Fine-tuning of atoms
https://reasonandscience.catsboard.com/t2763-fine-tuning-of-atoms
Planck: As a man who has devoted his whole life to the most clear-headed science, to the study of matter, I can tell you as a result of my research about atoms this much: There is no matter as such. All matter originates and exists only by virtue of a force that brings the particle of an atom to vibration and holds this most minute solar system of the atom together.
Leonard Susskind The Cosmic Landscape: String Theory and the Illusion of Intelligent Design 2006, page 176
Complex atomic nuclei are not likely to result from random collisions of particles, even in the early hot universe.
https://3lib.net/book/2472017/1d5be1
Why Fine-tuning Seems Designed
Free (three quarks) positive protons and electrons could just join up, and given their opposite electric charges. It could also have been that an equal number of electrons and protons had been formed post-Big Bang then the cosmos would be a soup on neutrons and perhaps neutrinos, but that would then be pretty much that. How is it that an electron, protons, and neutrons can arrange themselves just so as to eventually produce macro stuff, including us? How to go from particle physics to chemistry?
https://www.closertotruth.com/series/why-fine-tuning-seems-designed
Man Ho Chan The fine-tuned universe and the existence of God 5-24-2017
In the period called Big Bang Nucleosynthesis, hydrogen, helium, and a tiny amount of lithium were formed in the first three minutes since Big Bang.
https://philarchive.org/archive/CHATFU-2
A More Finely Tuned Universe
Why do fundamental particles possess the specific values of mass that they have? Presently, physicists have no explanation for this and similar questions. If the masses of particles or the values of fundamental constants were much different from what physicists have measured, carbon-based intelligent beings might not be here to measure them, because fundamental particles might not assemble into stable atoms, atoms might not form rocky planets and dying stars might not produce the chemical elements we find in our bodies.
https://www.insidescience.org/news/more-finely-tuned-universe
The mass of the proton and the neutron as an example of fine-tuning for life
Protons have a mass of 938.27 MeV. Neutrons have a mass of 939.56 MeV. The difference between them is small: a neutron is about 1.29 MeV heavier than a proton. There is no obvious reason why protons and neutrons should have just these masses, but if they were even slightly different, we wouldn’t be here.
http://www.focus.org.uk/proton_neutron.php
Paul Davies Yes, the universe looks like a fix. But that doesn't mean that a god fixed it 26 Jun 2007
For example, neutrons are just a tad heavier than protons. If it were the other way around, atoms couldn't exist, because all the protons in the universe would have decayed into neutrons shortly after the big bang. No protons, then no atomic nuclei, and no atoms. No atoms, no chemistry, no life. Like Baby Bear's porridge in the story of Goldilocks, the universe seems to be just right for life. So what's going on?
https://www.theguardian.com/commentisfree/2007/jun/26/spaceexploration.comment
Nigel Warburton: Is the Universe a conscious mind? August 2019.
The strong nuclear force (the force that binds together the elements in the nucleus of an atom) has a value of 0.007. If that value had been 0.006 or less, the Universe would have contained nothing but hydrogen. If it had been 0.008 or higher, the hydrogen would have fused to make heavier elements. In either case, any kind of chemical complexity would have been physically impossible. And without chemical complexity there can be no life.
https://aeon.co/essays/cosmopsychism-explains-why-the-universe-is-fine-tuned-for-life
Stephen C. Meyer: The return of the God hypothesis, page 185
For instance, to make life possible, the masses of the fundamental particles must meet an exacting combination of constraints. There is the fine-tuning of the masses of the two naturally occurring quarks, the up quark and down quark, in relation to the range of expected possible values. The fine-tuning of the masses of those quarks are considerable: 1 part in 10^21 . In addition, the difference in masses between the quarks cannot exceed one megaelectron volt, the equivalent of one-thousandth of 1 percent of the mass of the largest known quark, without producing either a neutron-only or a proton-only universe, both exceedingly boring and incompatible with life and even with simple chemistry. Equally problematic, increasing the mass of electrons by a factor of 2.5 would result in all the protons in all the atoms capturing all the orbiting electrons and turning them into neutrons. In that case, neither atoms, nor chemistry, nor life could exist. What’s more, the mass of the electron has to be less than the difference between the masses of the neutron and the proton and that difference represents fine-tuning of roughly 1 part in a 1000. In addition, if the mass of a special particle known as a neutrino were increased by a factor of 10, stars and galaxies would never have formed. The mass of a neutrino is about one-millionth that of an electron, so the allowable change is minuscule compared to its possible range. The combination of all these precisely fine-tuned conditions—including the fine-tuning of the laws and constants of physics, the initial arrangement of matter and energy, and various other contingent features of the universe —presents a remarkably restrictive set of criteria. These requirements for the existence of life, again defying our ability to describe their extreme improbability, have seemed to many physicists to require some explanation.
