2. Prebiotic Nucleotide Synthesis
DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) are the fundamental molecules that store and transmit genetic information in living organisms. These nucleic acids are at the core of life's information-driven processes, playing crucial roles in heredity, cellular function, and biological complexity. The study of nucleic acids began in 1871 when Friedrich Miescher first identified "nuclein" in his essay "Über die chemische Zusammensetzung der Eiterzellen" (About the chemical composition of pus cells). Miescher characterized this substance as nitrogen-containing and rich in phosphorous. Over the following decades, researchers worked to unravel the molecular structure of nucleic acids. A major breakthrough came in 1953 when James Watson and Francis Crick discovered the double-helix structure of DNA. Their work was significantly aided by X-ray crystallography data from Maurice Wilkins and Rosalind Franklin at King's College London. This collaborative effort, though not without controversy, led to our understanding of DNA's iconic double-helix structure. Both DNA and RNA are composed of three key components: a nitrogenous base, a five-carbon sugar (pentose), and a phosphate group. These elements combine to form nucleotides, the monomers of nucleic acids. DNA and RNA share a similar four-letter alphabet, with the main difference being that DNA uses thymine where RNA uses uracil. In the genome, DNA typically forms double strands with Watson-Crick base-pairing, while RNA is often single-stranded and more versatile in its functions. The central dogma of molecular biology describes the flow of genetic information from DNA to RNA to proteins in all known living organisms. DNA serves as the long-term storage of genetic information, while RNA plays multiple roles, including acting as a messenger in protein synthesis and serving regulatory functions. Some viruses even use RNA as their primary genetic material. Understanding the origin and evolution of these complex molecules is crucial for unraveling the mystery of life's beginnings on Earth. The prebiotic synthesis of nucleotides and their subsequent polymerization into functional nucleic acids remains one of the most challenging questions in origin of life research. As we delve deeper into nucleic acid chemistry, we continue to uncover the intricate processes that underlie life's information systems and their pivotal role in biological complexity.
2.1. Formation of Simple Prebiotic Chemicals for Nucleotide Synthesis
The formation of simple prebiotic chemicals necessary for nucleotide synthesis is a critical step in understanding the origin of life. This process involves the interplay of various elemental sources, atmospheric and aqueous chemistry, and energy inputs that drive prebiotic reactions.
2.1.1. Sources of carbon, nitrogen, and phosphorus
Carbon, nitrogen, and phosphorus are essential elements for the formation of nucleotides. In the prebiotic Earth, carbon was likely abundant in the form of carbon dioxide (CO2) in the atmosphere and dissolved in water bodies. Methane (CH4) may have also been present, particularly in reducing environments. Nitrogen was primarily available as molecular nitrogen (N2) in the atmosphere, with some conversion to ammonia (NH3) through various processes. Phosphorus, crucial for the phosphate groups in nucleotides, was less readily available. It may have been sourced from minerals such as apatite, or delivered by meteorites in the form of phosphides, which could be subsequently oxidized and solubilized.
2.1.2. Relevant atmospheric and aqueous chemistry
The early Earth's atmosphere is thought to have been weakly reducing or neutral, composed mainly of N2, CO2, and water vapor, with smaller amounts of CO, H2, and possibly CH4. This composition facilitated the formation of simple organic molecules through atmospheric chemistry. For instance, the reaction of methane with ammonia and water vapor, energized by lightning or UV radiation, could produce formaldehyde (CH2O) and hydrogen cyanide (HCN). These molecules are crucial precursors for more complex organic compounds.
In aqueous environments, such as primitive oceans, lakes, or hydrothermal systems, further chemical reactions could occur. The concentration of reactants through evaporation cycles or mineral adsorption might have played a significant role in driving these reactions forward. The formose reaction, which can produce sugars including ribose from formaldehyde, is an example of a potentially important aqueous reaction. However, the specificity and yield of such reactions under prebiotic conditions remain subjects of debate.
2.1.3. Energy sources for prebiotic reactions
Several energy sources have been proposed to drive prebiotic reactions:
UV radiation: The early Earth likely received more ultraviolet radiation due to the absence of an ozone layer. This high-energy radiation could have initiated photochemical reactions in the atmosphere and on exposed surfaces, potentially leading to the formation of simple organic molecules.
Lightning: Electrical discharges in the atmosphere could have provided localized, high-energy events capable of driving the synthesis of organic compounds from atmospheric gases. The Miller-Urey experiment famously demonstrated the production of amino acids through simulated lightning in a reducing atmosphere.
Hydrothermal vents: Submarine hydrothermal systems, both alkaline and acidic, have been proposed as potential sites for prebiotic chemistry. These environments provide thermal energy, mineral catalysts, and chemical gradients that could facilitate the formation and concentration of organic molecules.
Radioactivity: Natural radioactive decay from elements in Earth's crust could have provided another source of ionizing radiation, potentially driving chemical reactions in certain geological settings.
Impact events: During the early history of Earth, frequent impacts from asteroids and comets not only delivered organic materials but also provided localized, high-energy environments that could have driven complex chemical reactions.
Unresolved Challenges in the Formation of Simple Prebiotic Chemicals for Nucleotide Synthesis
1. Carbon Source Limitations
a) CO2 reduction hurdles:
- CO2 was likely abundant but its reduction to organic compounds is thermodynamically unfavorable
- No known efficient prebiotic catalysts for CO2 reduction under early Earth conditions
- Proposed mechanisms often require implausible concentrations of reducing agents
b) Methane utilization challenges:
- CH4 may have been present in reducing environments, but its direct incorporation into organic molecules is difficult
- Proposed atmospheric reactions with CH4 have low yields and specificity
- Lack of clear pathways from CH4 to more complex carbon compounds needed for nucleotides
Conceptual problem: Carbon Fixation
- No convincing prebiotic analogue to biological carbon fixation pathways
- Difficulty in explaining the transition from simple C1 compounds to complex organic molecules
2. Nitrogen Availability and Reactivity
a) N2 fixation barriers:
- N2 is chemically inert, requiring significant energy input for fixation
- Proposed prebiotic N2 fixation mechanisms (e.g., lightning, UV radiation) have low efficiency
- No clear path from N2 to biologically relevant nitrogen-containing compounds
b) Ammonia stability issues:
- NH3 is unstable under UV light, raising questions about its accumulation
- Atmospheric models suggest low NH3 concentrations in the prebiotic atmosphere
- Difficulty in maintaining sufficient NH3 levels for organic synthesis
Conceptual problem: Reactive Nitrogen Scarcity
- Lack of plausible mechanisms to generate and maintain high concentrations of reactive nitrogen species
- No known prebiotic routes to efficiently incorporate nitrogen into complex organic molecules
3. Phosphorus Accessibility and Reactivity
a) Mineral source limitations:
- Most phosphorus on early Earth was likely in insoluble mineral forms (e.g., apatite)
- Low solubility of phosphate minerals limits availability in aqueous environments
- No known efficient mechanisms for releasing and concentrating phosphate from minerals
b) Meteoritic phosphorus challenges:
- Proposed delivery of phosphides by meteorites faces issues of scarcity
- Oxidation and solubilization of phosphides under prebiotic conditions is poorly understood
- Lack of evidence for sufficient meteoritic phosphorus flux to sustain prebiotic chemistry
Conceptual problem: Phosphate Incorporation
- No clear prebiotic pathways for phosphorylation of organic molecules
- Difficulty in explaining the prevalence of phosphate in biological systems given its scarcity in prebiotic environments
4. Atmospheric and Aqueous Chemistry Complexities
a) Atmospheric composition uncertainties:
- Debate over the exact composition of the early Earth's atmosphere (reducing vs. neutral)
- Lack of consensus on the concentrations of key species like CH4, CO, and H2
- Difficulty in experimentally simulating accurate prebiotic atmospheric conditions
b) Formaldehyde and HCN formation challenges:
- Proposed mechanisms for CH2O and HCN synthesis often require specific atmospheric compositions
- Low yields and competing reactions in more realistic atmospheric models
- Stability and accumulation of these compounds in aqueous environments is questionable
c) Formose reaction limitations:
- Low selectivity for biologically relevant sugars like ribose
- Side reactions and degradation products dominate under most conditions
- No clear mechanism for the selection and stabilization of specific sugar products
Conceptual problem: Reaction Specificity
- Prebiotic reactions typically produce complex mixtures with low yields of desired products
- Lack of selective forces to drive the accumulation of specific, biologically relevant molecules
5. Energy Source Integration
a) UV radiation paradox:
- Can drive some synthetic reactions but also degrades organic molecules
- Difficulty in explaining how UV-sensitive compounds (e.g., nucleobases) could accumulate
b) Lightning event limitations:
- Provides intense but localized and infrequent energy input
- Challenge in explaining how products of lightning-driven chemistry could be preserved and concentrated
c) Hydrothermal system complexities:
- Varying conditions (temperature, pH, mineral composition) across different vent types
- Difficulty in reconciling the conditions required for different prebiotic reactions within a single hydrothermal setting
d) Radioactivity and impact event uncertainties:
- Sporadic and localized nature of these energy sources
- Lack of experimental evidence for efficient prebiotic synthesis under these conditions
Conceptual problem: Energy-Chemistry Coupling
- No known prebiotic mechanisms for efficiently channeling various energy inputs into specific, productive chemical pathways
- Difficulty in explaining how complex organic molecules could form and accumulate in high-energy environments without rapid degradation
These challenges highlight the significant gaps in our understanding of how the simple chemicals required for nucleotide synthesis could have formed and accumulated on the prebiotic Earth. The interplay between elemental sources, atmospheric and aqueous chemistry, and various energy inputs presents a complex system with many unresolved questions. The lack of plausible mechanisms to overcome these hurdles raises fundamental issues about the sufficiency of unguided processes to generate the precursors necessary for the origin of life.
2.2. Nucleobases: The Building Blocks of Genetic Information
Nucleobases are essential components of RNA and DNA, the molecules responsible for storing and transmitting genetic information. These bases are divided into two categories: purines and pyrimidines. Purines, which include adenine (A) and guanine (G), have a double-ring structure composed of nine atoms. Pyrimidines, comprising cytosine (C), thymine (T) in DNA, and uracil (U) in RNA, have a single six-atom ring structure.
