. This cellular separation from the surround pretty much builds around a simple and effective principle of divide et impera, i.e., divide the world into external environment and internal space and govern everything which goes into or out of the living cell/organism. Ca2+ permits binding reactions that are ~ 100 times faster than Mg2+( manganese ). Low Ca2+ in the cytosol of primeval cells is also compatible with energetics based around ATP and the usage of DNA/RNA for genetic encoding, because both cannot tolerate high Ca2+ concentrations; at the levels above 10 μMolar of Ca2+, this ion induces the precipitation of phosphates, causes aggregation of proteins and nucleic acids and disrupts lipid membranes . A Ca2+ homeostatic system assures a transmembrane Ca2+ gradient, which lies at the very base of Ca2+ signalling.
The maintenance of the stability of cells, osmotic, electrical and chemical, is life essential, and requires the cell to reject certain elements as ions, namely Na+ ( Sodium ), Ca2+ ( Calcium ) and cl ( Chlorine ), while retaining K+ ( potassium ions ) and Mg2+ ( magnesium ) . The levels of these simple ions are related to the cell's activities, both to metabolism and to the functioning of DNA. This requirement to reject Ca2+ in the initial stages of life is the pre-requisite of all its advanced functions. To maintain steady states of flow, cells have numerous signalling (circuit) systems employing carriers and messengers, amongst which co-enzymes are of major importance.
To describe cellular homeostasis , in total about twenty elements need to be regulated. 6
To get this is already a major feat. How did inanimated matter achieve this without guiding intelligence ? Origin of the calcium signaling machineryA complex Ca2+ signaling ‘toolkit’ might have evolved before the emergence of multicellular animals. 12 The basic principles of Ca2+ regulation emerged early in prokaryotes. 13
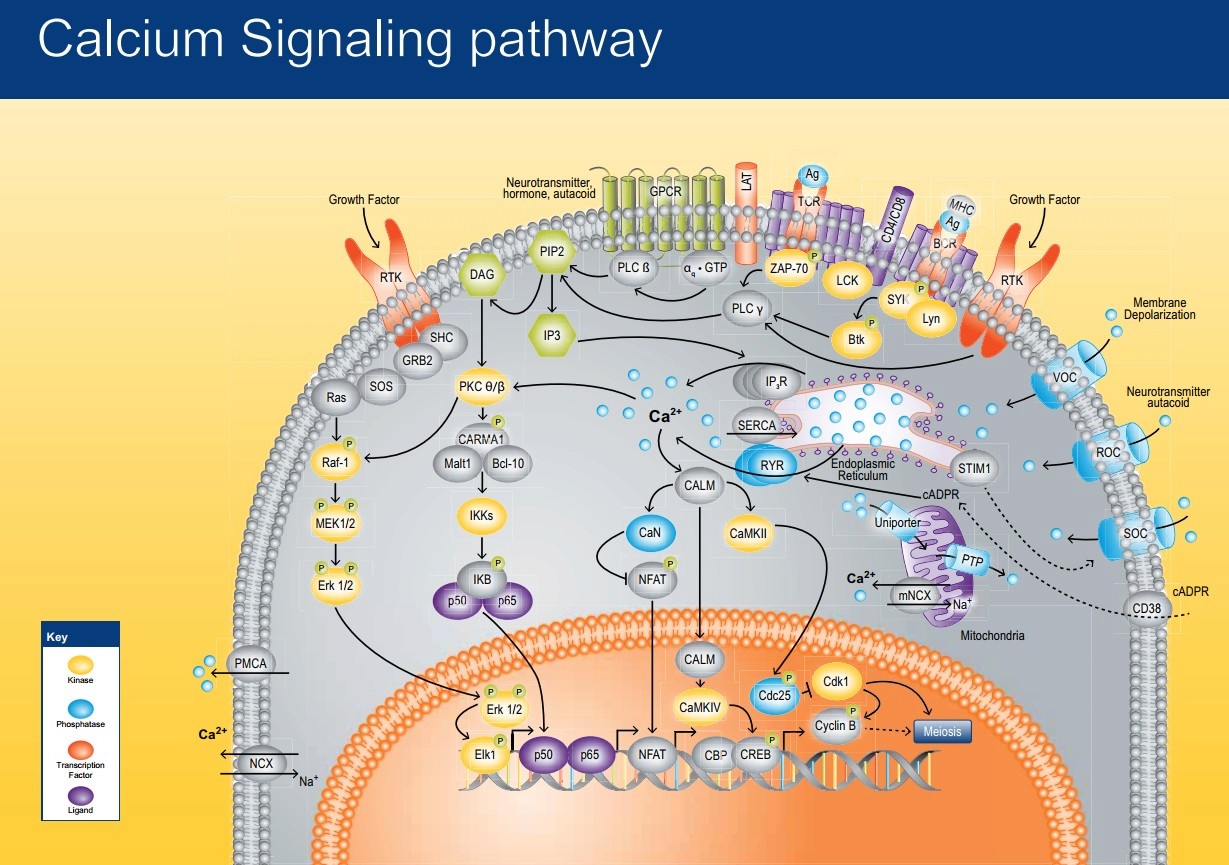
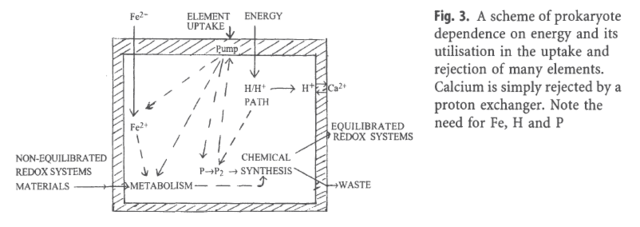 Figure 3
Figure 3Calcium (Ca2+) represents an important cytosolic signalling molecule as it can affect almost all cellular processes. 7 The flux of calcium across the plasma membrane and endomembranes, i.e. membranes demarcating internal organelles, critically relies on the operation of various calcium channels within the membranes. It is a critical element in all organisms; it is an essential nutrient which also has a conserved signaling role. Transporters that mediate the movement of this ion are therefore particularly important. These Ca2+ transporters impact upon cell division, growth, development, and adaptation to environmental conditions. 