Strikingly, the masses of “up quarks” and “down quarks,” the constituent parts of protons and neutrons, need to have precise values to allow for the production of the elements, including carbon, essential for a life-friendly universe. Indeed, the masses of these quarks must have simultaneously nine different conditions for the right nuclear reactions to have occurred in the early universe. The “right” reactions are ones that would produce the right elements (such as carbon and oxygen) in the right abundance necessary for life. The fine-tuning of the masses of these two naturally occurring quarks in relation to the range of expected possible values for the mass of any fundamental particle is exquisite. Physicists conceive of that range as extending between a mass of zero and the so-called Planck mass, an important unit of measure in quantum physics. But the value of the “up quark” must have a precise mass of between zero and just one billion trillionth of the Planck mass, corresponding to a fine-tuning of roughly 1 part in 10^21. The mass of the “down quark” must have a similarly precise fine-tuning.
https://3lib.net/book/15644088/9c418b
Brad Lemley Why is There Life? November 01, 2000
Of Rees's six numbers, two relate to basic forces, two determine the size and large-scale texture of the universe, and two fix the properties of space itself.
 , the .007 figure, which describes the strength of the force that binds atomic nuclei together and determines how all atoms on Earth are made.
, the .007 figure, which describes the strength of the force that binds atomic nuclei together and determines how all atoms on Earth are made.
https://web.archive.org/web/20140722210250/http://discovermagazine.com/2000/nov/cover/
Consider the ingredients you need:
The strong nuclear force particles;
The weak nuclear force particles;
The electromagnetic force particles;
Up-quarks and down-quarks
Luke A. Barnes The Fine-Tuning of the Universe for Intelligent Life June 11, 2012
https://arxiv.org/pdf/1112.4647.pdf
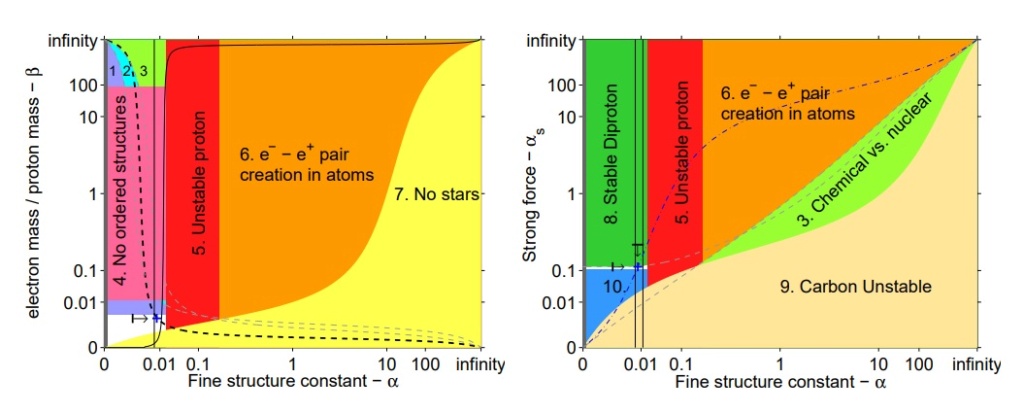
The life-permitting region (shown in white) in the (α, β) (left) and (α, αs) (right) parameter space, with other constants held at their values in our universe. Our universe is shown as a blue cross.
1. For hydrogen to exist — to power stars and form water and organic compounds — we must have mass electron < mass neutron − mass proton. Otherwise, the electron will be captured by the proton to form a neutron
2. For stable atoms, we need the radius of the electron orbit to be significantly larger than the nuclear radius.
3. We require that the typical energy of chemical reactions is much smaller than the typical energy of nuclear reactions. This ensures that the atomic constituents of chemical species maintain their identity in chemical reactions.
4. Unless β 1/4 << 1, stable ordered molecular structures (like chromosomes) are not stable. The atoms will too easily stray from their place in the lattice and the substance will spontaneously melt
5. The stability of the proton requires α . ( down quark md − up quark mu)/141 MeV, so that the extra electromagnetic mass-energy of a proton relative to a neutron is more than counter-balanced by the bare quark masses
6. Unless α << 1, the electrons in atoms and molecules are unstable to the creation of pairs. The limit shown is α < 0.2..
7. As in Equation 10, stars will not be stable unless β & α 2/100.
8. Unless αs/αs,0 . 1.003 + 0.031α/α0, the diproton has a bound state, which affects stellar burning and big bang nucleosynthesis.
9. Unless αs . 0.3α 1/2 , carbon and all larger elements are unstable
10. Unless αs/αs,0 & 0.91, the deuteron is unstable and the main nuclear reaction in stars (pp) does not proceed. A similar effect would be achieved35 unless md − mu + me < 3.4 MeV which makes the pp reaction energetically unfavourable. This region is numerically very similar to Region 1 in the left plot.
