9. Cellular Pluripotency
Cellular Pluripotency refers to the unique state of certain cells that possess the remarkable ability to differentiate into a wide range of cell types found in the body. These cells, known as pluripotent stem cells, hold immense significance in developmental biology, regenerative medicine, and basic research. Pluripotent stem cells are a type of stem cell that can give rise to almost any cell type found in the body, excluding those in the placenta and extraembryonic tissues. These cells can self-renew, maintaining their undifferentiated state, while also having the potential to differentiate into specialized cells like neurons, muscle cells, and blood cells. The study of pluripotency provides insights into the early stages of embryonic development. Understanding how cells transition from pluripotency to various specialized lineages helps unravel the complex process of how organisms develop and grow. Cellular pluripotency challenges conventional notions of cellular fate, highlighting the immense flexibility and potential of certain cells.
What are the molecular factors that confer cellular pluripotency and the ability to differentiate into various cell types?
Cellular pluripotency and the ability to differentiate into various cell types are conferred by a combination of molecular factors that regulate gene expression, epigenetic states, and signaling pathways. These factors work together to maintain a balance between self-renewal and differentiation. While the exact mechanisms can vary among different organisms, the following are some key molecular factors that play crucial roles in conferring cellular pluripotency:
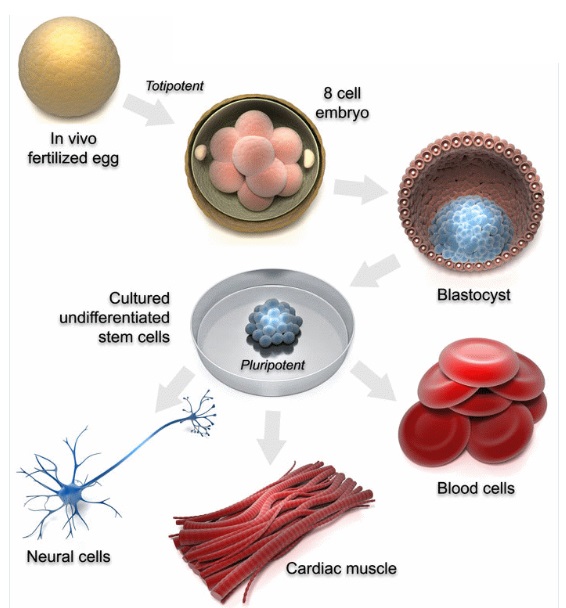
Pluripotency is a defining characteristic of certain stem cells, particularly embryonic stem cells. It refers to the ability of these stem cells to differentiate into cell types belonging to all three germ layers: the ectoderm, mesoderm, and endoderm. These germ layers are the fundamental cell lineages that give rise to the various tissues and organs in a developing organism.
Embryonic Stem Cells (ESCs): Pluripotency is most commonly associated with embryonic stem cells, which are derived from the inner cell mass of the blastocyst stage of the developing embryo. At this early stage, the cells are undifferentiated and possess the potential to give rise to any cell type in the body. This remarkable capability makes ESCs invaluable tools for research and regenerative medicine.
Three Germ Layers: During early embryonic development, the embryo undergoes gastrulation, a process in which the three germ layers are established. Each germ layer has the potential to differentiate into specific cell types and tissues:
Ectoderm: Gives rise to the skin, nervous system, and other external structures.
Mesoderm: Forms muscle, bone, blood, and other connective tissues.
Endoderm: Develops into the gut, respiratory tract, and other internal organs.
Versatility: Pluripotent stem cells can differentiate into a wide range of cell types, allowing them to contribute to the formation of various tissues and organs in the body. This versatility is crucial for the proper development and growth of an organism.
Differentiation: The process of pluripotent stem cells becoming specialized cells is called differentiation. As the cells differentiate, they progressively lose their pluripotency and commit to specific lineages. This process is tightly regulated by genetic and epigenetic mechanisms.
Appearance of Cellular Pluripotency in the evolutionary timeline
There are several hypotheses about how Cellular Pluripotency might have arisen.
Early Cellular Differentiation Mechanisms: One hypothesis is that early multicellular organisms would have possessed rudimentary mechanisms for cell differentiation. These mechanisms could have enabled certain cells to retain a level of plasticity, allowing them to differentiate into a variety of cell types as needed.
Emergence of Germ Layers: As multicellular organisms supposedly evolved, the emergence of germ layers (ectoderm, mesoderm, and endoderm) would have facilitated cellular specialization. Within these layers, certain cells would have retained pluripotency, allowing them to give rise to a range of cell types specific to their germ layer.
Evolution of Regulatory Networks: Over time, genetic and regulatory networks controlling cell fate determination would have become more complex and sophisticated. Regulatory genes that govern pluripotency-related factors would have emerged, allowing certain cells to maintain the potential for diverse differentiation outcomes.
Development of Developmental Pathways: Evolutionary developments in pathways such as Wnt, BMP, and Notch signaling would have contributed to the emergence of pluripotent cells. These pathways are known to play roles in cellular differentiation, and their evolution would have allowed for the preservation of pluripotency in certain cell populations.
Importance in Regeneration: Pluripotent cells would have provided an advantage in terms of regeneration and tissue repair. Organisms with cells capable of reverting to a pluripotent state would have been better equipped to regenerate lost or damaged tissues, leading to increased survival and reproductive success.
Benefit for Environmental Adaptation: Pluripotent cells would have facilitated adaptation to changing environments. Organisms with pluripotent cells would have been more flexible in responding to environmental challenges by producing specialized cell types suited to new conditions.
It's important to emphasize that the evolutionary emergence of pluripotency is complex and would have involved a combination of genetic, regulatory, and environmental factors. The gradual evolution of the molecular and genetic machinery necessary for maintaining pluripotency remains an area of active research and exploration.
De Novo Genetic Information necessary to instantiate Cellular Pluripotency
Creating the mechanisms of Cellular Pluripotency de novo would involve the precise generation and integration of genetic information into the existing genetic material. While the exact details are speculative, here's a hypothetical process of introducing new genetic information to establish cellular pluripotency:
Emergence of Regulatory Elements: New genetic elements, such as enhancers and promoters, would need to originate. These elements would regulate the expression of pluripotency-associated genes and coordinate their activity.
Pluripotency Master Regulators: New genes encoding master regulators of pluripotency would need to emerge. These genes would encode transcription factors that play pivotal roles in maintaining pluripotency by controlling the expression of numerous downstream genes.
Epigenetic Machinery: Genes encoding enzymes involved in epigenetic modifications, such as DNA methylation and histone modifications, would need to evolve. These modifications are critical for maintaining the open chromatin state required for pluripotency.
