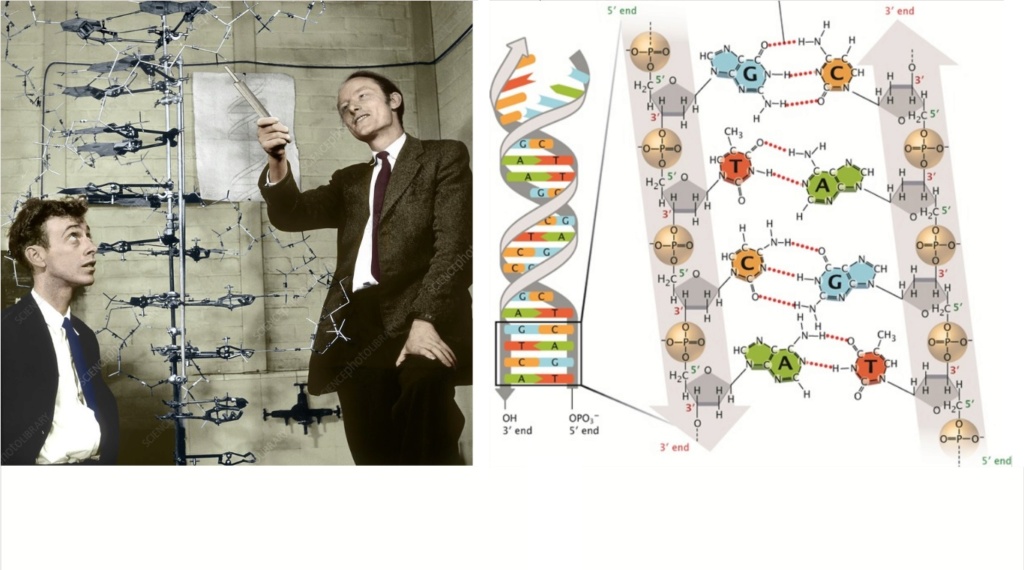
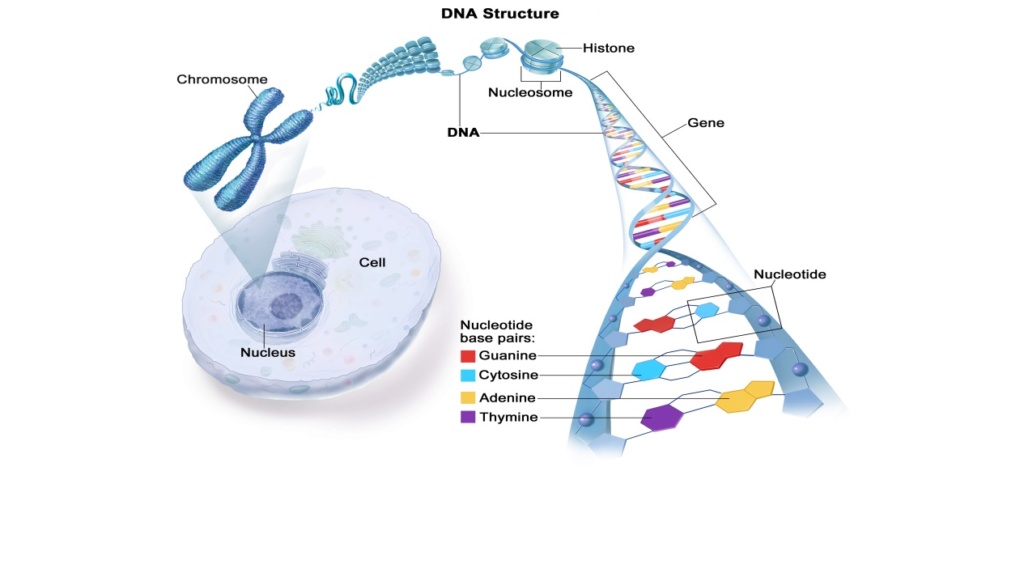
https://reasonandscience.catsboard.com/t2865-rna-dna-it-s-prebiotic-synthesis-impossible
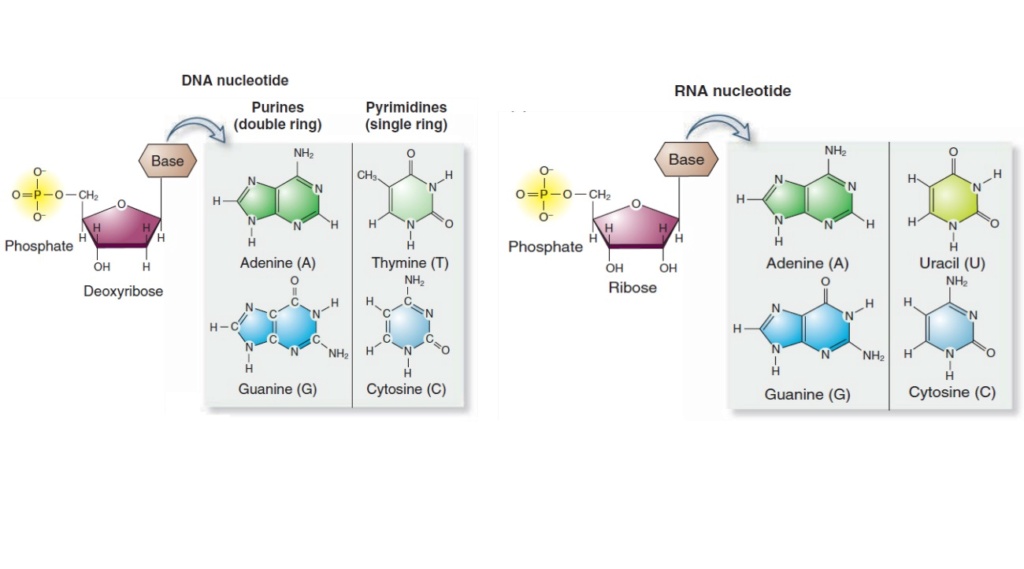
The synthesis of nucleobases, essential components of RNA and DNA, presents significant challenges in prebiotic chemistry. These challenges cast doubt on the hypothesis that life could have emerged spontaneously from simple chemical precursors on early Earth. The complexity of nucleobase synthesis is a primary obstacle. It requires a precise sequence of reactions, each with specific chemical requirements that are difficult to fulfill without biological catalysts. The probability of these intricate molecules forming spontaneously under natural conditions is exceedingly low, raising questions about their emergence without guidance. Certain nucleobases pose unique challenges. Cytosine and guanine lack viable natural pathways for their formation under prebiotic conditions. Despite extensive research, no plausible routes have been identified for their spontaneous synthesis. This gap in understanding is particularly problematic, as both molecules are crucial components of nucleic acids. The stability of nucleobases under prebiotic conditions is another major concern. These molecules exhibit short half-lives, rapidly degrading under conditions thought to resemble those of early Earth. This instability makes it unlikely that nucleobases could have accumulated in sufficient quantities to contribute to the formation of RNA or DNA.
Cytosine synthesis represents an especially formidable obstacle. To date, no natural route has been identified that could produce cytosine under plausible prebiotic conditions. This issue raises critical questions about how this essential component of nucleic acids could have emerged without guidance. Adenine synthesis requires extremely high concentrations of hydrogen cyanide, conditions unlikely to have existed on early Earth. Additionally, adenine is prone to rapid deamination, further complicating its stable accumulation and availability. Uracil, a key component of RNA, suffers from significant stability issues, particularly under the temperature conditions likely present on early Earth. Its short half-life at these temperatures complicates scenarios where uracil could have accumulated in sufficient quantities to participate in RNA formation. The phenomenon of nucleobase tautomerism presents another challenge. Controlling these structural forms to ensure proper base pairing is nearly impossible in a prebiotic environment without the regulatory mechanisms present in living systems. This lack of control could lead to errors in the formation of functional nucleic acids. Achieving the necessary concentrations of precursors for nucleobase formation is another formidable challenge. The dilute conditions presumed to have existed on early Earth would have made it nearly impossible to reach the concentrations required for these reactions to occur efficiently.
Identifying plausible prebiotic energy sources to drive the energetically unfavorable reactions involved in nucleobase synthesis remains an unresolved challenge. The absence of a reliable and consistent energy source raises significant doubts about how these reactions could have proceeded on early Earth. Controlling side reactions in a prebiotic environment poses another difficulty. The presence of various reactive species would have made it challenging to prevent or control unwanted side reactions that could interfere with nucleobase synthesis. Many of the reactions critical to nucleobase synthesis are thermodynamically unfavorable under prebiotic conditions. Without external intervention or highly specific conditions, the likelihood of these reactions occurring naturally is exceedingly low. The synthesis of nucleobases requires highly specific environmental conditions, including precise control over temperature, pH, and atmospheric composition. Achieving and maintaining these conditions over extended periods presents a significant challenge on early Earth. Water, while essential for many biochemical reactions, also promotes the rapid degradation of nucleobases and their precursors. This paradox presents a significant challenge for prebiotic nucleobase synthesis.
Ensuring the correct isomeric configuration of nucleobases is crucial for Watson-Crick base pairing, yet controlling this configuration without biological systems is extremely difficult. Incorrect isomers would result in faulty base pairing, hindering the formation of functional nucleic acids. The stereochemistry of the sugar components in nucleotides is critical for proper base pairing and the formation of stable nucleic acids. Achieving the correct stereochemistry in a prebiotic environment, without enzymatic guidance, is a significant challenge. Explaining the origin of homochirality in nucleotides remains one of the most significant unsolved problems in prebiotic chemistry. Without a mechanism to enforce chiral selection, the emergence of a uniform chirality required for functional nucleic acids is highly improbable. The stability of Watson-Crick base pairs relies heavily on precise bond energies within the nucleobases. Achieving the level of fine-tuning necessary for stable nucleic acids without biological regulatory systems is highly improbable. The specific sugar-phosphate backbone required for nucleic acid stability poses another significant challenge in prebiotic chemistry. Forming this backbone under early Earth conditions is highly difficult, and no clear natural pathway has been identified.
These challenges collectively paint a picture of immense complexity and improbability in the spontaneous formation of nucleobases and their subsequent assembly into functional nucleic acids. The precision required at each step, from the synthesis of individual nucleobases to their correct pairing and assembly, seems to defy the chaotic conditions of early Earth. The absence of plausible natural mechanisms to overcome these hurdles raises profound questions about the adequacy of purely naturalistic explanations for the origin of life. While the discovery of organic compounds, including nucleobases, in extraterrestrial environments has sparked interest in potential cosmic origins of life's building blocks, this scenario introduces its own set of challenges. The stability of nucleobases during space transit, their synthesis in harsh cosmic environments, and their integration into Earth's prebiotic chemistry remain problematic. These collective challenges underscore the limitations of current naturalistic explanations for the origin of life. The precise orchestration required for nucleobase synthesis, stability, and integration into functional biological systems seems improbable in an unguided scenario. Without invoking a guiding mechanism, the spontaneous emergence of such complex molecules and their successful organization into the first living systems remains a profound mystery in our understanding of life's origins.
