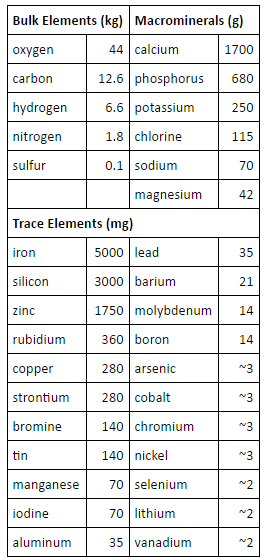
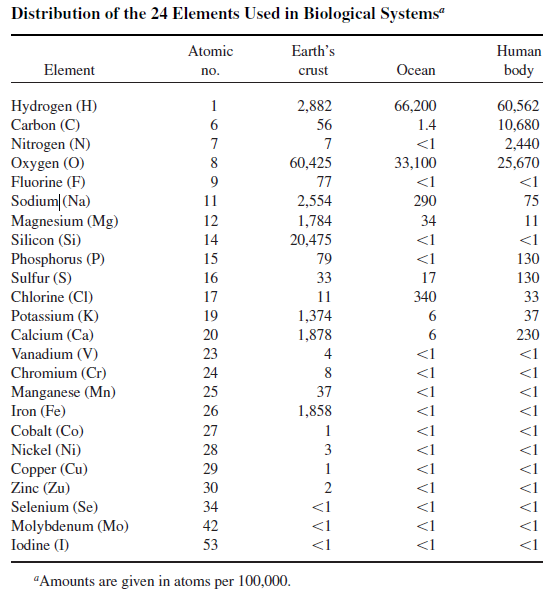
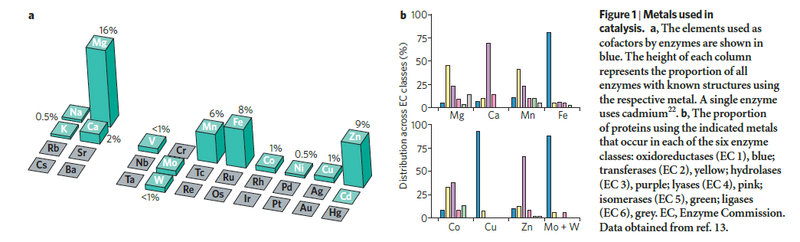
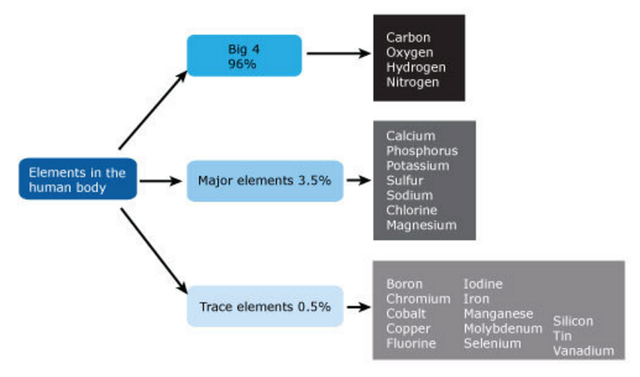
Oxygen
Our present atmosphere consists of 78% nitrogen (N2), 21% molecular oxygen (O2), and 1% of other gases, such as carbon dioxide CO2), argon (Ar), and water vapor H2O). An atmosphere containing free oxygen would be fatal to all origin of life schemes. While oxygen is necessary for life, free oxygen would oxidize and thus destroy all organic molecules required for the origin of life. Thus, in spite of much evidence that the earth has always had a significant quantity of free oxygen in the atmosphere, 3 proponents of evolution persist in declaring that there was no oxygen in the earth's early atmosphere. However, this would also be fatal to an evolutionary origin of life. If there were no oxygen there would be no protective layer of ozone surrounding the earth. Ozone is produced by radiation from the sun on the oxygen in the atmosphere, converting the diatomic oxygen(O2) we breathe to triatomic oxygen O3), which is ozone. Thus if there were no oxygen there would be no ozone. The deadly destructive ultraviolet light from the sun would pour down on the surface of the earth unimpeded, destroying those organic molecules required for life, reducing them to simple gases, such as nitrogen, carbon dioxide, and water. Thus, proponents of evolution face an irresolvable dilemma: in the presence of oxygen, life could not evolve; without oxygen, thus no ozone, life could not evolve or exist. 1
Carbon
Carbon is unique in its ability to combine with other atoms, forming a vast and unparalleled number of compounds in combination with hydrogen, oxygen and nitrogen. This universe of organic chemistry— with its huge diversity of chemical and physical properties—is precisely what is needed for the assembling of complex chemical systems. Furthermore, the general ‘metastability’ of carbon bonds and the consequent relative ease with which they can be assembled and rearranged by living systems contributes greatly to the fitness of carbon chemistry for biochemical life. No other atom is nearly as fit as carbon for the formation of complex biochemistry. Today, one century later, no one doubts these claims. Indeed the peerless fitness of the carbon atom to build chemical complexity and to partake in biochemistry has been affirmed by a host of researchers.