2 Ca2+ is a faster binding agent than any other available divalent ion from the environment. It reacts 103 times faster than Mg2+ ( manganese ) . 4 The calcium ion is arguably the most widely employed and important intracellular messenger in physiological and pathophysiological cellular processes. 5
Calcium plays a vital role in membrane integrity, catalysis, in the electrical properties of cells and, crucially, as a unique regulator of events inside cells. 3
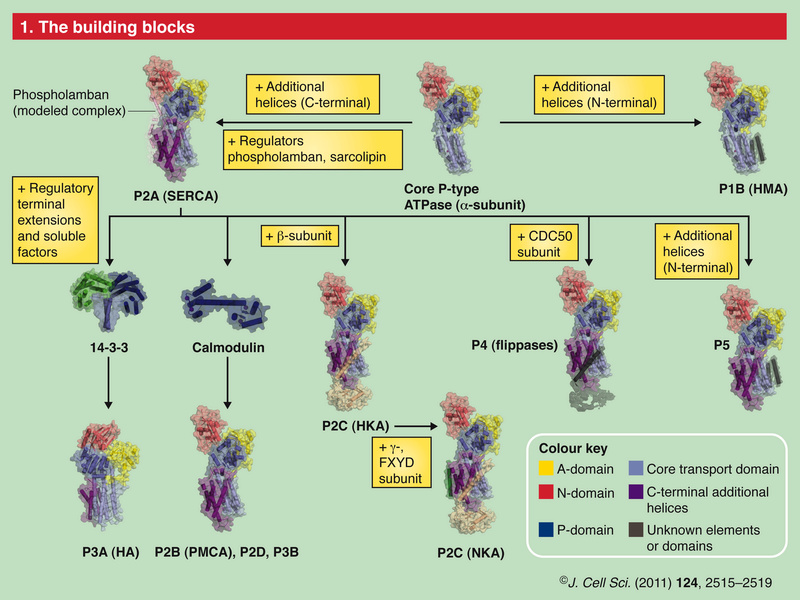
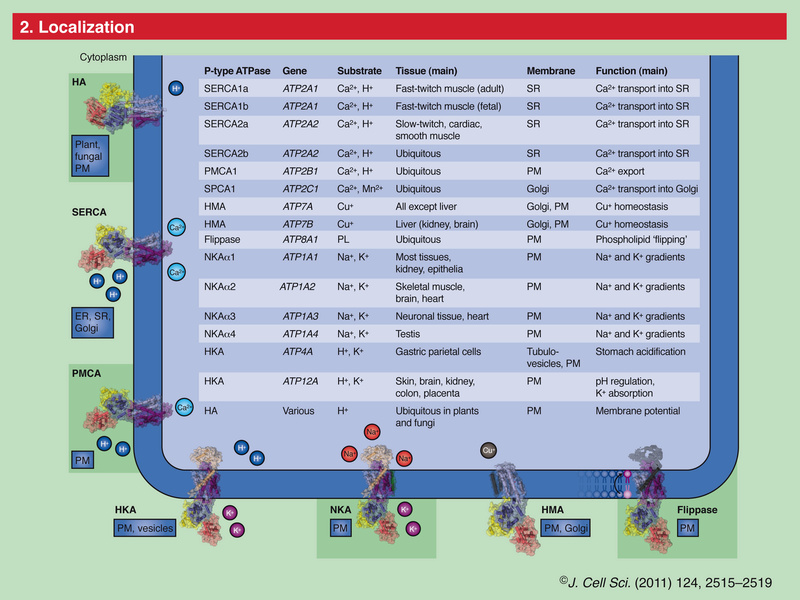
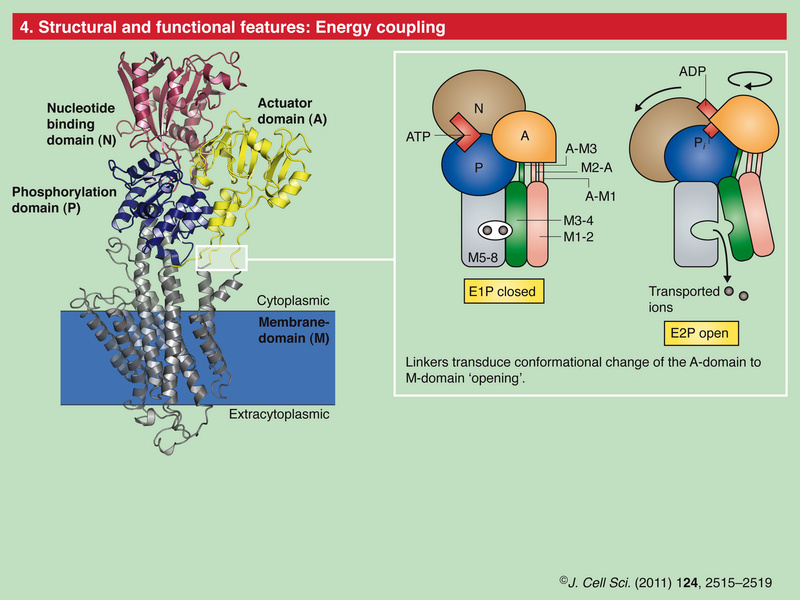
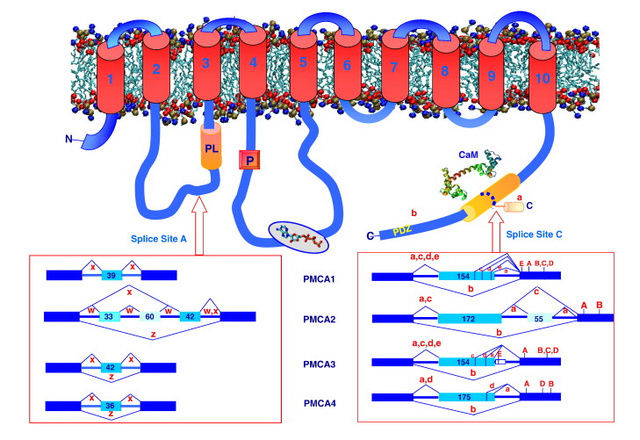
The ability of cells to maintain a large gradient of Ca2+ across their outer membrane is universal. All animal, plant and microbial cells have a low cytosolic free Ca2+ in the submicromolar range, and can keep this even when the free Ca2+ outside is as high as 1–10 mMolar ! Damage the ability of the plasma membrane to maintain this gradient and Ca2+ will flood into the cell, precipitating calcium phosphate, damaging the ATP-generating machinery, and even kill the cell. The evidence we have from molecular biology, together with the toxic nature of prolonged high Ca2+ levels inside cells, argues strongly that primeval cells must have had Ca2+ pumps to keep their free intracellular Ca2+ low, setting the scene for the ‘calcium pressure’ across then plasma membrane to be exploited to act as the source for cell activation.In order to maintain such a low cytosolic Ca2+ concentration, Ca2+ ions thus have to be transported against a steep concentration gradient. In addition, the positively charged molecules are often transported against a very negative membrane potential, contributing to a large electrochemical gradient for Ca2+ ions. 16 This function is carried out by (ACA)-encoded P-type ATPases. These ATP-driven pumps are members of a large protein family that are characterized by a phosphorylated (P) intermediate state of the protein during the pump cycle. Plasma membrane Ca2+ -ATPases belong to the group of P-type 2B proteins that share high homology to the plasma membrane calcium ATPase (PMCA) pumps from animals . . The 2B class of P-type ATPases is characterized by the presence of an N-terminus that acts as an auto-inhibitory domain. This N-terminal auto-inhibitory domain is released from the catalytic side of the ATPase after binding calmodulin in the presence of Ca2+.