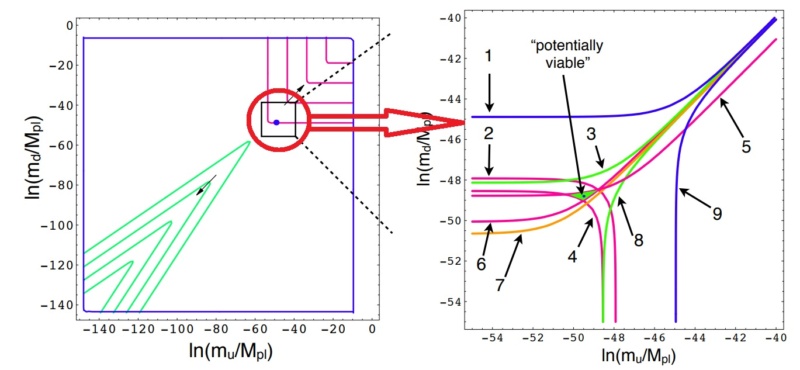
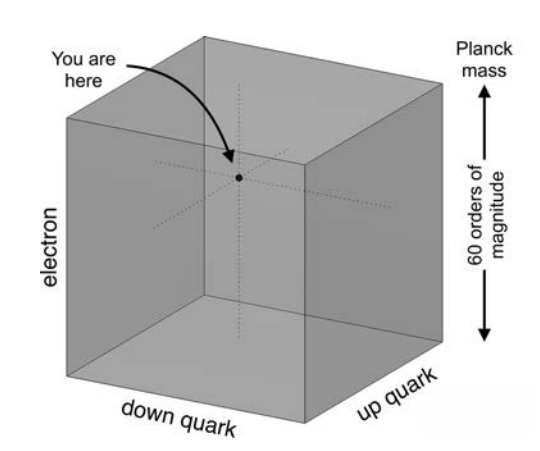
Parameter space of the masses of the up and down quark. The axes span ∼ 60 orders of magnitude.

The figure zooms in on a region of parameter space, showing boundaries of 9 independent life-permitting criteria
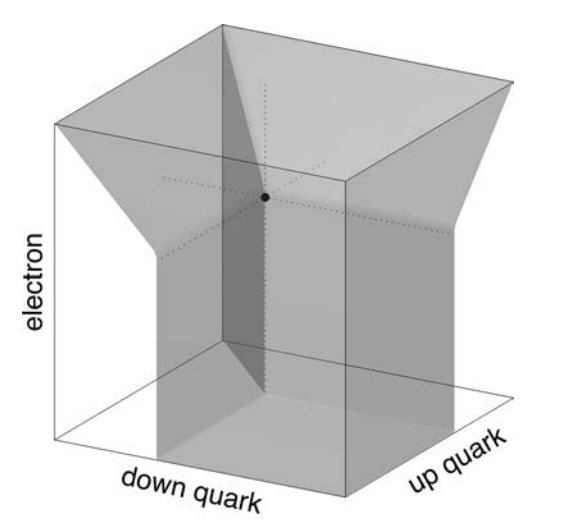
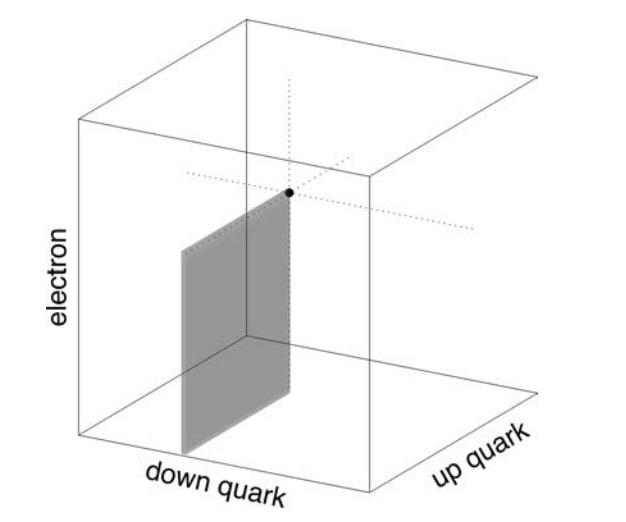
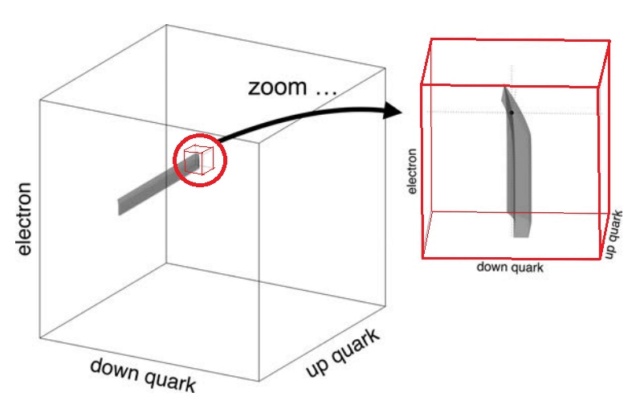
Each point on the graph corresponds to possible values for the masses of the up and down quarks (Mu , Md ). The masses are scaled by the Planck mass, Mpl , since Planck units are the most natural in cosmology. Each of the nine lines on the graph separate the regions corresponding to life-permitting and non-life-permitting universes for a specific criterion such as allowing for the existence of stable protons. In a universe capable of supporting life, all nine criteria must be met simultaneously, so the life-permitting region is the intersection of all nine life-permitting regions, marked in gray. That area corresponds to a minuscule proportion of all plausible values.
1. Above the blue line, there is only one stable element, which consists of a single particle ∆++. This element has the chemistry of helium, an inert, monatomic gas (above 4 K) with no known stable chemical compounds.
2. Above this red line, the deuteron is strongly unstable, decaying via the strong force. The first step in stellar nucleosynthesis in hydrogen-burning stars would fail.
