Kinesin and myosin motor proteins - amazing cargo carriers in the cell
https://reasonandscience.catsboard.com/t1448-kinesin-and-myosin-motor-proteins-amazing-cargo-carriers-in-the-cell
Two Types of Motor Proteins Move Along Microtubules
Torsion springs and lever arms: 2
a. “Myosin-Is are molecular motors that link cellular membranes to the actin cytoskeleton, where they play roles in mechano-signal transduction and membrane trafficking.”
b. “Some myosin-Is are proposed to act as force sensors, dynamically modulating their motile properties in response to changes in tension.”
c. “Tension sensing by myosin motors is important for numerous cellular processes, including control of force and energy utilization in contracting muscles, transport of cellular cargos, detection of auditory stimuli, and control of cell shape.”
d. The authors found that alternative splicing of the gene produces isoforms of the motor with lever arms of different lengths, with varying response to force. This “increases the range of force sensitivities of the proteins translated from the myo1b gene” and it “tunes the mechanical properties of myo1b for diverse mechanical challenges, while maintaining the protein’s basal kinetic and cargo-binding properties.”
e. How did these myosin machines arise? “Myosins have evolved different tension sensitivities tuned for these diverse cellular tasks,” the authors said. That was all they could say without giving any details of evolution.
Motor proteins
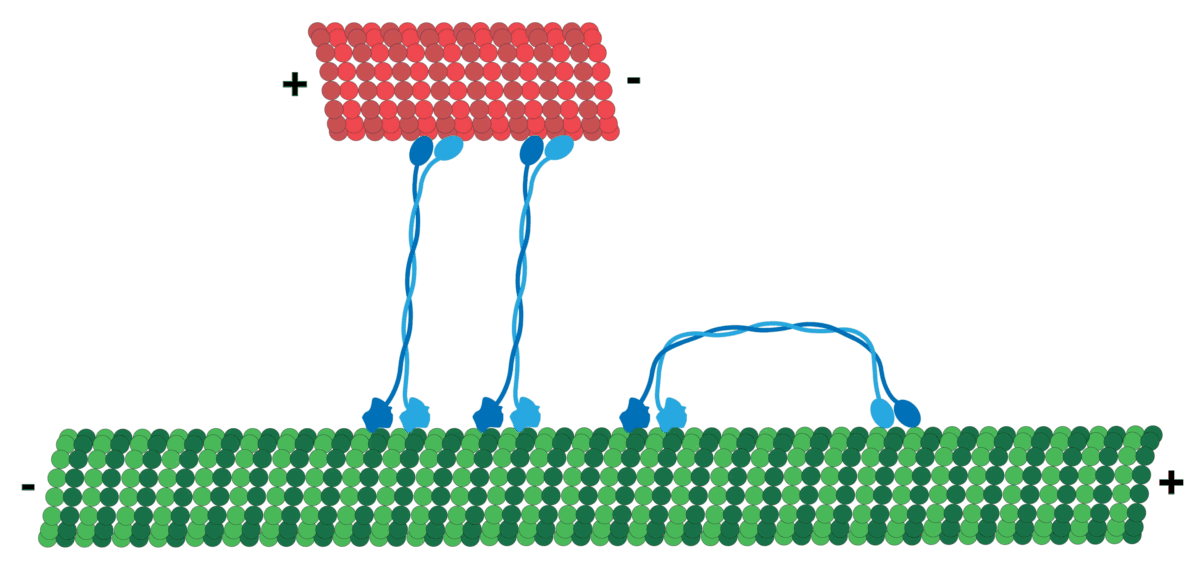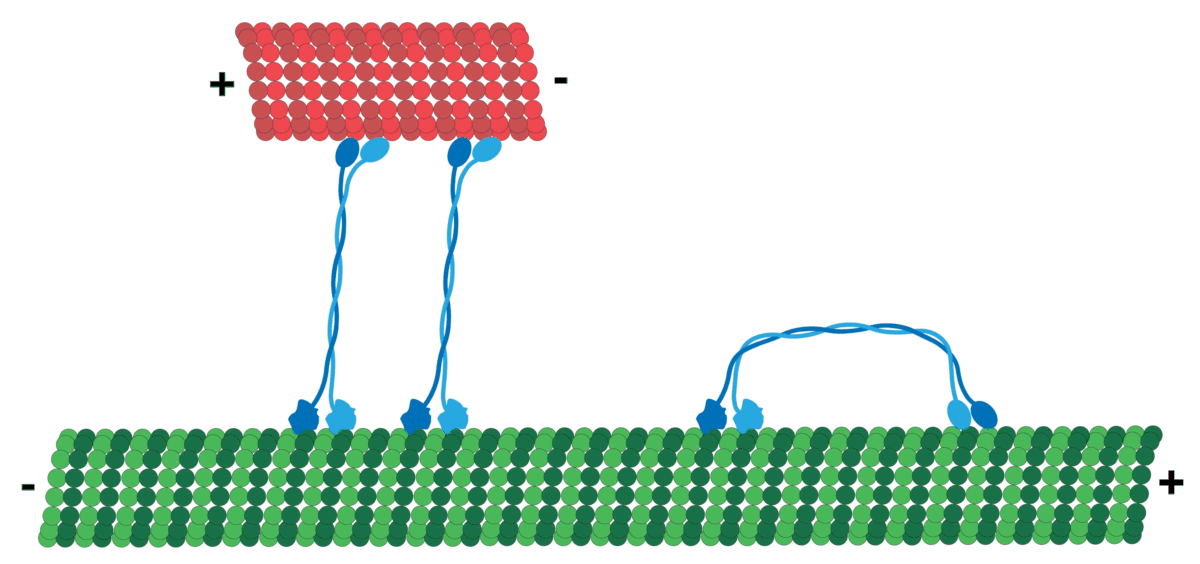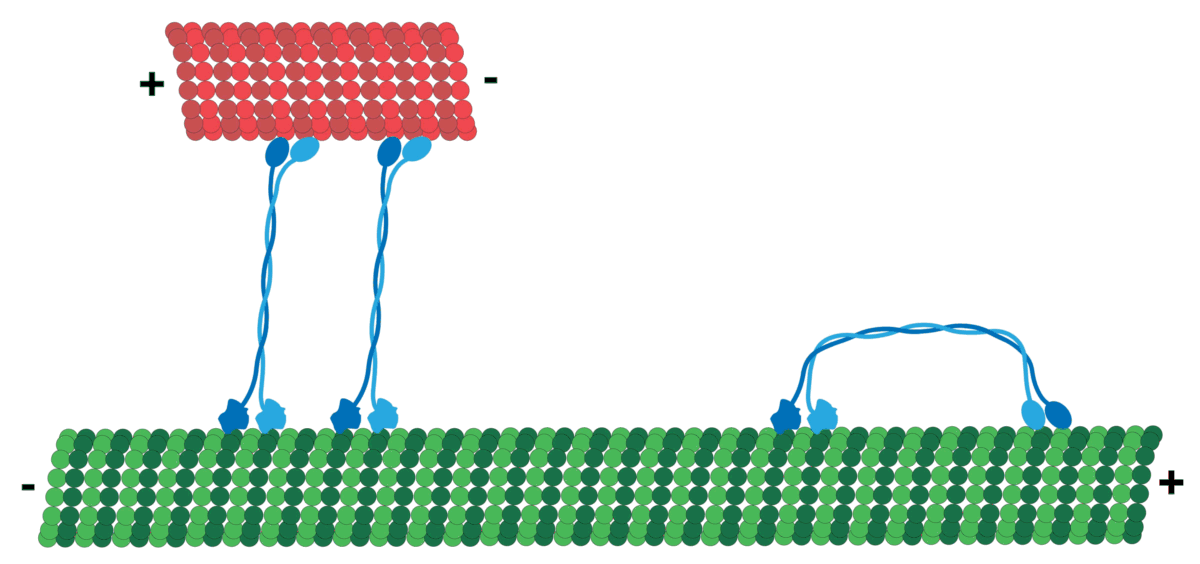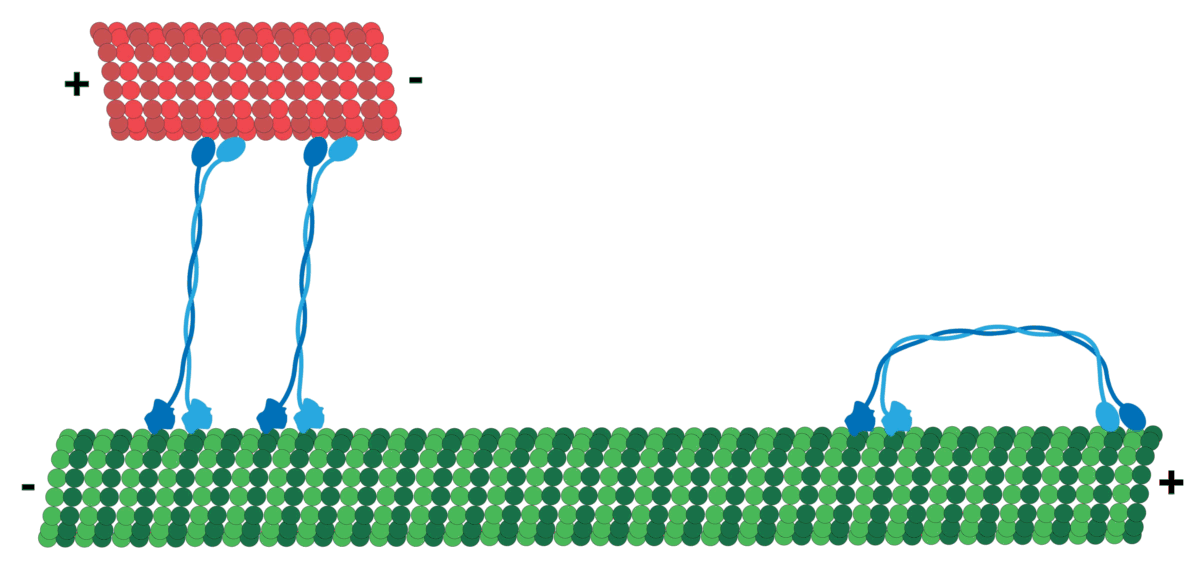
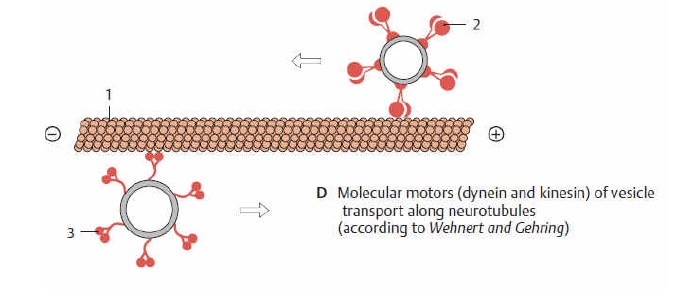
Microtubules can act as substrates for motor proteins that are involved in important cellular functions such as vesicle trafficking and cell division. Unlike other microtubule-associated proteins, motor proteins utilize the energy from ATP hydrolysis to generate mechanical work that moves the protein along the substrate. The major motor proteins that interact with microtubules are kinesin, which moves toward the (+) end of the microtubule, and dynein, which moves toward the (−) end.