Cell Signaling Pathways: Novel genes encoding components of signaling pathways (e.g., Wnt, BMP, FGF) that interact with the pluripotency network would need to arise. These pathways contribute to maintaining pluripotency and influencing cell fate decisions.
Cell-Cell Communication Genes: New genes related to cell-cell communication would be necessary. Pluripotent cells require signaling cues from their environment to maintain their undifferentiated state, so genes encoding receptors and ligands might evolve.
Genetic Network Development: The new genetic information would need to establish intricate networks of interactions. Cross-regulatory interactions between genes would need to evolve, ensuring the proper balance of pluripotency-related factors.
Chromatin Remodeling Factors: Genes encoding chromatin remodeling factors would be essential. These factors reshape the chromatin structure to allow access to key pluripotency genes and maintain the cellular identity.
Cell Division Control: Genes involved in regulating cell division rates and cycles would be important. Pluripotent cells must strike a balance between self-renewal and differentiation, which requires precise control over cell division.
DNA Repair and Replication: Genes encoding DNA repair and replication factors would need to be in place. The dynamic nature of pluripotency requires accurate DNA maintenance during cell division and replication.
Transcriptional Machinery: Components of the transcriptional machinery, including RNA polymerases and transcription factors, would need to evolve to interact with the new pluripotency-related genes.
The process of generating and integrating new genetic information to establish Cellular Pluripotency would involve the emergence of a complex network of genes, regulatory elements, and interactions. The simultaneous appearance of these components in a coordinated manner would be essential to ensure the functional establishment of pluripotency. It's important to note that this description is speculative and intended to highlight the complexity of creating the mechanisms for Cellular Pluripotency from scratch.
Manufacturing codes and languages that would have to emerge and be employed to instantiate Cellular Pluripotency
The establishment of Cellular Pluripotency involves a sophisticated interplay of various manufacturing codes and languages beyond genetic information. These codes and languages contribute to the development and maintenance of pluripotent cells within an organism.
Epigenetic Codes: The epigenetic code involves modifications to DNA and histone proteins that determine how genes are expressed. To establish pluripotency, new epigenetic codes would need to be instantiated to create an open chromatin structure, allowing access to pluripotency-related genes. Epigenetic modifications would regulate the balance between self-renewal and differentiation.
Protein Folding and Modification Codes: The correct folding and modification of proteins are crucial for pluripotency-related factors to function properly. Codes governing protein folding, post-translational modifications, and quality control mechanisms would need to evolve to ensure the proper functioning of pluripotency-associated proteins.
Cell Signaling Languages: Pluripotency requires intricate cell signaling networks. New signaling languages would need to emerge, allowing cells to communicate with each other and their environment. These languages would guide cellular responses to external cues, influencing pluripotency maintenance and differentiation decisions.
Metabolic Codes: Cellular metabolism plays a vital role in maintaining pluripotency. Evolving metabolic codes would be necessary to ensure the energy and nutrient requirements of pluripotent cells are met while maintaining their unique state.
Cytoskeletal Dynamics: Codes governing cytoskeletal dynamics would need to be instantiated to enable cell shape changes, migration, and interactions with the microenvironment. These codes contribute to the physical properties of pluripotent cells and their ability to respond to signals.
Extracellular Matrix (ECM) Codes: The ECM provides cues for cell adhesion, migration, and differentiation. New codes governing the synthesis and arrangement of ECM components would need to emerge to support pluripotency-related functions.
Membrane Receptor Codes: To interact with external signals, new membrane receptor codes would be essential. These codes would enable cells to sense and respond to specific ligands that influence pluripotency-related pathways.
Chemotactic Language: Pluripotent cells often migrate in response to chemical gradients. A chemotactic language would need to evolve, allowing cells to follow specific cues and migrate toward or away from certain regions to maintain pluripotency.
Cell Adhesion Codes: Codes governing cell adhesion molecules would be required for interactions between pluripotent cells and neighboring cells or the extracellular matrix. These codes would support the maintenance of pluripotency and cell-cell communication.
Stem Cell Niche Codes: Pluripotent cells reside in specific niches within tissues. Niche codes would need to evolve to create environments that support the self-renewal and differentiation of pluripotent cells.
These manufacturing codes and languages, operating in concert with genetic information, would need to be simultaneously instantiated and integrated into the organism's biological systems. Their coordinated emergence would be essential for the successful development of Cellular Pluripotency, highlighting the intricate design required to establish and maintain this complex cellular state.
Epigenetic Regulatory Mechanisms necessary to be instantiated to create Cellular Pluripotency
Creating Cellular Pluripotency would involve intricate epigenetic regulation to control gene expression patterns and maintain the pluripotent state. The systems and processes required to instantiate and maintain this regulation would work collaboratively.
Epigenetic Regulation: DNA Methylation Patterns: New DNA methylation patterns would need to emerge, allowing for the appropriate silencing and activation of genes associated with pluripotency and differentiation. These patterns would be established by DNA methyltransferases.
Histone Modifications: Specific histone modifications would need to be established, marking genes for either activation or repression. Histone acetyltransferases, methyltransferases, and other modifying enzymes would play a role in shaping the pluripotent epigenome.
Chromatin Accessibility: The establishment of an open chromatin structure in pluripotent cells would require changes in nucleosome positioning and chromatin remodeling. ATP-dependent chromatin remodelers and modifiers would be involved.
Non-Coding RNAs: Non-coding RNAs, such as microRNAs and long non-coding RNAs, would emerge to fine-tune gene expression and contribute to the maintenance of pluripotency-related pathways.
Collaborating Systems: Cell Signaling Pathways: Signaling pathways like Wnt, BMP, and FGF would interact with the epigenetic machinery to influence gene expression patterns and maintain pluripotency. They would provide external cues that guide epigenetic modifications.
Transcriptional Regulation: Transcription factors specific to pluripotency would collaborate with epigenetic regulators to control the expression of pluripotency-related genes. This collaboration would ensure the appropriate genes are activated or repressed.
Cell-Cell Communication: Intercellular communication would involve ligand-receptor interactions that transmit signals affecting epigenetic modifications. The collaboration between cells would contribute to maintaining a pluripotent environment.
Metabolic Regulation: Cellular metabolism and energy availability would influence epigenetic modifications. Metabolic pathways would interact with the epigenetic machinery to support pluripotency.
Quality Control Mechanisms: Cellular systems that ensure proper protein folding and quality control would collaborate with the epigenetic machinery. Correctly folded proteins would be essential for maintaining the pluripotent state.
Cell Division Control: The cell cycle and division control mechanisms would need to synchronize with epigenetic changes to ensure that pluripotent cells maintain their epigenetic identity during replication.