3. Above the green curve, neutrons in nuclei decay, so that hydrogen is the only stable element.
4. Below this red curve, the diproton is stable15. Two protons can fuse to helium-2 via a very fast electromagnetic reaction, rather than the much slower, weak nuclear pp-chain.
5. Above this red line, the production of deuterium in stars absorbs energy rather than releasing it. Also, the deuterium is unstable to weak decay.
6. Below this red line, a proton in a nucleus can capture an orbiting electron and become a neutron. Thus, atoms are unstable.
7. Below the orange curve, isolated protons are unstable, leaving no hydrogen left over from the early universe to power long-lived stars and play a crucial role in organic chemistry.
8. Below this green curve, protons in nuclei decay, so that any atoms that formed would disintegrate into a cloud of neutrons.
9. Below this blue line, the only stable element consists of a single particle ∆−, which can combine with a positron to produce an element with the chemistry of hydrogen. A handful of chemical reactions are possible, with their most complex product being (an analogue of) H2.
Why Cosmic Fine-tuning Demands Explanation
Fine-tuning is necessary in order to form the protons and the neutrons; and of course electrons. And all of these bits and pieces have to mesh like a clock - or even a watch. You can't just assemble these bits and pieces in just any old way and expect things to work out. These processes have nothing to do with the emergence of atoms from the fundamental particles, forces and fields that form the bedrock of the atomic realm. It's all governed by the laws, principles and relationships of quantum mechanics, all of which had to come from somewhere or from someone or from something. For example, there's the Pauli Exclusion Principle which requires that not more than two electrons can occupy exactly the same 'orbit' and then only if they have differing quantum values, like spin-up and spin-down. This prevents all electrons being together like commuters crowded into a Tokyo subway carriage at rush hour. Then there's the energy levels that electrons are allowed to have while 'orbiting' around the nucleus. That can be in this level or that level or the next level but not at in-between levels. This prevents electrons from spiraling down and impacting the positively charged nucleus which, being negatively charged, electrons would otherwise want to do. Design and fine-tuning by any other name still appears to be design and fine-tuning.
Consider further that the partial (fractional) electrical charges on the up-quarks and the down-quarks had to arrange themselves just-so such that a proton is a unity of positive electric charge and a neutron is a unity of electric charge neutrality. Then, the positive electric charge on the proton has to balance just so (to an infinite number of decimal places, at least as close to infinity as one can actually measure and calculate) the negative electric charge of the electron. How can the electric charge of the electron be EXACTLY equal and opposite to that of the proton when they otherwise share nothing in common?
If hydrogen atoms couldn't link up with oxygen atoms there could be no water and no water implies no life could arise. The same applies to dozens of other essential molecules that life requires.
On the other hand, why isn't there a universal solvent or acid that disassembles molecules? Everything can be stored in at least one kind of container. That too seems to be essential for life as is the requirement that some things need to be in solution some of the time. An atom is literally 99.99% empty space. And the part which supposedly is matter, might be just pure energy. And as such, the whole universe is simply held together by Gods power and his word: information.
Who fine-tuned the atomic parameters? Why fine-tuning? Well fine-tuning implies that something(s) exist against all the odds. Fine-tuning requires a tuner. The universe started with the design and fine-tuned engineering of the humble atom
https://www.closertotruth.com/series/why-cosmic-fine-tuning-demands-explanation
Sean D. Pitman, MD: The Detection of Intelligent Design Simple Stuff Mindless Nature Cannot Do
The proton mass is 1836 times that of an electron. If this ratio were off even slightly, molecules would not form properly. It is also interesting to note that although protons are very different in size and mass, the charges are exactly the same in opposite degree. If this were not the case, again, molecules necessary to support complex life could not form. The same is true of the electromagnetic coupling constant between protons and electrons - it is very precisely balanced to support complex life.
http://www.detectingdesign.com/detectingdesign.html
John Prytz: Is The Atom An Example Of Cosmic Design And Fine-Tuning? Feb.7.2016
The electron, the proton, and the quark are all entities within the realm of particle hence quantum physics. All three carry an electrical charge. All three have mass. After those observations, things get interesting, or messy, depending on your point of view. The electric charge of the proton is exactly equal and the opposite of the electric charge on the electron, despite the proton being nearly 2000 times more massive. There’s no set-in-concrete theoretical reason why this should be so. It cannot be determined from first principles, only experimentally measured. An electron has a negative charge exactly equal and opposite to that of a proton. Note: the charge is exactly equal, even though the proton has a far greater mass than the electron (some 2000 times heavier in fact, not that there has to be of necessity any relationship between mass and charge).
Now that’s strange since the electron is a fundamental particle but the positively charged proton is a composite particle, made up of a trio of quarks (as is the neutron with no net charge). The proton has two quarks each with a positive 2/3rds charge (up quark) and one quark with a negative 1/3rd charge (down quark) for an overall balance of one positive charge. (The neutron, on the other hand, has one up quark with a positive 2/3rds charge and two down quarks each with a negative 1/3rd charge, for an overall balance of zero charge – neither positive nor negative.)