Some viruses (including retroviruses, herpesviruses, parvoviruses, and adenoviruses) that require access to the nucleus to replicate their genomes attach to motor proteins.
Kinesin 6
Kinesins move along microtubule (MT) filaments and are powered by the hydrolysis of adenosine triphosphate (ATP) (thus kinesins are ATPases). The active movement of kinesins supports several cellular functions including mitosis, meiosis, and transport of cellular cargo, such as in axonal transport. Most kinesins walk towards the positive end of a microtubule, which, in most cells, entails transporting cargo from the center of the cell towards the periphery. This form of transport is known as anterograde transport. In contrast, dyneins are motor proteins that move toward the microtubules' negative end.
Question: What function could Kinesin and myosin motor proteins exercise inside the cell without microtubules? It's the same as to ask what function cars have without the roads upon to drive on. Motor proteins require the molecular highways to walk on. But the mitotic spindle also requires many of the kinesins for its formation, besides chromosome segregation during cell division. The interdependence is evident. One requires the other, so they had to arise together, at the same time.
In the cell, small molecules such as gases and glucose diffuse to where they are needed. Large molecules synthesized in the cell body, intracellular components such as vesicles, and organelles such as mitochondria are too large (and the cytosol too crowded) to diffuse to their destinations. Motor proteins fulfill the role of transporting large cargo about the cell to their required destinations.
Question: How could these large cargos be transported to the required destinations without microtubule nanomolecular highways, and without the cargo transport proteins? And how could the kinesins find the right destination, unless the microtubule code directs them to the final destination? Had the instruction of direction and destination not have to be programmed by an intelligence? Or is it more rational to infer that the code arose by chance, trial, and error until the Kinesins found their way to the right destination?