So inanimate chemistry had the innate drive of trials and errors to produce a cell membrane, and amongst tons of other things, a Ca+ gradient through highly complex Calcium channels to keep a 10 000-fold higher concentration of calcium outside the cell than inside the cytosol in order to create a environment suited for a protocell to keep its vital functions and not to die ? Why would chemical elements do that ? Did they have the innate drive and goal to become alive and keep a ambience prerequisite, homeostasis of various elements, to permit life ?The origin of life required two processes that dominated:(1) the generation of a proton gradient and
(2) linking this gradient to ATP production in part and in part to uptake of essential chemicals and rejection of others. The generation of a proton gradient required especially appropriate amounts of
iron (Fe2+), levels for electron transfer and the ATP production depended on controlling
H+, Mg2+ and phosphate in the cytoplasm.
The four elements had to be linked together at once through complex formation, Mg2 (magnesium )+ and H+ ( hydrogen ) with phosphates, while chemical redox reactions linked Fe ( Iron ) and H ( Hydrogen ), and there were also feed-back connections of all four required through metabolism of carbon compounds and genetic controls. Thus we can consider that a network of carriers of the fundamental building blocks of biological polymers and of energy, the co-enzymes, fed and controlled (through feedback) inter-communicating pathways of metabolism were required, so as to establish primitive homeostasis which at requisite energy input also set a low homeostatic ca+ (calcium) concentration. The whole required structural and catalytic units, proteins, which had to be supplied or restricted by feed-back to DNA and RNA coded production machineries. Each of the major pathways of the four elements, Fe, H, P, Mg, as well as of C, N, 0, S and Se, had therefore to be linked through genetic regulation as well as through chemical controls to one another and then to all other cell activities. The earliest cells must have relied on an internal homeostasis of at least H+, Fe2+, Mg2+ and phosphates apart from systems related to C/N/O/H/S/Se metabolism.
How do mainstream scientific papers explain how this gradient emerged ?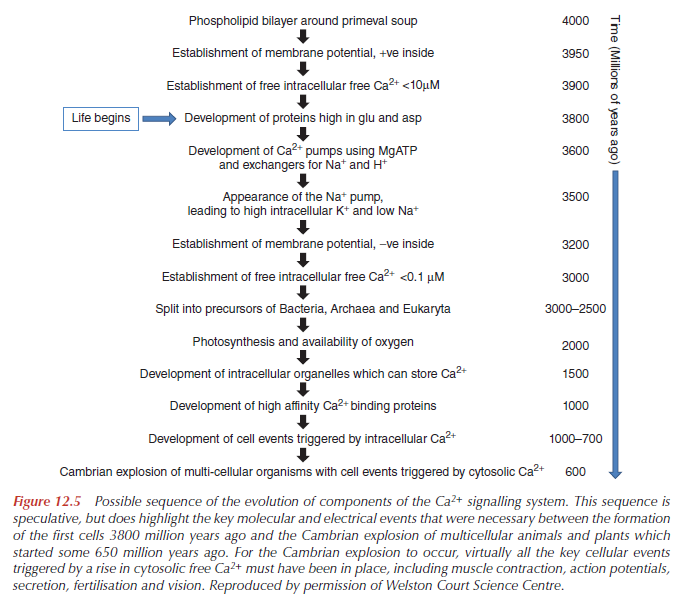
From the book, Intracellular Calcium, page 588
Calcium signalling and calcium channels: Evolution and general principlesWashout of Ca2+ ions from the Earth’s crust, in combination with a decreased alkalinisation of the ancient ocean, led to a continuous increase in Ca2+ concentration in the sea water, which in turn initiated the evolution of a Ca2+ homeostatic system that kept cytosolic Ca2+ at a low level. The molecules governing such homeostasis seem to evolve rather early in the genealogical tree as the most primitive bacteria were already in possession of Ca2+ pumps and Ca2+ exchangers. An increase in environmental Ca2+ concentration in combination with an evolving Ca2+ homeostatic system assured the build-up of a transmembrane Ca2+ gradient, which lies at the very base of Ca2+ signalling. This gradient soon was utilised by prokaryotes to develop Ca2+ permeable channels, which formed a pathway for a transmembrane Ca2+ influx and, thus, made Ca2+ signalling possible.
The evidence we have from molecular biology, together with the toxic nature of prolonged high Ca2+ levels inside cells, argues strongly that primeval cells must have had to develop Ca2+ pumps to keep their free intracellular Ca2+ low, setting the scene for the ‘calcium pressure’ across then plasma membrane to be exploited to act as the source for cell activation. A key chemical property of Ca2+ is that it comes on and off proteins fast, in milliseconds.
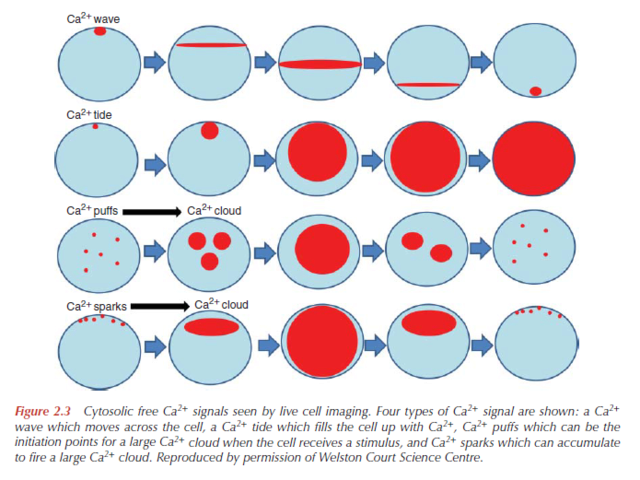
There are five main variations in the molecular processes responsible for Ca2+ signalling, which origin must be explained:
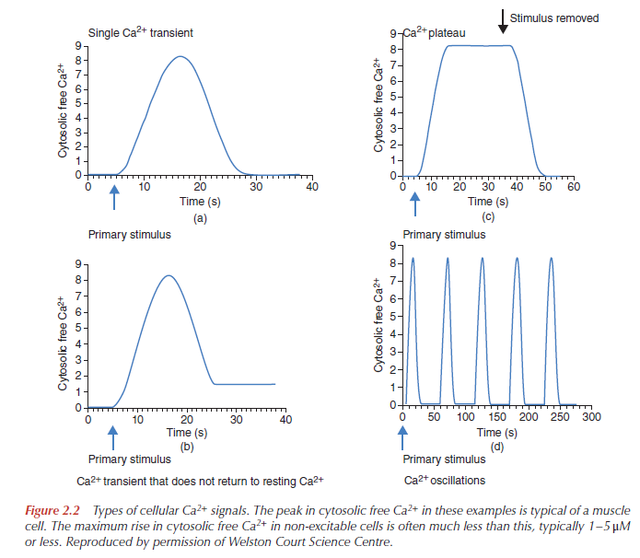
1. The type of Ca2+ signal, how big it is, how long it lasts and where in the cell it occurs.
2. The amino acid sequences, together with the gene sequences, of the components that produce the Ca2+ signal and those that are its targets.