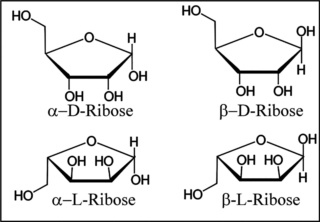
The structural difference between DNA and RNA lies in their sugar components. Ribonucleic acid (RNA) contains a hydroxyl (-OH) group, whereas deoxyribonucleic acid (DNA) has only a hydrogen atom in place of this hydroxyl group.
2.3. Prebiotic Synthesis of Nucleobases
Understanding how nucleobases could have formed under early Earth conditions is a crucial challenge in origin-of-life research. Scientists have explored various pathways for their synthesis, with varying degrees of success and plausibility.
2.3.1. Purines (Adenine and Guanine)
2.3.2. Hydrogen Cyanide (HCN) Polymerization
One of the earliest attempts to synthesize adenine was made by Oró in 1961. He reported the synthesis of adenine from aqueous solutions of ammonium cyanide at temperatures below 100°C. However, the yield was extremely low, at only 0.5%, with most of the cyanide forming an intractable polymer. This experiment highlighted a significant issue: there was no prebiotic natural selection mechanism to sort out the bases that could later be used as nucleobases from those with no function.
Shapiro critiqued this approach, pointing out that useful yields of adenine cannot be obtained except in the presence of 1.0 M or stronger ammonia, which is far higher than the estimated 0.01 M concentration that might have been present in primitive oceans and lakes. He also noted that adenine's instability on a geological time scale makes its widespread prebiotic accumulation unlikely.
Shapiro further elaborated on the challenges of adenine synthesis: "Adenine synthesis requires unreasonable hydrogen cyanide concentrations. Adenine plays an essential role in replication in all known living systems today and is prominent in many other aspects of biochemistry. Despite this, a consideration of its intrinsic chemical properties suggests that it did not play these roles at the very start of life. These properties include the low yields in known syntheses of adenine under authentic prebiotic conditions, its susceptibility to hydrolysis and to reaction with a variety of simple electrophiles, and its lack of specificity and strength in hydrogen bonding at the monomer and mixed oligomer level." 1
Regarding the possibility of an extraterrestrial source for adenine, Shapiro noted: "The isolation of adenine and guanine from meteorites has been cited as evidence that these substances might have been available as 'raw material' on prebiotic Earth. However, acid hydrolyses have been needed to release these materials, and the amounts isolated have been low." 2
For guanine, the situation is even more challenging. In 1984, Yuasa reported a mere 0.00017% yield of guanine after electrical discharge experiments. S.L. Miller and colleagues conducted experiments in 1999, yielding trace amounts of guanine (0.0007% to 0.0035%) from the polymerization of ammonium cyanide, suggesting that guanine could potentially arise in frozen regions of the primitive Earth. 3
In 2010, Abby Vogel Robinson reported: "For scientists attempting to understand how the building blocks of RNA originated on Earth, guanine -- the G in the four-letter code of life -- has proven to be a particular challenge. While the other three bases of RNA -- adenine (A), cytosine (C), and uracil (U) -- could be created by heating a simple precursor compound in the presence of certain naturally occurring catalysts, guanine had not been observed as a product of the same reactions."
2.3.3. Formamide-Based Synthesis
An alternative approach to purine synthesis involves formamide, a simpler and more versatile precursor. Studies have demonstrated that formamide can serve as a prebiotic source for purine synthesis, particularly when subjected to heat or mineral catalysis. Formamide can yield both adenine and guanine under high-temperature conditions without requiring extremely high concentrations of HCN. This pathway also has the advantage of producing higher yields of purines compared to HCN polymerization, with some studies reporting significant quantities of guanine in the presence of certain catalysts.
In a recent paper from 2018, Annabelle Biscans mentions other routes investigated: "Miyakama et al. suggest that purines have been formed in the atmosphere in the absence of hydrogen cyanide. They reported that guanine could have been generated from a gas mixture (nitrogen, carbon monoxide, and water) after cometary impacts. Also, it has been proposed that adenine was formed in the solar system (outside of Earth) and brought to Earth by meteorites, given the fact that adenine was found in significant quantity in carbonaceous chondrites."
Biscans concludes: "Despite great efforts and impressive advancements in the study of nucleoside and nucleotide abiogenesis, further investigation is necessary to explain the gaps in our understanding of the origin of RNA." 4
2.3.4. Pyrimidines (Cytosine and Uracil)
Pyrimidine bases are the second of the quartet that makes up DNA and RNA that stores genetic information. Uracil (Thymine in DNA) and cytosine are made of one nitrogen-containing ring. The prebiotic synthesis of pyrimidines presents its own set of challenges, particularly due to the instability of cytosine and the limited availability of plausible precursor molecules.
2.3.5. Cytosine Synthesis
Scientists have failed to produce cytosine in spark-discharge experiments. Robert Shapiro (1999) noted: "The formation of a substance in an electric spark discharge conducted in a simulated early atmosphere has also been regarded as a positive indication of its prebiotic availability. Again, low yields of adenine and guanine have been reported in such reactions, but no cytosine. The failure to isolate even traces of cytosine in these procedures signals the presence of some problem with its synthesis and/or stability. The deamination of cytosine and its destruction by other processes such as photochemical reactions place severe constraints on prebiotic cytosine syntheses." 1
Rich Deem (2001) summarized several issues with cytosine synthesis:
"Cytosine has never been found in any meteorites.
Cytosine is not produced in electric spark discharge experiments using simulated 'early Earth atmosphere.'
Synthesis based upon cyanoacetylene requires the presence of large amounts of methane and nitrogen, however, it is unlikely that significant amounts of methane were present at the time life originated.
Synthesis based upon cyanate is problematical, since it requires concentrations in excess of 1 M (molar). When concentrations of 0.1 M (still unrealistically high) are used, no cytosine is produced.
Synthesis based upon cyanoacetaldehyde and urea suffers from the problem of deamination of the cytosine in the presence of high concentrations of urea (low concentrations produce no cytosine). In addition, cyanoacetaldehyde is reactive with a number of prebiotic chemicals, so would never attain reasonable concentrations for the reaction to occur. Even without the presence of other chemicals, cyanoacetaldehyde has a half-life of only 31 years in water.
Cytosine deaminates with an estimated half-life of 340 years, so would not be expected to accumulate over time.
Ultraviolet light on the early earth would quickly convert cytosine to its photohydrate and cyclobutane photodimers (which rapidly deaminate)." 5
2.3.6. Uracil Synthesis
In 1961, Sidney Fox and colleagues synthesized uracil under: "thermal conditions which yield other materials of theoretical prebiochemical significance. The conditions studied in the synthesis of uracil included temperatures in the range of 100° to 140°C, heating periods of from 15 minutes to 2 hours." 6
2.3.7. Recent Advances in Pyrimidine Synthesis
In 2009, Sutherland and Szostak published a paper on a high-yielding route to activated pyrimidine nucleotides under conditions thought to be prebiotic, claiming to be "an encouraging step toward the greater goal of a plausible prebiotic pathway to RNA and the potential for an RNA world." 7
However, Robert Shapiro disagrees:
"Although as an exercise in chemistry this represents some very elegant work, this has nothing to do with the origin of life on Earth whatsoever. The chances that blind, undirected, inanimate chemistry would go out of its way in multiple steps and use of reagents in just the right sequence to form RNA is highly unlikely." 8
In 2019, Okamura and colleagues published a paper on pyrimidine nucleobase synthesis where their conclusion remarks are noteworthy:
"We show that the cascade reaction proceeds under one-pot conditions in a continuous manner to provide SMePy 6. Importantly, the key intermediate SMePy 6 gives rise not only to canonical but also to non-canonical bases, arguing for the simultaneous prebiotic formation of a diverse set of pyrimidines under prebiotically plausible conditions." 9
2.3.8. Stability and Decomposition of Nucleobases
The stability of nucleobases in prebiotic conditions is a significant challenge to their accumulation and eventual incorporation into early life forms. Adenine deaminates at 37°C with a half-life of 80 years. At 100°C, its half-life is 1 year. For guanine, at 100°C, its half-life is 10 months, uracil is 12 years, and thymine 56 years. For the decomposition of a nucleobase, this is very short. For nucleobases to accumulate in prebiotic environments, they must be synthesized at rates that exceed their decomposition. Therefore, adenine and the other nucleobases would never accumulate in any kind of "prebiotic soup." 1
A paper published in 2015 points out that:
"Nucleotide formation and stability are sensitive to temperature. Phosphorylation of nucleosides in the laboratory is slower at low temperatures, taking a few weeks at 65°C compared with a couple of hours at 100°C. The stability of nucleotides, on the other hand, is favored in warm conditions over high temperatures. If a WLP is too hot (>80°C), any newly formed nucleotides within it will hydrolyze in several days to a few years. At temperatures of 5°C to 35°C that either characterize more-temperate latitudes or a post snowball Earth, nucleotides can survive for thousand-to-million-year timescales. However, at such temperatures, nucleotide formation would be very slow." 10
This presents a significant paradox: in hot environments, nucleotides might form, but they decompose fast. On the other hand, in cold environments, they might not degrade that fast, but take a long time to form. Nucleotides would have to be generated by prebiotic environmental synthesis processes at a far higher rate than they are decomposed and destroyed, and accumulated and concentrated at one specific construction site.
2.3.9. Selection of Nucleobases Used in Life
The selection of specific nucleobases for life - adenine, guanine, cytosine, uracil, and thymine - is a topic of considerable scientific interest and debate. These nucleobases form the foundation of RNA and DNA, but their prevalence in biological systems raises questions about how they were selected from a vast "structure space" of possible molecules.
2.3.10.The Concept of Structure Space
H. James Cleaves (2015) introduced the concept of "structure space" to describe the number of molecular structures that could potentially exist given specific parameters 11. This space is incredibly vast - for example, the number of possible stable drug-like organic molecules may be on the order of 10^33 to 10^180. In comparison, as of July 2009, there were only about 49 million unique chemical substances registered with the Chemical Abstracts Service.
When considering nucleobases specifically, the structure space becomes even more complex. The number of molecules that could fulfill the minimal requirements of being "nucleic acid-like" is remarkably large and potentially limitless. This includes various structural isomers of RNA that could theoretically function as genetic platforms.
2.3.11. Prebiotic Chemistry and Nucleobase Formation
On the early Earth, a wide array of molecules could have been generated by natural processes such as lightning, hydrothermal vents, and volcanic eruptions. The Murchison meteorite, for instance, contains a complex set of organic compounds ranging from 100,000 to perhaps 10 million unique molecular species.