DNA Repair and Replication: Efficient DNA repair and replication processes would collaborate with the epigenetic machinery to preserve accurate epigenetic patterns during cell division.
Environmental Adaptation: The collaboration between epigenetic regulation and cellular responses to environmental cues would enable pluripotent cells to adapt to changing conditions while maintaining their identity.
The instantiation and maintenance of epigenetic regulation for Cellular Pluripotency would involve the coordinated interplay of these systems, ensuring that genes are expressed in a controlled manner to maintain the pluripotent state. The collaborative nature of these systems emphasizes the complexity and integrated design required for the development and functionality of pluripotent cells.
Signaling Pathways necessary to create, and maintain Cellular Pluripotency
The emergence of Cellular Pluripotency would involve the creation and orchestration of various signaling pathways that interact, collaborate, and crosstalk to establish and maintain the pluripotent state.
Wnt Signaling Pathway: The Wnt pathway would be crucial for the activation of pluripotency-related genes. It could promote the expression of key transcription factors, such as Oct4, contributing to the establishment of pluripotency. The Wnt pathway might crosstalk with the FGF and BMP pathways to fine-tune pluripotency maintenance.
FGF Signaling Pathway: Fibroblast Growth Factor (FGF) signaling would play a role in sustaining pluripotency. It might collaborate with the Wnt pathway to maintain self-renewal and block differentiation. The FGF pathway could crosstalk with the TGF-β pathway to balance self-renewal and differentiation signals.
BMP Signaling Pathway: Bone Morphogenetic Protein (BMP) signaling might drive the differentiation of non-pluripotent cells. However, in the context of pluripotency, it could collaborate with the FGF and Wnt pathways to establish a balance between pluripotency and differentiation cues.
Notch Signaling Pathway: Notch signaling might be involved in cell-cell communication and lineage commitment. In pluripotency, it could interact with the FGF and Wnt pathways to influence differentiation decisions and maintain the pluripotent state.
Hedgehog Signaling Pathway: Hedgehog signaling could contribute to cell-fate determination and differentiation. In the pluripotent context, it might cross-interact with the Wnt and FGF pathways to influence lineage specification.
TGF-β Signaling Pathway: Transforming Growth Factor-beta (TGF-β) signaling could play a role in differentiation. In pluripotency, it might interact with the FGF pathway to balance self-renewal and differentiation signals.
PI3K/AKT/mTOR Pathway: This pathway could regulate cellular growth and survival. It might collaborate with the FGF and Wnt pathways to support the pluripotent state by regulating cell cycle progression and nutrient availability.
Cell Adhesion Pathways: Signaling pathways related to cell adhesion, such as integrin-mediated signaling, could crosstalk with the above pathways to influence cell migration, communication, and the maintenance of pluripotency.
Metabolic Signaling: Metabolic pathways could cross-interact with various signaling pathways to ensure energy availability for pluripotent cells. Collaborations between metabolic and pluripotency-related pathways would contribute to self-renewal and pluripotency maintenance.
Environmental Sensing: The interplay between signaling pathways and the cell's environment would allow pluripotent cells to sense external cues and adapt their behavior accordingly. Collaboration with the immune system, tissue architecture, and developmental cues would influence pluripotency maintenance.
The interconnections and collaborations among these signaling pathways would create a highly intricate network that governs the establishment and maintenance of Cellular Pluripotency. The crosstalk among these pathways would ensure that pluripotent cells respond appropriately to internal and external cues, striking a balance between self-renewal and differentiation while interacting with other biological systems within the organism.
Regulatory codes necessary for maintenance and operation of Cellular Pluripotency
The maintenance and operation of Cellular Pluripotency would involve a complex interplay of regulatory codes and languages that control gene expression, cell behavior, and interactions. Here are some of the key regulatory codes and languages that would need to be instantiated and involved:
Transcriptional Regulatory Code: This code involves the binding of transcription factors to specific DNA sequences to activate or repress gene expression. Transcription factors like Oct4, Sox2, and Nanog would need to establish a regulatory network to maintain pluripotency-associated gene expression.
Cell Signaling Languages: Cell signaling pathways, including Wnt, FGF, and BMP, would require specific signaling languages to transmit information within pluripotent cells. These languages would guide cellular responses to maintain self-renewal and differentiation balance.
Epigenetic Language: The epigenetic language involves DNA methylation patterns, histone modifications, and chromatin structure that determine gene accessibility. This language would need to ensure the maintenance of an open chromatin state around pluripotency genes while repressing lineage-specific genes.
Chemotactic Language: A chemotactic language would guide the migration of pluripotent cells to specific niches or environments within tissues, ensuring proper self-renewal and differentiation cues.
Cell-Cell Communication Codes: Cell-cell communication would involve ligand-receptor interactions that convey information about the cellular environment and influence pluripotency maintenance.
Metabolic Code: The metabolic code would coordinate the energy and nutrient requirements of pluripotent cells, ensuring proper functioning and self-renewal.
Stem Cell Niche Code: Pluripotent cells reside in specific niches within tissues. The niche code would establish a microenvironment that supports self-renewal and prevents premature differentiation.
Quality Control Mechanisms: Regulatory codes related to protein folding, quality control, and cellular homeostasis would ensure that pluripotency-associated proteins are properly folded and functional.
Cell Cycle Code: The cell cycle regulatory code would coordinate cell division and replication with pluripotency maintenance, ensuring accurate transmission of genetic and epigenetic information to daughter cells.
DNA Repair and Replication Code: The code governing DNA repair and replication would safeguard the integrity of the genome and epigenome during cell division.
Differentiation Repression Code: To prevent premature differentiation, pluripotent cells would need a code that represses lineage-specific genes and pathways while maintaining pluripotency-related factors.
These regulatory codes and languages would collaboratively orchestrate the complex processes that maintain and operate Cellular Pluripotency. Their coordinated functioning would ensure the self-renewal, plasticity, and controlled differentiation potential of pluripotent cells within biological systems.
Is there scientific evidence supporting the idea that Cellular Pluripotency was brought about by the process of evolution?
The emergence of Cellular Pluripotency presents a confluence of complex regulatory systems, precise codes, and intricate interdependencies that operate harmoniously to maintain this unique cellular state. An evolutionary step-by-step process seems highly unlikely due to several reasons:
Interdependent Codes and Mechanisms: The codes, languages, signaling pathways, and proteins required for Cellular Pluripotency are deeply interdependent. Each component relies on the others to function properly. Without the simultaneous presence and coordination of these elements, any intermediate stages would lack functionality and would likely be eliminated by natural selection.
Functional Intermediate Stages: In the context of pluripotency, intermediate stages that lack the full set of required codes, languages, and mechanisms would not confer a survival advantage. Without the ability to maintain pluripotency, cells would be prone to spontaneous differentiation or loss of critical cellular functions, leading to non-viable outcomes.