Like actin filaments, microtubules also use motor proteins to transport cargo and perform a variety of other functions within the cell. There are two major classes of microtubule-based motors, kinesins, and dyneins. Kinesin-1, also called “conventional kinesin,” was first purified from squid neurons, where it carries membrane-enclosed organelles away from the cell body toward the axon terminal by walking toward the plus end of microtubules. Kinesin-1 is similar to myosin II in having two heavy chains per active motor; these form two globular head motor domains that are held together by an elongated coiled-coil tail that is responsible for heavy-chain dimerization. One kinesin-1 light chain associated with each heavy chain through its tail domain and mediates cargo binding. Like myosin, kinesin is a member of a large protein superfamily, for which the motor domain is the common element
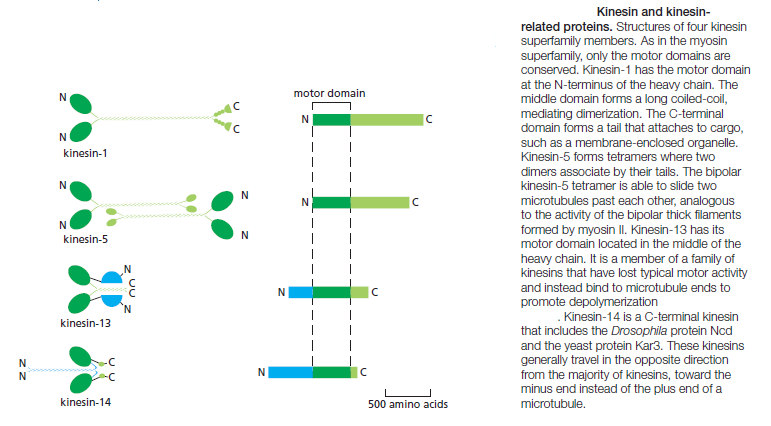
The yeast Saccharomyces cerevisiae has six distinct kinesins. The nematode C. elegans has 20 kinesins, and humans have 45. There are at least fourteen distinct families in the kinesin superfamily. Most of them have the motor domain at the N-terminus of the heavy chain and walk toward the plus end of the microtubule. One family has the motor domain at the C-terminus and walks in the opposite direction, toward the minus end of the microtubule, while kinesin-13 has a central motor domain and does not walk at all, but uses the
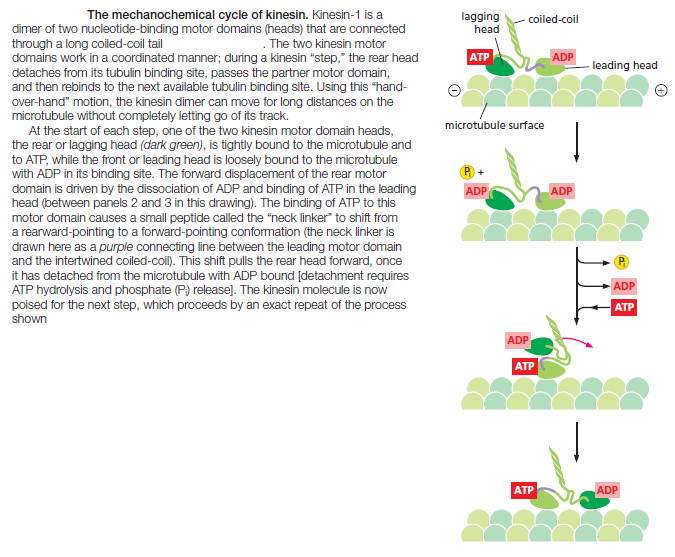
energy of ATP hydrolysis to depolymerize microtubule ends. Some kinesin heavy chains are homodimers, and others are heterodimers. Most kinesins have a binding site in the tail for another microtubule; alternatively, they may link the motor to a membrane-enclosed organelle via a light chain or an adaptor protein. Many of the kinesin superfamily members have specific roles in mitotic spindle formation and in chromosome segregation during cell division. In kinesin-1, instead of the rocking of a lever arm, small movements at the nucleotide-binding site regulate the docking and undocking of the motor head domain to a long linker region. This acts to throw the second head forward along the protofilament to a binding site 8 nm closer to the microtubule plus end, which is the distance between tubulin dimers of a protofilament. The nucleotide-hydrolysis cycles in the two heads are closely coordinated, so that this cycle of linker docking and undocking allows the two-headed motor to move in a hand-overhand (or head-over-head) stepwise manner
The dyneins are a family of minus-end directed microtubule motors unrelated to the kinesins. They are composed of one, two, or three heavy chains (that include the motor domain) and a large and variable number of associated intermediate, light-intermediate, and light chains. The dynein family has two major branches
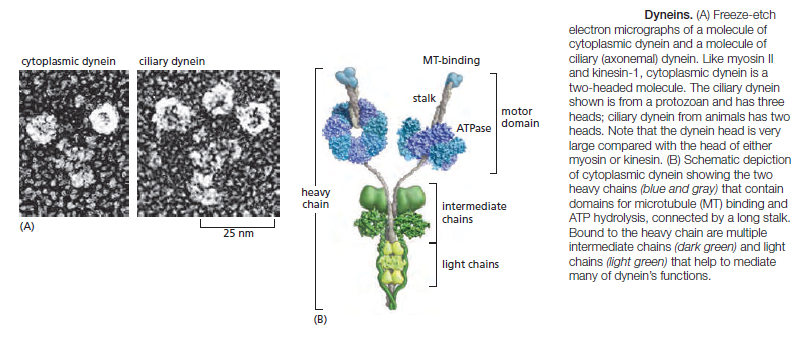
The first branch contains the cytoplasmic dyneins, which are homodimers of two heavy chains. Cytoplasmic dynein 1 is encoded by a single gene in almost all eukaryotic cells, but is missing from flowering plants and some algae. It is used for organelle and mRNA trafficking, for positioning the centrosome and nucleus during cell migration, and for construction of the microtubule spindle in mitosis and meiosis. Cytoplasmic dynein 2 is found only in eukaryotic organisms that have cilia and is used to transport material from the tip to the base of the cilia, a process called intraflagellar transport. Axonemal dyneins (also called ciliary dyneins) comprise the second branch and include monomers, heterodimers, and heterotrimers, with one, two, or three motor-containing heavy chains, respectively. They are highly specialized for the rapid and efficient sliding movements of microtubules that drive the beating of cilia and flagella Dyneins are the largest of the known molecular motors, and they are also among the fastest: axonemal dyneins attached to a glass slide can move microtubules at the rate of 14 μm/sec. The dynein motor is structurally unrelated to myosins and kinesins, but still follows the general rule of coupling nucleotide hydrolysis to microtubule binding and unbinding as well as to a force-generating conformational change
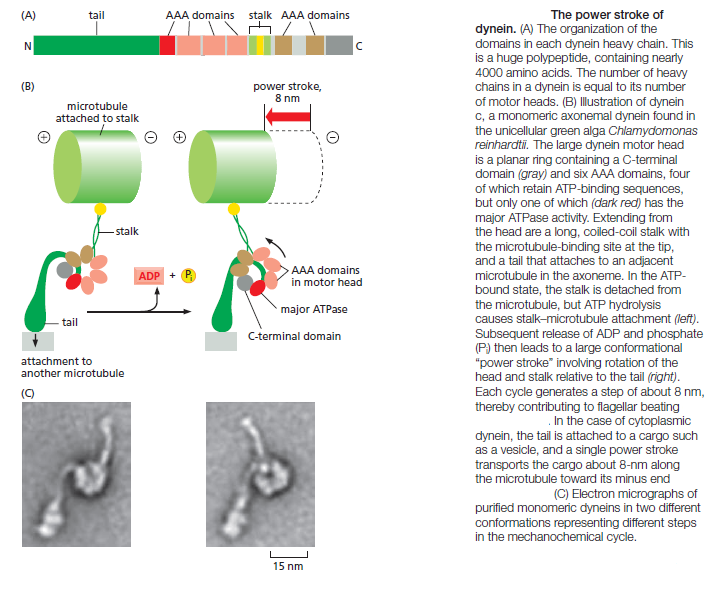
Microtubules and Motors Move Organelles and Vesicles
A major function of cytoskeletal motors in interphase cells is the transport and positioning of membrane-enclosed organelles . Kinesin was originally identified as the protein responsible for fast anterograde axonal transport, the rapid movement of mitochondria, secretory vesicle precursors, and various synapse components down the microtubule highways of the axon to the distant nerve terminals. Cytoplasmic dynein was identified as the motor responsible for transport in the opposite direction, retrograde axonal transport. Although organelles in most cells need not cover such long distances, their polarized transport is equally necessary. A typical microtubule array in an interphase cell is oriented with the minus ends near the center of the cell at the centrosome and the plus ends extending to the cell periphery. Thus, centripetal movements of organelles or vesicles toward the cell center require the action of minus-end directed cytoplasmic dynein motors, whereas centrifugal movements toward the periphery require plus-end directed kinesin motors. Interestingly, in animal cells, nearly all minus-end directed transport is driven by the single cytoplasmic dynein 1 motor, whereas 15 different kinesins are used for plus-end directed transport. A clear example of the effect of microtubules and microtubule motors on the behavior of intracellular membranes is their role in organizing the endoplasmic reticulum (ER) and the