RNA & DNA: It's prebiotic synthesis: Impossible !! Part 1
https://www.youtube.com/watch?v=-ZFlmL_BsXE
RNA & DNA: It's prebiotic synthesis: Impossible !! Part 2
https://www.youtube.com/watch?v=dv4mUjmuRRU
Main points addressed in the video
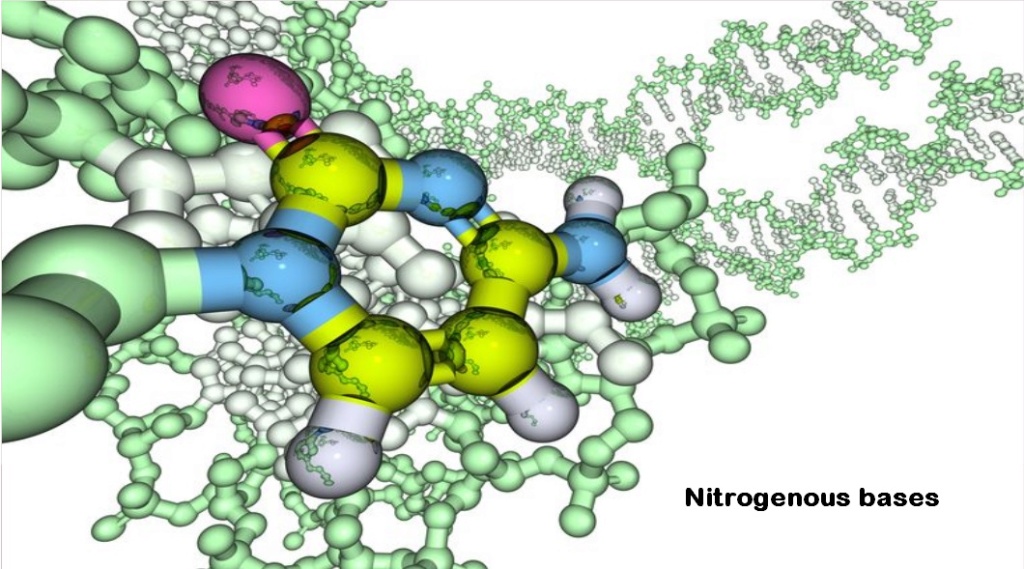
Synthesis of nitrogenous bases in prebiotic environments
- High-energy precursors to produce purines and pyrimidines would have had to be produced in a sufficiently concentrated form. There is no known prebiotic route to this.
- Scientists have failed to produce cytosine in spark-discharge experiments, nor has cytosine been recovered from meteorites or extraterrestrial sources. The deamination of cytosine and its destruction by other processes such as photochemical reactions place severe constraints on prebiotic cytosine syntheses.
- The origin of guanine bases has proven to be a particular challenge. While the other three bases of RNA could be created by heating a simple precursor compound in the presence of certain naturally occurring catalysts, guanine had not been observed as a product of the same reactions.
- Adenine synthesis requires unreasonable Hydrogen cyanide concentrations. Adenine deaminates 37°C with a half-life of 80 years. Therefore, adenine would never accumulate in any kind of "prebiotic soup." The adenine-uracil interaction is weak and nonspecific, and, therefore, would never be expected to function in any specific recognition scheme under the chaotic conditions of a "prebiotic soup."
- Uracil has also a half-life of only 12 years at 100◦C. For nucleobases to accumulate in prebiotic environments, they must be synthesized at rates that exceed their decomposition.
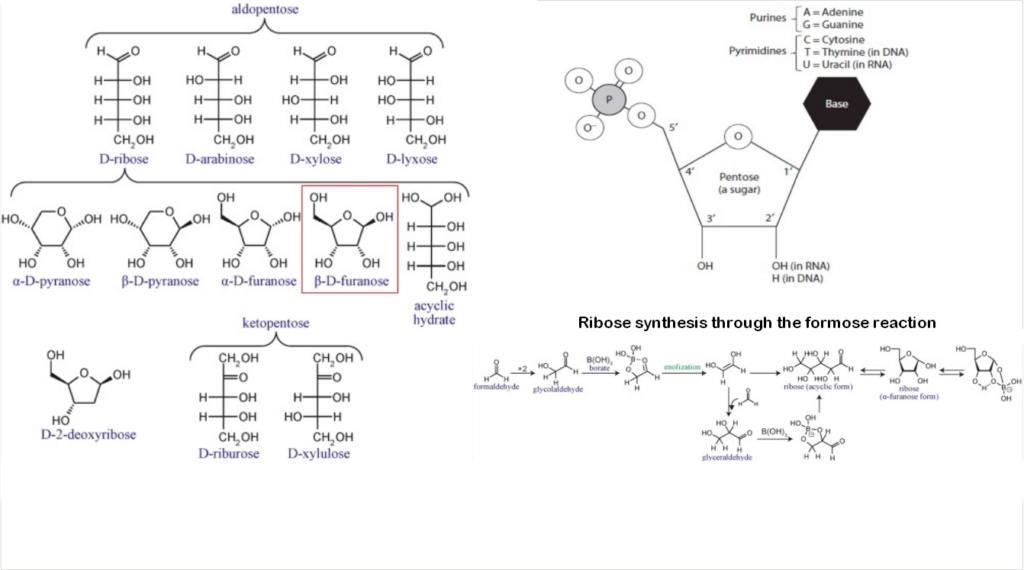
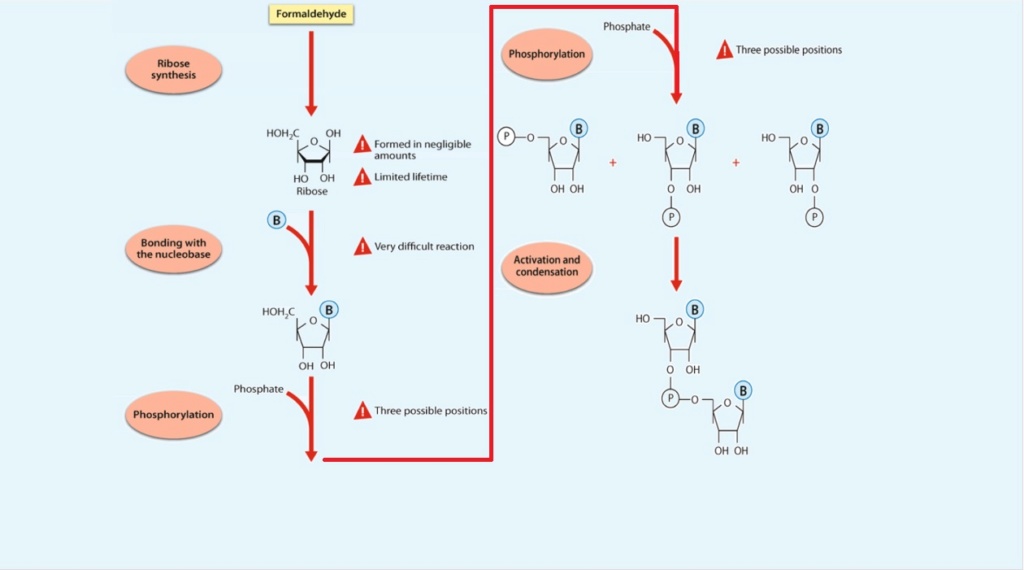
Ribose: Synthesis problems of the Pentose 5 carbon sugar ring
The best-studied mechanism relevant to the prebiotic synthesis of ribose is the formose reaction. Several problems have been recognized for the ribose synthesis via the formose reaction. The formose reaction is very complex. It depends on the presence of a suitable inorganic catalyst. Ribose is merely an intermediate product among a broad suite of compounds including sugars with more or fewer carbons.
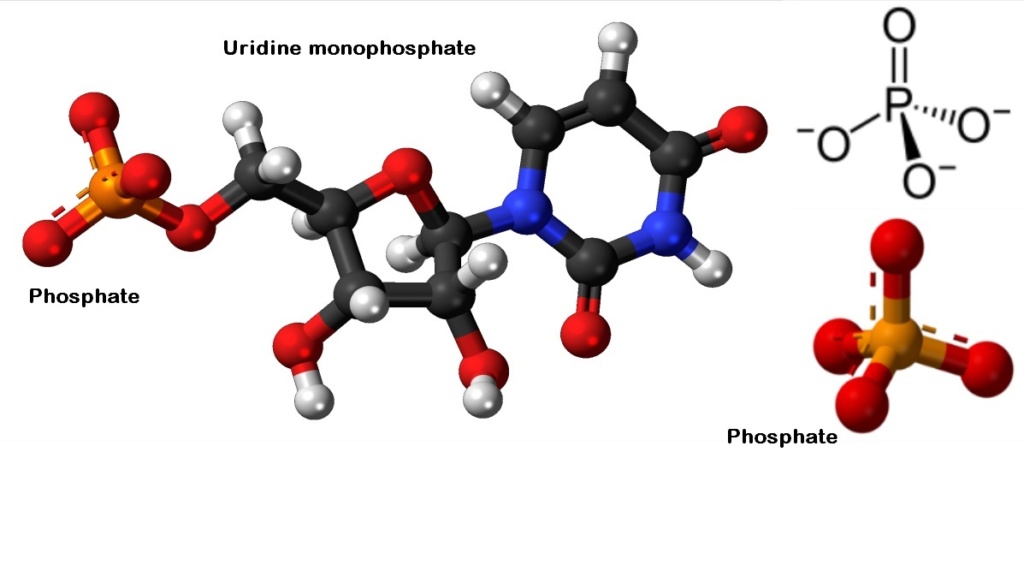
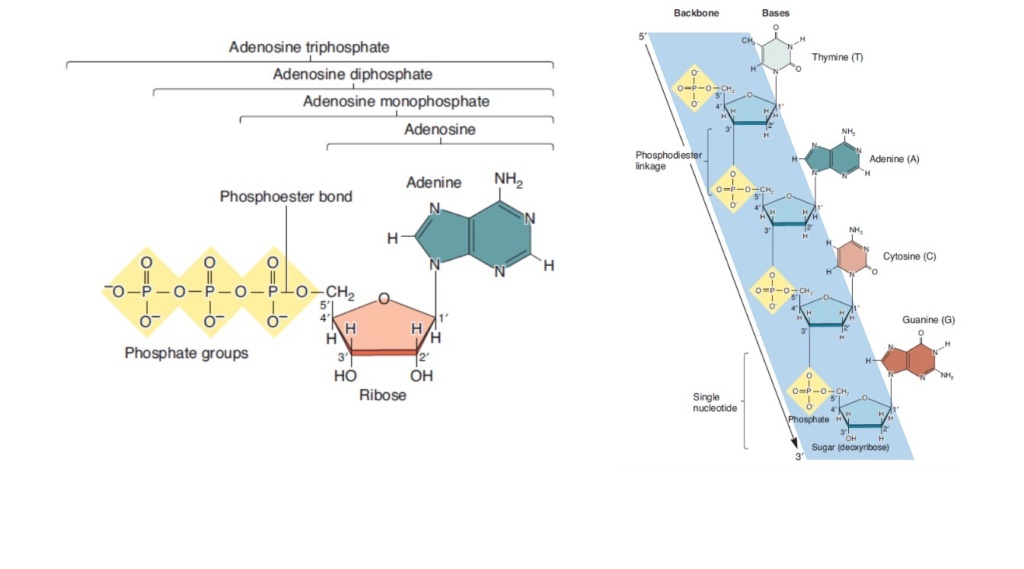
The phosphate group
Challenges in Prebiotic Nucleobase Synthesis
1. Complexity of Chemical Processes
The synthesis of nucleobases is governed by extremely intricate chemical reactions, which present formidable challenges under prebiotic conditions. These reactions necessitate a precise sequence of steps, each with stringent chemical requirements that are nearly impossible to fulfill without biological catalysts. The spontaneous formation of such complex molecules under natural conditions remains highly speculative.