Precision and Complexity: The intricate nature of pluripotency-related systems necessitates a high level of precision in their instantiation. The probability of these complex systems evolving sequentially and independently is extremely low. The simultaneous emergence of multiple interdependent components is more consistent with the concept of an orchestrated design.
Informational Requirements: The establishment of pluripotency requires the instantiation of sophisticated regulatory codes and languages that guide cellular behavior. These codes contain a vast amount of complex and specified information that is highly unlikely to arise through random, gradual processes.
Cellular Identity and Function: The unique nature of pluripotent cells, their ability to self-renew, and their differentiation potential point to a holistic and integrated design. A stepwise evolutionary process would struggle to explain how such intricate functions emerged gradually while maintaining cellular identity and viability.
Functional Irreducibility: The concept of irreducible complexity applies to Cellular Pluripotency, where removing even one essential component would render the system non-functional. This challenges the idea that such a complex trait could have evolved incrementally.
Considering the interwoven complexity, the simultaneous requirement for multiple codes, languages, and mechanisms, and the functional irreducibility of Cellular Pluripotency, it seems more reasonable to posit that this intricate system was instantiated and designed all at once. This viewpoint suggests that an intelligent agency played a role in creating the precise orchestration of elements necessary for the emergence and maintenance of Cellular Pluripotency.
Irreducibility and Interdependence of the systems to instantiate and operate Cellular Pluripotency
The systems involved in creating, developing, and operating Cellular Pluripotency are characterized by irreducibility and intricate interdependence. These manufacturing, signaling, and regulatory codes and languages are so tightly interwoven that the idea of their stepwise evolution becomes implausible.
Transcriptional Regulatory Code and Signaling Languages: The transcriptional regulatory network, comprised of transcription factors like Oct4, Sox2, and Nanog, communicates with cell signaling pathways such as Wnt, FGF, and BMP. These pathways activate or repress pluripotency-associated genes through complex language interactions. Without the simultaneous operation of both codes and languages, the precise gene expression patterns necessary for pluripotency would not emerge.
Epigenetic Language and Transcriptional Regulation: The epigenetic landscape, marked by DNA methylation, histone modifications, and chromatin structure, communicates with transcription factors. The epigenetic language determines gene accessibility, influencing which genes are activated or silenced. This interaction ensures proper gene expression profiles for pluripotency maintenance.
Cell Signaling Crosstalk: The cell signaling pathways—Wnt, FGF, BMP, etc.—crosstalk with each other to fine-tune pluripotency maintenance. These pathways communicate critical information about self-renewal, differentiation cues, and cell fate decisions. Disruption of any pathway or lack of crosstalk would disrupt the dynamic equilibrium required for pluripotency.
Feedback and Feedforward Loops: Regulatory codes, signaling languages, and pathways often include feedback and feedforward loops. These loops ensure the system's stability and responsiveness to internal and external cues. They also facilitate rapid adjustments to changing conditions, a feature unlikely to arise incrementally.
Functional Irreducibility: The irreducible nature of these systems means that removing any component or mechanism would render the whole system non-functional. In the absence of a complete set of codes, languages, and pathways, cellular pluripotency would not emerge. This functional irreducibility defies the notion of gradual, stepwise evolution.
Precision and Complexity: The precision and complexity required for these interdependent systems to arise simultaneously and function harmoniously point to an orchestrated design. A stepwise process would entail numerous non-functional intermediates, as one mechanism, language, or code system alone would lack utility without the others.
The intricate interdependence, precise communication, and irreducibility of these systems challenge the notion of a gradual evolution of Cellular Pluripotency. Instead, they align more coherently with the concept that these systems were instantaneously created and integrated, fully operational from the beginning, reflecting the work of designing intelligence.
Once Cellular Pluripotency is instantiated and operational, what other intra and extracellular systems is it interdependent with?
Once Cellular Pluripotency is instantiated and operational, it becomes interdependent with various intra and extracellular systems to ensure proper functioning and integration within the organism:
Intracellular Interdependencies
DNA Replication and Repair Systems: Pluripotent cells undergo frequent DNA replication and repair to maintain genomic integrity during self-renewal. The coordination between pluripotency and these systems prevents mutations and ensures accurate transmission of genetic information.
Epigenetic Maintenance Mechanisms: The proper maintenance of epigenetic marks, including DNA methylation and histone modifications, is critical for sustaining pluripotency-associated gene expression patterns. Epigenetic regulation and pluripotency are tightly interconnected.
Cell Cycle Regulation: The regulation of the cell cycle ensures that pluripotent cells maintain a balance between self-renewal and differentiation. Cell cycle checkpoints and regulatory factors coordinate with pluripotency-associated mechanisms.
Protein Quality Control: Pluripotent cells rely on proper protein folding and quality control mechanisms. Misfolded proteins or protein aggregates can disrupt pluripotency maintenance, making protein quality control systems essential.
Metabolic Pathways: The metabolic state of pluripotent cells influences their behavior. Energy production, nutrient utilization, and metabolic pathways are interdependent with pluripotency maintenance and differentiation.
Extracellular Interdependencies
Stem Cell Niches: Pluripotent cells reside within specialized niches that provide appropriate signals and microenvironment for their self-renewal. These niches contribute to the regulation of pluripotency and prevent premature differentiation.
Extracellular Matrix (ECM): The ECM provides physical support and signaling cues that influence pluripotent cell behavior. Integrin-mediated interactions with the ECM contribute to pluripotency maintenance and lineage commitment.
Cell-Cell Interactions: Communication between pluripotent cells and neighboring cells can influence pluripotency maintenance and differentiation decisions. Ligand-receptor interactions transmit signals that impact cell fate.
Immune System: Pluripotent cells interact with the immune system, as they can potentially elicit immune responses. Immune cells and cytokines in the microenvironment may influence pluripotency and differentiation.
Developmental and Tissue Context: Pluripotent cells play a role in embryonic development and tissue regeneration. Their behavior and differentiation potential are influenced by developmental cues and the specific tissue environment.
Extracellular Signaling Molecules: Secreted factors, such as growth factors and cytokines, influence pluripotency maintenance, self-renewal, and differentiation. The presence of appropriate signaling molecules is essential for proper pluripotency function.
The interdependence of Cellular Pluripotency with these intra and extracellular systems highlights its integration within the broader biological context. Successful pluripotency maintenance relies on the coordinated functioning of these interconnected systems, ensuring the cell's ability to self-renew and contribute to various developmental and regenerative processes.