Now you might suggest that an electron might be a fusion of a trio of down quarks, each with a negative 1/3rd charge, except the electron, again, isn’t a composite particle, and the mass is all wrong for that scenario. If an electron were a composite of a trio of down quarks, each with a minus 1/3rd charge, the electron would be thirty times more massive than it is – not something particle physicists would fail to take notice of.
Further, the force particle that governs the electron is the photon; that which governs the quarks inside the proton and the neutron is the gluon, which further differentiates the two things – quarks and electrons. In any event, if you could have a composite particle of a trio of negative 1/3rd down quarks, if that were the case, and it is the case, and it’s called the Negative Delta, you’d also need a composite particle that’s the fusion of a trio of positive 2/3rds up quarks for an overall charge of plus two. To the best of my knowledge, there is only one such critter in the particle zoo and it’s called the Doubly Positive Delta. I’m sure you’ve never heard of these Delta particles, which goes to show how much bearing or impact they have on life, the Universe, and everything.
In case you were wondering, there would be an anti-quark of minus 2/3rds charge, and an anti-quark of a positive 1/3rd charge, to yield an antiproton and an anti-neutron. The anti-proton would of course have an equal and opposite charge to the anti-electron (which has a formal name – the position). So things are equally as mysterious in the realm of the anti-world.
https://www.scientificexploration.org/forum/is-the-atom-an-example-of-cosmic-design-and-fine-tuning?fbclid=IwAR25Qb-8HnDFbuvaKqSfJBuRTJ0QahExap_tg93SwZnHljdZcgBJMYpTDy0
Question: How do you get 1/3rd or 2/3rds of an electric charge in any event? Of course one could just multiply by three and that does away with the fractions, but that doesn’t resolve the larger issues, like for that matter, what exactly is electric charge and how does it come to be?
Is the Universe Fine Tuned for Life?
The Right Atoms
In order for life to be possible, sufficient quantities of essential elements must be available – which means atoms of various sizes must be able to form. For that to occur, other delicate balances must exist among the constants of physics – the strong and weak nuclear forces, gravity, and nuclear ground state energies.*
The strong nuclear force is the force which governs he degree to which protons and neutrons “stick together” in atomic nuclei. If this force was weaker that it is, protons and neutrons would not stick together. In that case only one element would exist in the universe – hydrogen (the hydrogen atom has only one proton and no neutrons in its nuclei). If this force were too strong, however, protons and neutrons would have such an affinity for each other that not one would remain alone. In such a universe, there would be no hydrogen – only heavy elements. And life chemistry is impossible without hydrogen.
How delicate is the balance for the strong nuclear force? If it were just 2% weaker, or .3% stronger than it actually is, life would be impossible – and not just our form of life. We are talking about any form of life, at any time, anywhere in the universe.
There is also the weak nuclear force – the force that, among other things, governs the rate of radioactive decay. If this force were much stronger than it is, all matter in the universe would quickly be converted into “heavy” elements. On the other hand, if it were much weaker, then all matter in the universe would remain in the form of just the lightest elements. To have the elements that are essential for life chemistry – carbon, oxygen, nitrogen, phosphorus, for example – these forces must be delicately balanced.
The strength of gravity is responsible for determining how hot the nuclear furnaces in the cores of stars will burn. If the force of gravity were any stronger, then stars would be so hot tht they would burn up too quickly and too erratically for life to form. In addition, a planet that is capable of sustaining life (such as earth) must be supported by a start that is stable, and long burning. On the other hand, if the gravitational force weaker than it is, stars would never become hot enough to ignite nuclear fusion.*
The Right Nucleons
The universe is also fine tuned to the extent that there is just enough nucleons (protons and neutrons) to form the elements essential for life. After the “big bang”, all of the galaxies and stars that make up the universe today were form from left over nucleons from this initial singularity. Turns out that if the initial excess of nucleons over anti-nucleons were any smaller, there would not be enough matter for galaxies, stars and the heavy elements to form. If the excess were any greater, galaxies would form, but they would condense to the point that none of them would fragment to form stars and planets.*
The Right Electrons
Not must the universe have just the right number of nucleons – a precise number of electrons must also exist. Unless the number of electrons is equivalent to the number of protons to an accuracy of one part in 10(37) or better, electromagnetic forces in the universe would have so overcome gravitational forces that galaxies, stars, and planets never would have formed. *
https://evidencetobelieve.com/fine-tuning-of-the-universe-2/
ANIL ANANTHASWAMYIs the Universe Fine-Tuned for Life? MARCH 7, 2012
Take, for instance, the neutron. It is 1.00137841870 times heavier than the proton, which is what allows it to decay into a proton, electron and neutrino—a process that determined the relative abundances of hydrogen and helium after the big bang and gave us a universe dominated by hydrogen. If the neutron-to-proton mass ratio were even slightly different, we would be living in a very different universe: one, perhaps, with far too much helium, in which stars would have burned out too quickly for life to evolve, or one in which protons decayed into neutrons rather than the other way around, leaving the universe without atoms. So, in fact, we wouldn’t be living here at all—we wouldn’t exist.
