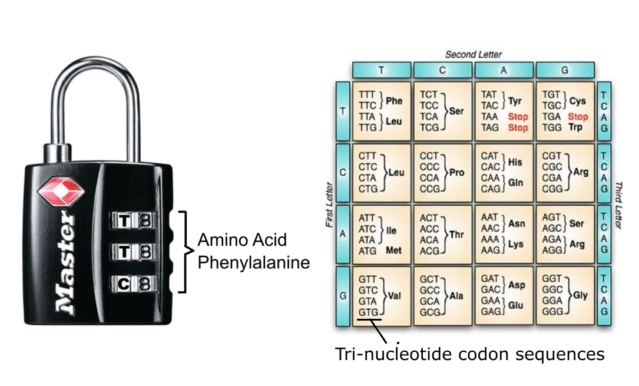
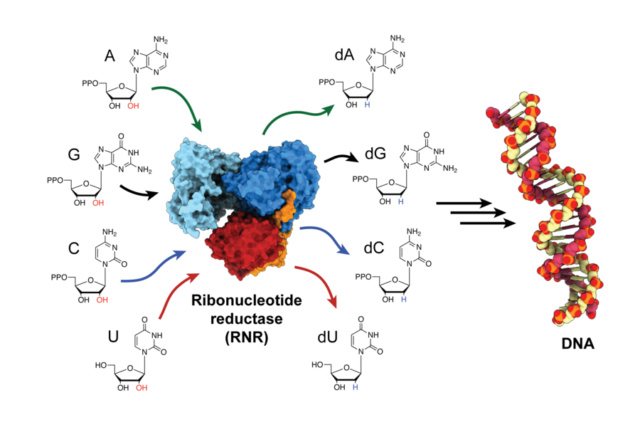
Biochemical fine-tuning - essential for life
https://reasonandscience.catsboard.com/t2591-biochemical-fine-tuning-essential-for-life
The fact that abiogenesis is not possible becomes evident and clear to anyone considering this: The basic building blocks that makeup life are four: nucleotides, amino acids, phospholipids, and carbohydrates. To illustrate my point, i will mention just nucleotides:
Nucleotides are composed of three distinctive chemical sub-units: a five-carbon sugar molecule, a nitrogenous base, and one phosphate group.
Ribose is sugar with five primes. Nucleobases must be always attached at the one prime end with glycosidic bonds, and phosphate groups at the 5 prime end, and joining the next nucleotide monomer for polymerization at the 3 prime positions.
There NO prebiotic natural selection that constraints the attachment of the phosphate group at these two positions, and worse, in a repetitive manner. It can be anywhere on the five primes.
The same problem is in regards to the correct position of nucleobases at the one prime position. Not only is there no prebiotic glycosidic bond formation process known, but there is no natural selection to attach the base at the one prime position, in a repetitive manner.
Nucleobases, pyrimidine, and purines require to have planar, flat shapes, in order to be able to bond together. The nitrogenous bases form hydrogen bonds between opposing DNA strands to form the rungs of the "twisted ladder" or double helix of DNA. If they were not complementary in the right way, they would not form the DNA ladder. But there is natural selection either to produce the right forms.
AmazingWatson–Crick base-pairing
The existence of Watson–Crick base-pairing in DNA and RNA is crucially dependent on the position of the chemical equilibria between tautomeric forms of the nucleobases.1 These equilibria in both purines and pyrimidines lie sharply on the side of amide- and imide-forms containing the (exocyclic) oxygen atoms in the form of carbonyl groups (C=O) and (exocyclic) nitrogen in the form of amino groups (NH2). The positions of these equilibria in a given environment are an intrinsic property of these molecules, determined by their Physico-chemical parameters (and thus, ultimately, by the fundamental physical constants of this universe). The chemist masters the Herculean task of grasping and classifying the boundless diversity of the constitution of organic molecules by using the concept of the “chemical bond.” He pragmatically deals with the differences in the thermodynamic stability of molecules by using individual energy parameters, which he empirically assigns to the various types of bonds in such a way that he can simply add up the number and kind of bonds present in the chemical formula of a molecule and use their associated average bond energies to estimate the relative energy content of essentially any given organic molecule.