One widely publicized coincidence is the ‘lucky’ fact that the nuclear resonances of the isotopes 12C and 16O are exactly what they need to be if carbon is to be synthesized and accumulate in any quantity in the interior of stars . The energy levels of these resonances ensure that 12C is first synthesized in stellar interiors from collisions between 8Be (beryllium) and He (helium) nuclei, and that the carbon synthesized is not depleted later. Hoyle made this discovery in 1953 while working at Caltech with William Fowler. An intriguing aspect of the discovery is that Hoyle made it based on a prediction from the anthropic principle . Hoyle himself
famously commented:
If you wanted to produce carbon and oxygen in roughly equal quantities by stellar nucleosynthesis, these are the two levels you would have to fix, and your fixing would have to be just about where these levels are found to be ... A common sense interpretation of the facts suggests that a super intellect has monkeyed with physics, as well as chemistry and biology, and that there are no blind forces worth speaking about in nature.
This discovery was acclaimed not only as a major scientific discovery but also as further evidence of the biocentricity of nature. Hoyle may have been one of the first to notice that the conditions necessary to permit carbon-based life require a very narrow range of basic physical constants, but the idea is now widely accepted. If those constants had been very slightly different, the universe would not have been conducive to the development of matter, astronomical structures, or elemental diversity, and thus the emergence of complex chemical systems.
Chlorine
This is a fascinating element that is found in all living tissue. Chlorine is essential for the function of cleansing the body of debris. It is also exchanged in the stomach to produce hydrochloric acid, a very necessary acid for protein digestion. Chlorine is a member of a group of elements called the halogens. Others in this group are fluoride, iodine, and bromine. The body maintains a delicate balance between all these elements. Today too much chlorine, bromine and fluoride are overwhelming the iodine and causing deficiencies in our bodies. Deficiency of this element is non-existent, unlike all the other electrolytes. The reason is that chlorine is part of salt (NaCl). Most people eat too much, rather than too little table salt, as it is found in almost all prepared and processed food items today. Thus we do not focus on this element in terms of deficiencies. In contrast, excessive exposure to chlorine is a severe problem. Too much table salt and chlorinated water are the main sources. Some bleached flour products are also sources. Environmental contamination of the food, water, and air are constant sources of this element, which is highly toxic in these forms. 3
Hydrogen
The most abundant element in the universe, hydrogen is also a promising source of "clean" fuel on Earth. Named after the Greek words hydro for "water" and genes for "forming," hydrogen makes up more than 90 percent of all of the atoms, which equals three-quarters of the mass of the universe, according to the Los Alamos National Laboratory. Hydrogen is essential for life, and it is present in nearly all the molecules in living things, according to the Royal Society of Chemistry. The element also occurs in the stars and powers the universe through the proton-proton reaction and carbon-nitrogen cycle. 4
Nitrogen
Nitrogen is one of the essential nutrients of life on Earth, with some organisms, such as the kinds of microbes found within the roots of legume plants, capable of converting nitrogen gas into molecules that other species can use. 1 Nitrogen fixation, as the process is called, involves breaking the powerful chemical bonds that hold nitrogen atoms in pairs in the atmosphere and using the resulting single nitrogen atoms to help create molecules such as ammonia, which is a building block of many complex organic molecules, such as proteins, DNA and RNA. Stüeken developed a model of abiotic nitrogen processes that could have played a role in early Earth. The results showed that such abiotic processes alone could not explain the nitrogen levels seen in the Isua rocks. 5
Potassium
Potassium is an essential mineral micronutrient and is the main intracellular ion for all types of cells. It is important in maintaining fluid and electrolyte balance in the bodies of humans and animals. Potassium is necessary for the function of all living cells and is thus present in all plant and animal tissues. It is found in especially high concentrations within plant cells, and in a mixed diet, it is most highly concentrated in fruits. The high concentration of potassium in plants, associated with comparatively very low amounts of sodium there, historically resulted in potassium first being isolated from the ashes of plants (potash), which in turn gave the element its modern name. The high concentration of potassium in plants means that heavy crop production rapidly depletes soils of potassium, and agricultural fertilizers consume 93% of the potassium chemical production of the modern world economy.