However, despite this chemical diversity, life on Earth uses a very specific set of nucleobases. To date, no one-pot reaction has yielded either purine or pyrimidine ribonucleosides directly from likely prevalent prebiotic starting materials, making the abiotic origin of these specific nucleobases a challenging problem to solve.
2.3.12. The RNA World Hypothesis and Alternative Nucleobases
Andro C. Rios (2014) suggested that the early RNA world may have included many types of nucleobases beyond those we see in modern life 12. This hypothesis is supported by the extensive use of non-canonical nucleobases in extant RNA and the similarity of many modified bases to heterocycles generated in simulated prebiotic chemistry experiments.
Nucleobase modification is a ubiquitous post-transcriptional activity found across all domains of life, vital to cellular function as it modulates genetic expression. This suggests that life may have initially used a wider variety of nucleobases before settling on the current set.
2.3.13. The Challenge of Selection
The central question remains: how did nature "decide" upon these specific heterocycles from the vast structure space of possible molecules? Several factors complicate this question:
1. The structure space of possible nucleobase-like molecules is essentially limitless, especially when considering different ring structures and isomeric conformations.
2. Modern cells synthesize nucleobases through complex metabolic pathways that were not present prebiotically.
3. Selecting a specific set of complex macromolecules out of unlimited "structure space" by unguided means is theoretically possible but extremely improbable.
Unresolved Challenges in Prebiotic Nucleobase Synthesis
1. Complexity of Chemical Processes
The synthesis of nucleobases under prebiotic conditions involves intricate, multi-step chemical reactions. Without biological catalysts or guided processes, replicating these reactions in a natural environment poses significant challenges. For instance, the formation of adenine from hydrogen cyanide requires specific concentrations and conditions that are unlikely to occur spontaneously.
Conceptual problem: Spontaneous Complexity
- No known natural mechanism to drive such complex, multi-step reactions without external guidance
- Extremely low probability of these reactions occurring in sequence without intervention
2. Specific Synthesis Challenges for Cytosine and Guanine
Despite extensive research, the synthesis of cytosine and guanine under plausible prebiotic conditions remains elusive. Cytosine, in particular, has never been produced in spark-discharge experiments simulating early Earth atmospheres. Guanine synthesis yields are extremely low, with reported yields as low as 0.00017% in electrical discharge experiments.
Conceptual problem: Lack of Natural Pathways
- No viable routes identified for cytosine formation under prebiotic conditions
- Extremely low yields for guanine synthesis raise questions about its availability on early Earth
3. Nucleobase Instability
Nucleobases degrade rapidly under conditions thought to be present on early Earth. For example, adenine deaminates at 37°C with a half-life of 80 years, and at 100°C, its half-life is reduced to just 1 year. This instability prevents the accumulation of nucleobases in sufficient concentrations for nucleic acid formation.
Conceptual problem: Molecular Instability
- Rapid degradation of nucleobases in prebiotic environments challenges their persistence
- Difficulty in explaining how unstable molecules could accumulate to form more complex structures
4. Cytosine Synthesis and Stability
Cytosine presents a unique challenge due to its synthesis difficulties and instability. It has not been detected in meteorites and is not produced in electric spark discharge experiments. Additionally, cytosine deaminates with an estimated half-life of 340 years, further complicating its accumulation over time.
Conceptual problem: Absence of Cytosine Pathway
- No plausible prebiotic route for cytosine formation identified
- Rapid deamination of cytosine challenges its role in early genetic systems
5. Guanine Formation Barriers
Guanine synthesis under prebiotic conditions has proven extremely challenging. Experiments have yielded only trace amounts (0.0007% to 0.0035%) from ammonium cyanide polymerization. The absence of a clear, high-yield pathway for guanine formation poses significant problems for prebiotic chemistry scenarios.
Conceptual problem: Guanine Synthesis Limitations
- Extremely low yields in prebiotic simulations question guanine's availability
- Lack of efficient synthesis pathways challenges guanine's inclusion in early genetic material
6. Adenine Synthesis Requirements
The synthesis of adenine requires unrealistically high concentrations of hydrogen cyanide (HCN). Useful yields are only obtained in the presence of 1.0 M or stronger ammonia, far exceeding the estimated 0.01 M concentration in primitive oceans and lakes.
Conceptual problem: Unrealistic Conditions
- Required HCN concentrations for adenine synthesis are implausible in natural settings
- Discrepancy between laboratory conditions and estimated prebiotic environments
7. Uracil Stability and Synthesis
While uracil synthesis has been demonstrated under thermal conditions, its stability under early Earth conditions remains problematic. At 100°C, uracil has a half-life of 12 years, which is relatively short on geological timescales.
Conceptual problem: Uracil Degradation
- Rapid degradation of uracil under likely early Earth conditions
- Challenge in explaining uracil's accumulation and incorporation into early genetic systems
8. Tautomeric Shifts in Nucleobases
Nucleobases can exist in multiple tautomeric forms, affecting their ability to form stable base pairs. In a prebiotic setting, controlling these tautomeric shifts to ensure proper base pairing would be extremely challenging.
Conceptual problem: Lack of Tautomeric Control
- Uncontrolled tautomeric shifts could lead to incorrect base pairing
- Absence of a regulatory mechanism to maintain proper tautomeric forms
9. Purity of Chemical Precursors
Prebiotic environments likely contained complex mixtures of chemicals, which would interfere with the synthesis of nucleobases. Achieving the necessary purity for precursor molecules in such environments is highly improbable.
Conceptual problem: Impurity and Contamination
- Impurities in prebiotic chemical pools would hinder nucleobase formation
- Difficulty in achieving required purity levels in natural settings
10. Concentration Problems
Achieving sufficient concentrations of nucleobase precursors is unlikely under the dilute conditions presumed to exist on early Earth. The synthesis of nucleobases typically requires concentrations far exceeding those plausible in prebiotic oceans or lakes.
Conceptual problem: Insufficient Concentrations
- Dilution of reactants in natural environments would prevent necessary nucleobase formation
- No known natural process capable of concentrating precursors sufficiently
11. Energy Source Deficit
The synthesis of nucleobases often requires significant energy input. Identifying a consistent and sufficient energy source to drive these endothermic reactions in a prebiotic setting remains an unresolved challenge.
Conceptual problem: Energy Source Identification
- Lack of a clear, continuous energy source for nucleobase synthesis reactions
- Difficulty in explaining how energy-intensive reactions could proceed naturally
12. Uncontrolled Side Reactions
In complex prebiotic environments, reactive species would likely interfere with nucleobase synthesis, causing unwanted side reactions. Controlling these side reactions without enzymatic guidance is problematic.
Conceptual problem: Side Reaction Control
- Side reactions would consume essential precursors, preventing nucleobase formation
- Absence of regulatory systems to direct specific reaction pathways
13. Thermodynamic Barriers
Many reactions needed to synthesize nucleobases are thermodynamically unfavorable. Overcoming these barriers without biological catalysts or highly specific conditions is improbable in a prebiotic setting.
Conceptual problem: Thermodynamic Challenges
- Thermodynamically unfavorable reactions unlikely to proceed without external intervention
- Difficulty in explaining how energy barriers were overcome in early Earth conditions
14. Environmental Condition Specificity
Nucleobase synthesis requires highly specific environmental conditions (e.g., pH, temperature) that are difficult to achieve and maintain in natural settings. The variability of early Earth environments poses a significant challenge to sustaining these conditions.
Conceptual problem: Environmental Control
- Maintaining consistent, favorable conditions for nucleobase synthesis is implausible
- Fluctuating early Earth conditions would disrupt synthesis processes
15. Water Paradox
While water is necessary for many prebiotic reactions, it also accelerates nucleobase degradation. This paradox presents a significant challenge in explaining how nucleobases could accumulate in aqueous environments.
Conceptual problem: Degradative Role of Water
- Water's dual role as solvent and degradation agent complicates nucleobase accumulation
- No known solution to this paradox in prebiotic environments
16. Correct Isomeric Configuration
Ensuring the correct isomeric forms of nucleobases is crucial for proper base pairing. Prebiotic environments lacked mechanisms for selecting the appropriate isomers, raising questions about how functional nucleic acids could form.
Conceptual problem: Isomeric Control
- No natural mechanism identified to control correct isomer selection
- Incorrect isomers would lead to faulty base pairing, hindering nucleic acid formation
17. Tautomeric Equilibria Control
Maintaining the correct tautomeric forms of nucleobases is essential for base pairing. Achieving this control in prebiotic environments without enzymatic regulation is highly improbable.
Conceptual problem: Tautomeric Imbalance
- Incorrect tautomeric forms would prevent functional base pairing
- High likelihood of tautomeric forms leading to nonfunctional nucleic acids
18. Stereochemistry of Sugar Components
The stereochemistry of sugar components in nucleotides is critical for functional nucleic acids. Achieving the correct stereochemistry without enzymatic control in a prebiotic setting is highly unlikely.
Conceptual problem: Stereochemical Control
- Incorrect stereochemistry would prevent the formation of functional nucleic acids
- Achieving right stereochemistry without enzymatic guidance is improbable
19. Fine-Tuning of Bond Energies
The hydrogen bond strengths in Watson-Crick base pairing are finely tuned for stability and function. Explaining how these precise bond energies emerged naturally is a significant challenge.
Conceptual problem: Bond Energy Fine-Tuning
- Precise bond energies required for stable nucleic acids cannot be explained through unguided processes
- Any deviation from necessary bond strengths would prevent stable nucleic acid formation
20. Hydrogen Bonding Specificity
The specificity of hydrogen bonding needed for Watson-Crick base pairing is unlikely to arise naturally. The probability of correct hydrogen bonding patterns occurring spontaneously is extremely low.
Conceptual problem: Hydrogen Bonding Specificity
- Low probability of correct hydrogen bonding patterns occurring naturally
- Incorrect hydrogen bonding would result in nonfunctional nucleic acids
21. Prevention of Alternative Base Pairs
In a prebiotic setting, non-Watson-Crick base pairs could form, disrupting nucleic acid formation. Explaining how only specific base pairs emerged without a guiding mechanism is problematic.
Conceptual problem: Alternative Base Pair Prevention
- Formation of incorrect base pairs would compromise emerging nucleic acids
- No known mechanism to prevent alternative base pairing in prebiotic conditions
22. Challenges in Backbone Chemistry
The formation of the sugar-phosphate backbone, essential for nucleic acid stability, is a complex process unlikely to occur naturally without guidance. No viable natural pathway for its spontaneous formation has been identified.