Conceptual problem: Spontaneous Complexity
- No natural mechanism is known to facilitate the assembly of intricate nucleobases without external guidance.
- The replication of necessary reaction conditions in a prebiotic environment is highly improbable, posing significant challenges to the hypothesis of spontaneous nucleobase synthesis.
2. Specific Synthesis Challenges
The natural synthesis of particular nucleobases, such as cytosine and guanine, is especially problematic. Despite extensive research efforts, no viable natural pathways have been identified that could lead to the formation of these molecules under prebiotic conditions. This gap in understanding casts serious doubt on the plausibility of their spontaneous formation.
Conceptual problem: Lack of Natural Pathways
- There is an absence of plausible, unguided routes for synthesizing cytosine and guanine, both essential components of nucleic acids.
- The improbability of these crucial molecules forming spontaneously raises unresolved questions about their origin.
3. Stability of Nucleobases
Under prebiotic conditions, nucleobases exhibit significant instability, characterized by short half-lives that impede their accumulation. This instability presents a major obstacle to the hypothesis that nucleobases could have been present in sufficient quantities on early Earth to contribute to the formation of RNA or DNA. The rapid degradation of these molecules under likely prebiotic conditions exacerbates the difficulty of explaining their role in early molecular evolution.
Conceptual problem: Molecular Instability
- Nucleobases degrade rapidly under conditions thought to resemble those of early Earth, making their sustained presence highly unlikely.
- Maintaining adequate concentrations of these unstable molecules for further chemical evolution poses a significant challenge.
4. Cytosine Synthesis Difficulty
Among all the nucleobases, cytosine represents a particularly formidable challenge for prebiotic synthesis. To date, no natural route has been identified that could produce cytosine under plausible prebiotic conditions. This issue raises critical questions about how this essential component of nucleic acids could have emerged without guidance.
Conceptual problem: Absence of Cytosine Pathway
- There is no known natural method for producing cytosine, a crucial building block of genetic material.
- The unresolved issues surrounding its spontaneous synthesis and accumulation highlight significant gaps in current prebiotic chemistry models.
5. Challenges in Guanine Formation
Guanine, a critical nucleobase for both RNA and DNA, presents substantial difficulties in prebiotic chemistry. Despite extensive research, no clear, natural pathway has been identified for its formation under early Earth conditions. The absence of such a pathway further complicates any scenario that proposes a spontaneous origin for nucleic acids.
Conceptual problem: Guanine Formation Barriers
- There is a significant lack of feasible prebiotic routes for guanine synthesis, making it improbable that guanine could emerge naturally without guided processes.
- This obstacle represents a major challenge in explaining how nucleic acids could have spontaneously emerged in a prebiotic context.
6. Adenine Synthesis Requirements
Adenine, another essential nucleobase, requires extremely high concentrations of hydrogen cyanide (HCN) for its synthesis, concentrations that are unlikely to have existed on early Earth. Additionally, adenine is prone to rapid deamination, which further complicates its stable accumulation and availability.
Conceptual problem: Unrealistic Conditions
- The required high concentrations of hydrogen cyanide for adenine synthesis are implausible in natural settings, raising questions about how adenine could have been formed in sufficient quantities.
- The deamination of adenine challenges its stability and availability, adding another layer of complexity to prebiotic nucleobase synthesis.
7. Uracil Stability Issues
Uracil, a key component of RNA, suffers from significant stability issues, particularly under the temperature conditions likely present on early Earth. Its short half-life at these temperatures complicates any scenario where uracil could have accumulated in sufficient quantities to participate in the formation of RNA.
Conceptual problem: Uracil Degradation
- The rapid degradation of uracil under relevant environmental conditions makes it unlikely that this nucleobase could have been present in the necessary concentrations for prebiotic processes.
- This degradation issue raises significant questions about how uracil could have contributed to the formation of functional RNA molecules.
8. Nucleobase Tautomerism
Tautomerism, the ability of nucleobases to exist in multiple structural forms, presents a significant challenge in the correct incorporation of these molecules into nucleic acids. In the absence of regulatory mechanisms that exist in living systems, controlling the tautomeric forms to ensure proper base pairing is nearly impossible in a prebiotic environment.
Conceptual problem: Lack of Tautomeric Control
- Without biological regulation, it is challenging to ensure the correct base pairing of nucleobases, leading to potential errors in the formation of functional nucleic acids.
- The risk of incorrect tautomeric forms interfering with nucleic acid formation highlights the difficulties in achieving the necessary specificity and stability under prebiotic conditions.
9. Purity of Chemical Precursors
The prebiotic environment likely consisted of impure and contaminated chemical pools, vastly different from the controlled conditions used in laboratory experiments. The presence of impurities would have significantly hindered the formation of nucleobases, which require high-purity precursors to form correctly.
Conceptual problem: Impurity and Contamination
- The contamination of chemical precursors in a prebiotic environment poses a major challenge to the spontaneous formation of nucleobases, as high-purity materials are typically required for successful synthesis.
- The difficulty in achieving the necessary purity in a natural setting further complicates the plausibility of unguided nucleobase formation.[/size]
10. Concentration Problems
Achieving the necessary concentrations of precursors for nucleobase formation represents a formidable challenge in prebiotic chemistry. The dilute conditions presumed to have existed on early Earth would have made it nearly impossible to reach the concentrations required for these reactions to occur efficiently. This dilution significantly hampers the likelihood of spontaneous nucleobase formation.
Conceptual problem: Insufficient Concentrations
- Dilute environmental conditions would have severely hindered nucleobase synthesis by preventing the accumulation of reactants to the necessary levels.
- There is no known natural process capable of concentrating these precursors to the levels required for nucleobase formation, making spontaneous synthesis highly improbable.
11. Energy Source Identification
Identifying plausible prebiotic energy sources to drive the energetically unfavorable reactions involved in nucleobase synthesis is a critical, unresolved challenge. The absence of a reliable and consistent energy source raises significant doubts about how these reactions could have proceeded on early Earth. Without such energy inputs, the formation of nucleobases would remain unexplained.
Conceptual problem: Energy Source Deficit
- The lack of a plausible prebiotic energy source to drive the necessary reactions casts doubt on the natural formation of nucleobases.
- The difficulty in explaining how these energetically unfavorable processes could occur naturally without external guidance further complicates the prebiotic scenario.
12. Controlling Side Reactions
In a prebiotic environment, the presence of various reactive species would have made it challenging to prevent or control unwanted side reactions that could interfere with nucleobase synthesis. These side reactions could consume essential precursors or produce alternative, non-functional compounds, further complicating the spontaneous formation of nucleobases.
Conceptual problem: Uncontrolled Reactions
- The challenge of preventing side reactions that could hinder nucleobase formation is significant, given the lack of regulatory mechanisms in a prebiotic setting.
- The absence of natural mechanisms to ensure the correct synthesis pathway is followed raises questions about how the required nucleobases could have emerged without guidance.
13. Thermodynamic Challenges
Many of the reactions critical to nucleobase synthesis are thermodynamically unfavorable under prebiotic conditions. Without external intervention or highly specific conditions, the likelihood of these reactions occurring naturally is exceedingly low. This thermodynamic barrier presents a significant obstacle to the hypothesis that nucleobases could have formed spontaneously.
Conceptual problem: Thermodynamic Barriers
- The challenge of overcoming energetically unfavorable reactions without any guided process is substantial, casting doubt on naturalistic explanations for nucleobase formation.
- The difficulty in explaining how these necessary reactions could proceed spontaneously, given the thermodynamic constraints, underscores the improbability of unguided nucleobase synthesis.[/size]
14. Environmental Condition Specificity
The synthesis of nucleobases requires highly specific environmental conditions, including precise control over temperature, pH, and atmospheric composition. Achieving and maintaining these conditions over the extended periods necessary for nucleobase accumulation presents a significant challenge on early Earth. The variability of natural settings makes it unlikely that these conditions could have been consistently favorable.
Conceptual problem: Environmental Precision
- The difficulty in achieving and maintaining the precise environmental conditions required for nucleobase synthesis is substantial.
- It is unlikely that consistent, favorable conditions could have been sustained in natural settings, raising doubts about the plausibility of spontaneous nucleobase formation.
15. The Water Paradox
Water, while essential for many biochemical reactions, also promotes the rapid degradation of nucleobases and their precursors. This paradox presents a significant challenge for prebiotic nucleobase synthesis, as the presence of water is both necessary for biochemical processes and detrimental to the stability of nucleobases.
Conceptual problem: Degradative Role of Water
- The dual role of water as both a necessary solvent and a destructive agent complicates the synthesis of nucleobases in aqueous environments.
- There is no known natural solution to prevent the degradation of nucleobases in the presence of water, further complicating prebiotic synthesis scenarios.
16. Correct Isomeric Configuration
Ensuring the correct isomeric configuration of nucleobases is crucial for Watson-Crick base pairing, yet controlling this configuration without biological systems is extremely difficult. Incorrect isomers would result in faulty base pairing, hindering the formation of functional nucleic acids and complicating the emergence of life.
Conceptual problem: Isomeric Control
- Achieving the correct isomeric forms naturally, without the guidance of biological systems, poses a significant challenge.
- The risk of incorrect isomeric configurations preventing proper base pairing raises doubts about the spontaneous emergence of functional nucleic acids.