Golgi apparatus. The network of ER membrane tubules aligns with microtubules and extends almost to the edge of the cell, whereas the Golgi apparatus is located near the centrosome. When cells are treated with a drug that depolymerizes microtubules, such as colchicine or nocodazole, the ER collapses to the center of the cell, while the Golgi apparatus fragments and disperses throughout the cytoplasm. In vitro, kinesins can tether ER-derived membranes to preformed microtubule tracks and walk toward the microtubule plus ends, dragging the ER membranes out into tubular protrusions and forming a membranous web that looks very much like the ER in cells. Conversely, dyneins are required for positioning the Golgi apparatus near the cell center of animal cells; they do this by moving Golgi vesicles along microtubule tracks toward the microtubules’ minus ends at the centrosome. The different tails and their associated light chains on specific motor proteins allow the motors to attach to their appropriate organelle cargo. Membrane-associated motor receptors that are sorted to specific membrane-enclosed compartments interact directly or indirectly with the tails of the appropriate kinesin family members. Many viruses take advantage of microtubule motor-based transport during infection and use kinesin to move from their site of replication and assembly to the plasma membrane, from which they are poised to infect neighboring cells. An outer-membrane protein of Vaccinia virus, for example, contains an amino acid motif that mediates binding to kinesin-1 light chain and transport along microtubules to the plasma membrane. Interestingly, this motif is present in over 450 human proteins, one-third of which are associated with human diseases. Thus, kinesin transports a diverse set of cargoes involved in a wide range of important cellular functions. For dynein, a large macromolecular assembly often mediates attachment to membranes. Cytoplasmic dynein, itself a huge protein complex, requires association with a second large protein complex called dynactin to translocate organelles effectively. The dynactin complex includes a short, actin-like filament that forms from the actin-related protein Arp1 (distinct from Arp2 and Arp3, the components of the Arp 2/3 complex involved in the nucleation of conventional actin filaments)
Kinesin motor proteins - amazing cargo carriers in the cell
http://reasonandscience.heavenforum.org/t1448-kinesin-motor-proteins-amazing-cargo-carriers-in-the-cell
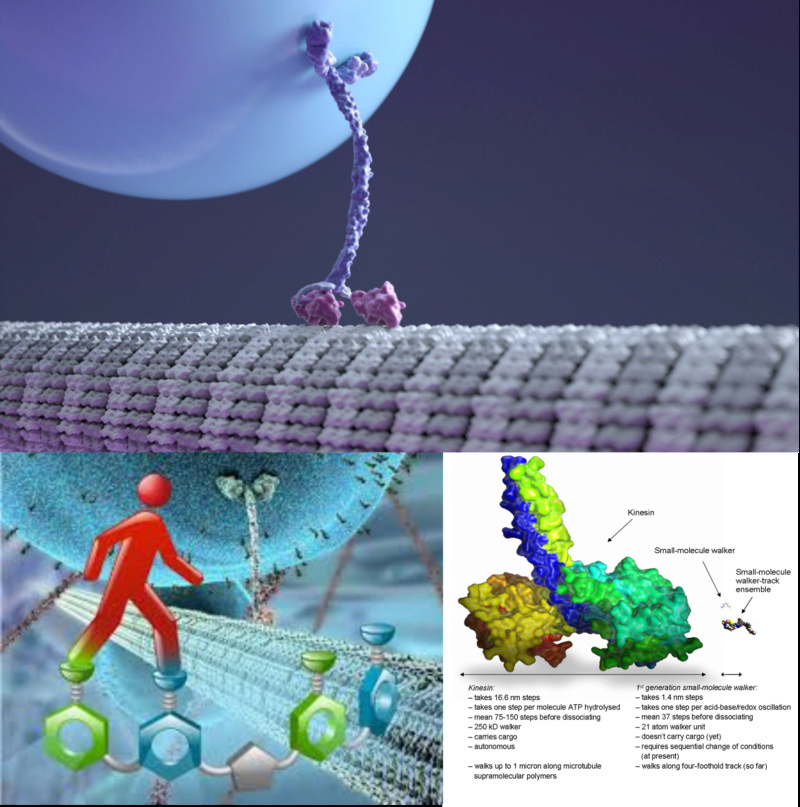
The Kinesin motor protein is the cargo carrier in the cell and helps in cell division. Its lifes smallest motor. They look like a post man, marching and holding the cargo with two " arms ", almost like humans. They know exactly where to catch the cargo, where to go in the cell, and where to drop it.
Micro tubule highways built up before they walk on them, assembled by proteins, each manufactured in accordance with the coded instructions of DNA, and once the cargo has been delivered at the right place, the nano highway dismantles. Until recently, scientists did not have a idea of how ATP fuel propels its walking like movement . The action happens at atomic level. That is one more example of the amazing engineering science has discovered in the cell. How do you want to explain these motors, which exist inside the cell ? They had all to be arise before life existed, since they make part of the cells functions. There is no selective advantage to have a " half " nanomotor. So how could it then arise in a step up fashion, just by natural means ? why should chance, physical necessity, or natural selection, provide a better explanation, than intelligent design ?
https://www.youtube.com/watch?v=FJ4N0iSeR8U
http://iaincarstairs.wordpress.com/2013/03/25/as-smart-as-molecules/
The kinesin illustrated above is from a Harvard animation and shows in correct proportion the transport of a sack of proteins from one place in the cell to another on one of the hundreds of thousands of highways which mysteriously build and unravel within every cell. Kinesin actually walks, step over step, travelling exclusively outwards from the centre of the cell, in strides of 8 nanometres. It can cope with obstacles in its path while holding onto its bag of goodies.
It covers 1 millimetre in 125,000 steps, making at most a few hundred steps in each journey, at a rate of 100 steps/second. The 14 variant groups of these motor proteins have been the subject of ingenious research, some of which precisely measured their force in PicoNewtons while striding over nanometre bridges. The graph is a straight line, meaning they’re as strong at the end of the journey as the beginning: given a surrounding supply of ATP they are literally tireless.
Kinesin’s fuel efficiency is nearly 50% – much more than a gasoline engine, and scaled up, exerts an incredible amount of torque. It requires no supernatural intervention: in vitro it behaves exactly as in the cell, given ATP, simply due to the arrangement of the amino acid components. Its step is precisely calibrated to the microtubule segments; the two components are part of one system.
Therefore the distance walked by one of these little Santas in a day is 69 million nanometres, or 6.9 centimetres – small wonder you need your own body weight in ATP each day. Scale him up to our size so his stride is 80 cm rather than 8 Nm with a factor of 100m and he could be covering 4,200 miles in a day.
With perhaps 100,000 at work in a single cell, and with 100 trillion cells, that’s a total distance travelled per day of just under 7 metres per cell, 691 billion km all told. Even if they only worked half the elapsed time, and then, only for one night – that’s easily enough to visit every house on the planet.