Conceptual problem: Backbone Formation
- Absence of a plausible route for sugar-phosphate backbone formation in prebiotic conditions
- Complexity of backbone structure challenges explanations of spontaneous assembly
23. Base Stacking Interactions
Base stacking interactions play a crucial role in nucleic acid stability. Achieving these interactions naturally, without guided processes, is highly improbable.
Conceptual problem: Base Stacking Stability
- No known natural mechanism for forming stable base stacking arrangements
- Absence of correct base stacking would compromise nucleic acid structural integrity
24. Selection of Nucleobase Analogs
It remains unclear why only specific nucleobases capable of Watson-Crick pairing were selected from numerous potential analogs in a prebiotic environment. The absence of selective pressure in a prebiotic world challenges explanations of how only specific nucleobases emerged.
Conceptual problem: Analog Selection
- No identified natural process to explain selection of Watson-Crick compatible nucleobases
- Lack of selective pressure in prebiotic conditions challenges nucleobase specificity
25. Formation of Stable Nucleotides
The formation of nucleosides and nucleotides in aqueous solutions presents significant hurdles under prebiotic conditions. Current research has not identified a prebiotic method for stable nucleotide formation.
Conceptual problem: Nucleotide Formation
- No known prebiotic method for stable nucleotide formation
- Improbability of controlled combination of nucleobases, sugars, and phosphate groups without biological systems
26. Role of Environmental Conditions
Nucleobase synthesis depends on specific environmental factors such as pH, temperature, and ion concentrations. Maintaining consistently favorable conditions on early Earth for nucleobase formation is highly improbable.
Conceptual problem: Environmental Specificity
- Achieving and maintaining precise environmental conditions required for nucleobase synthesis is unlikely in natural settings
- Variability in Earth's early environments would have prevented sustained conditions needed for successful nucleobase formation
2.3.10. Prebiotic Nucleobase Synthesis - Extraterrestrial Sources
The discovery of organic compounds, including nucleobases, in extraterrestrial environments has been one of the most exciting developments in the field of astrobiology and origin of life studies. Nucleobases, the fundamental building blocks of RNA and DNA, have been detected in various cosmic settings, including interstellar space, comets, and meteorites that have fallen to Earth. In 1969, the Murchison meteorite, which fell in Australia, became a landmark in this area of research. Analysis of this carbonaceous chondrite revealed the presence of various organic compounds, including purine and pyrimidine bases. Since then, numerous studies have confirmed the presence of nucleobases in other meteorites, such as the Tagish Lake meteorite and the Antarctic meteorites. Furthermore, space-based observations and laboratory simulations of interstellar ice analogues have suggested that nucleobases could form in the harsh conditions of space. These findings have led some researchers to propose that the essential ingredients for life might have been delivered to early Earth through extraterrestrial sources, potentially jumpstarting the emergence of life. This scenario, often referred to as panspermia or exogenesis, has gained attention as a potential solution to some of the challenges faced in explaining the prebiotic synthesis of these crucial biomolecules on Earth. However, while the presence of nucleobases in space and meteorites is intriguing, it introduces its own set of challenges and does not necessarily solve the fundamental problems of prebiotic nucleobase availability and subsequent RNA or DNA formation. The following points outline why the extraterrestrial source of nucleobases, despite its initial promise, does not fully address the challenges in prebiotic nucleobase synthesis:
Challenges in Prebiotic Nucleobase Synthesis - Addressing Extraterrestrial Sources
1. Stability and Delivery of Nucleobases
The hypothesis that nucleobases were delivered to Earth via meteorites or comets raises significant questions regarding the stability of these molecules during transit. Space is an environment characterized by intense radiation, extreme temperatures, and vacuum conditions, all of which could degrade delicate organic compounds. The survival of nucleobases from their formation in interstellar space to their delivery to Earth remains an unresolved issue. For instance, purine and pyrimidine bases detected in meteorites like the Murchison have undergone intense scrutiny, yet their preservation under such harsh conditions is not fully understood.
Conceptual problem: Nucleobase Stability
- Uncertainty about how nucleobases could remain stable over long cosmic journeys
- Lack of a natural mechanism that could protect these molecules from degradation in space
2. Synthesis in Extraterrestrial Environments
The formation of nucleobases in space introduces additional challenges. Laboratory simulations of interstellar ice analogs suggest that nucleobases can form under specific conditions, but these simulations often require highly controlled environments that may not reflect the chaotic nature of space. The complexity of synthesizing these molecules under natural, unguided conditions, such as in the vast and varied regions of interstellar space, remains a daunting challenge. This issue is further complicated by the fact that nucleobases require precise conditions for their formation, which raises doubts about the likelihood of such processes occurring spontaneously in space.
Conceptual problem: Spontaneous Synthesis
- Difficulty in replicating space conditions conducive to nucleobase formation in the laboratory
- Improbability of spontaneous nucleobase synthesis in uncontrolled, natural space environments
3. Integration into Prebiotic Chemistry
Even if nucleobases were successfully delivered to Earth, integrating them into the prebiotic chemistry required for life is another unsolved problem. Nucleobases would need to not only survive the conditions of early Earth but also integrate into a functional system capable of RNA or DNA formation. The spontaneous assembly of nucleobases into these complex macromolecules, without the guidance of enzymatic processes or an existing template, poses a significant conceptual barrier. The precise order and structure of nucleotides in RNA and DNA are critical for their function, yet there is no known natural mechanism that could have organized these molecules into the correct sequences in the absence of life.
Conceptual problem: Molecular Integration
- Challenge in explaining how nucleobases could self-assemble into functional nucleic acids
- Lack of a known process that could ensure the correct sequencing of nucleotides without guidance
4. Alternative Pathways and Polyphyly
The existence of alternative nucleobase synthesis pathways in different environments, which often share no homology, presents evidence for polyphyly—the notion that life may have originated from multiple independent sources. The Murchison meteorite and other extraterrestrial findings suggest that nucleobases could form in a variety of ways, yet these pathways do not converge on a single, universal mechanism. This divergence undermines the concept of a universal common ancestor and suggests that life, if it emerged from these extraterrestrial sources, did so in a polyphyletic manner. The lack of shared ancestry between these pathways further complicates the narrative of a singular, natural origin of life.
Conceptual problem: Independent Origins
- Evidence of multiple, distinct pathways for nucleobase synthesis challenges the idea of a single origin
- Polyphyly suggests life may have emerged from different sources, contradicting universal common ancestry
5. Naturalistic Explanations and Their Limits
The challenges associated with extraterrestrial nucleobase synthesis and delivery highlight the limitations of naturalistic explanations for the origin of life. The precise conditions required for nucleobase stability, synthesis, and integration into prebiotic chemistry seem improbably orchestrated in a purely unguided scenario. This raises fundamental questions about the adequacy of naturalistic frameworks to account for the emergence of life’s building blocks. Without invoking a guiding mechanism, the spontaneous appearance of such complex molecules and their successful integration into functional biological systems remains unexplained.
Conceptual problem: Adequacy of Naturalistic Explanations
- Inadequacy of naturalistic mechanisms to fully explain nucleobase synthesis, stability, and integration
- Lack of a coherent natural process that could account for the coordinated emergence of life’s building blocks
References
1. Oró, J. (1961). Mechanism of synthesis of adenine from hydrogen cyanide under possible primitive earth conditions. Nature, 191(4794), 1193-1194. Link. (This pioneering work reports on the prebiotic synthesis of adenine from hydrogen cyanide, suggesting a possible mechanism for nucleobase formation on early Earth.)
2. Shapiro, R. (2000). A replicator was not involved in the origin of life. IUBMB Life, 49(3), 173-176. Link. (This paper challenges the RNA world hypothesis, arguing that a self-replicating molecule was not necessary for the origin of life.)
3. Yuasa, S. (1984). Electric discharge synthesis of guanine and its role in the origin of life. Origins of Life and Evolution of the Biosphere, 14(1), 79-85. Link. (This study reports on the synthesis of guanine through electric discharge experiments, exploring its potential role in life's origins.)
4. Biscans, A. (2018). Exploring the emergence of RNA nucleosides and nucleotides on the early Earth. Life, 8(4), 57. Link. (This comprehensive review examines various pathways for the prebiotic synthesis of RNA components, discussing recent advancements and challenges.)
5. Chyba, C., & Sagan, C. (1992). Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life. Nature, 355(6356), 125-132. Link. (This paper explores multiple sources of organic molecules on early Earth, including terrestrial synthesis, delivery by comets and meteorites, and impact-induced synthesis.)
6. Fox, S. W., & Harada, K. (1961). Synthesis of uracil under conditions of a thermal model of prebiological chemistry. Science, 133(3468), 1923-1924. Link. (This study reports on the thermal synthesis of uracil under simulated prebiotic conditions, contributing to our understanding of pyrimidine formation.)
7. Powner, M. W., Gerland, B., & Sutherland, J. D. (2009). Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature, 459(7244), 239-242. Link. (This paper presents a novel pathway for the synthesis of pyrimidine ribonucleotides under prebiotic conditions, addressing a key challenge in the RNA world hypothesis.)
8. Sanderson, K. (2009). Insight into RNA origins. Chemistry World. Link. (This article reports on the work of Sutherland and colleagues, discussing the implications of their findings for understanding RNA origins.)
9. Okamura, H., Crisp, A., P., & Carell, T. (2019). A one-pot, water compatible synthesis of pyrimidine nucleobases under plausible prebiotic conditions. Chemical Communications, 55(13), 1939-1942. Link. (This paper describes a novel, efficient method for synthesizing pyrimidine nucleobases under conditions that could have existed on early Earth.)
10. Pearce, B. K., Pudritz, R. E., Semenov, D. A., & Henning, T. K. (2017). Origin of the RNA world: The fate of nucleobases in warm little ponds. Proceedings of the National Academy of Sciences, 114(43), 11327-11332. Link. (This study investigates the formation and accumulation of RNA nucleobases in warm little ponds on early Earth, considering various environmental factors.)
11. Cleaves, H. J. (2015). The origin of the biologically coded amino acids. Journal of Theoretical Biology, 382, 9-17. Link. (This paper examines the selection of the 20 canonical amino acids, providing insights into the chemical evolution that led to the current genetic code.)
12. Rios, A. C., & Tor, Y. (2013). On the origin of the canonical nucleobases: an assessment of selection pressures across chemical and early biological evolution. Israel Journal of Chemistry, 53(6-7), 469-483. Link. (This study analyzes the factors that may have influenced the selection of the canonical nucleobases, considering both chemical and early biological evolution.)
DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) are the fundamental molecules that store and transmit genetic information in living organisms. These nucleic acids are at the core of life's information-driven processes, playing crucial roles in heredity, cellular function, and biological complexity. The study of nucleic acids began in 1871 when Friedrich Miescher first identified "nuclein" in his essay "Über die chemische Zusammensetzung der Eiterzellen" (About the chemical composition of pus cells). Miescher characterized this substance as nitrogen-containing and rich in phosphorous. Over the following decades, researchers worked to unravel the molecular structure of nucleic acids. A major breakthrough came in 1953 when James Watson and Francis Crick discovered the double-helix structure of DNA. Their work was significantly aided by X-ray crystallography data from Maurice Wilkins and Rosalind Franklin at King's College London. This collaborative effort, though not without controversy, led to our understanding of DNA's iconic double-helix structure. Both DNA and RNA are composed of three key components: a nitrogenous base, a five-carbon sugar (pentose), and a phosphate group. These elements combine to form nucleotides, the monomers of nucleic acids. DNA and RNA share a similar four-letter alphabet, with the main difference being that DNA uses thymine where RNA uses uracil. In the genome, DNA typically forms double strands with Watson-Crick base-pairing, while RNA is often single-stranded and more versatile in its functions. The central dogma of molecular biology describes the flow of genetic information from DNA to RNA to proteins in all known living organisms. DNA serves as the long-term storage of genetic information, while RNA plays multiple roles, including acting as a messenger in protein synthesis and serving regulatory functions. Some viruses even use RNA as their primary genetic material. Understanding the origin and evolution of these complex molecules is crucial for unraveling the mystery of life's beginnings on Earth. The prebiotic synthesis of nucleotides and their subsequent polymerization into functional nucleic acids remains one of the most challenging questions in origin of life research. As we delve deeper into nucleic acid chemistry, we continue to uncover the intricate processes that underlie life's information systems and their pivotal role in biological complexity.
2.1. Formation of Simple Prebiotic Chemicals for Nucleotide Synthesis
The formation of simple prebiotic chemicals necessary for nucleotide synthesis is a critical step in understanding the origin of life. This process involves the interplay of various elemental sources, atmospheric and aqueous chemistry, and energy inputs that drive prebiotic reactions.
2.1.1. Sources of carbon, nitrogen, and phosphorus
Carbon, nitrogen, and phosphorus are essential elements for the formation of nucleotides. In the prebiotic Earth, carbon was likely abundant in the form of carbon dioxide (CO2) in the atmosphere and dissolved in water bodies. Methane (CH4) may have also been present, particularly in reducing environments. Nitrogen was primarily available as molecular nitrogen (N2) in the atmosphere, with some conversion to ammonia (NH3) through various processes. Phosphorus, crucial for the phosphate groups in nucleotides, was less readily available. It may have been sourced from minerals such as apatite, or delivered by meteorites in the form of phosphides, which could be subsequently oxidized and solubilized.
2.1.2. Relevant atmospheric and aqueous chemistry
The early Earth's atmosphere is thought to have been weakly reducing or neutral, composed mainly of N2, CO2, and water vapor, with smaller amounts of CO, H2, and possibly CH4. This composition facilitated the formation of simple organic molecules through atmospheric chemistry. For instance, the reaction of methane with ammonia and water vapor, energized by lightning or UV radiation, could produce formaldehyde (CH2O) and hydrogen cyanide (HCN). These molecules are crucial precursors for more complex organic compounds.
In aqueous environments, such as primitive oceans, lakes, or hydrothermal systems, further chemical reactions could occur. The concentration of reactants through evaporation cycles or mineral adsorption might have played a significant role in driving these reactions forward. The formose reaction, which can produce sugars including ribose from formaldehyde, is an example of a potentially important aqueous reaction. However, the specificity and yield of such reactions under prebiotic conditions remain subjects of debate.
2.1.3. Energy sources for prebiotic reactions
Several energy sources have been proposed to drive prebiotic reactions:
UV radiation: The early Earth likely received more ultraviolet radiation due to the absence of an ozone layer. This high-energy radiation could have initiated photochemical reactions in the atmosphere and on exposed surfaces, potentially leading to the formation of simple organic molecules.
Lightning: Electrical discharges in the atmosphere could have provided localized, high-energy events capable of driving the synthesis of organic compounds from atmospheric gases. The Miller-Urey experiment famously demonstrated the production of amino acids through simulated lightning in a reducing atmosphere.
Hydrothermal vents: Submarine hydrothermal systems, both alkaline and acidic, have been proposed as potential sites for prebiotic chemistry. These environments provide thermal energy, mineral catalysts, and chemical gradients that could facilitate the formation and concentration of organic molecules.
Radioactivity: Natural radioactive decay from elements in Earth's crust could have provided another source of ionizing radiation, potentially driving chemical reactions in certain geological settings.
Impact events: During the early history of Earth, frequent impacts from asteroids and comets not only delivered organic materials but also provided localized, high-energy environments that could have driven complex chemical reactions.
Unresolved Challenges in the Formation of Simple Prebiotic Chemicals for Nucleotide Synthesis
1. Carbon Source Limitations
a) CO2 reduction hurdles:
- CO2 was likely abundant but its reduction to organic compounds is thermodynamically unfavorable
- No known efficient prebiotic catalysts for CO2 reduction under early Earth conditions
- Proposed mechanisms often require implausible concentrations of reducing agents
b) Methane utilization challenges:
- CH4 may have been present in reducing environments, but its direct incorporation into organic molecules is difficult
- Proposed atmospheric reactions with CH4 have low yields and specificity
- Lack of clear pathways from CH4 to more complex carbon compounds needed for nucleotides
Conceptual problem: Carbon Fixation
- No convincing prebiotic analogue to biological carbon fixation pathways
- Difficulty in explaining the transition from simple C1 compounds to complex organic molecules
2. Nitrogen Availability and Reactivity
a) N2 fixation barriers:
- N2 is chemically inert, requiring significant energy input for fixation
- Proposed prebiotic N2 fixation mechanisms (e.g., lightning, UV radiation) have low efficiency
- No clear path from N2 to biologically relevant nitrogen-containing compounds
b) Ammonia stability issues:
- NH3 is unstable under UV light, raising questions about its accumulation
- Atmospheric models suggest low NH3 concentrations in the prebiotic atmosphere
- Difficulty in maintaining sufficient NH3 levels for organic synthesis
Conceptual problem: Reactive Nitrogen Scarcity
- Lack of plausible mechanisms to generate and maintain high concentrations of reactive nitrogen species
- No known prebiotic routes to efficiently incorporate nitrogen into complex organic molecules
3. Phosphorus Accessibility and Reactivity
a) Mineral source limitations:
- Most phosphorus on early Earth was likely in insoluble mineral forms (e.g., apatite)
- Low solubility of phosphate minerals limits availability in aqueous environments
- No known efficient mechanisms for releasing and concentrating phosphate from minerals
b) Meteoritic phosphorus challenges:
- Proposed delivery of phosphides by meteorites faces issues of scarcity
- Oxidation and solubilization of phosphides under prebiotic conditions is poorly understood
- Lack of evidence for sufficient meteoritic phosphorus flux to sustain prebiotic chemistry
Conceptual problem: Phosphate Incorporation
- No clear prebiotic pathways for phosphorylation of organic molecules
- Difficulty in explaining the prevalence of phosphate in biological systems given its scarcity in prebiotic environments
4. Atmospheric and Aqueous Chemistry Complexities
a) Atmospheric composition uncertainties:
- Debate over the exact composition of the early Earth's atmosphere (reducing vs. neutral)
- Lack of consensus on the concentrations of key species like CH4, CO, and H2
- Difficulty in experimentally simulating accurate prebiotic atmospheric conditions
b) Formaldehyde and HCN formation challenges:
- Proposed mechanisms for CH2O and HCN synthesis often require specific atmospheric compositions
- Low yields and competing reactions in more realistic atmospheric models
- Stability and accumulation of these compounds in aqueous environments is questionable
c) Formose reaction limitations:
- Low selectivity for biologically relevant sugars like ribose
- Side reactions and degradation products dominate under most conditions
- No clear mechanism for the selection and stabilization of specific sugar products
Conceptual problem: Reaction Specificity
- Prebiotic reactions typically produce complex mixtures with low yields of desired products
- Lack of selective forces to drive the accumulation of specific, biologically relevant molecules
5. Energy Source Integration
a) UV radiation paradox:
- Can drive some synthetic reactions but also degrades organic molecules
- Difficulty in explaining how UV-sensitive compounds (e.g., nucleobases) could accumulate
b) Lightning event limitations:
- Provides intense but localized and infrequent energy input
- Challenge in explaining how products of lightning-driven chemistry could be preserved and concentrated
c) Hydrothermal system complexities:
- Varying conditions (temperature, pH, mineral composition) across different vent types
- Difficulty in reconciling the conditions required for different prebiotic reactions within a single hydrothermal setting
d) Radioactivity and impact event uncertainties:
- Sporadic and localized nature of these energy sources
- Lack of experimental evidence for efficient prebiotic synthesis under these conditions
Conceptual problem: Energy-Chemistry Coupling
- No known prebiotic mechanisms for efficiently channeling various energy inputs into specific, productive chemical pathways
- Difficulty in explaining how complex organic molecules could form and accumulate in high-energy environments without rapid degradation
These challenges highlight the significant gaps in our understanding of how the simple chemicals required for nucleotide synthesis could have formed and accumulated on the prebiotic Earth. The interplay between elemental sources, atmospheric and aqueous chemistry, and various energy inputs presents a complex system with many unresolved questions. The lack of plausible mechanisms to overcome these hurdles raises fundamental issues about the sufficiency of unguided processes to generate the precursors necessary for the origin of life.
2.2. Nucleobases: The Building Blocks of Genetic Information
Nucleobases are essential components of RNA and DNA, the molecules responsible for storing and transmitting genetic information. These bases are divided into two categories: purines and pyrimidines. Purines, which include adenine (A) and guanine (G), have a double-ring structure composed of nine atoms. Pyrimidines, comprising cytosine (C), thymine (T) in DNA, and uracil (U) in RNA, have a single six-atom ring structure.
The structural difference between DNA and RNA lies in their sugar components. Ribonucleic acid (RNA) contains a hydroxyl (-OH) group, whereas deoxyribonucleic acid (DNA) has only a hydrogen atom in place of this hydroxyl group.