17. Tautomeric Equilibria Control
The control of tautomeric equilibria is essential to ensure correct base pairing, yet this control is highly sensitive to environmental conditions. The variability of these conditions on early Earth, coupled with the absence of regulatory mechanisms in prebiotic chemistry, raises significant doubts about the feasibility of maintaining correct nucleobase pairing.
Conceptual problem: Tautomeric Imbalance
- Maintaining the necessary tautomeric equilibria without biological intervention is highly challenging.
- The potential for incorrect base pairing due to uncontrolled tautomeric shifts presents a major obstacle to the formation of functional nucleic acids.
18. Stereochemistry of Sugar Components
The stereochemistry of the sugar components in nucleotides is critical for proper base pairing and the formation of stable nucleic acids. Achieving the correct stereochemistry in a prebiotic environment, without enzymatic guidance, is a significant challenge, as incorrect configurations could prevent the formation of stable and functional nucleic acids.
Conceptual problem: Stereochemical Control
- Ensuring correct stereochemistry in a prebiotic environment is highly improbable without guided processes.
- The absence of natural mechanisms to guide the correct formation of sugar components raises questions about the spontaneous formation of nucleotides.
19. Chiral Selection Origins
Explaining the origin of homochirality in nucleotides, a necessary condition for proper base pairing, remains one of the most significant unsolved problems in prebiotic chemistry. Without a mechanism to enforce chiral selection, the emergence of a uniform chirality required for functional nucleic acids is highly improbable, further complicating the naturalistic origins of life.
Conceptual problem: Homochirality Emergence
- The unexplained origin of uniform chirality in prebiotic environments represents a major challenge to the naturalistic origin of nucleic acids.
- The lack of a plausible natural mechanism to consistently select one chiral form raises significant doubts about the spontaneous emergence of life.[/size]
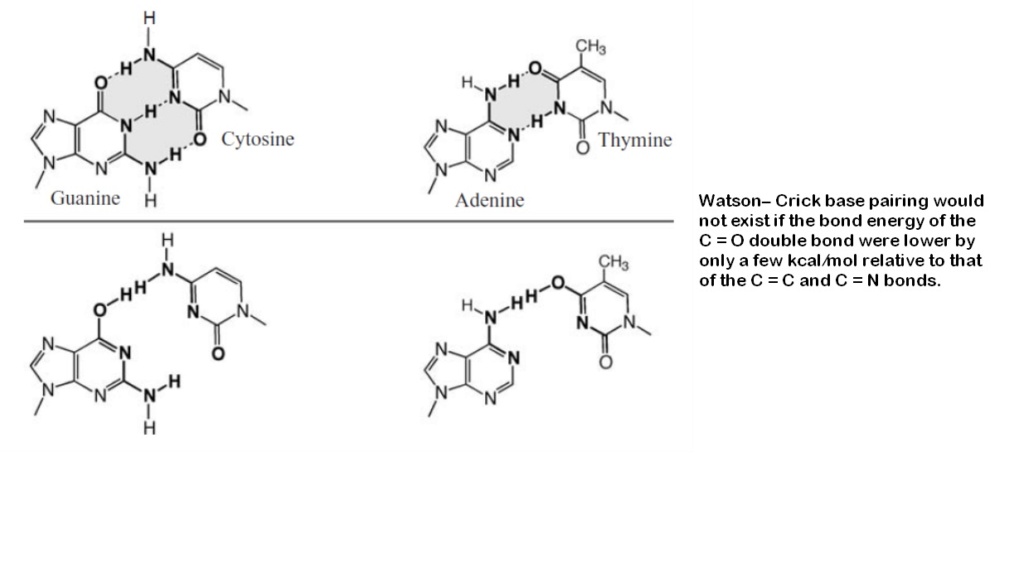
20. Bond Energy Fine-Tuning
The stability of Watson-Crick base pairs relies heavily on the precise bond energies within the nucleobases, particularly in carbon-oxygen double bonds. Achieving the level of fine-tuning necessary for stable nucleic acids without the regulatory oversight found in biological systems is highly improbable, making spontaneous nucleic acid formation under prebiotic conditions unlikely.
Conceptual problem: Bond Energy Regulation
- The natural environment lacks the precise control needed to fine-tune bond energies critical for stable nucleic acid structures.
- Without guided processes, the stability required for the formation of functional nucleic acids cannot be ensured.
21. Hydrogen Bonding Specificity
The specificity of hydrogen bonding is crucial for Watson-Crick base pairing in nucleic acids. Achieving this specificity naturally, without biological regulatory mechanisms, is highly challenging, making errors in base pairing likely and hindering the formation of functional nucleic acids.
Conceptual problem: Specificity of Hydrogen Bonds
- Ensuring precise hydrogen bonding in natural settings is difficult without regulatory systems.
- The potential for incorrect hydrogen bonding patterns poses a significant obstacle to the correct formation of nucleic acids.
22. Preventing Alternative Base Pairs
In a prebiotic environment, the formation of alternative, non-Watson-Crick base pairs could interfere with the correct assembly of nucleic acids. Without regulatory mechanisms to prevent these alternative pairings, the spontaneous origin of life becomes even more implausible.
Conceptual problem: Alternative Pairing Prevention
- Natural settings lack the mechanisms required to prevent incorrect base pairing.
- The formation of stable, non-functional alternative base pairs could disrupt nucleic acid assembly.
23. Challenges in Backbone Chemistry
The specific sugar-phosphate backbone required for nucleic acid stability poses another significant challenge in prebiotic chemistry. Forming this backbone under early Earth conditions is highly difficult, and no clear natural pathway has been identified, complicating the scenario of spontaneous nucleic acid formation.
Conceptual problem: Backbone Formation
- The absence of plausible prebiotic pathways to form the sugar-phosphate backbone challenges the naturalistic origin of nucleic acids.
- Achieving the precise chemical requirements for a stable nucleic acid backbone without guidance appears highly unlikely.
24. Base Stacking Interactions
Base stacking interactions contribute to the stability of the nucleic acid double helix, but achieving these interactions naturally, without the guidance of biological systems, is highly challenging. This raises doubts about the spontaneous formation of a stable nucleic acid structure under prebiotic conditions.
Conceptual problem: Base Stacking Instability
- The natural environment lacks the specific conditions needed to achieve correct base stacking interactions.
- The resulting instability could prevent the formation of functional nucleic acid structures.
25. Selection of Nucleobase Analogs
A significant challenge in prebiotic chemistry is explaining why only certain nucleobases capable of Watson-Crick pairing were selected from numerous possible analogs. The natural selection process that led to the exclusive use of these specific nucleobases remains unexplained, further complicating the scenario of a naturalistic origin.
Conceptual problem: Analog Selection Process
- No natural mechanism has been identified to explain the exclusive selection of Watson-Crick compatible nucleobases.
- The specific choice of these nucleobases over other potential analogs remains a significant challenge to the spontaneous emergence of life.
26. Formation of Stable Nucleotides
The prebiotic formation of nucleosides, particularly in aqueous solutions, presents a significant hurdle. Current research has not identified successful natural methods for combining pyrimidine bases and ribose to form stable nucleotides, which is a critical step in nucleic acid formation.
Conceptual problem: Nucleoside Formation Barriers
- The natural environment lacks the processes necessary to combine pyrimidine bases with ribose efficiently.
- The absence of a viable prebiotic method for nucleoside formation raises significant doubts about the natural origin of nucleotides.
27. Role of Environmental Conditions
For nucleobase synthesis to proceed, the physical and chemical environment, including pH, temperature, and metal ion concentrations, must be precisely controlled. The likelihood of maintaining such conditions consistently over time on early Earth is low, posing a major challenge to prebiotic nucleobase synthesis scenarios.
Conceptual problem: Environmental Control
- The natural environment is unlikely to have consistently maintained the precise conditions necessary for nucleobase synthesis.
- Without controlled conditions, the spontaneous synthesis of nucleobases under prebiotic conditions becomes highly improbable.
Challenges in Prebiotic Nucleobase Synthesis - Addressing Extraterrestrial Sources
The discovery of organic compounds, including nucleobases, in extraterrestrial environments has been one of the most exciting developments in the field of astrobiology and origin of life studies. Nucleobases, the fundamental building blocks of RNA and DNA, have been detected in various cosmic settings, including interstellar space, comets, and meteorites that have fallen to Earth. In 1969, the Murchison meteorite, which fell in Australia, became a landmark in this area of research. Analysis of this carbonaceous chondrite revealed the presence of various organic compounds, including purine and pyrimidine bases. Since then, numerous studies have confirmed the presence of nucleobases in other meteorites, such as the Tagish Lake meteorite and the Antarctic meteorites. Furthermore, space-based observations and laboratory simulations of interstellar ice analogues have suggested that nucleobases could form in the harsh conditions of space. These findings have led some researchers to propose that the essential ingredients for life might have been delivered to early Earth through extraterrestrial sources, potentially jumpstarting the emergence of life. This scenario, often referred to as panspermia or exogenesis, has gained attention as a potential solution to some of the challenges faced in explaining the prebiotic synthesis of these crucial biomolecules on Earth. However, while the presence of nucleobases in space and meteorites is intriguing, it introduces its own set of challenges and does not necessarily solve the fundamental problems of prebiotic nucleobase availability and subsequent RNA or DNA formation. The following points outline why the extraterrestrial source of nucleobases, despite its initial promise, does not fully address the challenges in prebiotic nucleobase synthesis:
1. Stability and Delivery of Nucleobases
The hypothesis that nucleobases were delivered to Earth via meteorites or comets raises significant questions regarding the stability of these molecules during transit. Space is an environment characterized by intense radiation, extreme temperatures, and vacuum conditions, all of which could degrade delicate organic compounds. The survival of nucleobases from their formation in interstellar space to their delivery to Earth remains an unresolved issue. For instance, purine and pyrimidine bases detected in meteorites like the Murchison have undergone intense scrutiny, yet their preservation under such harsh conditions is not fully understood.
Conceptual problem: Nucleobase Stability
- Uncertainty about how nucleobases could remain stable over long cosmic journeys
- Lack of a natural mechanism that could protect these molecules from degradation in space
2. Synthesis in Extraterrestrial Environments
The formation of nucleobases in space introduces additional challenges. Laboratory simulations of interstellar ice analogs suggest that nucleobases can form under specific conditions, but these simulations often require highly controlled environments that may not reflect the chaotic nature of space. The complexity of synthesizing these molecules under natural, unguided conditions, such as in the vast and varied regions of interstellar space, remains a daunting challenge. This issue is further complicated by the fact that nucleobases require precise conditions for their formation, which raises doubts about the likelihood of such processes occurring spontaneously in space.