1. Systems Based on Semiotic Codes: The systems governing Cellular Pluripotency rely on complex regulatory codes, epigenetic languages, and signaling pathways to orchestrate cellular behavior and maintain pluripotency.
2. Irreducible Interdependence: The irreducible and interdependent nature of these systems implies that they must have emerged together, fully functional, to ensure successful pluripotency. Disrupting any one system would compromise the delicate balance required for pluripotency maintenance.
3. Integrated Design: The simultaneous existence of interdependent systems, each contributing to the overall pluripotency functionality, aligns with the concept of a coordinated design rather than a gradual accumulation of components through evolutionary steps.
Cellular Pluripotency refers to the unique state of certain cells that possess the remarkable ability to differentiate into a wide range of cell types found in the body. These cells, known as pluripotent stem cells, hold immense significance in developmental biology, regenerative medicine, and basic research. Pluripotent stem cells are a type of stem cell that can give rise to almost any cell type found in the body, excluding those in the placenta and extraembryonic tissues. These cells can self-renew, maintaining their undifferentiated state, while also having the potential to differentiate into specialized cells like neurons, muscle cells, and blood cells. The study of pluripotency provides insights into the early stages of embryonic development. Understanding how cells transition from pluripotency to various specialized lineages helps unravel the complex process of how organisms develop and grow. Cellular pluripotency challenges conventional notions of cellular fate, highlighting the immense flexibility and potential of certain cells.
What are the molecular factors that confer cellular pluripotency and the ability to differentiate into various cell types?
Cellular pluripotency and the ability to differentiate into various cell types are conferred by a combination of molecular factors that regulate gene expression, epigenetic states, and signaling pathways. These factors work together to maintain a balance between self-renewal and differentiation. While the exact mechanisms can vary among different organisms, the following are some key molecular factors that play crucial roles in conferring cellular pluripotency:

Pluripotency is a defining characteristic of certain stem cells, particularly embryonic stem cells. It refers to the ability of these stem cells to differentiate into cell types belonging to all three germ layers: the ectoderm, mesoderm, and endoderm. These germ layers are the fundamental cell lineages that give rise to the various tissues and organs in a developing organism.
Embryonic Stem Cells (ESCs): Pluripotency is most commonly associated with embryonic stem cells, which are derived from the inner cell mass of the blastocyst stage of the developing embryo. At this early stage, the cells are undifferentiated and possess the potential to give rise to any cell type in the body. This remarkable capability makes ESCs invaluable tools for research and regenerative medicine.
Three Germ Layers: During early embryonic development, the embryo undergoes gastrulation, a process in which the three germ layers are established. Each germ layer has the potential to differentiate into specific cell types and tissues:
Ectoderm: Gives rise to the skin, nervous system, and other external structures.
Mesoderm: Forms muscle, bone, blood, and other connective tissues.
Endoderm: Develops into the gut, respiratory tract, and other internal organs.
Versatility: Pluripotent stem cells can differentiate into a wide range of cell types, allowing them to contribute to the formation of various tissues and organs in the body. This versatility is crucial for the proper development and growth of an organism.
Differentiation: The process of pluripotent stem cells becoming specialized cells is called differentiation. As the cells differentiate, they progressively lose their pluripotency and commit to specific lineages. This process is tightly regulated by genetic and epigenetic mechanisms.
Appearance of Cellular Pluripotency in the evolutionary timeline
There are several hypotheses about how Cellular Pluripotency might have arisen.
Early Cellular Differentiation Mechanisms: One hypothesis is that early multicellular organisms would have possessed rudimentary mechanisms for cell differentiation. These mechanisms could have enabled certain cells to retain a level of plasticity, allowing them to differentiate into a variety of cell types as needed.
Emergence of Germ Layers: As multicellular organisms supposedly evolved, the emergence of germ layers (ectoderm, mesoderm, and endoderm) would have facilitated cellular specialization. Within these layers, certain cells would have retained pluripotency, allowing them to give rise to a range of cell types specific to their germ layer.
Evolution of Regulatory Networks: Over time, genetic and regulatory networks controlling cell fate determination would have become more complex and sophisticated. Regulatory genes that govern pluripotency-related factors would have emerged, allowing certain cells to maintain the potential for diverse differentiation outcomes.
Development of Developmental Pathways: Evolutionary developments in pathways such as Wnt, BMP, and Notch signaling would have contributed to the emergence of pluripotent cells. These pathways are known to play roles in cellular differentiation, and their evolution would have allowed for the preservation of pluripotency in certain cell populations.
Importance in Regeneration: Pluripotent cells would have provided an advantage in terms of regeneration and tissue repair. Organisms with cells capable of reverting to a pluripotent state would have been better equipped to regenerate lost or damaged tissues, leading to increased survival and reproductive success.
Benefit for Environmental Adaptation: Pluripotent cells would have facilitated adaptation to changing environments. Organisms with pluripotent cells would have been more flexible in responding to environmental challenges by producing specialized cell types suited to new conditions.
It's important to emphasize that the evolutionary emergence of pluripotency is complex and would have involved a combination of genetic, regulatory, and environmental factors. The gradual evolution of the molecular and genetic machinery necessary for maintaining pluripotency remains an area of active research and exploration.
De Novo Genetic Information necessary to instantiate Cellular Pluripotency
Creating the mechanisms of Cellular Pluripotency de novo would involve the precise generation and integration of genetic information into the existing genetic material. While the exact details are speculative, here's a hypothetical process of introducing new genetic information to establish cellular pluripotency:
Emergence of Regulatory Elements: New genetic elements, such as enhancers and promoters, would need to originate. These elements would regulate the expression of pluripotency-associated genes and coordinate their activity.
Pluripotency Master Regulators: New genes encoding master regulators of pluripotency would need to emerge. These genes would encode transcription factors that play pivotal roles in maintaining pluripotency by controlling the expression of numerous downstream genes.
Epigenetic Machinery: Genes encoding enzymes involved in epigenetic modifications, such as DNA methylation and histone modifications, would need to evolve. These modifications are critical for maintaining the open chromatin state required for pluripotency.
Cell Signaling Pathways: Novel genes encoding components of signaling pathways (e.g., Wnt, BMP, FGF) that interact with the pluripotency network would need to arise. These pathways contribute to maintaining pluripotency and influencing cell fate decisions.
Cell-Cell Communication Genes: New genes related to cell-cell communication would be necessary. Pluripotent cells require signaling cues from their environment to maintain their undifferentiated state, so genes encoding receptors and ligands might evolve.
Genetic Network Development: The new genetic information would need to establish intricate networks of interactions. Cross-regulatory interactions between genes would need to evolve, ensuring the proper balance of pluripotency-related factors.