https://www.pbs.org/wgbh/nova/article/is-the-universe-fine-tuned-for-life/
Dr. Walter L. Bradley: Is There Scientific Evidence for the Existence of God? How the Recent Discoveries Support a Designed Universe 20 August 2010
The Rest Mass of Subatomic Particles - Key to Universe Rich in Elemental Diversity
Scientists have been surprised to discover the extraordinary tuning of the masses of the elementary particles to each other and to the forces in nature. Stephen Hawking has noted that the difference in the rest mass of the neutron and the rest mass of the proton must be approximately equal to twice the mass of the electron. The mass-energy of the proton is 938.28 MeV and the mass-energy of the neutron is 939.57 MeV. The mass-energy of the electron is 0.51 MeV, or approximately half of the difference in neutron and proton mass-energies, just as Hawking indicated it must be. If the mass-energy of the proton plus the mass-energy of the electron were not slightly smaller than the mass-energy of the neutron, then electrons would combine with protons to form neutrons, with all atomic structure collapsing, leaving an inhospitable world composed only of neutrons.
On the other hand, if this difference were larger, then neutrons would all decay into protons and electrons, leaving a world of pure hydrogen, since neutrons are necessary for protons to combine to build heavier nuclei and the associated elements. As things stand, the neutron is just heavy enough to ensure that the Big Bang would yield one neutron to every seven protons, allowing for an abundant supply of hydrogen for star fuel and enough neutrons to build up the heavier elements in the universe.
The Nuclear Weak Coupling Force - Tuned to Give an Ideal Balance Between Hydrogen (as Fuel for Sun) and Heavier Elements as Building Blocks for Life
The weak force governs certain interactions at the subatomic or nuclear level. If the weak force coupling constant were slightly larger, neutrons would decay more rapidly, reducing the production of deuterons, and thus of helium and elements with heavier nuclei. On the other hand, if the weak force coupling constant were slightly weaker, the Big Bang would have burned almost all of the hydrogen into helium, with the ultimate outcome being a universe with little or no hydrogen and many heavier elements instead. This would leave no long-lived stars and no hydrogen-containing compounds, especially water. In 1991, Breuer noted that the appropriate mix of hydrogen and helium to provide hydrogen-containing compounds, long-term stars, and heavier elements is approximately 75 percent hydrogen and 25 percent helium, which is just what we find in our universe.{32}
This is obviously only an illustrative--but not exhaustive--list of cosmic "coincidences." Clearly, the four forces in nature and the universal constants must be very carefully calibrated or scaled to provide a universe that satisfies the key requirements for life that we enumerated in our initial "needs statement": for example, elemental diversity, an abundance of oxygen and carbon, and a long-term energy source (our sun) that is precisely matched to the bonding strength of organic molecules, with minimal absorption by water or Earth's terrestrial atmosphere.
John Wheeler, formerly Professor of Physics at Princeton, in discussing these observations asks:
Is man an unimportant bit of dust on an unimportant planet in an unimportant galaxy somewhere in the vastness of space? No! The necessity to produce life lies at the center of the universe's whole machinery and design.....Slight variations in physical laws such as gravity or electromagnetism would make life impossible
https://web.archive.org/web/20110805203154/http://www.leaderu.com/real/ri9403/evidence.html#ref21
John D. Barrow The Anthropic Cosmological Principle 1986 page 295
Remarkably, it transpires that the gross properties of all atomic and molecular systems are controlled by only two dimension-less physical parameters the fine structure constant,(1/137: It is the "coupling constant" or measure of the strength of the electromagnetic force that governs how electrically charged elementary particles (e.g., electron, muon) and light (photons) interact. ), and the electron to proton mass ratio, where the protons is 1836 heavier than the electron. No physical theory has yet been able to explain the numerical values of these two pure numbers that determine, to within an order of magnitude or so, all the qualitative features of bound states of the electromagnetic interaction. The difference between simple order of magnitude estimates for physical parameters involving powers of alpha and beta and the exact calculations of the quantum theory (which agree remarkably with observation) consists only of geometrical factors like 4pi/3 and integral quantum numbers. We shall see also that the particular values of alpha and beta are responsible for various 'coincidences' of Nature on which the possibility of our own existence is contingent. The first physicist to stress the all-encompassing role of alpha and beta in determining the inevitable structure of atomic systems seems to have been Max Born. In 1935 he delivered a lecture to the Indian Scientific Association entitled 'The Mysterious Number 137' which highlighted the importance of the fine structure constant in atomic physics. Electrons moving in orbits of quantized angular momentum about a central nucleus. The centripedal force required to sustain the rotational motion is supplied by the electromagnetic attraction between the positively charged nucleus and the negatively charged electron). The hydrogen atom can be modelled by two particles: a nuclear proton bound by the Coulomb force to an orbiting electron.
Our universe is determined by the fact that only the choice mN / mc = 1837 guarantees that there are long chain molecules of the right kinds and size as to make biological phenomena possible. It could be for instance that the slightest variation in these parameters would change critically the size and length of the rings in the DNA helix as to invalidate its typical way of replicating itself. In this sense we could say that mN / me = 1837 just because we are here.