3. The biochemical and electrical characteristics of these components (e.g. binding constants, kinetics, affinities, specificities, conductances).
4. The level of expression of the components.
5. The number of cells expressing particular components.
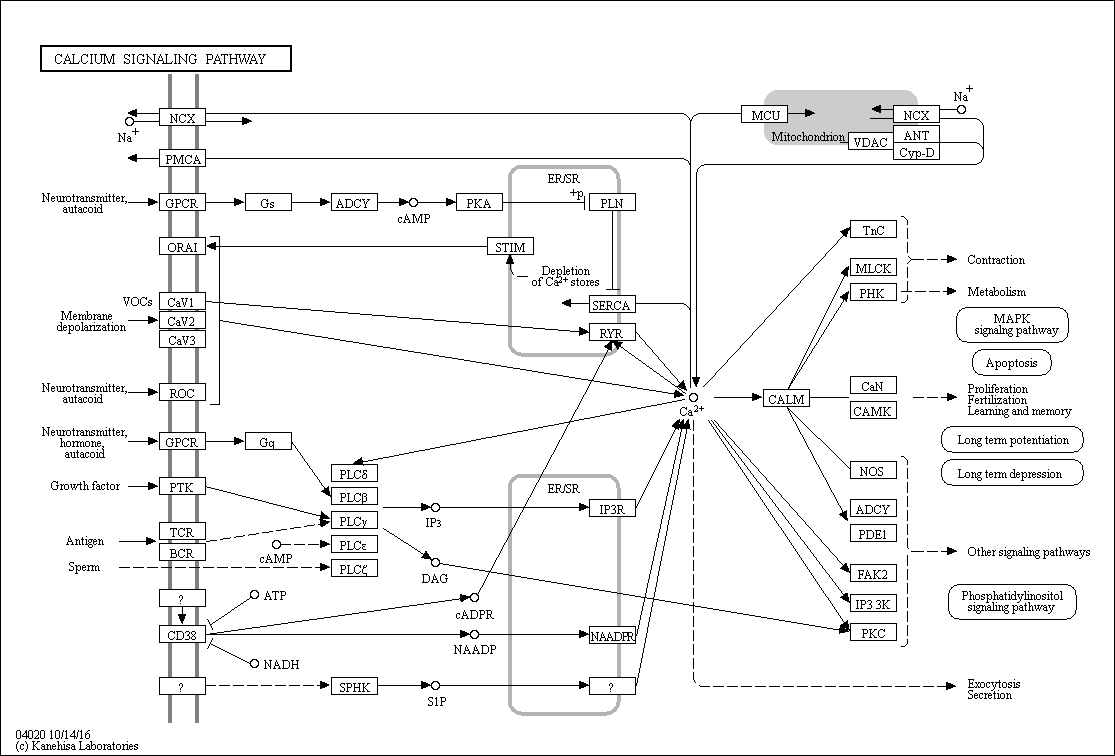
There are at least six types of Ca2+ pumps in the plasma membrane of cells:
four Ca2+-MgATPases,
two Na+/Ca2+ exchangers and other
Ca2+/H+ exchangers.
Three types of Ca2+ pumps in the Endoplasmic Reticulum (ER) (SERCA1, 2 and 3)
three types of each receptor (IP3 and ryanodine) in the ER that cause Ca2+ to be released into the cytosol.
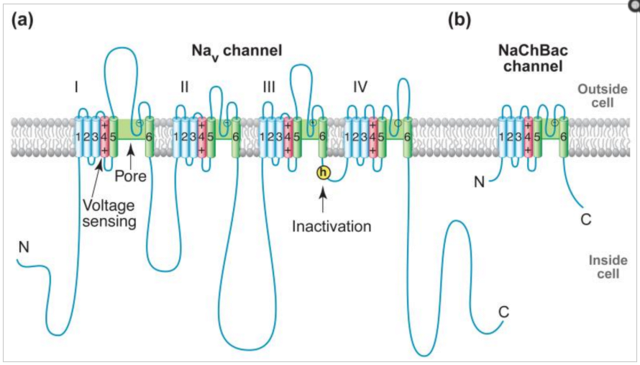
And there are a plethora of Ca2+ channels in the plasma membrane opened by voltage, ligands outside and inside cells, and events inside the ER.
Questions : Is the process analogue or digital and how does calcium determine this? Whether a cell fires or not can be determined by calcium reaching its target or not, by the level the Ca2+ reached, or by the
thermodynamic and morphological properties of the Ca2+ target. Yet there are analogue processes superimposed on the digital ones.
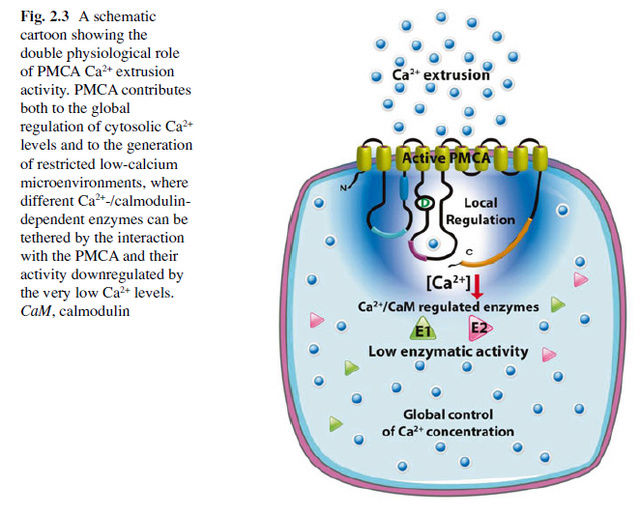
We have here a classic example of pseudo scientific just so stories, full of assertions, but no scientific evidence that evolution explains the feat. P-type Ca2+ pumps require Calmodulin (CaM) activators. They work in a interdependent way. Since Calcium carries messages to virtually all important functions of cells, the setup had to emerge prior dna replication began , and evolution could not have been a driving force. How was the strict regulation setup ? No regulation, no homeostasis, no calcium intracellular signaling, no life. Intracellular calcium homeostasis and signalingCa(2+) is a universal carrier of biological information: it controls cell life from its origin at fertilization to its end in the process of programmed cell death. Ca(2+) is a conventional diffusible second messenger released inside cells by the interaction of first messengers with plasma membrane receptors. However, it can also penetrate directly into cells to deliver information without the intermediation of first or second messengers. Even more distinctively, Ca(2+) can act as a first messenger, by interacting with a plasma membrane receptor to set in motion intracellular signaling pathways that involve Ca(2+) itself.