As for the elves, hammering away – clearly a metaphor for the ribosomes! And Rudolph.. well, perhaps his nose is a phosphate molecule.
http://creation.com/incredible-kinesin
Fast and efficient
Not only do these incredible kinesin robots perform a variety of tasks, they also do so with incredible efficiency! Check out these ‘state of the art’ features:
Power—“Not only is it tiny, but kinesin’s motor is about 50 percent efficient, which is about twice as good as a gasoline engine. And pound for pound, kinesin produces nearly 15 times more power than that man-made engine.”5
Speed—The kinesin motor is impressively fast, capable of 100 steps per second. “Scaled up to our own dimensions, a motor with corresponding properties would travel at similar speeds and produce as much horsepower per unit weight as the jet engines of the Thrust supersonic car6, which recently broke the sound barrier.”7 (This would be proportional to a person moving 600 meters per second or 1,300 miles per hour!)
Energy efficient—Kinesins are powered by the universal energy compound known as ATP (which is produced by another incredible molecular motor called ATP synthase—see animation, below right. Each molecule of ATP “fuel” that kinesin encounters triggers precisely one 8-nanometer step of the ‘postman’, but kinesins go into ‘sleep mode’ when cargo isn’t attached to prevent ATP from being wasted. Similar to how modern computers shut down after a period of un-use to conserve energy, kinesin have a hibernation feature as well. (Although scientists know that the motor folds over in an “autoinhibited” 8 state when resting, the molecular mechanism remains unclear.)
Team players—Kinesin molecules also work together when the going gets tough! If the load needing transport is too heavy for one ‘postman’ to handle, there is “ … significant evidence that cargoes in-vivo are transported by multiple motors.”9
They also demonstrate ‘multiple handling’ of their cargo. Similar to runners in a relay race, kinesins can ‘hand off’ their cargo to a ‘fresh’ bystander after delivering it a certain distance, and the other kinesin will finish the delivery process.
Flexible planning—Kinesins also have a ‘bypass mode’ ability that allows them to navigate around obstructions they may encounter. Similar to a GPS system ‘re-computing’, kinesins have demonstrated the remarkable ability to re-route automatically when needed.
Recycling—The most ardent champion of the ‘green’ movement would be jealous of the kinesin’s conservation and recycling capability. There is good evidence they are either transported back to the cell center in groups by large transport units (like mass transit in cities) or alternatively dismantled and their parts recycled when done their tasks.10
http://www.evolutionnews.org/2011/08/molecular_motors_enter_into_en049491.html
http://creationrevolution.com/walking-molecules-%E2%80%93-simple-cell-%E2%80%93-part-4/
http://creationsensation.blogspot.com.br/2012/11/the-kinesin-linear-motor.html
http://newscenter.lbl.gov/feature-stories/2010/02/17/kinesin-seesaw/
http://valelab.ucsf.edu/publications/1998kulljmusrescellm.pdf
Recent studies have shown surprising structural and functional similarities between the motor domains of kinesin and
myosin. Common features have also been described for motor proteins and G proteins. Despite these similarities, the
evolutionary relationships between these proteins, even among the motor proteins, has not been obvious, since the
topological connectivities of the core overlapping structural elements in these transducing proteins are not identical to one
another. Using secondary structure topology, comparison of functional domains and active site chemistry as criteria for
relatedness, we propose a set of rules for determining potential evolutionary relationships between proteins showing little or
no sequence identity.
http://www.evolutionnews.org/2014/06/kinesin_fast_ef_1086631.html
How do the proteins and organelles get from where they are made to where they are used? And how do things that need to be recycled return to the cell body? It's by means of kinesin (for outward bound travel) and another motor protein called dynein (for inward bound travel). Remarkably, dynein and kinesin cooperate, rather than compete -- otherwise there would be a constant tug of war in the cell. They "know" where packages are meant to go, and which motor protein should do the job. How that happens is a whole other story.
This isn't just abstract stuff. Many neurodegenerative diseases have been linked to mutations of kinesin, including Alzheimer's disease. So this little workhorse of the cell is not only a marvel, but essential.
Finally, in order for kinesin to work, we need the motor protein itself, the microtubule highway it walks along, and some mysterious means of connecting and directing where its cargos get carried. This system appears to be essential for a variety of eukaryotic cell functions. At least 11 kinesin families appear to have existed in the putative last common ancestor for all eukaryotes.

Life’s smallest motor, a protein that shuttles cargo within cells and helps cells divide, does so by rocking up and down like a seesaw, according to research conducted by scientists at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory and Brandeis University.
A molecular motor gives up its secrets. Electron microscope images of kinesin, one of life's smallest molecular motors, have been used to derive new, highly detailed 3-D maps
A molecular motor gives up its secrets. Electron microscope images of kinesin, one of life's smallest molecular motors, have been used to derive new, highly detailed 3-D maps (transparent surface) of the motor frozen in action. By fitting atomic models (colored ribbons) into the 3-D maps, a detailed mechanism has been derived for how a single molecule of biological fuel, called ATP, initiates motor movement. (Image by Charles Sindelar, Brandeis University)
The researchers created high-resolution snapshots of a protein motor, called kinesin, as it walked along a microtubule, which are tube-shaped structures that form a cell’s “skeleton.” The result is the closest look yet at the structural changes kinesin proteins undergo as they ferry molecules within cells.
“We see for the first time how kinesin’s atomic-scale moving parts allow it to pull itself and its cargo along a microtubule,” says Ken Downing, a biophysicist with Berkeley Lab’s Life Sciences Division. He conducted the research with postdoctoral fellow Charles Sindelar, now at Brandeis University.
“We found that there is a pivot point, where the kinesin motor attaches to the microtubule, which acts like a fulcrum and causes kinesin to rock up and down like a seesaw as it moves along the microtubule,” adds Downing.
Their research is reported this week in the online early edition of the Proceedings of the National Academy of Sciences.
The first-ever glimpse of kinesin’s seesaw motion offers key insights into one of life’s most fundamental processes. Fueled by an energy-giving compound called ATP, kinesin proteins motor along microtubules like trains on a railroad track, towing cargo to various locations within cells and assisting in cell division. Microtubules are a cylindrical weave of proteins found throughout cells that serve as cellular scaffolding.
Until now, scientists did not have a clear picture of what happens when ATP binds with kinesin, and especially how this process triggers structural changes in kinesin that propel the protein along microtubules.
Extremely high-resolution crystallography images of kinesin motors have enabled researchers to piece together the protein’s three-dimensional structure. But these images don’t reveal how it works.
“The problem is that it is not until the protein motor binds to a microtubule that structural rearrangements occur that enable ATP hydrolysis, the process that transfers energy from
ATP to kinesin,” says Downing.
To image kinesin at this critical stage, Downing and Sindelar turned to cryoelectron microscopy, which is a type of electron microscopy in which the sample is studied at extremely low temperatures. The technology is used by structural biologists to image proteins and other molecules as they appear in real-world conditions, in this case a kinesin protein attached to a microtubule.
The technique yielded 8 to 9 angstrom-resolution snapshots of the kinesin motor at four stages of the motor’s cycle as it moves along a microtubule. One angstrom is one-ten billionth of a meter. Using these images as a guide, the researchers then “dropped in” even higher resolution crystallographic images of kinesin’s components. This step enabled them to derive atomic-level structural models of kinesin in action.
“Collectively, this work provides a detailed molecular explanation for kinesin’s microtubule-attached power stroke,” says Downing. “In other words, we can see it how it works in real life. We looked at kinesin in different phases, and learned what causes it to move from one conformation to another, which is how it pulls cargo along the microtubule.”
In addition to further elucidating a key biological process, Downing and Sindelar’s research may inform the development of disease-fighting drugs. One of kinesin’s main jobs is moving chromosomes apart during cell division. Anything that blocks this process will lead to cell death, which is the basis of several cancer therapies such as taxol.