The Exclusion Principle plays a key role in Nature. Aside from guaranteeing the stability of matter and the 'large' size of atomic and molecular structures, it creates the shell structure of atomic electrons. These electronic heirarchies are responsible for the existence and enormous diversity of chemical properties. One could imagine a world in which the Exclusion Principle did not exist or one in which electrons were bosons but it would be a world of compact, superdense bodies with little scope for complex structures or living organisms and any two molecules that encountered one another would release huge quantities of binding energy.
There is an aspect of physics that is essential for the existence and stability of atomic systems—quantization. In 1913 Niels Bohr proposed the radical revision of the naive atomic models that imagined the electrons to orbit a nucleus in the manner of a mini solar system. The quantization principle he used restricted the energy of the orbital electrons to certain discrete values: multiples of a universal energy quantum fixed by Planck's constant. In the non-quantum atom, electrons can possess all possible energies. They can reside at any orbital radius so long as their velocity is sufficient to establish an equilibrium between centrifugal and Coulomb forces. All atoms would be different under these circumstances and, worse still, the continuous buffeting of electrons by photons and other particles would cause a steady change in electron orbit (and hence chemistry). The quantum principle avoids this: if one electron is added to a proton there is only one orbital radius available to it in quantum theory and consequently all hydrogen atoms are identical. This could not be the case in a non-quantum theory. Likewise, tiny environmental perturbations do not upset the structure of the atom because an entire quantum of energy must be added before the electron orbital is altered. Thus, despite its traditional reputation as the harbinger of chance and indeterminism, quantum theory is the basis for the fidelity and large-scale stability of Nature.
https://3lib.net/book/1131892/9a639b

1. https://www.scientificexploration.org/forum/is-the-atom-an-example-of-cosmic-design-and-fine-tuning?fbclid=IwAR25Qb-8HnDFbuvaKqSfJBuRTJ0QahExap_tg93SwZnHljdZcgBJMYpTDy0
3. https://aeon.co/essays/cosmopsychism-explains-why-the-universe-is-fine-tuned-for-life
4. http://www.detectingdesign.com/detectingdesign.html
More links:
https://www.tapatalk.com/groups/vixra/electric-charge-an-example-of-fine-tuning-t121.html
Thayer Watkins What holds the nucleus of an atom together?
https://www.sjsu.edu/faculty/watkins/nucleus.htm
https://reasonandscience.catsboard.com/t2763-fine-tuning-of-atoms
Planck: As a man who has devoted his whole life to the most clear-headed science, to the study of matter, I can tell you as a result of my research about atoms this much: There is no matter as such. All matter originates and exists only by virtue of a force that brings the particle of an atom to vibration and holds this most minute solar system of the atom together.
Leonard Susskind The Cosmic Landscape: String Theory and the Illusion of Intelligent Design 2006, page 176
Complex atomic nuclei are not likely to result from random collisions of particles, even in the early hot universe.
https://3lib.net/book/2472017/1d5be1
Why Fine-tuning Seems Designed
Free (three quarks) positive protons and electrons could just join up, and given their opposite electric charges. It could also have been that an equal number of electrons and protons had been formed post-Big Bang then the cosmos would be a soup on neutrons and perhaps neutrinos, but that would then be pretty much that. How is it that an electron, protons, and neutrons can arrange themselves just so as to eventually produce macro stuff, including us? How to go from particle physics to chemistry?
https://www.closertotruth.com/series/why-fine-tuning-seems-designed
Man Ho Chan The fine-tuned universe and the existence of God 5-24-2017
In the period called Big Bang Nucleosynthesis, hydrogen, helium, and a tiny amount of lithium were formed in the first three minutes since Big Bang.
https://philarchive.org/archive/CHATFU-2
A More Finely Tuned Universe
Why do fundamental particles possess the specific values of mass that they have? Presently, physicists have no explanation for this and similar questions. If the masses of particles or the values of fundamental constants were much different from what physicists have measured, carbon-based intelligent beings might not be here to measure them, because fundamental particles might not assemble into stable atoms, atoms might not form rocky planets and dying stars might not produce the chemical elements we find in our bodies.
https://www.insidescience.org/news/more-finely-tuned-universe
The mass of the proton and the neutron as an example of fine-tuning for life
Protons have a mass of 938.27 MeV. Neutrons have a mass of 939.56 MeV. The difference between them is small: a neutron is about 1.29 MeV heavier than a proton. There is no obvious reason why protons and neutrons should have just these masses, but if they were even slightly different, we wouldn’t be here.
http://www.focus.org.uk/proton_neutron.php
Paul Davies Yes, the universe looks like a fix. But that doesn't mean that a god fixed it 26 Jun 2007
For example, neutrons are just a tad heavier than protons. If it were the other way around, atoms couldn't exist, because all the protons in the universe would have decayed into neutrons shortly after the big bang. No protons, then no atomic nuclei, and no atoms. No atoms, no chemistry, no life. Like Baby Bear's porridge in the story of Goldilocks, the universe seems to be just right for life. So what's going on?
https://www.theguardian.com/commentisfree/2007/jun/26/spaceexploration.comment
Nigel Warburton: Is the Universe a conscious mind? August 2019.