Perhaps the most distinctive property of the Ca(2+) signal is its ambivalence: while essential to the correct functioning of cells, Ca(2+) becomes an agent that mediates cell distress, or even (toxic) cell death, if its concentration and movements inside cells are not carefully tuned. Ca(2+) is controlled by reversible complexation to specific proteins, which could be pure Ca(2+) buffers, or which, in addition to buffering Ca(2+), also decode its signal to pass it on to targets. The most important actors in the buffering of cell Ca(2+) are proteins that transport it across the plasma membrane and the membrane of the organelles: some have high Ca(2+) affinity and low transport capacity (e.g., Ca(2+) pumps), others have opposite properties (e.g., the Ca(2+) uptake system of mitochondria). Between the initial event of fertilization, and the terminal event of programmed cell death, the Ca(2+) signal regulates the most important activities of the cell, from the expression of genes, to heart and muscle contraction and other motility processes, to diverse metabolic pathways involved in the generation of cell fuels.
Inseparable tandem: evolution chooses ATP and Ca2+ to control life, death and cellular signalling.From the very dawn of biological evolution, ATP was selected as a multipurpose energy-storing molecule.
Had a adequate energy supply in the cell not to be established prior when life began ? And so, had the origin of energy supply not have to be setup without having evolution at hand as driving force, since dna replication was not setup yet ? Metabolism of ATP required intracellular free Ca(2+) to be set at exceedingly low concentrations, which in turn provided the background for the role of Ca(2+) as a universal signalling molecule. The early-eukaryote life forms also evolved functional compartmentalization and vesicle trafficking, which used Ca(2+) as a universal signalling ion; similarly, Ca(2+) is needed for regulation of ciliary and flagellar beat, amoeboid movement, intracellular transport, as well as of numerous metabolic processes. Thus, during evolution, exploitation of atmospheric oxygen and increasingly efficient ATP production via oxidative phosphorylation by bacterial endosymbionts were a first step for the emergence of complex eukaryotic cells. Simultaneously, Ca(2+) started to be exploited for short-range signalling, despite restrictions by the preset phosphate-based energy metabolism, when both phosphates and Ca(2+) interfere with each other because of the low solubility of calcium phosphates. The need to keep cytosolic Ca(2+) low forced cells to restrict Ca(2+) signals in space and time and to develop energetically favourable Ca(2+) signalling and Ca(2+) microdomains. These steps in tandem dominated further evolution.
The ATP molecule (often released by Ca(2+)-regulated exocytosis) rapidly grew to be the universal chemical messenger for intercellular communication;
ATP effects are mediated by an extended family of purinoceptors often linked to Ca(2+) signalling. Similar to atmospheric oxygen, Ca(2+) must have been reverted from a deleterious agent to a most useful (intra- and extracellular) signalling molecule. Invention of intracellular trafficking further increased the role for Ca(2+) homeostasis that became critical for regulation of cell survival and cell death.
Several mutually interdependent effects of Ca(2+) and ATP have been exploited in evolution, thus turning an originally unholy alliance into a fascinating success story.This article is part of the themed issue 'Evolution brings Ca(2+) and ATP together to control life and death'.
Interdependece is evidence AGAINST evolution. Its remarkable how the authors implicitly overlook this obvious fact. Interdependence means, one molecule has no use without the other. But how could and would evolution produce it, if it had no use in the organism by its own ? The Regulation of a Cell’s Ca 2+ Signaling Toolkit: The Ca 2+ Homeostasome 9
The Ca 2+ ion serves as a ubiquitous second messenger in eukaryotic cells and changes in the intracellular Ca 2+ concentration regulate many responses within a cell, but also communication between cells.
In order to make use of such an apparently simple signal, i.e. a change in the intracellular Ca 2+ concentration, cells are equipped with sophisticated machinery to precisely regulate the shape (amplitude, duration) of Ca 2+ signals in a localization-specific manner. To ascertain such a precise regulation, cells rely on the components of the Ca 2+ signaling toolkit. This embraces Ca 2+ entry systems including
Ca 2+ channels in the plasma membrane and organellar membranes, and Ca 2+ extrusion/uptake systems including
Ca 2+ -ATPases (Ca 2+ pumps) and
Na + /Ca 2+ exchangers. The Ca 2+ -signaling components not only orchestrate their activity as to ascertain the high accuracy of intracellular Ca 2+ signaling, but they are also implicated in the regulation of their own expression. The total of the molecules that build the network of Ca 2+ signaling components, and that are involved in their own regulation as to maintain physiological Ca 2+ homeostasis resulting in phenotypic stability is named the Ca 2+ homeostasome.
The cytosolic free Ca 2+ concentration is tightly regulated by Ca 2+ -binding proteins, Ca 2+ pumps and other transporters to maintain low [Ca 2+ ] i levels of about 100nM in order to avoid toxic calcium phosphate precipitation within the cell. 15
A bimodular mechanism of calcium control in eukaryotesHow Resting Cells Maintain Their Ca2+ BalanceIn order to maintain the very large electrochemical gradient of Ca2+ across the plasma membrane, with a submicromolar cytosolic free Ca2+, in the presence of an extracellular free Ca2+ in the millimolar range, there has therefore to be a mechanism that continuously ‘pumps’ Ca2+ out of the cell. Without it, with a membrane potential on tens of millivolts, negative inside, the cytosolic free Ca2+ would eventually rise to hundreds of millimolar or even molar concentrations. Such a rise in free Ca2+ could not be prevented by internal stores such as the ER or mitochondria. As the Ca2+ has to be pumped out against a large electrochemical gradient, Ca2+ transport has to be coupled to another process with a ‘positive’ electrochemical gradient. Cells therefore exploit two sources of potential energy to pump Ca2+ out of cells against the electrochemical gradient across the plasma membrane:
1. ATP hydrolysis.
2. Another ion gradient, such as Na+ or H+.
The first of these to be discovered was the Ca2+ pump in red cells (Schatzmann, 1966, 1975). Even red cells can maintain a submicromolar cytosolic free Ca2+ in the presence of millimolar extracellular Ca2+ (Campbell and Dormer, 1975, 1978).
The universal need for Ca2+ by all cells falls into four distinct functional categories:
1. Structural (in both hard and soft tissues).
2. Electrical.
3. Cofactor.
4. Intracellular signal.
1. StructuralCa2+ bound to inorganic anions, proteins and phospholipids is vital in maintaining the soft structures of most cells. Ca2+ bound to proteins holds cells in organs together and, on the outside of cells, helps to maintain the stability of the plasma membrane, together with its semipermeable properties.