Chromatin Remodeling Factors: Genes encoding chromatin remodeling factors would be essential. These factors reshape the chromatin structure to allow access to key pluripotency genes and maintain the cellular identity.
Cell Division Control: Genes involved in regulating cell division rates and cycles would be important. Pluripotent cells must strike a balance between self-renewal and differentiation, which requires precise control over cell division.
DNA Repair and Replication: Genes encoding DNA repair and replication factors would need to be in place. The dynamic nature of pluripotency requires accurate DNA maintenance during cell division and replication.
Transcriptional Machinery: Components of the transcriptional machinery, including RNA polymerases and transcription factors, would need to evolve to interact with the new pluripotency-related genes.
The process of generating and integrating new genetic information to establish Cellular Pluripotency would involve the emergence of a complex network of genes, regulatory elements, and interactions. The simultaneous appearance of these components in a coordinated manner would be essential to ensure the functional establishment of pluripotency. It's important to note that this description is speculative and intended to highlight the complexity of creating the mechanisms for Cellular Pluripotency from scratch.
Manufacturing codes and languages that would have to emerge and be employed to instantiate Cellular Pluripotency
The establishment of Cellular Pluripotency involves a sophisticated interplay of various manufacturing codes and languages beyond genetic information. These codes and languages contribute to the development and maintenance of pluripotent cells within an organism.
Epigenetic Codes: The epigenetic code involves modifications to DNA and histone proteins that determine how genes are expressed. To establish pluripotency, new epigenetic codes would need to be instantiated to create an open chromatin structure, allowing access to pluripotency-related genes. Epigenetic modifications would regulate the balance between self-renewal and differentiation.
Protein Folding and Modification Codes: The correct folding and modification of proteins are crucial for pluripotency-related factors to function properly. Codes governing protein folding, post-translational modifications, and quality control mechanisms would need to evolve to ensure the proper functioning of pluripotency-associated proteins.
Cell Signaling Languages: Pluripotency requires intricate cell signaling networks. New signaling languages would need to emerge, allowing cells to communicate with each other and their environment. These languages would guide cellular responses to external cues, influencing pluripotency maintenance and differentiation decisions.
Metabolic Codes: Cellular metabolism plays a vital role in maintaining pluripotency. Evolving metabolic codes would be necessary to ensure the energy and nutrient requirements of pluripotent cells are met while maintaining their unique state.
Cytoskeletal Dynamics: Codes governing cytoskeletal dynamics would need to be instantiated to enable cell shape changes, migration, and interactions with the microenvironment. These codes contribute to the physical properties of pluripotent cells and their ability to respond to signals.
Extracellular Matrix (ECM) Codes: The ECM provides cues for cell adhesion, migration, and differentiation. New codes governing the synthesis and arrangement of ECM components would need to emerge to support pluripotency-related functions.
Membrane Receptor Codes: To interact with external signals, new membrane receptor codes would be essential. These codes would enable cells to sense and respond to specific ligands that influence pluripotency-related pathways.
Chemotactic Language: Pluripotent cells often migrate in response to chemical gradients. A chemotactic language would need to evolve, allowing cells to follow specific cues and migrate toward or away from certain regions to maintain pluripotency.
Cell Adhesion Codes: Codes governing cell adhesion molecules would be required for interactions between pluripotent cells and neighboring cells or the extracellular matrix. These codes would support the maintenance of pluripotency and cell-cell communication.
Stem Cell Niche Codes: Pluripotent cells reside in specific niches within tissues. Niche codes would need to evolve to create environments that support the self-renewal and differentiation of pluripotent cells.
These manufacturing codes and languages, operating in concert with genetic information, would need to be simultaneously instantiated and integrated into the organism's biological systems. Their coordinated emergence would be essential for the successful development of Cellular Pluripotency, highlighting the intricate design required to establish and maintain this complex cellular state.
Epigenetic Regulatory Mechanisms necessary to be instantiated to create Cellular Pluripotency
Creating Cellular Pluripotency would involve intricate epigenetic regulation to control gene expression patterns and maintain the pluripotent state. The systems and processes required to instantiate and maintain this regulation would work collaboratively.
Epigenetic Regulation: DNA Methylation Patterns: New DNA methylation patterns would need to emerge, allowing for the appropriate silencing and activation of genes associated with pluripotency and differentiation. These patterns would be established by DNA methyltransferases.
Histone Modifications: Specific histone modifications would need to be established, marking genes for either activation or repression. Histone acetyltransferases, methyltransferases, and other modifying enzymes would play a role in shaping the pluripotent epigenome.
Chromatin Accessibility: The establishment of an open chromatin structure in pluripotent cells would require changes in nucleosome positioning and chromatin remodeling. ATP-dependent chromatin remodelers and modifiers would be involved.
Non-Coding RNAs: Non-coding RNAs, such as microRNAs and long non-coding RNAs, would emerge to fine-tune gene expression and contribute to the maintenance of pluripotency-related pathways.
Collaborating Systems: Cell Signaling Pathways: Signaling pathways like Wnt, BMP, and FGF would interact with the epigenetic machinery to influence gene expression patterns and maintain pluripotency. They would provide external cues that guide epigenetic modifications.
Transcriptional Regulation: Transcription factors specific to pluripotency would collaborate with epigenetic regulators to control the expression of pluripotency-related genes. This collaboration would ensure the appropriate genes are activated or repressed.
Cell-Cell Communication: Intercellular communication would involve ligand-receptor interactions that transmit signals affecting epigenetic modifications. The collaboration between cells would contribute to maintaining a pluripotent environment.
Metabolic Regulation: Cellular metabolism and energy availability would influence epigenetic modifications. Metabolic pathways would interact with the epigenetic machinery to support pluripotency.
Quality Control Mechanisms: Cellular systems that ensure proper protein folding and quality control would collaborate with the epigenetic machinery. Correctly folded proteins would be essential for maintaining the pluripotent state.
Cell Division Control: The cell cycle and division control mechanisms would need to synchronize with epigenetic changes to ensure that pluripotent cells maintain their epigenetic identity during replication.
DNA Repair and Replication: Efficient DNA repair and replication processes would collaborate with the epigenetic machinery to preserve accurate epigenetic patterns during cell division.
Environmental Adaptation: The collaboration between epigenetic regulation and cellular responses to environmental cues would enable pluripotent cells to adapt to changing conditions while maintaining their identity.
The instantiation and maintenance of epigenetic regulation for Cellular Pluripotency would involve the coordinated interplay of these systems, ensuring that genes are expressed in a controlled manner to maintain the pluripotent state. The collaborative nature of these systems emphasizes the complexity and integrated design required for the development and functionality of pluripotent cells.