“New insights into how kinesin works could allow scientists to develop drugs that target and block particular kinesin movements,” says Downing.
http://faculty.washington.edu/casbury/pdfs/Asbury%202005%20Curr%20Opin%20Cell%20Biol.pdf
2) http://crev.info/2010/01/molecular_machines_use_moving_parts/
https://reasonandscience.catsboard.com/t1448-kinesin-and-myosin-motor-proteins-amazing-cargo-carriers-in-the-cell
Two Types of Motor Proteins Move Along Microtubules
Torsion springs and lever arms: 2
a. “Myosin-Is are molecular motors that link cellular membranes to the actin cytoskeleton, where they play roles in mechano-signal transduction and membrane trafficking.”
b. “Some myosin-Is are proposed to act as force sensors, dynamically modulating their motile properties in response to changes in tension.”
c. “Tension sensing by myosin motors is important for numerous cellular processes, including control of force and energy utilization in contracting muscles, transport of cellular cargos, detection of auditory stimuli, and control of cell shape.”
d. The authors found that alternative splicing of the gene produces isoforms of the motor with lever arms of different lengths, with varying response to force. This “increases the range of force sensitivities of the proteins translated from the myo1b gene” and it “tunes the mechanical properties of myo1b for diverse mechanical challenges, while maintaining the protein’s basal kinetic and cargo-binding properties.”
e. How did these myosin machines arise? “Myosins have evolved different tension sensitivities tuned for these diverse cellular tasks,” the authors said. That was all they could say without giving any details of evolution.
Motor proteins
Microtubules can act as substrates for motor proteins that are involved in important cellular functions such as vesicle trafficking and cell division. Unlike other microtubule-associated proteins, motor proteins utilize the energy from ATP hydrolysis to generate mechanical work that moves the protein along the substrate. The major motor proteins that interact with microtubules are kinesin, which moves toward the (+) end of the microtubule, and dynein, which moves toward the (−) end.
- Dynein is composed of two identical heavy chains, which make up two large globular head domains, and a variable number of intermediate and light chains. Dynein-mediated transport takes place from the (+) end towards the (-) end of the microtubule. ATP hydrolysis occurs in the globular head domains, which share similarities with the AAA+ (ATPase associated with various cellular activities) protein family. ATP hydrolysis in these domains is coupled to movement along the microtubule via the microtubule-binding domains. Dynein transports vesicles and organelles throughout the cytoplasm. In order to do this, dynein molecules bind organelle membranes via a protein complex that contains a number of elements including dynactin.
- Kinesin has a similar structure to dynein. Kinesin is involved in the transport of a variety of intracellular cargoes, including vesicles, organelles, protein complexes, and mRNAs toward the microtubule (+) end.[34] The majority of the kinesins are involved in the transport of vesicles from a microtubule’s (-) end toward the (+) end, that is toward the distal region of a cell or neural axon.
Some viruses (including retroviruses, herpesviruses, parvoviruses, and adenoviruses) that require access to the nucleus to replicate their genomes attach to motor proteins.
Kinesin 6
Kinesins move along microtubule (MT) filaments and are powered by the hydrolysis of adenosine triphosphate (ATP) (thus kinesins are ATPases). The active movement of kinesins supports several cellular functions including mitosis, meiosis, and transport of cellular cargo, such as in axonal transport. Most kinesins walk towards the positive end of a microtubule, which, in most cells, entails transporting cargo from the center of the cell towards the periphery. This form of transport is known as anterograde transport. In contrast, dyneins are motor proteins that move toward the microtubules' negative end.
Question: What function could Kinesin and myosin motor proteins exercise inside the cell without microtubules? It's the same as to ask what function cars have without the roads upon to drive on. Motor proteins require the molecular highways to walk on. But the mitotic spindle also requires many of the kinesins for its formation, besides chromosome segregation during cell division. The interdependence is evident. One requires the other, so they had to arise together, at the same time.
In the cell, small molecules such as gases and glucose diffuse to where they are needed. Large molecules synthesized in the cell body, intracellular components such as vesicles, and organelles such as mitochondria are too large (and the cytosol too crowded) to diffuse to their destinations. Motor proteins fulfill the role of transporting large cargo about the cell to their required destinations.
Question: How could these large cargos be transported to the required destinations without microtubule nanomolecular highways, and without the cargo transport proteins? And how could the kinesins find the right destination, unless the microtubule code directs them to the final destination? Had the instruction of direction and destination not have to be programmed by an intelligence? Or is it more rational to infer that the code arose by chance, trial, and error until the Kinesins found their way to the right destination?
Like actin filaments, microtubules also use motor proteins to transport cargo and perform a variety of other functions within the cell. There are two major classes of microtubule-based motors, kinesins, and dyneins. Kinesin-1, also called “conventional kinesin,” was first purified from squid neurons, where it carries membrane-enclosed organelles away from the cell body toward the axon terminal by walking toward the plus end of microtubules. Kinesin-1 is similar to myosin II in having two heavy chains per active motor; these form two globular head motor domains that are held together by an elongated coiled-coil tail that is responsible for heavy-chain dimerization. One kinesin-1 light chain associated with each heavy chain through its tail domain and mediates cargo binding. Like myosin, kinesin is a member of a large protein superfamily, for which the motor domain is the common element

The yeast Saccharomyces cerevisiae has six distinct kinesins. The nematode C. elegans has 20 kinesins, and humans have 45. There are at least fourteen distinct families in the kinesin superfamily. Most of them have the motor domain at the N-terminus of the heavy chain and walk toward the plus end of the microtubule. One family has the motor domain at the C-terminus and walks in the opposite direction, toward the minus end of the microtubule, while kinesin-13 has a central motor domain and does not walk at all, but uses the
energy of ATP hydrolysis to depolymerize microtubule ends. Some kinesin heavy chains are homodimers, and others are heterodimers. Most kinesins have a binding site in the tail for another microtubule; alternatively, they may link the motor to a membrane-enclosed organelle via a light chain or an adaptor protein. Many of the kinesin superfamily members have specific roles in mitotic spindle formation and in chromosome segregation during cell division. In kinesin-1, instead of the rocking of a lever arm, small movements at the nucleotide-binding site regulate the docking and undocking of the motor head domain to a long linker region. This acts to throw the second head forward along the protofilament to a binding site 8 nm closer to the microtubule plus end, which is the distance between tubulin dimers of a protofilament. The nucleotide-hydrolysis cycles in the two heads are closely coordinated, so that this cycle of linker docking and undocking allows the two-headed motor to move in a hand-overhand (or head-over-head) stepwise manner

The dyneins are a family of minus-end directed microtubule motors unrelated to the kinesins. They are composed of one, two, or three heavy chains (that include the motor domain) and a large and variable number of associated intermediate, light-intermediate, and light chains. The dynein family has two major branches