But Ca2+ also plays a structural role inside the cell. Although Mg2+ is the main cation bound to the negatively charged phosphate groups inDNA and RNA, Ca2+ also appears to bind. For example, Ca2+ bound to DNA may play an important role in the structure the chromosome in bacteria. Nucleotides such as ATP, GTP, CTP and TTP are all mostly bound to a divalent cation inside the cell. With a free Mg2+ estimated at 1–2mM, this is the favoured cation. And the form that reacts with virtually all kinases and pumps is ATPMg2– . However, in secretory vesicles Ca2+ bound to nucleotides and other molecules plays a role in maintaining the structural role of the vesicle. It also enables the contents to dissolve very quickly when released from the vesicle after it fuses with the plasma membrane.
2.ElectricalElectrophysiology of Intracellular Ca2+Electrophysiology is the study of the electrical properties of cells. These electrical properties depend on the movement of ions, rather than electrons, across lipid bilayers. A crucial feature of Ca2+ as an intracellular signal is how it interacts with these electrical properties. Across the outer membrane of the cell (i.e. the plasma membrane) the electrochemical gradient is made up of the membrane potential, which is negative inside, thereby attracting Ca2+ in, and the huge concentration difference of Ca2+, some 10000- to 100 000-fold between the outside and inside.
In a house, electricity is carried by electrons along a wire. In contrast, much of the electrical activity in living systems is carried by ions: K+, Na+, Ca2+ and Cl– . Since Ca2+ is a positively charged ion, it will move towards a negative potential. Ca2+ will itself generate an electrical potential if it moves across a semipermeable membrane down a concentration gradient. Many cells have specific Ca2+ channels to exploit this electrical activity. All cells maintain an electrical potential difference across their outer membrane. This is usually of the order of 40–100mV, negative inside, depending on cell type, though red blood cells have a membrane potential less than this. The membrane potential occurs because of the selective permeability of the plasma membrane to particular cations and anions. Pure phospholipids bilayers are poorly permeable to ions. But as soon as proteins are inserted across the bilayer, ions are able to diffuse across. But we now know that the selective permeability of biological membranes to particular ions is caused by ion channels, which are usually, but not always, proteins. In 1902, Bernstein suggested that K+ ions ( potassium ) were responsible for the resting potential of cells and that the action potential of electrically excitable cells might be due to loss in the selective permeability of the cell membrane to potassium. It is true that the resting membrane potential of most cells is caused by K+ ions. However, thanks to pioneering studies using giant nerve cells, such as those from the squid Loligo forbesi, Curtis and Cole at Woods Hole in the United States and Hodgkin and Huxley at Plymouth in the United Kingdom showed that the action potential in many nerve axons starts as a result of a sudden increase in the permeability to Na+ ( Sodium ). This rapidly depolarises the cell, which then repolarises as a result of increased permeability to K+. This is the so-called ‘sodium theory’ of the action potential, and resulted in Alan Hodgkin and Andrew Huxley being awarded the Nobel Prize in 1963. Soon after their pioneering work it was realised that many excitable cells also have Ca2+ channels that are sensitive to the potential across the plasma membrane. Thus, the contraction of a barnaclemuscle, to hold the plates shut when the tide goes out, is provoked by the transmitter glutamate initiating an action potential dependent on Ca2+ ions moving into the cell. Interestingly, our own heart beat depends on the electrical activity of Na+, Ca2+ and K+. The electrical activity of Ca2+ often may lead to, or contribute significantly to, the rise in cytosolic free Ca2+ which is responsible for the cell event (e.g. a heart cell beat). However, the movement of Ca2+ through ion channels does not inevitably lead to a global increase in cytosolic free Ca2+. Thus, the electrical role of Ca2+ can be considered distinct from its role as an intracellular regulator, even though these two functions interact closely with each other.
3. CofactorCa2+ regulates the activity of many extracellular proteins in the blood and the gut. This role is one of a cofactor and is different from the role of Ca2+ inside cells as an intracellular regulator. In the case of Ca2+ as an intracellular regulator it is a change in free Ca2+ inside the cell which triggers a cell event. But with enzymes and other proteins outside cells, there is no change in free Ca2+ associated with their activation. Only by removing Ca2+ artificially (e.g. using a chelator) can the effect of Ca2+ be prevented. Collect a fewmillilitres of blood in glass tube, tilt it to and fro, and within a fewminutes a clot will form. A scheme to explain this was proposed by Morawitz in 1903. The key protein, thrombin, is released by platelets and damaged tissue. Thrombin provokes blood plasma to clot, as first shown by Kühne in 1864. But if the Ca2+ is removed from the plasma by addition of citrate, first isolated by the Swedish chemist Scheele in 1784, then the clot will not form. Prevention of a blood clot, using a Ca2+ chelator or heparin, is standard medical practice if clinical analysis is to be carried out on a plasma sample (i.e. the total blood fluid minus all the cells). Ca2+ is essential for the prothrombin to thrombin reaction via cleavage of a small peptide and for several other reactions in the blood-clotting cascade. The binding sites for Ca2+ have been identified and shown in three dimensions by X-ray crystallography.Yet Ca2+ is not a ‘physiological’ regulator in the blood-clotting process. Yes, it is an essential cofactor, but changes in blood Ca2+ are not responsible for initiating or propagating the blood-clotting cascade. Nor are changes in free Ca2+ in blood thought to significantly regulate the rate of clot formation. Similarly, an essential role for Ca2+ was discovered in the classical pathway of the complement cascade. This pathway was discovered by Jules Bordet (1870–1961).When complement is activated the first component complex, C1, attaches to a cell, it then binds other components leading to formation of the membrane attack complex (C5b6789n). This complex causes the cell to burst, unless the cell can remove the potentially lethal complex and its associated pore. This can easily be demonstrated using sheep erythrocytes sensitised with an antibody. Addition of serum to these antibody-coated cells results in attachment of complement factor C1q to the antibody, which requires Ca2+. This is followed by binding of C1r and s which activate a proteolytic cascade forming ultimately C5b678 with multiple C9s. It is this that forms a pore causing the erythrocyte to explode. Cell lysis can be quantified by measuring release of haemoglobin into the extracellular fluid. But, as with the blood-clotting cascade, Ca2+ is not a ‘physiological’ regulator of the complement cascade. It is an essential cofactor for this so-called ‘classical’ pathway, but Ca2+ does not initiate it nor are changes in blood Ca2+ thought to regulate it significantly. Several other biological process, proteins and enzymes also have an absolute requirement for Ca2+ (Table 1.9). Examples in animals include the enzyme
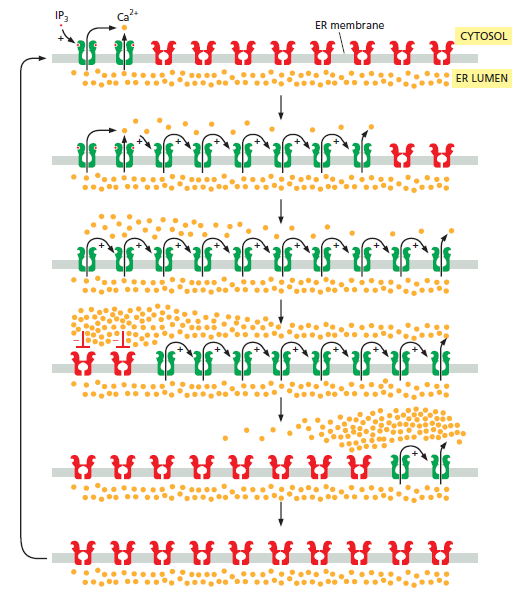
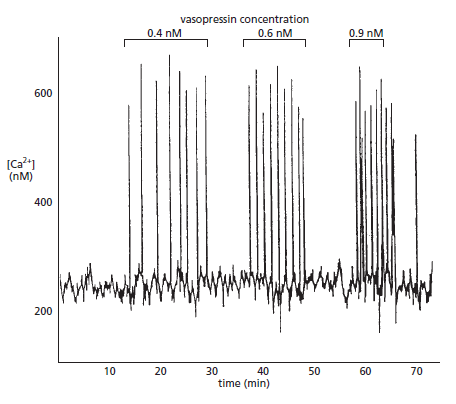
4. Intracellular RegulatorThe key feature of Ca2+ as a universal intracellular regulator in animal, plant and microbial cells is that a change in free Ca2+, somewhere inside the cell, must occur prior to a cell event and that this change in free Ca2+ is responsible for initiating the cell event (Figure below).