Conceptual problem: Spontaneous Synthesis
- Difficulty in replicating space conditions conducive to nucleobase formation in the laboratory
- Improbability of spontaneous nucleobase synthesis in uncontrolled, natural space environments
3. Integration into Prebiotic Chemistry
Even if nucleobases were successfully delivered to Earth, integrating them into the prebiotic chemistry required for life is another unsolved problem. Nucleobases would need to not only survive the conditions of early Earth but also integrate into a functional system capable of RNA or DNA formation. The spontaneous assembly of nucleobases into these complex macromolecules, without the guidance of enzymatic processes or an existing template, poses a significant conceptual barrier. The precise order and structure of nucleotides in RNA and DNA are critical for their function, yet there is no known natural mechanism that could have organized these molecules into the correct sequences in the absence of life.
Conceptual problem: Molecular Integration
- Challenge in explaining how nucleobases could self-assemble into functional nucleic acids
- Lack of a known process that could ensure the correct sequencing of nucleotides without guidance
4. Alternative Pathways and Polyphyly
The existence of alternative nucleobase synthesis pathways in different environments, which often share no homology, presents evidence for polyphyly—the notion that life may have originated from multiple independent sources. The Murchison meteorite and other extraterrestrial findings suggest that nucleobases could form in a variety of ways, yet these pathways do not converge on a single, universal mechanism. This divergence undermines the concept of a universal common ancestor and suggests that life, if it emerged from these extraterrestrial sources, did so in a polyphyletic manner. The lack of shared ancestry between these pathways further complicates the narrative of a singular, natural origin of life.
Conceptual problem: Independent Origins
- Evidence of multiple, distinct pathways for nucleobase synthesis challenges the idea of a single origin
- Polyphyly suggests life may have emerged from different sources, contradicting universal common ancestry
5. Naturalistic Explanations and Their Limits
The challenges associated with extraterrestrial nucleobase synthesis and delivery highlight the limitations of naturalistic explanations for the origin of life. The precise conditions required for nucleobase stability, synthesis, and integration into prebiotic chemistry seem improbably orchestrated in a purely unguided scenario. This raises fundamental questions about the adequacy of naturalistic frameworks to account for the emergence of life’s building blocks. Without invoking a guiding mechanism, the spontaneous appearance of such complex molecules and their successful integration into functional biological systems remains unexplained.
Conceptual problem: Adequacy of Naturalistic Explanations
- Inadequacy of naturalistic mechanisms to fully explain nucleobase synthesis, stability, and integration
- Lack of a coherent natural process that could account for the coordinated emergence of life’s building blocks
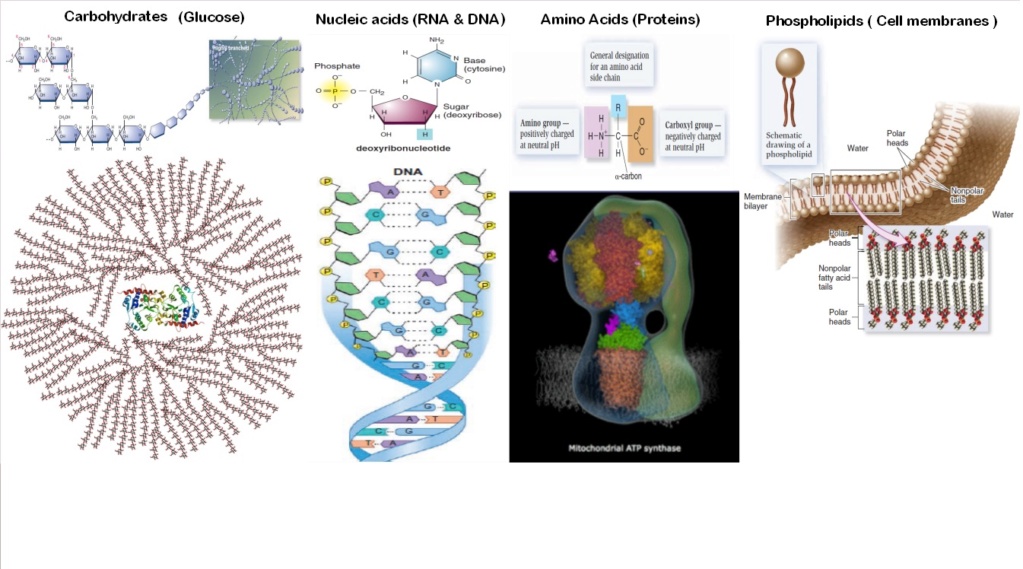
1.1.4. Sugars
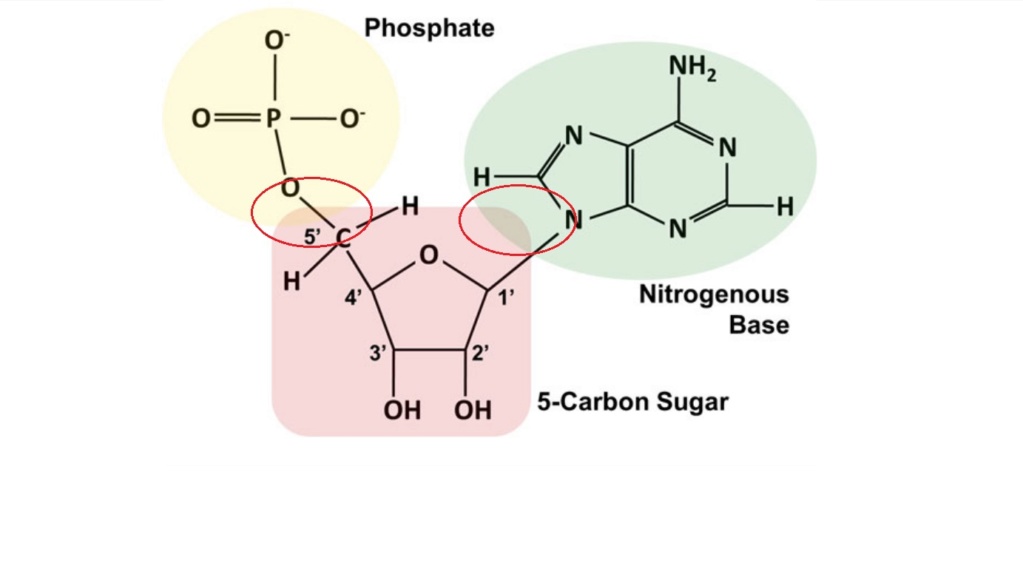
Sugars play crucial roles in the chemistry of life, particularly in the formation of nucleic acids and energy metabolism. For the origin of life, certain sugars are especially significant due to their involvement in the formation of RNA and DNA. The key sugars essential for the origin of life are:
1. Ribose: A five-carbon sugar that forms the backbone of RNA. It's critical for:
Genetic information: As part of RNA, it's crucial for the RNA World hypothesis, where RNA may have been the first genetic material.
Prebiotic chemistry: Its formation under prebiotic conditions is a key area of study in origin of life research.
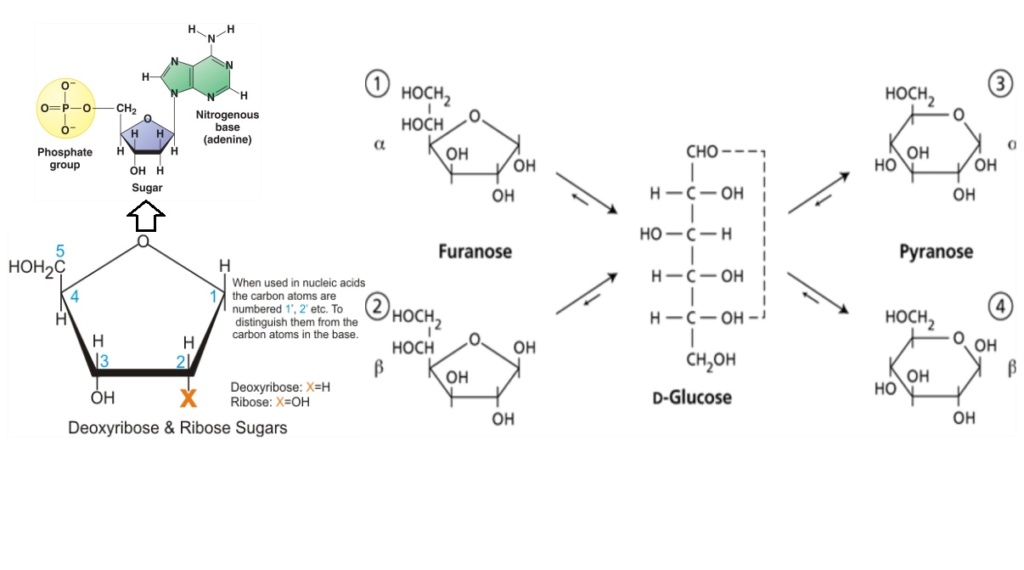
2. Deoxyribose: A modified form of ribose that lacks one oxygen atom. It's vital for:
DNA structure: Forms the sugar-phosphate backbone of DNA, which eventually became the primary carrier of genetic information.
Evolutionary transition: Its emergence may represent a critical step in the evolution of genetic systems.
3. Glucose: While not directly involved in nucleic acid formation, glucose is significant for:
Energy source: Potentially one of the earliest energy sources for primitive metabolic systems.
Precursor molecule: Can serve as a starting point for the synthesis of other important biological molecules, including ribose.
These sugars are fundamental to the origin of life:
1. RNA and DNA formation: Ribose and deoxyribose are essential components of RNA and DNA respectively, which are central to genetic information storage and transmission.
2. Energy storage and transfer: Sugars like glucose could have served as early energy sources in prebiotic chemical systems.
3. Prebiotic synthesis: The formation of these sugars under prebiotic conditions is a critical area of study in origin of life research.