The first branch contains the cytoplasmic dyneins, which are homodimers of two heavy chains. Cytoplasmic dynein 1 is encoded by a single gene in almost all eukaryotic cells, but is missing from flowering plants and some algae. It is used for organelle and mRNA trafficking, for positioning the centrosome and nucleus during cell migration, and for construction of the microtubule spindle in mitosis and meiosis. Cytoplasmic dynein 2 is found only in eukaryotic organisms that have cilia and is used to transport material from the tip to the base of the cilia, a process called intraflagellar transport. Axonemal dyneins (also called ciliary dyneins) comprise the second branch and include monomers, heterodimers, and heterotrimers, with one, two, or three motor-containing heavy chains, respectively. They are highly specialized for the rapid and efficient sliding movements of microtubules that drive the beating of cilia and flagella Dyneins are the largest of the known molecular motors, and they are also among the fastest: axonemal dyneins attached to a glass slide can move microtubules at the rate of 14 μm/sec. The dynein motor is structurally unrelated to myosins and kinesins, but still follows the general rule of coupling nucleotide hydrolysis to microtubule binding and unbinding as well as to a force-generating conformational change

Microtubules and Motors Move Organelles and Vesicles
A major function of cytoskeletal motors in interphase cells is the transport and positioning of membrane-enclosed organelles . Kinesin was originally identified as the protein responsible for fast anterograde axonal transport, the rapid movement of mitochondria, secretory vesicle precursors, and various synapse components down the microtubule highways of the axon to the distant nerve terminals. Cytoplasmic dynein was identified as the motor responsible for transport in the opposite direction, retrograde axonal transport. Although organelles in most cells need not cover such long distances, their polarized transport is equally necessary. A typical microtubule array in an interphase cell is oriented with the minus ends near the center of the cell at the centrosome and the plus ends extending to the cell periphery. Thus, centripetal movements of organelles or vesicles toward the cell center require the action of minus-end directed cytoplasmic dynein motors, whereas centrifugal movements toward the periphery require plus-end directed kinesin motors. Interestingly, in animal cells, nearly all minus-end directed transport is driven by the single cytoplasmic dynein 1 motor, whereas 15 different kinesins are used for plus-end directed transport. A clear example of the effect of microtubules and microtubule motors on the behavior of intracellular membranes is their role in organizing the endoplasmic reticulum (ER) and the Golgi apparatus. The network of ER membrane tubules aligns with microtubules and extends almost to the edge of the cell, whereas the Golgi apparatus is located near the centrosome. When cells are treated with a drug that depolymerizes microtubules, such as colchicine or nocodazole, the ER collapses to the center of the cell, while the Golgi apparatus fragments and disperses throughout the cytoplasm. In vitro, kinesins can tether ER-derived membranes to preformed microtubule tracks and walk toward the microtubule plus ends, dragging the ER membranes out into tubular protrusions and forming a membranous web that looks very much like the ER in cells. Conversely, dyneins are required for positioning the Golgi apparatus near the cell center of animal cells; they do this by moving Golgi vesicles along microtubule tracks toward the microtubules’ minus ends at the centrosome. The different tails and their associated light chains on specific motor proteins allow the motors to attach to their appropriate organelle cargo. Membrane-associated motor receptors that are sorted to specific membrane-enclosed compartments interact directly or indirectly with the tails of the appropriate kinesin family members. Many viruses take advantage of microtubule motor-based transport during infection and use kinesin to move from their site of replication and assembly to the plasma membrane, from which they are poised to infect neighboring cells. An outer-membrane protein of Vaccinia virus, for example, contains an amino acid motif that mediates binding to kinesin-1 light chain and transport along microtubules to the plasma membrane. Interestingly, this motif is present in over 450 human proteins, one-third of which are associated with human diseases. Thus, kinesin transports a diverse set of cargoes involved in a wide range of important cellular functions. For dynein, a large macromolecular assembly often mediates attachment to membranes. Cytoplasmic dynein, itself a huge protein complex, requires association with a second large protein complex called dynactin to translocate organelles effectively. The dynactin complex includes a short, actin-like filament that forms from the actin-related protein Arp1 (distinct from Arp2 and Arp3, the components of the Arp 2/3 complex involved in the nucleation of conventional actin filaments)
Kinesin motor proteins - amazing cargo carriers in the cell
http://reasonandscience.heavenforum.org/t1448-kinesin-motor-proteins-amazing-cargo-carriers-in-the-cell
The Kinesin motor protein is the cargo carrier in the cell and helps in cell division. Its lifes smallest motor. They look like a post man, marching and holding the cargo with two " arms ", almost like humans. They know exactly where to catch the cargo, where to go in the cell, and where to drop it.
Micro tubule highways built up before they walk on them, assembled by proteins, each manufactured in accordance with the coded instructions of DNA, and once the cargo has been delivered at the right place, the nano highway dismantles. Until recently, scientists did not have a idea of how ATP fuel propels its walking like movement . The action happens at atomic level. That is one more example of the amazing engineering science has discovered in the cell. How do you want to explain these motors, which exist inside the cell ? They had all to be arise before life existed, since they make part of the cells functions. There is no selective advantage to have a " half " nanomotor. So how could it then arise in a step up fashion, just by natural means ? why should chance, physical necessity, or natural selection, provide a better explanation, than intelligent design ?
https://www.youtube.com/watch?v=FJ4N0iSeR8U
http://iaincarstairs.wordpress.com/2013/03/25/as-smart-as-molecules/
The kinesin illustrated above is from a Harvard animation and shows in correct proportion the transport of a sack of proteins from one place in the cell to another on one of the hundreds of thousands of highways which mysteriously build and unravel within every cell. Kinesin actually walks, step over step, travelling exclusively outwards from the centre of the cell, in strides of 8 nanometres. It can cope with obstacles in its path while holding onto its bag of goodies.
It covers 1 millimetre in 125,000 steps, making at most a few hundred steps in each journey, at a rate of 100 steps/second. The 14 variant groups of these motor proteins have been the subject of ingenious research, some of which precisely measured their force in PicoNewtons while striding over nanometre bridges. The graph is a straight line, meaning they’re as strong at the end of the journey as the beginning: given a surrounding supply of ATP they are literally tireless.
Kinesin’s fuel efficiency is nearly 50% – much more than a gasoline engine, and scaled up, exerts an incredible amount of torque. It requires no supernatural intervention: in vitro it behaves exactly as in the cell, given ATP, simply due to the arrangement of the amino acid components. Its step is precisely calibrated to the microtubule segments; the two components are part of one system.
Therefore the distance walked by one of these little Santas in a day is 69 million nanometres, or 6.9 centimetres – small wonder you need your own body weight in ATP each day. Scale him up to our size so his stride is 80 cm rather than 8 Nm with a factor of 100m and he could be covering 4,200 miles in a day.
With perhaps 100,000 at work in a single cell, and with 100 trillion cells, that’s a total distance travelled per day of just under 7 metres per cell, 691 billion km all told. Even if they only worked half the elapsed time, and then, only for one night – that’s easily enough to visit every house on the planet.
As for the elves, hammering away – clearly a metaphor for the ribosomes! And Rudolph.. well, perhaps his nose is a phosphate molecule.
http://creation.com/incredible-kinesin
Fast and efficient
Not only do these incredible kinesin robots perform a variety of tasks, they also do so with incredible efficiency! Check out these ‘state of the art’ features:
Power—“Not only is it tiny, but kinesin’s motor is about 50 percent efficient, which is about twice as good as a gasoline engine. And pound for pound, kinesin produces nearly 15 times more power than that man-made engine.”5
Speed—The kinesin motor is impressively fast, capable of 100 steps per second. “Scaled up to our own dimensions, a motor with corresponding properties would travel at similar speeds and produce as much horsepower per unit weight as the jet engines of the Thrust supersonic car6, which recently broke the sound barrier.”7 (This would be proportional to a person moving 600 meters per second or 1,300 miles per hour!)