Potassium, another solvent mineral, and a heart mineral. It is also essential for regulation of the heartbeat, fluid balance and to maintain blood pressure. It is also needed for buffering the blood, and cell membrane effects including nerve transmission and muscular contraction. Deficiency can cause cramps, fatigue and heart irregularities. Good sources are herring, sardines, halibut, goose, most nuts and seeds, watercress, garlic, lentils, spinach, artichokes, lima beans, Swiss chard, avocados, buckwheat, wheat bran, molasses, and kelp. Be sure to drink the water in which you cook vegetables to obtain the potassium from the vegetables. [url=http://drlwilson.com/articles/minerals for life.htm]6[/url]
Calcium
In view of the importance of calcium (Ca2+) as a universal intracellular regulator, its essential role in cell signaling and communication in many biological Intra and extracellular processes, it is surprising how little it is mentioned in the origins ( evolution/ID) debate. Most discussions about the origin of life start with RNA worlds versus metabolism-first scenarios, panspermia, hydrothermal vent theory etc. The origin of life cannot be elucidated, without taking into consideration and explaining how the calcium signaling machinery and cell homeostasis appeared.
Calcium, the structural element, is found mainly in our bones. Calcium also regulates cell membrane permeability to control nerve impulse transmission and muscle contraction. It is important for blood clotting, and it regulates hormonal secretion and cell division. Good food sources are dairy products such as cheese and yogurt. Smaller amounts are in milk, sardines, egg yolks, almonds, sesame seeds, seaweed and dark green vegetables. Goat cheese is better than cow’s milk cheese for most people because cows are often fed or injected with antibiotics, female hormones, and growth hormones. 6
Molybdenum
Minerals containing the elements boron and molybdenum are key in assembling atoms into life-forming molecules. 11 The researcher points out that boron minerals help carbohydrate rings to form from pre-biotic chemicals, and then molybdenum takes that intermediate molecule and rearranges it to form ribose, and hence RNA. This raises problems for how life began on Earth, since the early Earth is thought to have been unsuitable for the formation of the necessary boron and molybdenum minerals. It is thought that the boron minerals needed to form RNA from pre-biotic soups were not available on early Earth in sufficient quantity, and the molybdenum minerals were not available in the correct chemical form. "It’s only when molybdenum becomes highly oxidised that it is able to influence how early life formed. "This form of molybdenum couldn’t have been available on Earth at the time life first began, because three billion years ago, the surface of the Earth had very little oxygen. 7
Sulfur
Sulfur, a fiery cleansing and joining mineral. It is an important element for digestion and detoxification in the liver. It is needed for the joints and in all connective tissue. This includes the hair, skin and nails. Most dietary sulfur comes from sulfur-containing amino acids found mainly in animal protein foods. Good sources are eggs, meats, and often smelly foods like garlic and onions. Other sources are kale, watercress, Brussels sprouts, horseradish, cabbage cauliflower, and cranberries. Vegetarians can easily become deficient in sulfur if they do not eat eggs. Deficiency can affect hair, nails, skin, joints, energy and the ability to detoxify poisons. Today, plenty of organic or usable sulfur is needed to oppose excess copper in the body. Most people today have too much biounavailable copper in their bodies, and sulfur is needed to help remove it. Good sources are animal proteins such as eggs, particularly the egg yolk. [url=http://drlwilson.com/articles/minerals for life.htm]6[/url]
Iron
The origin of life required two processes that dominated:
(1) the generation of a proton gradient and
(2) linking this gradient to ATP production in part and in part to uptake of essential chemicals and rejection of others. The generation of a proton gradient required especially appropriate amounts of iron (Fe2+), levels for electron transfer and the ATP production depended on controlling H+, Mg2+ and phosphate in the cytoplasm. 8
Iron serves essential functions in both prokaryotes and eukaryotes, and cells have highly specialized mechanisms for acquiring and handling this metal.