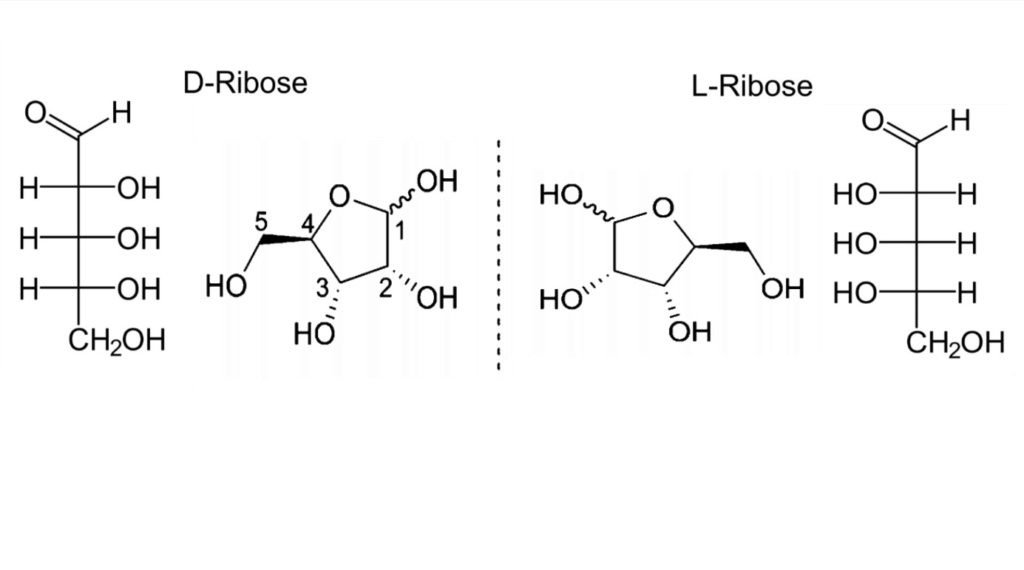
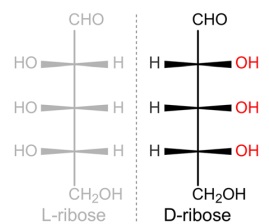
4. Chirality: The specific stereochemistry of these sugars is crucial for the function of nucleic acids, presenting challenges and clues for understanding life's origins.
Understanding the prebiotic synthesis and selection of these specific sugars is crucial for unraveling how the first self-replicating molecules may have formed. This area of study continues to be at the forefront of research into life's origins, with implications for astrobiology and our understanding of what constitutes the minimum requirements for life.
On prebiotic earth, however, there would have been no way to activate phosphate somehow, in order to promote the energy dispendious reaction.
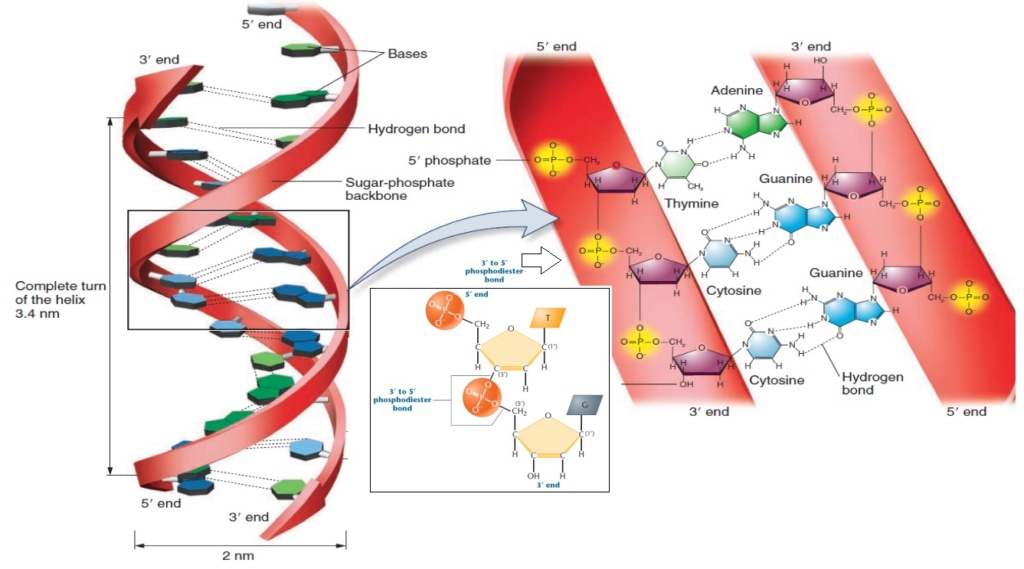
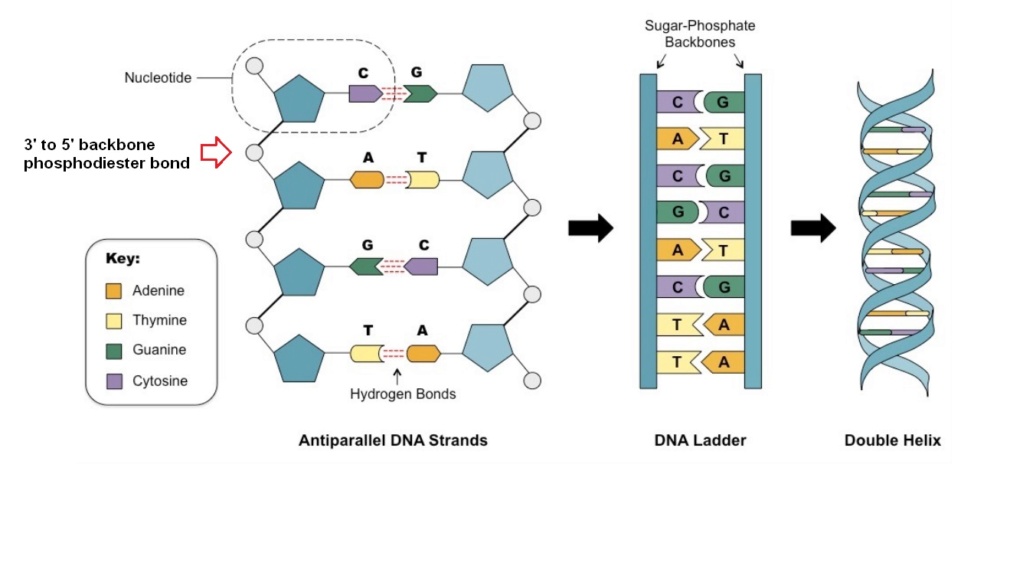
Prebiotic RNA and DNA synthesis
1. No prebiotic mechanism is known to select:
- Right-handed configurations of RNA and DNA
- The right backbone sugar
- How to get size complementarity of the nucleotide bases to form a DNA strand and strands of the DNA molecule running in the opposite directions
2. Bringing all the parts together and joining them in the right position
- Attach the nucleic bases to the ribose and in a repetitive manner at the same, correct place, and the backbone being a repetitive homopolymer
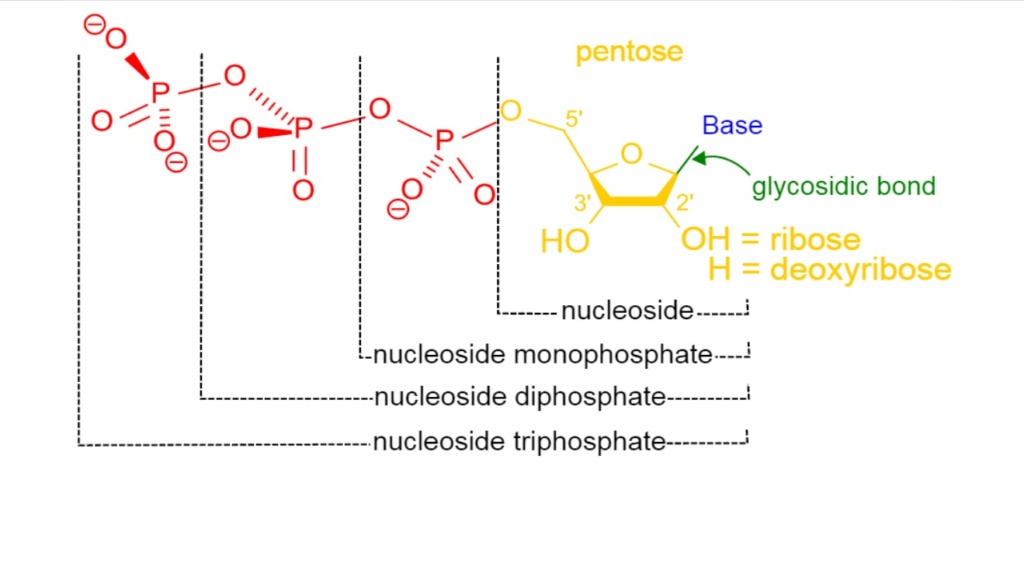
- Prebiotic glycosidic bond formation between nucleosides and the base
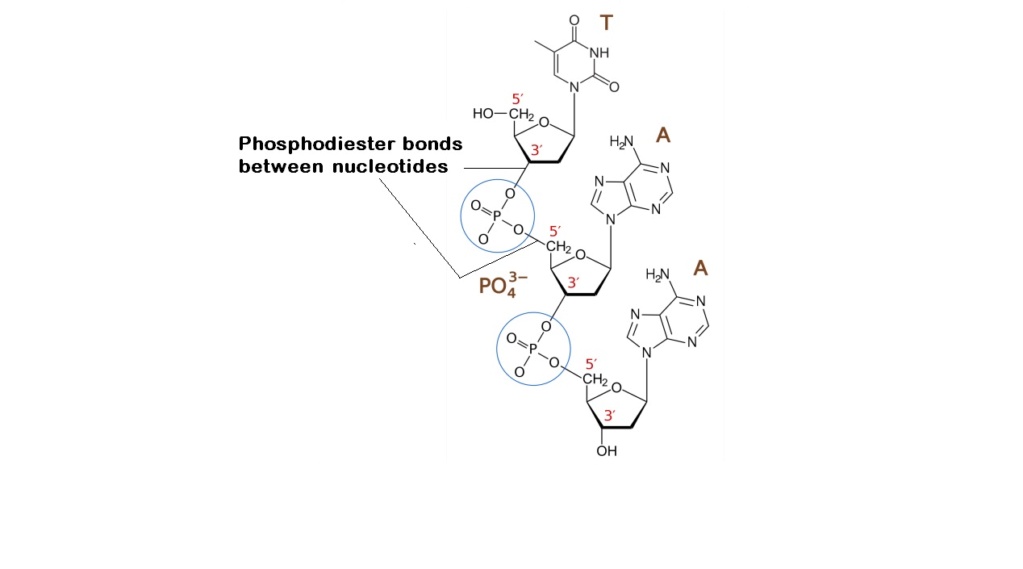
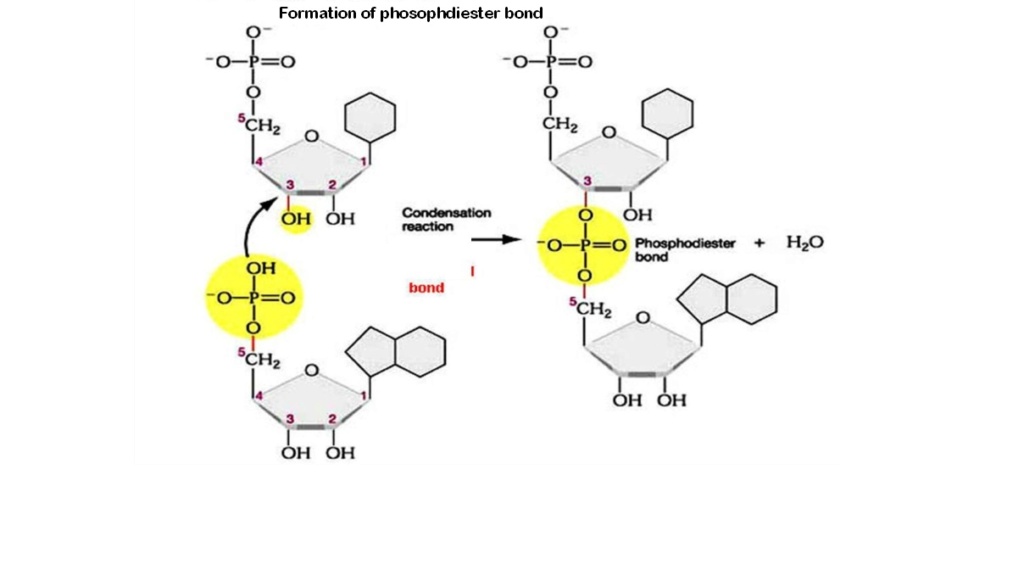
- Prebiotic phosphodiester bond formation
- Fine-tuning of the strength of the hydrogen base pairing forces
3. The instability, degradation, and asphalt problem
- Bonds that are thermodynamically unstable in water, and overall intrinsic instability. RNA’s nucleotide building blocks degrade at warm temperatures in time periods ranging from nineteen days to twelve years. These extremely short survival rates for the four RNA nucleotide building blocks suggest why life’s origin would have to be virtually instantaneous—all the necessary RNA molecules would have to be assembled before any of the nucleotide building blocks decayed.