2.3. Prebiotic Synthesis of Nucleobases
Understanding how nucleobases could have formed under early Earth conditions is a crucial challenge in origin-of-life research. Scientists have explored various pathways for their synthesis, with varying degrees of success and plausibility.
2.3.1. Purines (Adenine and Guanine)
2.3.2. Hydrogen Cyanide (HCN) Polymerization
One of the earliest attempts to synthesize adenine was made by Oró in 1961. He reported the synthesis of adenine from aqueous solutions of ammonium cyanide at temperatures below 100°C. However, the yield was extremely low, at only 0.5%, with most of the cyanide forming an intractable polymer. This experiment highlighted a significant issue: there was no prebiotic natural selection mechanism to sort out the bases that could later be used as nucleobases from those with no function.
Shapiro critiqued this approach, pointing out that useful yields of adenine cannot be obtained except in the presence of 1.0 M or stronger ammonia, which is far higher than the estimated 0.01 M concentration that might have been present in primitive oceans and lakes. He also noted that adenine's instability on a geological time scale makes its widespread prebiotic accumulation unlikely.
Shapiro further elaborated on the challenges of adenine synthesis: "Adenine synthesis requires unreasonable hydrogen cyanide concentrations. Adenine plays an essential role in replication in all known living systems today and is prominent in many other aspects of biochemistry. Despite this, a consideration of its intrinsic chemical properties suggests that it did not play these roles at the very start of life. These properties include the low yields in known syntheses of adenine under authentic prebiotic conditions, its susceptibility to hydrolysis and to reaction with a variety of simple electrophiles, and its lack of specificity and strength in hydrogen bonding at the monomer and mixed oligomer level." 1
Regarding the possibility of an extraterrestrial source for adenine, Shapiro noted: "The isolation of adenine and guanine from meteorites has been cited as evidence that these substances might have been available as 'raw material' on prebiotic Earth. However, acid hydrolyses have been needed to release these materials, and the amounts isolated have been low." 2
For guanine, the situation is even more challenging. In 1984, Yuasa reported a mere 0.00017% yield of guanine after electrical discharge experiments. S.L. Miller and colleagues conducted experiments in 1999, yielding trace amounts of guanine (0.0007% to 0.0035%) from the polymerization of ammonium cyanide, suggesting that guanine could potentially arise in frozen regions of the primitive Earth. 3
In 2010, Abby Vogel Robinson reported: "For scientists attempting to understand how the building blocks of RNA originated on Earth, guanine -- the G in the four-letter code of life -- has proven to be a particular challenge. While the other three bases of RNA -- adenine (A), cytosine (C), and uracil (U) -- could be created by heating a simple precursor compound in the presence of certain naturally occurring catalysts, guanine had not been observed as a product of the same reactions."
2.3.3. Formamide-Based Synthesis
An alternative approach to purine synthesis involves formamide, a simpler and more versatile precursor. Studies have demonstrated that formamide can serve as a prebiotic source for purine synthesis, particularly when subjected to heat or mineral catalysis. Formamide can yield both adenine and guanine under high-temperature conditions without requiring extremely high concentrations of HCN. This pathway also has the advantage of producing higher yields of purines compared to HCN polymerization, with some studies reporting significant quantities of guanine in the presence of certain catalysts.
In a recent paper from 2018, Annabelle Biscans mentions other routes investigated: "Miyakama et al. suggest that purines have been formed in the atmosphere in the absence of hydrogen cyanide. They reported that guanine could have been generated from a gas mixture (nitrogen, carbon monoxide, and water) after cometary impacts. Also, it has been proposed that adenine was formed in the solar system (outside of Earth) and brought to Earth by meteorites, given the fact that adenine was found in significant quantity in carbonaceous chondrites."
Biscans concludes: "Despite great efforts and impressive advancements in the study of nucleoside and nucleotide abiogenesis, further investigation is necessary to explain the gaps in our understanding of the origin of RNA." 4
2.3.4. Pyrimidines (Cytosine and Uracil)
Pyrimidine bases are the second of the quartet that makes up DNA and RNA that stores genetic information. Uracil (Thymine in DNA) and cytosine are made of one nitrogen-containing ring. The prebiotic synthesis of pyrimidines presents its own set of challenges, particularly due to the instability of cytosine and the limited availability of plausible precursor molecules.
2.3.5. Cytosine Synthesis
Scientists have failed to produce cytosine in spark-discharge experiments. Robert Shapiro (1999) noted: "The formation of a substance in an electric spark discharge conducted in a simulated early atmosphere has also been regarded as a positive indication of its prebiotic availability. Again, low yields of adenine and guanine have been reported in such reactions, but no cytosine. The failure to isolate even traces of cytosine in these procedures signals the presence of some problem with its synthesis and/or stability. The deamination of cytosine and its destruction by other processes such as photochemical reactions place severe constraints on prebiotic cytosine syntheses." 1
Rich Deem (2001) summarized several issues with cytosine synthesis:
"Cytosine has never been found in any meteorites.
Cytosine is not produced in electric spark discharge experiments using simulated 'early Earth atmosphere.'
Synthesis based upon cyanoacetylene requires the presence of large amounts of methane and nitrogen, however, it is unlikely that significant amounts of methane were present at the time life originated.
Synthesis based upon cyanate is problematical, since it requires concentrations in excess of 1 M (molar). When concentrations of 0.1 M (still unrealistically high) are used, no cytosine is produced.
Synthesis based upon cyanoacetaldehyde and urea suffers from the problem of deamination of the cytosine in the presence of high concentrations of urea (low concentrations produce no cytosine). In addition, cyanoacetaldehyde is reactive with a number of prebiotic chemicals, so would never attain reasonable concentrations for the reaction to occur. Even without the presence of other chemicals, cyanoacetaldehyde has a half-life of only 31 years in water.
Cytosine deaminates with an estimated half-life of 340 years, so would not be expected to accumulate over time.
Ultraviolet light on the early earth would quickly convert cytosine to its photohydrate and cyclobutane photodimers (which rapidly deaminate)." 5
2.3.6. Uracil Synthesis
In 1961, Sidney Fox and colleagues synthesized uracil under: "thermal conditions which yield other materials of theoretical prebiochemical significance. The conditions studied in the synthesis of uracil included temperatures in the range of 100° to 140°C, heating periods of from 15 minutes to 2 hours." 6
2.3.7. Recent Advances in Pyrimidine Synthesis
In 2009, Sutherland and Szostak published a paper on a high-yielding route to activated pyrimidine nucleotides under conditions thought to be prebiotic, claiming to be "an encouraging step toward the greater goal of a plausible prebiotic pathway to RNA and the potential for an RNA world." 7
However, Robert Shapiro disagrees:
"Although as an exercise in chemistry this represents some very elegant work, this has nothing to do with the origin of life on Earth whatsoever. The chances that blind, undirected, inanimate chemistry would go out of its way in multiple steps and use of reagents in just the right sequence to form RNA is highly unlikely." 8
In 2019, Okamura and colleagues published a paper on pyrimidine nucleobase synthesis where their conclusion remarks are noteworthy:
"We show that the cascade reaction proceeds under one-pot conditions in a continuous manner to provide SMePy 6. Importantly, the key intermediate SMePy 6 gives rise not only to canonical but also to non-canonical bases, arguing for the simultaneous prebiotic formation of a diverse set of pyrimidines under prebiotically plausible conditions." 9
2.3.8. Stability and Decomposition of Nucleobases
The stability of nucleobases in prebiotic conditions is a significant challenge to their accumulation and eventual incorporation into early life forms. Adenine deaminates at 37°C with a half-life of 80 years. At 100°C, its half-life is 1 year. For guanine, at 100°C, its half-life is 10 months, uracil is 12 years, and thymine 56 years. For the decomposition of a nucleobase, this is very short. For nucleobases to accumulate in prebiotic environments, they must be synthesized at rates that exceed their decomposition. Therefore, adenine and the other nucleobases would never accumulate in any kind of "prebiotic soup." 1
A paper published in 2015 points out that:
"Nucleotide formation and stability are sensitive to temperature. Phosphorylation of nucleosides in the laboratory is slower at low temperatures, taking a few weeks at 65°C compared with a couple of hours at 100°C. The stability of nucleotides, on the other hand, is favored in warm conditions over high temperatures. If a WLP is too hot (>80°C), any newly formed nucleotides within it will hydrolyze in several days to a few years. At temperatures of 5°C to 35°C that either characterize more-temperate latitudes or a post snowball Earth, nucleotides can survive for thousand-to-million-year timescales. However, at such temperatures, nucleotide formation would be very slow." 10
This presents a significant paradox: in hot environments, nucleotides might form, but they decompose fast. On the other hand, in cold environments, they might not degrade that fast, but take a long time to form. Nucleotides would have to be generated by prebiotic environmental synthesis processes at a far higher rate than they are decomposed and destroyed, and accumulated and concentrated at one specific construction site.
2.3.9. Selection of Nucleobases Used in Life
The selection of specific nucleobases for life - adenine, guanine, cytosine, uracil, and thymine - is a topic of considerable scientific interest and debate. These nucleobases form the foundation of RNA and DNA, but their prevalence in biological systems raises questions about how they were selected from a vast "structure space" of possible molecules.
2.3.10.The Concept of Structure Space
H. James Cleaves (2015) introduced the concept of "structure space" to describe the number of molecular structures that could potentially exist given specific parameters 11. This space is incredibly vast - for example, the number of possible stable drug-like organic molecules may be on the order of 10^33 to 10^180. In comparison, as of July 2009, there were only about 49 million unique chemical substances registered with the Chemical Abstracts Service.
When considering nucleobases specifically, the structure space becomes even more complex. The number of molecules that could fulfill the minimal requirements of being "nucleic acid-like" is remarkably large and potentially limitless. This includes various structural isomers of RNA that could theoretically function as genetic platforms.
2.3.11. Prebiotic Chemistry and Nucleobase Formation
On the early Earth, a wide array of molecules could have been generated by natural processes such as lightning, hydrothermal vents, and volcanic eruptions. The Murchison meteorite, for instance, contains a complex set of organic compounds ranging from 100,000 to perhaps 10 million unique molecular species.
However, despite this chemical diversity, life on Earth uses a very specific set of nucleobases. To date, no one-pot reaction has yielded either purine or pyrimidine ribonucleosides directly from likely prevalent prebiotic starting materials, making the abiotic origin of these specific nucleobases a challenging problem to solve.