Organisms use a variety of transition metals as catalytic centers in proteins, including iron, copper, manganese, and zinc. Iron is well suited to redox reactions due to its capability to act as both an electron donor and acceptor. In eukaryotic cells, iron is a cofactor for a wide variety of metalloproteins involved in energy metabolism, oxygen binding, DNA biosynthesis and repair, synthesis of biopolymers, cofactors, and vitamins, drug metabolism, antioxidant function, and many others. Because iron is so important for survival, organisms utilize several techniques to optimize uptake and storage to ensure maintenance of sufficient levels for cellular requirements. However, the redox properties of iron also make it extremely toxic if cells have excessive amounts. Free iron can catalyze the formation of reactive oxygen species such as the hydroxyl radical, which in turn can damage proteins, lipids, membranes, and DNA. Cells must maintain a delicate balance between iron deficiency and iron overload that involves coordinated control at the transcriptional, post-transcriptional, and post-translational levels to help fine tune iron utilization and
iron trafficking.
Iron, the oxygen carrier and an energy mineral as well. It is required in hemoglobin for transporting oxygen in the blood, for detoxification and for energy production in the cells. Iron is found in lean meats, organ meats, shellfish, molasses, beans, whole-grain cereals, and dark green vegetables. Menstruating women and children on poor diets are most commonly low in iron. For much more information about iron, read Chronic Iron Toxicity.
Magnesium
There are 24 metal and nonmetal elements, that are essential for life, amongst them magnesium, which plays a critical role in cellular metabolism, DNA repair, its also present in all deoxyribonucleic acid (DNA) and RNA activation processes, stabilizing macromolecular complexes and membranes. As activator of over 300 different enzymes, magnesium participates in many metabolic processes, such as glycolysis, Krebs cycle, β-oxidation or ion transport across cell membranes. Cells must have mechanisms to maintain physiological levels of Mg2+. It is indispensable for the nucleus ( in eukaryotes ) to function as a whole and for the maintenance of physical stability as well as aggregation of rybosomes into polysomes able to initiate protein synthesis. All these different essential roles elucidate that life could not have had a first go without magnesium.
But in order for the cell to be able to make use of it, Magnesium like other metal ions has to be transported inside cells across the cell membrane by specific membrane proteins. Three distinct classes of Mg2+ transporters have been identified in bacteria. MgtA transporter proteins can sense magnesium ions down to micromolar concentrations, which is the equivalent to a pinch (1 gram) of magnesium salt in 10,000 liters of water. Wow ! This detection system depends on a specific lipid molecule in the membrane called cardiolipin. MgtA and cardiolipin have to work together in an interdependent manner.
Organisms must maintain physiological levels of Mg2+ because this divalent cation is critical for the stabilization of membranes and ribosomes, the neutralization of nucleic acids, and as a cofactor in a variety of enzymatic reactions. Furthermore, specialized biosynthesis pathways and specialized proteins exist to make these import proteins and cardiolipin.