4. The energy problem
- Doing things costs energy. There has to be a ready source of energy to produce RNA. In modern cells, energy is consumed to make RNA.
5. The minimal nucleotide quantity problem.
- The prebiotic conditions would have had to be right for reactions to give perceptible yields of bases that could pair with each other.
6. The Water Paradox
- The hydrolytic deamination of DNA and RNA nucleobases is rapid and irreversible, as is the base-catalyzed cleavage of RNA in water. This leads to a paradox: RNA requires water to do its job, but RNA cannot emerge in water and cannot replicate with sufficient fidelity in water without sophisticated repair mechanisms in place.
7.The transition problem from prebiotic to biochemical synthesis
- Even if all this in a freaky accident occurred by random events, that still says nothing about the huge gap and enormous transition that would be still ahead to arrive at a fully functional interlocked and interdependent metabolic network, where complex biosynthesis pathways produce nucleotides in modern cells.
Unguided prebiotic synthesis of RNA and DNA: an unsolved riddle!
I think, to say that on average the 14 hurdles that it would take to make primed nucleotides would each take 10 unit operations - that at least 140 little events would have to be appropriately sequenced. Unguided, the appropriate thing happened at each point on one occasion in six.The odds against a successful unguided synthesis of a batch of primed nucleotide on the primitive Earth would be a huge number, represented approximately by a 1 followed by 109 zeros ( 10^109). 'The odds are enormous against its being coincidence. No figures could express them.'
Synthesis of nitrogenous bases in prebiotic environments
- High-energy precursors to produce purines and pyrimidines would have had to be produced in a sufficiently concentrated form. There is no known prebiotic route to this.
- Scientists have failed to produce cytosine in spark-discharge experiments, nor has cytosine been recovered from meteorites or extraterrestrial sources. The deamination of cytosine and its destruction by other processes such as photochemical reactions place severe constraints on prebiotic cytosine syntheses.
- The origin of guanine bases has proven to be a particular challenge. While the other three bases of RNA could be created by heating a simple precursor compound in the presence of certain naturally occurring catalysts, guanine had not been observed as a product of the same reactions.
- Adenine synthesis requires unreasonable Hydrogen cyanide concentrations. Adenine deaminates 37°C with a half-life of 80 years. Therefore, adenine would never accumulate in any kind of "prebiotic soup." The adenine-uracil interaction is weak and nonspecific, and, therefore, would never be expected to function in any specific recognition scheme under the chaotic conditions of a "prebiotic soup."
- Uracil has also a half-life of only 12 years at 100◦C. For nucleobases to accumulate in prebiotic environments, they must be synthesized at rates that exceed their decomposition.
Ribose: Synthesis problems of the Pentose 5 carbon sugar ring
The best-studied mechanism relevant to the prebiotic synthesis of ribose is the formose reaction. Several problems have been recognized for the ribose synthesis via the formose reaction. The formose reaction is very complex. It depends on the presence of a suitable inorganic catalyst. Ribose is merely an intermediate product among a broad suite of compounds including sugars with more or fewer carbons.
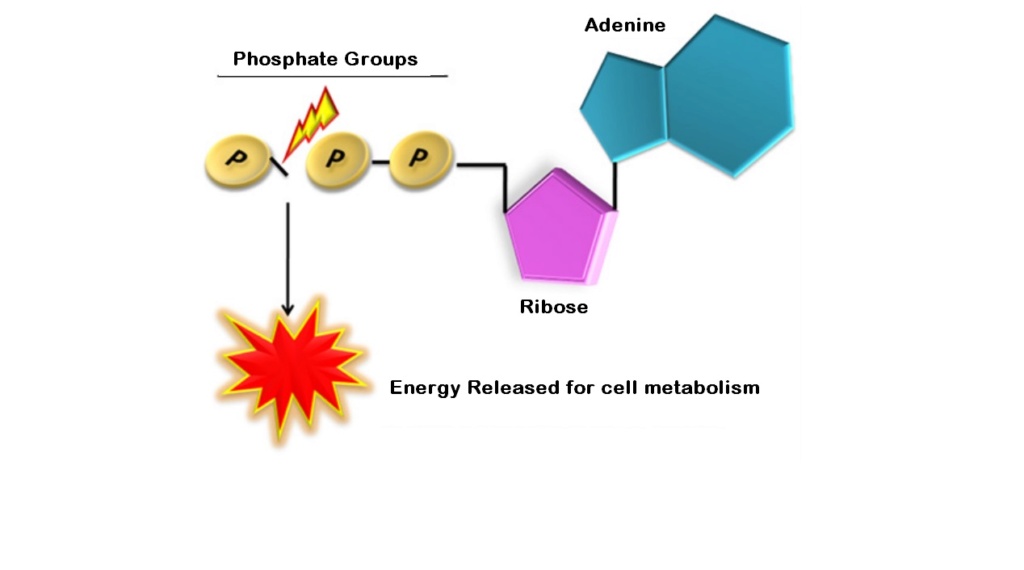
The phosphate group
On prebiotic earth, however, there would have been no way to activate phosphate somehow, in order to promote the energy dispendious reaction.
1. No prebiotic mechanism is known to select:
- Right-handed configurations of RNA and DNA
- The right backbone sugar
- How to get size complementarity of the nucleotide bases to form a DNA strand and strands of the DNA molecule running in the opposite directions
2. Bringing all the parts together and joining them in the right position
- Attach the nucleic bases to the ribose and in a repetitive manner at the same, correct place, and the backbone being a repetitive homopolymer
- Prebiotic glycosidic bond formation between nucleosides and the base
- Prebiotic phosphodiester bond formation
- Fine-tuning of the strength of the hydrogen base pairing forces
3. The instability, degradation, and asphalt problem
- Bonds that are thermodynamically unstable in water, and overall intrinsic instability. RNA’s nucleotide building blocks degrade at warm temperatures in time periods ranging from nineteen days to twelve years. These extremely short survival rates for the four RNA nucleotide building blocks suggest why life’s origin would have to be virtually instantaneous—all the necessary RNA molecules would have to be assembled before any of the nucleotide building blocks decayed.
4. The energy problem
- Doing things costs energy. There has to be a ready source of energy to produce RNA. In modern cells, energy is consumed to make RNA.
5. The minimal nucleotide quantity problem.
- The prebiotic conditions would have had to be right for reactions to give perceptible yields of bases that could pair with each other.
6. The Water Paradox
- The hydrolytic deamination of DNA and RNA nucleobases is rapid and irreversible, as is the base-catalyzed cleavage of RNA in water. This leads to a paradox: RNA requires water to do its job, but RNA cannot emerge in water and cannot replicate with sufficient fidelity in water without sophisticated repair mechanisms in place.
7.The transition problem from prebiotic to biochemical synthesis
- Even if all this in a freaky accident occurred by random events, that still says nothing about the huge gap and enormous transition that would be still ahead to arrive at a fully functional interlocked and interdependent metabolic network, where complex biosynthesis pathways produce nucleotides in modern cells.
Synthesis of nitrogenous bases in prebiotic environments
- High-energy precursors to produce purines and pyrimidines would have had to be produced in a sufficiently concentrated form. There is no known prebiotic route to this.
- Scientists have failed to produce cytosine in spark-discharge experiments, nor has cytosine been recovered from meteorites or extraterrestrial sources. The deamination of cytosine and its destruction by other processes such as photochemical reactions place severe constraints on prebiotic cytosine syntheses.
- The origin of guanine bases has proven to be a particular challenge. While the other three bases of RNA could be created by heating a simple precursor compound in the presence of certain naturally occurring catalysts, guanine had not been observed as a product of the same reactions.
- Adenine synthesis requires unreasonable Hydrogen cyanide concentrations. Adenine deaminates 37°C with a half-life of 80 years. Therefore, adenine would never accumulate in any kind of "prebiotic soup." The adenine-uracil interaction is weak and nonspecific, and, therefore, would never be expected to function in any specific recognition scheme under the chaotic conditions of a "prebiotic soup."
- Uracil has also a half-life of only 12 years at 100◦C. For nucleobases to accumulate in prebiotic environments, they must be synthesized at rates that exceed their decomposition.
Ribose: Synthesis problems of the Pentose 5 carbon sugar ring
The best-studied mechanism relevant to the prebiotic synthesis of ribose is the formose reaction. Several problems have been recognized for the ribose synthesis via the formose reaction. The formose reaction is very complex. It depends on the presence of a suitable inorganic catalyst. Ribose is merely an intermediate product among a broad suite of compounds including sugars with more or fewer carbons.
The phosphate group
On prebiotic earth, however, there would have been no way to activate phosphate somehow, in order to promote the energy dispendious reaction.
Unguided prebiotic synthesis of RNA and DNA: an unsolved riddle!
The origin of the RNA and DNA molecule is an origin of life problem, not evolution.
Steve Benner, one of the world’s leading authorities on abiogenesis: The “origins problem” CANNOT be solved.
Graham Cairns-Smith: The odds against a successful unguided synthesis of a batch of primed nucleotide on the primitive Earth would be a huge number, represented approximately by a 1 followed by 109 zeros ( 10^109). 'The odds are enormous against its being coincidence. No figures could express them.'