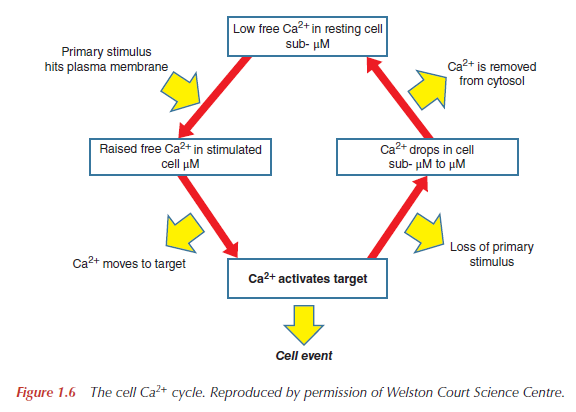
It is this which distinguishes the ‘active’ role for Ca2+ as an intracellular regulator from its ‘passive’ role as a cofactor of many proteins. The selection of Ca2+ as a universal intracellular regulator has depended on two critical features. First,
all cells have mechanisms to maintain a very low cytosolic free Ca2+, with the consequent large gradient of Ca2+ across the plasma membrane, as well as between the inside of organelles such as the ER and the cytosol. These gradients, typically 10 000-fold across the plasma membrane, generate a Ca2+ pressure, which is exploited by physiological and pharmacological stimuli to provoke a cellular event. Secondly, the chemistry of Ca2+ makes it ideal as an intracellular regulator. Oxygen ligands provide high-affinity Ca2+-binding sites which enable Ca2+ to bind, and come off, proteins quickly at micromolar concentrations of Ca2+, in the presence of millimolar Mg2+. Binding of Mg2+ to proteins occurs, but this is not able to produce the necessary structural change to initiate a cellular event nor is it possible to generate the necessary gradient of Mg2+ across membranes that is possible with Ca2+. Cations such as Zn2+, Mn2+, Fe2+/Fe3+ or Cu+/Cu2+ have not been selected because they come off proteins too slowly to allow a bee to buzz or an Olympic athlete to run 100m in less than 10 s. Furthermore, Ca2+ has only one ionisation state, unlike Fe2+/Fe3+ or Cu+/Cu2+, which are involved in redox reactions.
It is the special electrical and chemical properties of Ca2+ that have allowed Natural Selection biological cells to generate the electrochemical gradients and intracellular targets which are required to exploit these in such a wide range of biological processes. Life
evolved emerged in an environment containing many different cations, including Ca2+. The role that Ca2+ came to play in the early cells may have been conserved during subsequent evolution. The attributes of specific cations suited them to specific roles. Cations of related elements often are associated in pairs, with one needed for intracellular nutrition and the other not used intracellularly. Unlike Mg2+, which functions in many intracellular roles and must be transported inward and carefully regulated,
Ca2+ frequently functions extracellularly, rather than intracellularly, in biological processes and is maintained at low intracellular levels by efflux transport pathways. Many of the functions that Ca2+ performs in eukaryotes may therefore be expected to be present in prokaryotes. The common evolutionary origin of the prokaryotes and eukaryotes and the many examples of evolutionary conservation of structure and function that have been shown to exist between them, support the concept that such evolutionary conservation should extend to the role of Ca2+.
Extensive investigations on Ca2+ in eukaryotic cells have shown its important role in signal transduction, as a signal transmitter in membrane depolarization events, as an intracellular second messenger and as an effector of actin–myosin contraction. Recently, however, there has been an increased interest in the role of Ca2+ in prokaryotes and a number of investigations have reported data demonstrating a
clear Ca2+ contribution to structure and regulatory functions of the prokaryotic cell. The evidence in favor of Ca2+ as a mediator of regulatory phenomena in prokaryotes continues to accumulate.
There is evidence that calcium is involved in a number of bacterial processes such as maintenance of cell structure, motility, cell division, gene expression, and cell differentiation processes such as sporulation, heterocyst formation, and fruiting body development.Since Ca2+ plays a pivotal role in numerous biological processes in both prokaryotes and eukaryotes, its intracellular concentration must be strictly regulated and maintained to constant values. The free intracellular Ca2+ concentration in bacteria is tightly regulated ranging from 100 to 300 nM, against large changes in external Ca2+concentrations. This is also necessary to avoid toxic effects of free Ca2+ excess and irreversible damage to the cells, such as formation of calcium salts relatively insoluble. Thus
the maintenance of low intracellular concentrations of calcium is essential both for the survival of an organism and for calcium to function as a secondary messenger.The ability to control Ca2+ content, or at least intracellular free Ca2+ concentration, may thus be a fundamental attribute of all cells. A wide array of mechanisms participate in attaining such a homeostatic condition. Calcium regulation in bacterial cells comprises influxes and effluxes dependent on active and passive transport mechanisms. Due to the fact that normal intracellular calcium concentrations are usually up to 103 times lower than extracellular concentrations, passive transport usually accounts for calcium influx. A net Ca2+ concentration gradient across the cell membrane is formed which by present-day values would be of the order of 10000 - 100000.