Magnesium is the bright and shining mineral. Magnesium is named after the Greek city of Magnesia, where large deposits of magnesium carbonate were found centuries ago. It is required for over 500 enzymes that regulate sugar metabolism, energy production, cell membrane permeability, and muscle and nerve conduction. Foods high in magnesium include milk, almonds, brazil nuts, cashews, whole soybeans (but not tofu, tempeh or soy protein), parsnips, wheat bran, whole grains, green vegetables, seafood, kelp and molasses. Most people need more magnesium than they are eating because food refining strips away magnesium. Deficiency causes muscle cramps, weakness, depression, and fatigue. Magnesium works closely with potassium and is a calcium antagonist. 9
Magnesium and magnesium transporters, another example of cell interdependence comes to light
http://reasonandscience.heavenforum.org/t2440-magnesium-and-magnesium-transporters-another-example-of-interdependence-comes-to-light
Phosphate
The short supply of phosphorus poses a significant problem for a naturalistic origin of life because so much of this ingredient is required to make replicator molecules. Phosphates are part of the backbone of both DNA and RNA. A phosphate molecule must accompany every nucleoside in them. Possible precursors to DNA and RNA molecules would seem to require similar phosphate richness. Without life molecules (already assembled and operating), no known natural process can harvest the amounts of phosphorus necessary for life from the environment. All the phosphate-rich deposits on Earth are produced by life.
Phosphorus, the fiercest energy mineral. It is required for energy production, DNA synthesis, and protein synthesis. It is also needed for calcium metabolism, muscle contraction, and cell membrane structure. Excellent sources include all meats, along with eggs, fish and other animal proteins. All proteins have some phosphorus in them. However, red meats and high purine proteins tend to have the most. These include organ meats, sardines, and anchovies. The latter two are not bad fish to eat. Other fish tend to be too high in mercury to make them good foods for regular use. Other decent food sources are most nuts and seeds, chickpeas, garlic, lentils, popcorn, soybeans, and some cheeses. Animal-based sources of phosphorus are often absorbed better than grains and beans that contain phytates. These are phosphorus compounds that are not well-absorbed and that actually interfere with the absorption of calcium, magnesium, and zinc, in particular. They are found in most grains and beans. This is why proper cooking and preparation of bread, beans, and other foods is extremely important. Eating these foods raw eating unleavened bread is not wise for this reason.
Sodium
the volatility and the solvent mineral. It helps regulate blood pressure, fluid balance, transport of carbon dioxide, and affects cell membrane permeability and other cell membrane functions. Deficiency causes fatigue and fluid imbalances such as low blood pressure. Food sources include sea salt, seafood, eggs, beet greens, Swiss chard, olives, peas, and butter. Table salt is a refined junk food. Most of the minerals have been stripped away, and aluminum is often added as a flowing agent. Use natural sea salt instead.
Zinc
It is vital for the functionality of more than 300 enzymes, for the stabilization of DNA, and for gene expression. Helps generate cells Important for growth and brain development Key for immune system Humans need up to 15 mg of zinc per day. Zinc Deficiency is 5th Leading Cause of Death and Disease in the Developing World
Zinc is required for hundreds of enzymes in the human body. These include the sense of taste and smell, vision, growth, sexual development, digestive enzyme production, male potency, prostate gland health, blood sugar regulation and processing of alcohol.Zinc is very important for the joints, the skin, wound healing, and to prevent birth defects. Zinc helps prevent diabetes, acne, epilepsy and childhood hyperactivity, and helps detoxify heavy metals. Adequate zinc has a calming effect and is needed to regenerate all body tissues.Refined food is very low in zinc. There are very few excellent sources of zinc today. Among the best are red meats, organ meats and some seafood. Other sources that are not quite as good are poultry such as chicken and turkey, eggs, wheat, oatmeal, pumpkin and sunflower seeds, wheat germ and colostrum.