Tan, Change; Stadler, Rob. The Stairway To Life
The longest chains (up to fifty monomers) and the highest production of molecules with the correct bonds have been achieved in the presence of montmorillonite clay. With purine nucleotides (adenosine and guanine), the percentage of correct phosphodiester bonds actually exceeded that of the incorrect bonds. However, the addition of each monomer to the chain comes with a probability of incorrect bonding, and one incorrect bond irreversibly destroys the homolinkage of the growing polymer, just as a train with one derailed boxcar can destroy the entire train. Therefore, the synthetic yield of biopolymers with the desired homolinkage decreases exponentially as the length of the biopolymer increases—even when starting only with pure building blocks.
In the presence of montmorillonite, polymerization of purine nucleotides (i.e., adenine and guanine) is favored over pyrimidine nucleotides (i.e., cytosine, thymine, and uracil) . This would constrain the potential information-carrying capacity of the resulting polymers, somewhat like requiring an author to have the letters A through M appear in their writing twice as often as the letters N through Z.
Another issue with the montmorillonite-catalyzed reaction is that the most successful polymerization occurred with an inosine nitrogenous base, which is not used to synthesize natural DNA or RNA. Also, the resulting oligomers decompose in the presence of water and the clay accelerates this decomposition.
Production of DNA with perfect homolinkage throughout the length of a genome (for example, there are approximately 500,000 nucleotides in the simplest known free-living organism’s genome) is impossible without the molecular machinery that is available only in living organisms.
An E. coli cell is about two micrometers long (two millionths of a meter) and one micrometer in diameter, and it contains a circular DNA molecule with 4.6 million base pairs. If fully extended, the DNA molecule would measure about 1.4 millimeters, or about 700 times longer than the E. coli cell. Picture your car, representing an E. coli, containing a rope that represents the DNA. Scaling up the E. coli to become the size of your car (about five meters or sixteen feet in length), the DNA would correspondingly scale up to approximately 3.5 kilometers or 2.2 miles of rope with a diameter of six millimeters or ¼ inch, contained in your car. Indeed, all of that DNA has to be compressed to fit within each bacterial cell. Now, imagine the E. coli cell duplicating this DNA before replication and needing to separate the two interlinked copies before cell division. Genomic DNAs are so long that they cannot fit into any cells without being highly compacted with the help of multiple proteins. Also, such a length of rope cannot be manipulated without kinking and supercoiling, especially when DNA is unwound for reproduction. Additional topoisomerase enzymes and structural maintenance of chromosome (SMC) proteins are essential for this purpose.
Prof. Steven A. Benner: When Did Life Likely Emerge on Earth in an RNA-First Process? 24 September 2019
All of these path‐hypotheses involve relatively reduced organic molecules that serve as the precursors of the four standard RNA nucleobases (guanine, adenine, cytosine, and uracil) or “grandfather′s axe” heterocycles (not shown). Thus, all assume the production of substantial amounts of reduced primary precursors, likely in the Hadean atmosphere (before 4 billion years ago). These primary precursors include hydrogen cyanide (HCN), cyanamide (H2NCN), cyanoacetylene (HCCCN), cyanogen (NCCN), ammonia (NH3), and cyanic acid (HCNO). Further, they all assume that these (or their downstream products) avoided dilution into a global ocean, perhaps by adsorbing on solids, or by delivery to sub‐aerial land with a constrained aquifer. Most chemical path‐hypotheses to create RNA prebiologically all but require an atmosphere that is more reducing than what planetary accretion models suggest was the norm above the Hadean Earth.
https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/syst.201900035
Prof. Steven A. Benner: Asphalt, Water, and the Prebiotic Synthesis of Ribose, Ribonucleosides, and RNA 2012
Some bonds in RNA appear to be “impossible” to form under any conditions considered plausible for early Earth
https://pubs.acs.org/doi/abs/10.1021/ar200332w
Henderson James Cleaves: One Among Millions: The Chemical Space of Nucleic Acid-Like Molecules September 9, 2019
Various types of nucleic acid-like molecules have been enumerated and synthesized, but this is the first systematic attempt to enumerate, quantify and describe this chemical space. This space is surprisingly large, though its size appears predictable by typical isomerism studies. It is remarkable, given the existence of this structure space, that biology found a solution to the need for information storage. Is the solution life found to genetic molecular information storage optimal? In one sense obviously yes: it works very well and has managed to robustly support biological evolution over 3.5-4 Ga of planetary change. In another sense, from the standpoint of xeno- and synthetic biology, could other, perhaps equally good, or even better genetic systems be devised? The answer to this question will require sophisticated and protracted chemical experimentation. Studies to date suggest that the answer could be no. Many nearly as good, some equally good, and a few stronger base-pairing analogue systems are known.
https://pubs.acs.org/doi/10.1021/acs.jcim.9b00632
Graham Cairns-Smith: Genetic takeover, page 66:
Now you may say that there are alternative ways of building up nucleotides, and perhaps there was some geochemical way on the early Earth. But what we know of the experimental difficulties in nucleotide synthesis speaks strongly against any such supposition. However it is to be put together, a nucleotide is too complex and metastable a molecule for there to be any reason to expect an easy synthesis. If you were to consider in more detail a process such as the purification of an intermediate ( to form amide bonds between amino acids and nucleotides ) you would find many subsidiary operations — washings, pH changes and so on. (Remember Merrifield’s machine: for one overall reaction, making one peptide bond, there were about 90 distinct operations required.)
https://3lib.net/book/2797668/0495a0
A. Graham Cairns-Smith: Chemistry and the Missing Era of Evolution
What is missing from this story of the evolution of life on earth is the original means of producing such sophisticated materials as RNA. The main problem is that the replication of RNA depends on a clean supply of rather complicated monomers—activated nucleotides. What was required to set the scene for an RNA world was a highly competent, long-term means of production of at least two nucleotides. In practice the discrimination required to make nucleotide parts cleanly, or to assemble them correctly, still seems insufficient.
https://sci-hub.ren/10.1002/chem.200701215
Let us consider some of the difficulties to make RNA & DNA
1. as we have seen, it is not even clear that the primitive Earth would have generated and maintained organic molecules. All that we can say is that there might have been prevital organic chemistry going on, at least in special locations.
2. highenergy precursors of purines and pyrimidines had to be produced in a sufficiently concentrated form (for example at least 0.01 M HCN).
3. the conditions must now have been right for reactions to give perceptible yields of at least two bases that could pair with each other.
4. these bases must then have been separated from the confusing jumble of similar molecules that would also have been made, and the solutions must have been sufficiently concentrated.
5. in some other location a formaldehyde concentration of above 0.01 M must have built up.
6. this accumulated formaldehyde had to oligomerise to sugars.
7. somehow the sugars must have been separated and resolved, so as to give a moderately good concentration of, for example, D-ribose.
8. bases and sugars must now have come together.
9. Ninth, they must have been induced to react to make nucleosides. (There are no known ways of bringing about this thermo dynamically uphill reaction in aqueous solution: purine nucleosides have been made by dry phase synthesis, but not even this method has been successful for condensing pyrimidine bases and ribose to give nucleosides
10. Whatever the mode of joining base and sugar it had to be between the correct nitrogen atom of the base and the correct carbon atom of the sugar. This junction will fix the pentose sugar as either the a- or fl-anomer of either the furanose or pyranose forms. For nucleic acids it has to be the fl-furanose. (In the dry-phase purine nucleoside syntheses referred to above, all four of these isomers were present with never more than 8 ‘Z, of the correct structure.)
11. phosphate must have been, or must now come to have been, present at reasonable concentrations. (The concentrations in the oceans would have been very low, so we must think about special situations—evaporating lagoons and such things
12. the phosphate must be activated in some way — for example as a linear or cyclic polyphosphate — so that (energetically uphill) phosphorylation of the nucleoside is possible.
13. to make standard nucleotides only the 5’- hydroxyl of the ribose should be phosphorylated. (In solid-state reactions with urea and inorganic phosphates as a phosphorylating agent, this was the dominant species to begin with. Longer heating gave the nucleoside cyclic 2’,3’-phosphate as the major product although various dinucleotide derivatives and nucleoside polyphosphates are also formed
14. if not already activated — for example as the cyclic 2’,3’-phosphate — the nucleotides must now be activated (for example with polyphosphate) and a reasonably pure solution of these species created of reasonable concentration. Alternatively, a suitable coupling agent must now have been fed into the system.
15. the activated nucleotides (or the nucleotides with coupling agent) must now have polymerised. Initially this must have happened without a pre-existing polynucleotide template (this has proved very difficult to simulate ; but more important, it must have come to take place on pre-existing polynucleotides if the key function of transmitting information to daughter molecules was to be achieved by abiotic means. This has proved difficult too. Orgel & Lohrmann give three main classes of problem.
(i) While it has been shown that adenosine derivatives form stable helical structures with poly(U) — they are in fact triple helixes — and while this enhances the condensation of adenylic acid with either adenosine or another adenylic acid — mainly to di(A) - stable helical structures were not formed when either poly(A) or poly(G) Were used as templates.
(ii) It was difficult to find a suitable means of making the internucleotide bonds. Specially designed water-soluble carbodiimides were used in the experiments described above, but the obvious pre-activated nucleotides — ATP or cyclic 2’,3’-phosphates — were unsatisfactory. Nucleoside 5'-phosphorimidazolides, for example: N/\ n K/N/P-r’o%OHN/\N were more successful, but these now involve further steps and a supply of imidazole, for their synthesis.
(iii) Internucleotide bonds formed on a template are usually a mixture of 2’—5’ and the normal 3’—5’ types. Often the 2’—5’ bonds predominate although it has been found that Zn“, as well as acting as an eflicient catalyst for the templatedirected oligomerisation of guanosine 5’-phosphorimidazolide also leads to a preference for the 3’—5’ bonds.
16. the physical and chemical environment must at all times have been suitable — for example the pH, the temperature, the M2+ concentrations.
17. all reactions must have taken place well out of the ultraviolet sunlight; that is, not only away from its direct, highly destructive effects on nucleic acid-like molecules, but away too from the radicals produced by the sunlight, and from the various longer lived reactive species produced by these radicals.
18. unlike polypeptides, where you can easily imagine functions for imprecisely made products (for capsules, ionexchange materials, etc), a genetic material must work rather well to be any use at all — otherwise it will quickly let slip any information that it has managed to accumulate.
19. what is required here is not some wild one-off freak of an event: it is not true to say ‘it only had to happen once’. A whole set-up had to be maintained for perhaps millions of years: a reliable means of production of activated nucleotides at the least.
As the difficulties accumulate the stakes get higher: success would be all the more resounding, but it becomes less likely. Sooner or later it becomes wiser to put your money elsewhere.

Last edited by Otangelo on Thu Sep 19, 2024 7:45 am; edited 165 times in total