Signaling Pathways necessary to create, and maintain Cellular Pluripotency
The emergence of Cellular Pluripotency would involve the creation and orchestration of various signaling pathways that interact, collaborate, and crosstalk to establish and maintain the pluripotent state.
Wnt Signaling Pathway: The Wnt pathway would be crucial for the activation of pluripotency-related genes. It could promote the expression of key transcription factors, such as Oct4, contributing to the establishment of pluripotency. The Wnt pathway might crosstalk with the FGF and BMP pathways to fine-tune pluripotency maintenance.
FGF Signaling Pathway: Fibroblast Growth Factor (FGF) signaling would play a role in sustaining pluripotency. It might collaborate with the Wnt pathway to maintain self-renewal and block differentiation. The FGF pathway could crosstalk with the TGF-β pathway to balance self-renewal and differentiation signals.
BMP Signaling Pathway: Bone Morphogenetic Protein (BMP) signaling might drive the differentiation of non-pluripotent cells. However, in the context of pluripotency, it could collaborate with the FGF and Wnt pathways to establish a balance between pluripotency and differentiation cues.
Notch Signaling Pathway: Notch signaling might be involved in cell-cell communication and lineage commitment. In pluripotency, it could interact with the FGF and Wnt pathways to influence differentiation decisions and maintain the pluripotent state.
Hedgehog Signaling Pathway: Hedgehog signaling could contribute to cell-fate determination and differentiation. In the pluripotent context, it might cross-interact with the Wnt and FGF pathways to influence lineage specification.
TGF-β Signaling Pathway: Transforming Growth Factor-beta (TGF-β) signaling could play a role in differentiation. In pluripotency, it might interact with the FGF pathway to balance self-renewal and differentiation signals.
PI3K/AKT/mTOR Pathway: This pathway could regulate cellular growth and survival. It might collaborate with the FGF and Wnt pathways to support the pluripotent state by regulating cell cycle progression and nutrient availability.
Cell Adhesion Pathways: Signaling pathways related to cell adhesion, such as integrin-mediated signaling, could crosstalk with the above pathways to influence cell migration, communication, and the maintenance of pluripotency.
Metabolic Signaling: Metabolic pathways could cross-interact with various signaling pathways to ensure energy availability for pluripotent cells. Collaborations between metabolic and pluripotency-related pathways would contribute to self-renewal and pluripotency maintenance.
Environmental Sensing: The interplay between signaling pathways and the cell's environment would allow pluripotent cells to sense external cues and adapt their behavior accordingly. Collaboration with the immune system, tissue architecture, and developmental cues would influence pluripotency maintenance.
The interconnections and collaborations among these signaling pathways would create a highly intricate network that governs the establishment and maintenance of Cellular Pluripotency. The crosstalk among these pathways would ensure that pluripotent cells respond appropriately to internal and external cues, striking a balance between self-renewal and differentiation while interacting with other biological systems within the organism.
Regulatory codes necessary for maintenance and operation of Cellular Pluripotency
The maintenance and operation of Cellular Pluripotency would involve a complex interplay of regulatory codes and languages that control gene expression, cell behavior, and interactions. Here are some of the key regulatory codes and languages that would need to be instantiated and involved:
Transcriptional Regulatory Code: This code involves the binding of transcription factors to specific DNA sequences to activate or repress gene expression. Transcription factors like Oct4, Sox2, and Nanog would need to establish a regulatory network to maintain pluripotency-associated gene expression.
Cell Signaling Languages: Cell signaling pathways, including Wnt, FGF, and BMP, would require specific signaling languages to transmit information within pluripotent cells. These languages would guide cellular responses to maintain self-renewal and differentiation balance.
Epigenetic Language: The epigenetic language involves DNA methylation patterns, histone modifications, and chromatin structure that determine gene accessibility. This language would need to ensure the maintenance of an open chromatin state around pluripotency genes while repressing lineage-specific genes.
Chemotactic Language: A chemotactic language would guide the migration of pluripotent cells to specific niches or environments within tissues, ensuring proper self-renewal and differentiation cues.
Cell-Cell Communication Codes: Cell-cell communication would involve ligand-receptor interactions that convey information about the cellular environment and influence pluripotency maintenance.
Metabolic Code: The metabolic code would coordinate the energy and nutrient requirements of pluripotent cells, ensuring proper functioning and self-renewal.
Stem Cell Niche Code: Pluripotent cells reside in specific niches within tissues. The niche code would establish a microenvironment that supports self-renewal and prevents premature differentiation.
Quality Control Mechanisms: Regulatory codes related to protein folding, quality control, and cellular homeostasis would ensure that pluripotency-associated proteins are properly folded and functional.
Cell Cycle Code: The cell cycle regulatory code would coordinate cell division and replication with pluripotency maintenance, ensuring accurate transmission of genetic and epigenetic information to daughter cells.
DNA Repair and Replication Code: The code governing DNA repair and replication would safeguard the integrity of the genome and epigenome during cell division.
Differentiation Repression Code: To prevent premature differentiation, pluripotent cells would need a code that represses lineage-specific genes and pathways while maintaining pluripotency-related factors.
These regulatory codes and languages would collaboratively orchestrate the complex processes that maintain and operate Cellular Pluripotency. Their coordinated functioning would ensure the self-renewal, plasticity, and controlled differentiation potential of pluripotent cells within biological systems.
Is there scientific evidence supporting the idea that Cellular Pluripotency was brought about by the process of evolution?
The emergence of Cellular Pluripotency presents a confluence of complex regulatory systems, precise codes, and intricate interdependencies that operate harmoniously to maintain this unique cellular state. An evolutionary step-by-step process seems highly unlikely due to several reasons:
Interdependent Codes and Mechanisms: The codes, languages, signaling pathways, and proteins required for Cellular Pluripotency are deeply interdependent. Each component relies on the others to function properly. Without the simultaneous presence and coordination of these elements, any intermediate stages would lack functionality and would likely be eliminated by natural selection.
Functional Intermediate Stages: In the context of pluripotency, intermediate stages that lack the full set of required codes, languages, and mechanisms would not confer a survival advantage. Without the ability to maintain pluripotency, cells would be prone to spontaneous differentiation or loss of critical cellular functions, leading to non-viable outcomes.
Precision and Complexity: The intricate nature of pluripotency-related systems necessitates a high level of precision in their instantiation. The probability of these complex systems evolving sequentially and independently is extremely low. The simultaneous emergence of multiple interdependent components is more consistent with the concept of an orchestrated design.
Informational Requirements: The establishment of pluripotency requires the instantiation of sophisticated regulatory codes and languages that guide cellular behavior. These codes contain a vast amount of complex and specified information that is highly unlikely to arise through random, gradual processes.