2.3.12. The RNA World Hypothesis and Alternative Nucleobases
Andro C. Rios (2014) suggested that the early RNA world may have included many types of nucleobases beyond those we see in modern life 12. This hypothesis is supported by the extensive use of non-canonical nucleobases in extant RNA and the similarity of many modified bases to heterocycles generated in simulated prebiotic chemistry experiments.
Nucleobase modification is a ubiquitous post-transcriptional activity found across all domains of life, vital to cellular function as it modulates genetic expression. This suggests that life may have initially used a wider variety of nucleobases before settling on the current set.
2.3.13. The Challenge of Selection
The central question remains: how did nature "decide" upon these specific heterocycles from the vast structure space of possible molecules? Several factors complicate this question:
1. The structure space of possible nucleobase-like molecules is essentially limitless, especially when considering different ring structures and isomeric conformations.
2. Modern cells synthesize nucleobases through complex metabolic pathways that were not present prebiotically.
3. Selecting a specific set of complex macromolecules out of unlimited "structure space" by unguided means is theoretically possible but extremely improbable.
Unresolved Challenges in Prebiotic Nucleobase Synthesis
1. Complexity of Chemical Processes
The synthesis of nucleobases under prebiotic conditions involves intricate, multi-step chemical reactions. Without biological catalysts or guided processes, replicating these reactions in a natural environment poses significant challenges. For instance, the formation of adenine from hydrogen cyanide requires specific concentrations and conditions that are unlikely to occur spontaneously.
Conceptual problem: Spontaneous Complexity
- No known natural mechanism to drive such complex, multi-step reactions without external guidance
- Extremely low probability of these reactions occurring in sequence without intervention
2. Specific Synthesis Challenges for Cytosine and Guanine
Despite extensive research, the synthesis of cytosine and guanine under plausible prebiotic conditions remains elusive. Cytosine, in particular, has never been produced in spark-discharge experiments simulating early Earth atmospheres. Guanine synthesis yields are extremely low, with reported yields as low as 0.00017% in electrical discharge experiments.
Conceptual problem: Lack of Natural Pathways
- No viable routes identified for cytosine formation under prebiotic conditions
- Extremely low yields for guanine synthesis raise questions about its availability on early Earth
3. Nucleobase Instability
Nucleobases degrade rapidly under conditions thought to be present on early Earth. For example, adenine deaminates at 37°C with a half-life of 80 years, and at 100°C, its half-life is reduced to just 1 year. This instability prevents the accumulation of nucleobases in sufficient concentrations for nucleic acid formation.
Conceptual problem: Molecular Instability
- Rapid degradation of nucleobases in prebiotic environments challenges their persistence
- Difficulty in explaining how unstable molecules could accumulate to form more complex structures
4. Cytosine Synthesis and Stability
Cytosine presents a unique challenge due to its synthesis difficulties and instability. It has not been detected in meteorites and is not produced in electric spark discharge experiments. Additionally, cytosine deaminates with an estimated half-life of 340 years, further complicating its accumulation over time.
Conceptual problem: Absence of Cytosine Pathway
- No plausible prebiotic route for cytosine formation identified
- Rapid deamination of cytosine challenges its role in early genetic systems
5. Guanine Formation Barriers
Guanine synthesis under prebiotic conditions has proven extremely challenging. Experiments have yielded only trace amounts (0.0007% to 0.0035%) from ammonium cyanide polymerization. The absence of a clear, high-yield pathway for guanine formation poses significant problems for prebiotic chemistry scenarios.
Conceptual problem: Guanine Synthesis Limitations
- Extremely low yields in prebiotic simulations question guanine's availability
- Lack of efficient synthesis pathways challenges guanine's inclusion in early genetic material
6. Adenine Synthesis Requirements
The synthesis of adenine requires unrealistically high concentrations of hydrogen cyanide (HCN). Useful yields are only obtained in the presence of 1.0 M or stronger ammonia, far exceeding the estimated 0.01 M concentration in primitive oceans and lakes.
Conceptual problem: Unrealistic Conditions
- Required HCN concentrations for adenine synthesis are implausible in natural settings
- Discrepancy between laboratory conditions and estimated prebiotic environments
7. Uracil Stability and Synthesis
While uracil synthesis has been demonstrated under thermal conditions, its stability under early Earth conditions remains problematic. At 100°C, uracil has a half-life of 12 years, which is relatively short on geological timescales.
Conceptual problem: Uracil Degradation
- Rapid degradation of uracil under likely early Earth conditions
- Challenge in explaining uracil's accumulation and incorporation into early genetic systems
8. Tautomeric Shifts in Nucleobases
Nucleobases can exist in multiple tautomeric forms, affecting their ability to form stable base pairs. In a prebiotic setting, controlling these tautomeric shifts to ensure proper base pairing would be extremely challenging.
Conceptual problem: Lack of Tautomeric Control
- Uncontrolled tautomeric shifts could lead to incorrect base pairing
- Absence of a regulatory mechanism to maintain proper tautomeric forms
9. Purity of Chemical Precursors
Prebiotic environments likely contained complex mixtures of chemicals, which would interfere with the synthesis of nucleobases. Achieving the necessary purity for precursor molecules in such environments is highly improbable.
Conceptual problem: Impurity and Contamination
- Impurities in prebiotic chemical pools would hinder nucleobase formation
- Difficulty in achieving required purity levels in natural settings
10. Concentration Problems
Achieving sufficient concentrations of nucleobase precursors is unlikely under the dilute conditions presumed to exist on early Earth. The synthesis of nucleobases typically requires concentrations far exceeding those plausible in prebiotic oceans or lakes.
Conceptual problem: Insufficient Concentrations
- Dilution of reactants in natural environments would prevent necessary nucleobase formation
- No known natural process capable of concentrating precursors sufficiently
11. Energy Source Deficit
The synthesis of nucleobases often requires significant energy input. Identifying a consistent and sufficient energy source to drive these endothermic reactions in a prebiotic setting remains an unresolved challenge.
Conceptual problem: Energy Source Identification
- Lack of a clear, continuous energy source for nucleobase synthesis reactions
- Difficulty in explaining how energy-intensive reactions could proceed naturally
12. Uncontrolled Side Reactions
In complex prebiotic environments, reactive species would likely interfere with nucleobase synthesis, causing unwanted side reactions. Controlling these side reactions without enzymatic guidance is problematic.
Conceptual problem: Side Reaction Control
- Side reactions would consume essential precursors, preventing nucleobase formation
- Absence of regulatory systems to direct specific reaction pathways
13. Thermodynamic Barriers
Many reactions needed to synthesize nucleobases are thermodynamically unfavorable. Overcoming these barriers without biological catalysts or highly specific conditions is improbable in a prebiotic setting.
Conceptual problem: Thermodynamic Challenges
- Thermodynamically unfavorable reactions unlikely to proceed without external intervention
- Difficulty in explaining how energy barriers were overcome in early Earth conditions
14. Environmental Condition Specificity
Nucleobase synthesis requires highly specific environmental conditions (e.g., pH, temperature) that are difficult to achieve and maintain in natural settings. The variability of early Earth environments poses a significant challenge to sustaining these conditions.
Conceptual problem: Environmental Control
- Maintaining consistent, favorable conditions for nucleobase synthesis is implausible
- Fluctuating early Earth conditions would disrupt synthesis processes
15. Water Paradox
While water is necessary for many prebiotic reactions, it also accelerates nucleobase degradation. This paradox presents a significant challenge in explaining how nucleobases could accumulate in aqueous environments.
Conceptual problem: Degradative Role of Water
- Water's dual role as solvent and degradation agent complicates nucleobase accumulation
- No known solution to this paradox in prebiotic environments
16. Correct Isomeric Configuration
Ensuring the correct isomeric forms of nucleobases is crucial for proper base pairing. Prebiotic environments lacked mechanisms for selecting the appropriate isomers, raising questions about how functional nucleic acids could form.
Conceptual problem: Isomeric Control
- No natural mechanism identified to control correct isomer selection
- Incorrect isomers would lead to faulty base pairing, hindering nucleic acid formation
17. Tautomeric Equilibria Control
Maintaining the correct tautomeric forms of nucleobases is essential for base pairing. Achieving this control in prebiotic environments without enzymatic regulation is highly improbable.
Conceptual problem: Tautomeric Imbalance
- Incorrect tautomeric forms would prevent functional base pairing
- High likelihood of tautomeric forms leading to nonfunctional nucleic acids
18. Stereochemistry of Sugar Components
The stereochemistry of sugar components in nucleotides is critical for functional nucleic acids. Achieving the correct stereochemistry without enzymatic control in a prebiotic setting is highly unlikely.
Conceptual problem: Stereochemical Control
- Incorrect stereochemistry would prevent the formation of functional nucleic acids
- Achieving right stereochemistry without enzymatic guidance is improbable
19. Fine-Tuning of Bond Energies
The hydrogen bond strengths in Watson-Crick base pairing are finely tuned for stability and function. Explaining how these precise bond energies emerged naturally is a significant challenge.
Conceptual problem: Bond Energy Fine-Tuning
- Precise bond energies required for stable nucleic acids cannot be explained through unguided processes
- Any deviation from necessary bond strengths would prevent stable nucleic acid formation
20. Hydrogen Bonding Specificity
The specificity of hydrogen bonding needed for Watson-Crick base pairing is unlikely to arise naturally. The probability of correct hydrogen bonding patterns occurring spontaneously is extremely low.
Conceptual problem: Hydrogen Bonding Specificity
- Low probability of correct hydrogen bonding patterns occurring naturally
- Incorrect hydrogen bonding would result in nonfunctional nucleic acids
21. Prevention of Alternative Base Pairs
In a prebiotic setting, non-Watson-Crick base pairs could form, disrupting nucleic acid formation. Explaining how only specific base pairs emerged without a guiding mechanism is problematic.
Conceptual problem: Alternative Base Pair Prevention
- Formation of incorrect base pairs would compromise emerging nucleic acids
- No known mechanism to prevent alternative base pairing in prebiotic conditions
22. Challenges in Backbone Chemistry
The formation of the sugar-phosphate backbone, essential for nucleic acid stability, is a complex process unlikely to occur naturally without guidance. No viable natural pathway for its spontaneous formation has been identified.
Conceptual problem: Backbone Formation
- Absence of a plausible route for sugar-phosphate backbone formation in prebiotic conditions
- Complexity of backbone structure challenges explanations of spontaneous assembly
23. Base Stacking Interactions
Base stacking interactions play a crucial role in nucleic acid stability. Achieving these interactions naturally, without guided processes, is highly improbable.