Vegetarians run a high risk of zinc deficiency because they avoid red meats, in most cases. Low zinc, especially in vegetarians, tends to cause a worsening of copper toxicity.
Chromium
a blood sugar mineral . It is also an energy mineral. A desert rodent called the sand rat develops diabetes when fed a laboratory diet. When returned to the desert, the diabetes goes away. Extensive research indicates the problem with the laboratory food is a lack of chromium.
Chromium is essential to for insulin metabolism. It can also help lower cholesterol. Chromium deficiency is very common, especially in middle-aged and older people. Food sources of chromium are brewers yeast, liver, kidney, beef, whole wheat bread, wheat germ, beets, mushrooms and beer. Unfortunately, most of these foods are not recommended for various reasons. Chromium can be obtained from supplements, and this is usually the best way to make sure you get enough each day.
Selenium
is required for the development of certain higher brain centers. Selenium is vital for detoxification and for thyroid activity in the human body, among its many functions. It is also needed for protein synthesis, helps the body get rid of toxic cadmium and mercury, and is needed for antioxidant production (glutathione peroxidase). As an anti-oxidant, it may help prevent cancer and birth defects.
Lithium
It is also a more advanced spiritual mineral for the future. It has a calming, balancing and protective effect on the brain and the entire nervous system. It is found in many natural foods so it is not necessary to supplement it in many cases. However, anyone who is taking an anti-depressant or any brain-altering drug, or is suffering from any brain-related problem may benefit from a natural lithium supplement such as lithium orotate. The lithium used by medical doctors for bipolar disorder is quite toxic and should be avoided if at all possible. The natural product is far less potent, but is better absorbed and much less toxic or perhaps totally non-toxic.
Cobalt
vitamin B12 mineral. Cobalt is essential for life as part of the vitamin B12 molecule. Vitamin B12 is required for the nervous system and blood formation. It is found in animal products. Deficiency causes anemia and a very severe dementia that can be irreversible.
Deficiency occurs mainly in strict vegetarians and in those with impaired digestion or any disorder of the stomach. It is deficient in some elderly people whose stomach does not absorb it very well.
Iodine
a cleanser and a thyroid mineral (along with manganese). Iodine, however, it is required for all the cells of the body. It is somewhat more important for women. It is needed to make thyroid hormones, and for the regulation of metabolism. It is important for women’s breast health, cancer prevention and many other body functions in somewhat mysterious ways.
Boron
It is very essential for plants, though perhaps less so for human beings. Boron can help maintain female hormone production and bone integrity.
Silicon
along with selenium, is important for the bones and skin. Food sources include lettuce, parsnips, asparagus, dandelion greens, rice bran, horseradish, onion, spinach and cucumbers, and in herbs such as horsetail. Since it is in many foods, supplements are usually not needed. Silicon and selenium also are both spiritual minerals needed for higher brain activity.
Trace minerals often work in pairs or triplets. The interaction of minerals in the body is a complex and interesting subject. There are many other trace minerals such as molybdenum, vanadium, bromine, germanium, nickel, tin, cesium, rubidium, strontium, gold, silver, titanium, tritium and others. 10
1. http://www.icr.org/article/few-reasons-evolutionary-origin-life-impossible/
2. http://thisquantumworld.com/wp/a-critique-of-quantum-mechanics/fine-tuning/
3. http://www.warriormatrix.com/about13509.html&sid=0a2ba2e8e17096632a536b1d8d85f852
4. https://www.livescience.com/28466-hydrogen.html
5. https://www.astrobio.net/news-exclusive/nitrogen-ancient-rocks-sign-early-life/
6. http://drlwilson.com/articles/minerals%20for%20life.htm
7. http://www.universityherald.com/articles/4375/20130829/earth-life-began-mars-red-planet-building-blocks-rna-dna.htm
8. https://link.springer.com/chapter/10.1007/978-3-642-58306-3_1
9. http://www.living-well.info/minerals-for-life/
10. http://drlwilson.com/Articles/MINERALS%20FOR%20LIFE.htm