This gradient has to be maintained by continuous exclusion of Ca2+ from the cell. The removal of Ca2+ by active extrusion requires energy to pump the Ca2+ against the electrochemical gradient. The metabolic apparatus that serves this function involves Ca2+ protein-based and non-proteinaceous channels, Ca2+ antiporters (Ca2+/2H+, Ca2+/Na+), and ATP-dependent Ca2+ pumps. ATP-driven calcium efflux appears to play a pivotal role in microbial calcium regulation. Another way to remove Ca2+ is to bind it into a harmless form by Ca2+ chelating materials.
Evolution of calcium homeostasis: From birth of the first cell to an omnipresent signalling systemSpecific Ca2+ homeostatic system appeared very early in the history of the cell, as a survival system preventing Ca2+-mediated cell damage. This homeostatic system produced a steep (∼20,000 times) concentration gradient between extracellular and intracellular compartments, which has both survival importance (even relatively short increases in cytosolic Ca2+ concentrations higher then 100 nM are incompatible with life) and signalling function.
Evolution Cells utilise this gradient together with an ability of Ca2+ to interact with many biological molecules to create the most widespread and versatile signalling system, controlling the majority of cellular processes and executing complex routines of intercellular communications.
The first cell, therefore, was defined by a membrane, which contained ion conducting pores and ion pump(s) combined with some source of energy. Failure of any of these components would swamp the cytosol with ocean water and eliminate life forever. “.. Control over Ca2+ ions was the most crucial because Ca2+ ions interact eagerly with biological molecules due to numerous specific properties (flexible coordination chemistry, high affinity for carboxylate oxygen, which is the most frequent motif in amino acids, rapid binding kinetics, etc. At high concentrations, however, Ca2+ causes aggregation of proteins and nucleic acids, affects the integrity of lipid membranes and initiates the precipitation of phosphates.
High intracellular Ca2+ concentration therefore is incompatible with life; at all phylogenetic stages, from the most ancient bacteria to the most specialised eukaryotic cells, an increase in cytosolic Ca2+ is always cytotoxic. As a result,
the first forms of life, however primitive, required an effective Ca2+ homeostatic system, which maintained intracellular Ca2+ at comfortably low concentrations—somewhere around 100 nM, this being ∼10,000–20,000 times lower than that in the extracellular milieu. Indeed, even the most primitive bacteria are endowed with
plasmalemmal Ca2+ pumps. The ancient roots of calcium signalling evolutionary treeMolecular cascades of calcium homeostasis and signalling (Ca2+ pumps, channels, cation exchangers, and Ca2+-binding proteins) emerged in prokaryotes and further developed at the unicellular stage of eukaryote evolution. With progressive evolution, mechanisms of signalling became diversified reflecting multiplication and specialisation of Ca2+-regulated cellular activities. Recent genomic analysis of organisms from different systematic positions, combined with proteomic and functional probing invigorated expansion in our understanding of the evolution of Ca2+ signalling. Particularly impressive is the consistent role of Ca2+-ATPases/pumps, calmodulin and calcineurin from very early stages of eukaryotic evolution, although with interspecies differences. Deviations in Ca2+ handling and signalling are observed between vertebrates and flowering plants as well as between protists at the basis of the two systematic categories, Unikonta (for example choanoflagellates) and Bikonta (for example ciliates). Only the B-subunit of calcineurin, for instance, is maintained to regulate highly diversified protein kinases for stress defence in flowering plants, whereas the complete dimeric protein, in vertebrates up to humans, regulates gene transcription, immune-defence and plasticity of the brain. Calmodulin is similarly maintained
throughout evolution, but in plants a calmoldulin-like domain is integrated into protein kinase molecules. The eukaryotic cell has inherited and invented many mechanisms to exploit the advantages of signalling by Ca2+, and there is considerable overall similarity in basic processes of Ca2+ regulation and signalling during evolution, although some details may vary.
The huge Ca2+ concentration gradient seemingly accompanies every life form from the very first day of its existence. However, its maintenance demands substantial energy consumption. Thus it would have been surprising if evolution had not led to adaptation of the Ca2+ homeostatic system, whose initial function was to protect the cell against massive and incessant “Ca2+ pressure”, for more positive ends. And so it was: the components of mechanism initially designed for survival set the stage for the appearance of the Ca2+ signalling system that triumphantly advanced, through many phylogenetic stages to the most ubiquitous and versatile regulatory machinery cells have ever had in their possession
Calcium binding proteins and calcium signaling in prokaryotesCa2+ triggers life at fertilization, and controls the development and differentiation of cells into specialized types. It mediates the subsequent activity of these cells and, finally, is invariably involved in cell death. To coordinate all of these functions, Ca2+ signals need to be flexible yet precisely regulated. This incredible versatility arises through the use of a Ca2+- signalling ‘tool kit’, whereby the ion can act in the various contexts of space, time and amplitude. Different cell types then select combinations of Ca2+ signals with the precise parameters to fit their physiology. 1
Evolution of Ca2+-Binding Sites 2
An essential step in the evolution of Ca2+ signalling was the appearance of Ca2+-binding sites in target proteins. At least four types of high-affinity Ca2+-binding site have been identified, and which satisfy the eight, or sometimes seven, oxygen coordination required for micromolar affinity for Ca2+ in the presence of millimolar Mg2+. These are:
1. EF-hand (type I), first found in parvalbumin, and its modifications in copines and the S-100 family.
2. C2, first found at the C-terminus of synaptotagmin.
3. Types II, III and AB, found in annexin
1. Molecular Biomineralization: Aquatic Organisms Forming Extraordinary Materials, page 1272. Protein Phylogenetic Analysis of Ca2+/cation Antiporters and Insights into their Evolution in Plants3. Intracellular Calcium, page 224. The evolution of calcium biochemistry
5. Calcium and evolutionary aspects of aging , page 1
6. Calcium homeostasis, page 6
7. Calcium signalling and calcium channels: Evolution and general principles
8. Evolution of the Calcium-Based Intracellular Signaling System
9. Calcium Signaling, ADVANCES IN EXPERIMENTAL MEDICINE AND BIOLOGY page 15
10. https://www.ncbi.nlm.nih.gov/pubmed/25063443
11. https://designmatrix.wordpress.com/2009/01/31/the-calcium-toolkit/
12. https://www.ncbi.nlm.nih.gov/pubmed/18385221
13. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4355082/
14. https://www.researchgate.net/publication/7657470_Exporting_calcium_from_cells
15. https://academic.oup.com/jxb/article/67/13/3997/1749466/Calcium-impacts-carbon-and-nitrogen-balance-in-the
16. http://onlinelibrary.wiley.com/doi/10.1111/j.1365-3040.2009.02075.x/pdf
17. The functions of Ca2+ in bacteria: a role for EF-hand proteins?
18. Calcium in the Early Evolution of Living Systems: A Biohistorical Approach
19. http://www.sciencedirect.com/science/article/pii/S0092867407015310