Cellular Identity and Function: The unique nature of pluripotent cells, their ability to self-renew, and their differentiation potential point to a holistic and integrated design. A stepwise evolutionary process would struggle to explain how such intricate functions emerged gradually while maintaining cellular identity and viability.
Functional Irreducibility: The concept of irreducible complexity applies to Cellular Pluripotency, where removing even one essential component would render the system non-functional. This challenges the idea that such a complex trait could have evolved incrementally.
Considering the interwoven complexity, the simultaneous requirement for multiple codes, languages, and mechanisms, and the functional irreducibility of Cellular Pluripotency, it seems more reasonable to posit that this intricate system was instantiated and designed all at once. This viewpoint suggests that an intelligent agency played a role in creating the precise orchestration of elements necessary for the emergence and maintenance of Cellular Pluripotency.
Irreducibility and Interdependence of the systems to instantiate and operate Cellular Pluripotency
The systems involved in creating, developing, and operating Cellular Pluripotency are characterized by irreducibility and intricate interdependence. These manufacturing, signaling, and regulatory codes and languages are so tightly interwoven that the idea of their stepwise evolution becomes implausible.
Transcriptional Regulatory Code and Signaling Languages: The transcriptional regulatory network, comprised of transcription factors like Oct4, Sox2, and Nanog, communicates with cell signaling pathways such as Wnt, FGF, and BMP. These pathways activate or repress pluripotency-associated genes through complex language interactions. Without the simultaneous operation of both codes and languages, the precise gene expression patterns necessary for pluripotency would not emerge.
Epigenetic Language and Transcriptional Regulation: The epigenetic landscape, marked by DNA methylation, histone modifications, and chromatin structure, communicates with transcription factors. The epigenetic language determines gene accessibility, influencing which genes are activated or silenced. This interaction ensures proper gene expression profiles for pluripotency maintenance.
Cell Signaling Crosstalk: The cell signaling pathways—Wnt, FGF, BMP, etc.—crosstalk with each other to fine-tune pluripotency maintenance. These pathways communicate critical information about self-renewal, differentiation cues, and cell fate decisions. Disruption of any pathway or lack of crosstalk would disrupt the dynamic equilibrium required for pluripotency.
Feedback and Feedforward Loops: Regulatory codes, signaling languages, and pathways often include feedback and feedforward loops. These loops ensure the system's stability and responsiveness to internal and external cues. They also facilitate rapid adjustments to changing conditions, a feature unlikely to arise incrementally.
Functional Irreducibility: The irreducible nature of these systems means that removing any component or mechanism would render the whole system non-functional. In the absence of a complete set of codes, languages, and pathways, cellular pluripotency would not emerge. This functional irreducibility defies the notion of gradual, stepwise evolution.
Precision and Complexity: The precision and complexity required for these interdependent systems to arise simultaneously and function harmoniously point to an orchestrated design. A stepwise process would entail numerous non-functional intermediates, as one mechanism, language, or code system alone would lack utility without the others.
The intricate interdependence, precise communication, and irreducibility of these systems challenge the notion of a gradual evolution of Cellular Pluripotency. Instead, they align more coherently with the concept that these systems were instantaneously created and integrated, fully operational from the beginning, reflecting the work of designing intelligence.
Once Cellular Pluripotency is instantiated and operational, what other intra and extracellular systems is it interdependent with?
Once Cellular Pluripotency is instantiated and operational, it becomes interdependent with various intra and extracellular systems to ensure proper functioning and integration within the organism:
Intracellular Interdependencies
DNA Replication and Repair Systems: Pluripotent cells undergo frequent DNA replication and repair to maintain genomic integrity during self-renewal. The coordination between pluripotency and these systems prevents mutations and ensures accurate transmission of genetic information.
Epigenetic Maintenance Mechanisms: The proper maintenance of epigenetic marks, including DNA methylation and histone modifications, is critical for sustaining pluripotency-associated gene expression patterns. Epigenetic regulation and pluripotency are tightly interconnected.
Cell Cycle Regulation: The regulation of the cell cycle ensures that pluripotent cells maintain a balance between self-renewal and differentiation. Cell cycle checkpoints and regulatory factors coordinate with pluripotency-associated mechanisms.
Protein Quality Control: Pluripotent cells rely on proper protein folding and quality control mechanisms. Misfolded proteins or protein aggregates can disrupt pluripotency maintenance, making protein quality control systems essential.
Metabolic Pathways: The metabolic state of pluripotent cells influences their behavior. Energy production, nutrient utilization, and metabolic pathways are interdependent with pluripotency maintenance and differentiation.
Extracellular Interdependencies
Stem Cell Niches: Pluripotent cells reside within specialized niches that provide appropriate signals and microenvironment for their self-renewal. These niches contribute to the regulation of pluripotency and prevent premature differentiation.
Extracellular Matrix (ECM): The ECM provides physical support and signaling cues that influence pluripotent cell behavior. Integrin-mediated interactions with the ECM contribute to pluripotency maintenance and lineage commitment.
Cell-Cell Interactions: Communication between pluripotent cells and neighboring cells can influence pluripotency maintenance and differentiation decisions. Ligand-receptor interactions transmit signals that impact cell fate.
Immune System: Pluripotent cells interact with the immune system, as they can potentially elicit immune responses. Immune cells and cytokines in the microenvironment may influence pluripotency and differentiation.
Developmental and Tissue Context: Pluripotent cells play a role in embryonic development and tissue regeneration. Their behavior and differentiation potential are influenced by developmental cues and the specific tissue environment.
Extracellular Signaling Molecules: Secreted factors, such as growth factors and cytokines, influence pluripotency maintenance, self-renewal, and differentiation. The presence of appropriate signaling molecules is essential for proper pluripotency function.
The interdependence of Cellular Pluripotency with these intra and extracellular systems highlights its integration within the broader biological context. Successful pluripotency maintenance relies on the coordinated functioning of these interconnected systems, ensuring the cell's ability to self-renew and contribute to various developmental and regenerative processes.
1. Systems Based on Semiotic Codes: The systems governing Cellular Pluripotency rely on complex regulatory codes, epigenetic languages, and signaling pathways to orchestrate cellular behavior and maintain pluripotency.
2. Irreducible Interdependence: The irreducible and interdependent nature of these systems implies that they must have emerged together, fully functional, to ensure successful pluripotency. Disrupting any one system would compromise the delicate balance required for pluripotency maintenance.
3. Integrated Design: The simultaneous existence of interdependent systems, each contributing to the overall pluripotency functionality, aligns with the concept of a coordinated design rather than a gradual accumulation of components through evolutionary steps.
Last edited by Otangelo on Sun 3 Sep 2023 - 23:22; edited 1 time in total