Conceptual problem: Base Stacking Stability
- No known natural mechanism for forming stable base stacking arrangements
- Absence of correct base stacking would compromise nucleic acid structural integrity
24. Selection of Nucleobase Analogs
It remains unclear why only specific nucleobases capable of Watson-Crick pairing were selected from numerous potential analogs in a prebiotic environment. The absence of selective pressure in a prebiotic world challenges explanations of how only specific nucleobases emerged.
Conceptual problem: Analog Selection
- No identified natural process to explain selection of Watson-Crick compatible nucleobases
- Lack of selective pressure in prebiotic conditions challenges nucleobase specificity
25. Formation of Stable Nucleotides
The formation of nucleosides and nucleotides in aqueous solutions presents significant hurdles under prebiotic conditions. Current research has not identified a prebiotic method for stable nucleotide formation.
Conceptual problem: Nucleotide Formation
- No known prebiotic method for stable nucleotide formation
- Improbability of controlled combination of nucleobases, sugars, and phosphate groups without biological systems
26. Role of Environmental Conditions
Nucleobase synthesis depends on specific environmental factors such as pH, temperature, and ion concentrations. Maintaining consistently favorable conditions on early Earth for nucleobase formation is highly improbable.
Conceptual problem: Environmental Specificity
- Achieving and maintaining precise environmental conditions required for nucleobase synthesis is unlikely in natural settings
- Variability in Earth's early environments would have prevented sustained conditions needed for successful nucleobase formation
2.3.10. Prebiotic Nucleobase Synthesis - Extraterrestrial Sources
The discovery of organic compounds, including nucleobases, in extraterrestrial environments has been one of the most exciting developments in the field of astrobiology and origin of life studies. Nucleobases, the fundamental building blocks of RNA and DNA, have been detected in various cosmic settings, including interstellar space, comets, and meteorites that have fallen to Earth. In 1969, the Murchison meteorite, which fell in Australia, became a landmark in this area of research. Analysis of this carbonaceous chondrite revealed the presence of various organic compounds, including purine and pyrimidine bases. Since then, numerous studies have confirmed the presence of nucleobases in other meteorites, such as the Tagish Lake meteorite and the Antarctic meteorites. Furthermore, space-based observations and laboratory simulations of interstellar ice analogues have suggested that nucleobases could form in the harsh conditions of space. These findings have led some researchers to propose that the essential ingredients for life might have been delivered to early Earth through extraterrestrial sources, potentially jumpstarting the emergence of life. This scenario, often referred to as panspermia or exogenesis, has gained attention as a potential solution to some of the challenges faced in explaining the prebiotic synthesis of these crucial biomolecules on Earth. However, while the presence of nucleobases in space and meteorites is intriguing, it introduces its own set of challenges and does not necessarily solve the fundamental problems of prebiotic nucleobase availability and subsequent RNA or DNA formation. The following points outline why the extraterrestrial source of nucleobases, despite its initial promise, does not fully address the challenges in prebiotic nucleobase synthesis:
Challenges in Prebiotic Nucleobase Synthesis - Addressing Extraterrestrial Sources
1. Stability and Delivery of Nucleobases
The hypothesis that nucleobases were delivered to Earth via meteorites or comets raises significant questions regarding the stability of these molecules during transit. Space is an environment characterized by intense radiation, extreme temperatures, and vacuum conditions, all of which could degrade delicate organic compounds. The survival of nucleobases from their formation in interstellar space to their delivery to Earth remains an unresolved issue. For instance, purine and pyrimidine bases detected in meteorites like the Murchison have undergone intense scrutiny, yet their preservation under such harsh conditions is not fully understood.
Conceptual problem: Nucleobase Stability
- Uncertainty about how nucleobases could remain stable over long cosmic journeys
- Lack of a natural mechanism that could protect these molecules from degradation in space
2. Synthesis in Extraterrestrial Environments
The formation of nucleobases in space introduces additional challenges. Laboratory simulations of interstellar ice analogs suggest that nucleobases can form under specific conditions, but these simulations often require highly controlled environments that may not reflect the chaotic nature of space. The complexity of synthesizing these molecules under natural, unguided conditions, such as in the vast and varied regions of interstellar space, remains a daunting challenge. This issue is further complicated by the fact that nucleobases require precise conditions for their formation, which raises doubts about the likelihood of such processes occurring spontaneously in space.
Conceptual problem: Spontaneous Synthesis
- Difficulty in replicating space conditions conducive to nucleobase formation in the laboratory
- Improbability of spontaneous nucleobase synthesis in uncontrolled, natural space environments
3. Integration into Prebiotic Chemistry
Even if nucleobases were successfully delivered to Earth, integrating them into the prebiotic chemistry required for life is another unsolved problem. Nucleobases would need to not only survive the conditions of early Earth but also integrate into a functional system capable of RNA or DNA formation. The spontaneous assembly of nucleobases into these complex macromolecules, without the guidance of enzymatic processes or an existing template, poses a significant conceptual barrier. The precise order and structure of nucleotides in RNA and DNA are critical for their function, yet there is no known natural mechanism that could have organized these molecules into the correct sequences in the absence of life.
Conceptual problem: Molecular Integration
- Challenge in explaining how nucleobases could self-assemble into functional nucleic acids
- Lack of a known process that could ensure the correct sequencing of nucleotides without guidance
4. Alternative Pathways and Polyphyly
The existence of alternative nucleobase synthesis pathways in different environments, which often share no homology, presents evidence for polyphyly—the notion that life may have originated from multiple independent sources. The Murchison meteorite and other extraterrestrial findings suggest that nucleobases could form in a variety of ways, yet these pathways do not converge on a single, universal mechanism. This divergence undermines the concept of a universal common ancestor and suggests that life, if it emerged from these extraterrestrial sources, did so in a polyphyletic manner. The lack of shared ancestry between these pathways further complicates the narrative of a singular, natural origin of life.
Conceptual problem: Independent Origins
- Evidence of multiple, distinct pathways for nucleobase synthesis challenges the idea of a single origin
- Polyphyly suggests life may have emerged from different sources, contradicting universal common ancestry
5. Naturalistic Explanations and Their Limits
The challenges associated with extraterrestrial nucleobase synthesis and delivery highlight the limitations of naturalistic explanations for the origin of life. The precise conditions required for nucleobase stability, synthesis, and integration into prebiotic chemistry seem improbably orchestrated in a purely unguided scenario. This raises fundamental questions about the adequacy of naturalistic frameworks to account for the emergence of life’s building blocks. Without invoking a guiding mechanism, the spontaneous appearance of such complex molecules and their successful integration into functional biological systems remains unexplained.
Conceptual problem: Adequacy of Naturalistic Explanations
- Inadequacy of naturalistic mechanisms to fully explain nucleobase synthesis, stability, and integration
- Lack of a coherent natural process that could account for the coordinated emergence of life’s building blocks
References
1. Oró, J. (1961). Mechanism of synthesis of adenine from hydrogen cyanide under possible primitive earth conditions. Nature, 191(4794), 1193-1194. Link. (This pioneering work reports on the prebiotic synthesis of adenine from hydrogen cyanide, suggesting a possible mechanism for nucleobase formation on early Earth.)
2. Shapiro, R. (2000). A replicator was not involved in the origin of life. IUBMB Life, 49(3), 173-176. Link. (This paper challenges the RNA world hypothesis, arguing that a self-replicating molecule was not necessary for the origin of life.)
3. Yuasa, S. (1984). Electric discharge synthesis of guanine and its role in the origin of life. Origins of Life and Evolution of the Biosphere, 14(1), 79-85. Link. (This study reports on the synthesis of guanine through electric discharge experiments, exploring its potential role in life's origins.)
4. Biscans, A. (2018). Exploring the emergence of RNA nucleosides and nucleotides on the early Earth. Life, 8(4), 57. Link. (This comprehensive review examines various pathways for the prebiotic synthesis of RNA components, discussing recent advancements and challenges.)
5. Chyba, C., & Sagan, C. (1992). Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life. Nature, 355(6356), 125-132. Link. (This paper explores multiple sources of organic molecules on early Earth, including terrestrial synthesis, delivery by comets and meteorites, and impact-induced synthesis.)
6. Fox, S. W., & Harada, K. (1961). Synthesis of uracil under conditions of a thermal model of prebiological chemistry. Science, 133(3468), 1923-1924. Link. (This study reports on the thermal synthesis of uracil under simulated prebiotic conditions, contributing to our understanding of pyrimidine formation.)
7. Powner, M. W., Gerland, B., & Sutherland, J. D. (2009). Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature, 459(7244), 239-242. Link. (This paper presents a novel pathway for the synthesis of pyrimidine ribonucleotides under prebiotic conditions, addressing a key challenge in the RNA world hypothesis.)
8. Sanderson, K. (2009). Insight into RNA origins. Chemistry World. Link. (This article reports on the work of Sutherland and colleagues, discussing the implications of their findings for understanding RNA origins.)
9. Okamura, H., Crisp, A., P., & Carell, T. (2019). A one-pot, water compatible synthesis of pyrimidine nucleobases under plausible prebiotic conditions. Chemical Communications, 55(13), 1939-1942. Link. (This paper describes a novel, efficient method for synthesizing pyrimidine nucleobases under conditions that could have existed on early Earth.)
10. Pearce, B. K., Pudritz, R. E., Semenov, D. A., & Henning, T. K. (2017). Origin of the RNA world: The fate of nucleobases in warm little ponds. Proceedings of the National Academy of Sciences, 114(43), 11327-11332. Link. (This study investigates the formation and accumulation of RNA nucleobases in warm little ponds on early Earth, considering various environmental factors.)
11. Cleaves, H. J. (2015). The origin of the biologically coded amino acids. Journal of Theoretical Biology, 382, 9-17. Link. (This paper examines the selection of the 20 canonical amino acids, providing insights into the chemical evolution that led to the current genetic code.)
12. Rios, A. C., & Tor, Y. (2013). On the origin of the canonical nucleobases: an assessment of selection pressures across chemical and early biological evolution. Israel Journal of Chemistry, 53(6-7), 469-483. Link. (This study analyzes the factors that may have influenced the selection of the canonical nucleobases, considering both chemical and early biological evolution.)
Last edited by Otangelo on Mon Oct 07, 2024 11:21 am; edited 6 times in total