Now comes the striking interpretation of the Darwinism-inclined and indoctrinated mind :
Whatever biological phenomena appear fine-tuned can be interpreted in principle as the result of life having finetuned itself to the properties of matter through natural selection. Indeed, to interpret in this way what we observe in the living world is mainstream thinking within contemporary biology and biological chemistry.
Sometimes it strikes me how un-imaginative these folks are. They cannot imagine anything else besides NATURAL SELECTION. So the hero on the block strikes again. The multi-versatile mechanism propagated by Darwin explains and solves practically any issue and arising question of origins. Can't explain a phenomenon in question? NS did it..... huh...
This alone is a real KILLER for abiogenesis. If scientists would consider it, they would move on to other scientific fields, and forget about attempting to solve the riddle of the origin of life by natural unguided means. Its, simply stated, IMPOSSIBLE !!
https://reasonandscience.catsboard.com/t2591-biochemical-fine-tuning-essential-for-life
The fact that abiogenesis is not possible becomes evident and clear to anyone considering this: The basic building blocks that makeup life are four: nucleotides, amino acids, phospholipids, and carbohydrates. To illustrate my point, i will mention just nucleotides:
Nucleotides are composed of three distinctive chemical sub-units: a five-carbon sugar molecule, a nitrogenous base, and one phosphate group.
Ribose is sugar with five primes. Nucleobases must be always attached at the one prime end with glycosidic bonds, and phosphate groups at the 5 prime end, and joining the next nucleotide monomer for polymerization at the 3 prime positions.
There NO prebiotic natural selection that constraints the attachment of the phosphate group at these two positions, and worse, in a repetitive manner. It can be anywhere on the five primes.
The same problem is in regards to the correct position of nucleobases at the one prime position. Not only is there no prebiotic glycosidic bond formation process known, but there is no natural selection to attach the base at the one prime position, in a repetitive manner.
Nucleobases, pyrimidine, and purines require to have planar, flat shapes, in order to be able to bond together. The nitrogenous bases form hydrogen bonds between opposing DNA strands to form the rungs of the "twisted ladder" or double helix of DNA. If they were not complementary in the right way, they would not form the DNA ladder. But there is natural selection either to produce the right forms.
AmazingWatson–Crick base-pairing
The existence of Watson–Crick base-pairing in DNA and RNA is crucially dependent on the position of the chemical equilibria between tautomeric forms of the nucleobases.1 These equilibria in both purines and pyrimidines lie sharply on the side of amide- and imide-forms containing the (exocyclic) oxygen atoms in the form of carbonyl groups (C=O) and (exocyclic) nitrogen in the form of amino groups (NH2). The positions of these equilibria in a given environment are an intrinsic property of these molecules, determined by their Physico-chemical parameters (and thus, ultimately, by the fundamental physical constants of this universe). The chemist masters the Herculean task of grasping and classifying the boundless diversity of the constitution of organic molecules by using the concept of the “chemical bond.” He pragmatically deals with the differences in the thermodynamic stability of molecules by using individual energy parameters, which he empirically assigns to the various types of bonds in such a way that he can simply add up the number and kind of bonds present in the chemical formula of a molecule and use their associated average bond energies to estimate the relative energy content of essentially any given organic molecule.
Now comes the striking interpretation of the Darwinism-inclined and indoctrinated mind :
Whatever biological phenomena appear fine-tuned can be interpreted in principle as the result of life having finetuned itself to the properties of matter through natural selection. Indeed, to interpret in this way what we observe in the living world is mainstream thinking within contemporary biology and biological chemistry.
Sometimes it strikes me how un-imaginative these folks are. They cannot imagine anything else besides NATURAL SELECTION. So the hero on the block strikes again. The multi-versatile mechanism propagated by Darwin explains and solves practically any issue and arising question of origins. Can't explain a phenomenon in question? NS did it..... huh...
This alone is a real KILLER for abiogenesis. If scientists would consider it, they would move on to other scientific fields, and forget about attempting to solve the riddle of the origin of life by natural unguided means. Its, simply stated, IMPOSSIBLE !!