Energy efficient—Kinesins are powered by the universal energy compound known as ATP (which is produced by another incredible molecular motor called ATP synthase—see animation, below right. Each molecule of ATP “fuel” that kinesin encounters triggers precisely one 8-nanometer step of the ‘postman’, but kinesins go into ‘sleep mode’ when cargo isn’t attached to prevent ATP from being wasted. Similar to how modern computers shut down after a period of un-use to conserve energy, kinesin have a hibernation feature as well. (Although scientists know that the motor folds over in an “autoinhibited” 8 state when resting, the molecular mechanism remains unclear.)
Team players—Kinesin molecules also work together when the going gets tough! If the load needing transport is too heavy for one ‘postman’ to handle, there is “ … significant evidence that cargoes in-vivo are transported by multiple motors.”9
They also demonstrate ‘multiple handling’ of their cargo. Similar to runners in a relay race, kinesins can ‘hand off’ their cargo to a ‘fresh’ bystander after delivering it a certain distance, and the other kinesin will finish the delivery process.
Flexible planning—Kinesins also have a ‘bypass mode’ ability that allows them to navigate around obstructions they may encounter. Similar to a GPS system ‘re-computing’, kinesins have demonstrated the remarkable ability to re-route automatically when needed.
Recycling—The most ardent champion of the ‘green’ movement would be jealous of the kinesin’s conservation and recycling capability. There is good evidence they are either transported back to the cell center in groups by large transport units (like mass transit in cities) or alternatively dismantled and their parts recycled when done their tasks.10
http://www.evolutionnews.org/2011/08/molecular_motors_enter_into_en049491.html
http://creationrevolution.com/walking-molecules-%E2%80%93-simple-cell-%E2%80%93-part-4/
http://creationsensation.blogspot.com.br/2012/11/the-kinesin-linear-motor.html
http://newscenter.lbl.gov/feature-stories/2010/02/17/kinesin-seesaw/
http://valelab.ucsf.edu/publications/1998kulljmusrescellm.pdf
Recent studies have shown surprising structural and functional similarities between the motor domains of kinesin and
myosin. Common features have also been described for motor proteins and G proteins. Despite these similarities, the
evolutionary relationships between these proteins, even among the motor proteins, has not been obvious, since the
topological connectivities of the core overlapping structural elements in these transducing proteins are not identical to one
another. Using secondary structure topology, comparison of functional domains and active site chemistry as criteria for
relatedness, we propose a set of rules for determining potential evolutionary relationships between proteins showing little or
no sequence identity.
http://www.evolutionnews.org/2014/06/kinesin_fast_ef_1086631.html
How do the proteins and organelles get from where they are made to where they are used? And how do things that need to be recycled return to the cell body? It's by means of kinesin (for outward bound travel) and another motor protein called dynein (for inward bound travel). Remarkably, dynein and kinesin cooperate, rather than compete -- otherwise there would be a constant tug of war in the cell. They "know" where packages are meant to go, and which motor protein should do the job. How that happens is a whole other story.
This isn't just abstract stuff. Many neurodegenerative diseases have been linked to mutations of kinesin, including Alzheimer's disease. So this little workhorse of the cell is not only a marvel, but essential.
Finally, in order for kinesin to work, we need the motor protein itself, the microtubule highway it walks along, and some mysterious means of connecting and directing where its cargos get carried. This system appears to be essential for a variety of eukaryotic cell functions. At least 11 kinesin families appear to have existed in the putative last common ancestor for all eukaryotes.

Life’s smallest motor, a protein that shuttles cargo within cells and helps cells divide, does so by rocking up and down like a seesaw, according to research conducted by scientists at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory and Brandeis University.
A molecular motor gives up its secrets. Electron microscope images of kinesin, one of life's smallest molecular motors, have been used to derive new, highly detailed 3-D maps
A molecular motor gives up its secrets. Electron microscope images of kinesin, one of life's smallest molecular motors, have been used to derive new, highly detailed 3-D maps (transparent surface) of the motor frozen in action. By fitting atomic models (colored ribbons) into the 3-D maps, a detailed mechanism has been derived for how a single molecule of biological fuel, called ATP, initiates motor movement. (Image by Charles Sindelar, Brandeis University)
The researchers created high-resolution snapshots of a protein motor, called kinesin, as it walked along a microtubule, which are tube-shaped structures that form a cell’s “skeleton.” The result is the closest look yet at the structural changes kinesin proteins undergo as they ferry molecules within cells.
“We see for the first time how kinesin’s atomic-scale moving parts allow it to pull itself and its cargo along a microtubule,” says Ken Downing, a biophysicist with Berkeley Lab’s Life Sciences Division. He conducted the research with postdoctoral fellow Charles Sindelar, now at Brandeis University.
“We found that there is a pivot point, where the kinesin motor attaches to the microtubule, which acts like a fulcrum and causes kinesin to rock up and down like a seesaw as it moves along the microtubule,” adds Downing.
Their research is reported this week in the online early edition of the Proceedings of the National Academy of Sciences.
The first-ever glimpse of kinesin’s seesaw motion offers key insights into one of life’s most fundamental processes. Fueled by an energy-giving compound called ATP, kinesin proteins motor along microtubules like trains on a railroad track, towing cargo to various locations within cells and assisting in cell division. Microtubules are a cylindrical weave of proteins found throughout cells that serve as cellular scaffolding.
Until now, scientists did not have a clear picture of what happens when ATP binds with kinesin, and especially how this process triggers structural changes in kinesin that propel the protein along microtubules.
Extremely high-resolution crystallography images of kinesin motors have enabled researchers to piece together the protein’s three-dimensional structure. But these images don’t reveal how it works.
“The problem is that it is not until the protein motor binds to a microtubule that structural rearrangements occur that enable ATP hydrolysis, the process that transfers energy from
ATP to kinesin,” says Downing.
To image kinesin at this critical stage, Downing and Sindelar turned to cryoelectron microscopy, which is a type of electron microscopy in which the sample is studied at extremely low temperatures. The technology is used by structural biologists to image proteins and other molecules as they appear in real-world conditions, in this case a kinesin protein attached to a microtubule.
The technique yielded 8 to 9 angstrom-resolution snapshots of the kinesin motor at four stages of the motor’s cycle as it moves along a microtubule. One angstrom is one-ten billionth of a meter. Using these images as a guide, the researchers then “dropped in” even higher resolution crystallographic images of kinesin’s components. This step enabled them to derive atomic-level structural models of kinesin in action.
“Collectively, this work provides a detailed molecular explanation for kinesin’s microtubule-attached power stroke,” says Downing. “In other words, we can see it how it works in real life. We looked at kinesin in different phases, and learned what causes it to move from one conformation to another, which is how it pulls cargo along the microtubule.”
In addition to further elucidating a key biological process, Downing and Sindelar’s research may inform the development of disease-fighting drugs. One of kinesin’s main jobs is moving chromosomes apart during cell division. Anything that blocks this process will lead to cell death, which is the basis of several cancer therapies such as taxol.
“New insights into how kinesin works could allow scientists to develop drugs that target and block particular kinesin movements,” says Downing.
http://faculty.washington.edu/casbury/pdfs/Asbury%202005%20Curr%20Opin%20Cell%20Biol.pdf
2) http://crev.info/2010/01/molecular_machines_use_moving_parts/
Last edited by Otangelo on Fri Nov 05, 2021 6:14 am; edited 13 times in total