The strong nuclear force (the force that binds together the elements in the nucleus of an atom) has a value of 0.007. If that value had been 0.006 or less, the Universe would have contained nothing but hydrogen. If it had been 0.008 or higher, the hydrogen would have fused to make heavier elements. In either case, any kind of chemical complexity would have been physically impossible. And without chemical complexity there can be no life.
https://aeon.co/essays/cosmopsychism-explains-why-the-universe-is-fine-tuned-for-life
Stephen C. Meyer: The return of the God hypothesis, page 185
For instance, to make life possible, the masses of the fundamental particles must meet an exacting combination of constraints. There is the fine-tuning of the masses of the two naturally occurring quarks, the up quark and down quark, in relation to the range of expected possible values. The fine-tuning of the masses of those quarks are considerable: 1 part in 10^21 . In addition, the difference in masses between the quarks cannot exceed one megaelectron volt, the equivalent of one-thousandth of 1 percent of the mass of the largest known quark, without producing either a neutron-only or a proton-only universe, both exceedingly boring and incompatible with life and even with simple chemistry. Equally problematic, increasing the mass of electrons by a factor of 2.5 would result in all the protons in all the atoms capturing all the orbiting electrons and turning them into neutrons. In that case, neither atoms, nor chemistry, nor life could exist. What’s more, the mass of the electron has to be less than the difference between the masses of the neutron and the proton and that difference represents fine-tuning of roughly 1 part in a 1000. In addition, if the mass of a special particle known as a neutrino were increased by a factor of 10, stars and galaxies would never have formed. The mass of a neutrino is about one-millionth that of an electron, so the allowable change is minuscule compared to its possible range. The combination of all these precisely fine-tuned conditions—including the fine-tuning of the laws and constants of physics, the initial arrangement of matter and energy, and various other contingent features of the universe —presents a remarkably restrictive set of criteria. These requirements for the existence of life, again defying our ability to describe their extreme improbability, have seemed to many physicists to require some explanation.
Strikingly, the masses of “up quarks” and “down quarks,” the constituent parts of protons and neutrons, need to have precise values to allow for the production of the elements, including carbon, essential for a life-friendly universe. Indeed, the masses of these quarks must have simultaneously nine different conditions for the right nuclear reactions to have occurred in the early universe. The “right” reactions are ones that would produce the right elements (such as carbon and oxygen) in the right abundance necessary for life. The fine-tuning of the masses of these two naturally occurring quarks in relation to the range of expected possible values for the mass of any fundamental particle is exquisite. Physicists conceive of that range as extending between a mass of zero and the so-called Planck mass, an important unit of measure in quantum physics. But the value of the “up quark” must have a precise mass of between zero and just one billion trillionth of the Planck mass, corresponding to a fine-tuning of roughly 1 part in 10^21. The mass of the “down quark” must have a similarly precise fine-tuning.
https://3lib.net/book/15644088/9c418b
Brad Lemley Why is There Life? November 01, 2000
Of Rees's six numbers, two relate to basic forces, two determine the size and large-scale texture of the universe, and two fix the properties of space itself.
 , the .007 figure, which describes the strength of the force that binds atomic nuclei together and determines how all atoms on Earth are made.
, the .007 figure, which describes the strength of the force that binds atomic nuclei together and determines how all atoms on Earth are made.https://web.archive.org/web/20140722210250/http://discovermagazine.com/2000/nov/cover/
Consider the ingredients you need:
The strong nuclear force particles;
The weak nuclear force particles;
The electromagnetic force particles;
Up-quarks and down-quarks
Luke A. Barnes The Fine-Tuning of the Universe for Intelligent Life June 11, 2012
https://arxiv.org/pdf/1112.4647.pdf

The life-permitting region (shown in white) in the (α, β) (left) and (α, αs) (right) parameter space, with other constants held at their values in our universe. Our universe is shown as a blue cross.
1. For hydrogen to exist — to power stars and form water and organic compounds — we must have mass electron < mass neutron − mass proton. Otherwise, the electron will be captured by the proton to form a neutron
2. For stable atoms, we need the radius of the electron orbit to be significantly larger than the nuclear radius.
3. We require that the typical energy of chemical reactions is much smaller than the typical energy of nuclear reactions. This ensures that the atomic constituents of chemical species maintain their identity in chemical reactions.
4. Unless β 1/4 << 1, stable ordered molecular structures (like chromosomes) are not stable. The atoms will too easily stray from their place in the lattice and the substance will spontaneously melt
5. The stability of the proton requires α . ( down quark md − up quark mu)/141 MeV, so that the extra electromagnetic mass-energy of a proton relative to a neutron is more than counter-balanced by the bare quark masses
6. Unless α << 1, the electrons in atoms and molecules are unstable to the creation of pairs. The limit shown is α < 0.2..
7. As in Equation 10, stars will not be stable unless β & α 2/100.
8. Unless αs/αs,0 . 1.003 + 0.031α/α0, the diproton has a bound state, which affects stellar burning and big bang nucleosynthesis.
9. Unless αs . 0.3α 1/2 , carbon and all larger elements are unstable
10. Unless αs/αs,0 & 0.91, the deuteron is unstable and the main nuclear reaction in stars (pp) does not proceed. A similar effect would be achieved35 unless md − mu + me < 3.4 MeV which makes the pp reaction energetically unfavourable. This region is numerically very similar to Region 1 in the left plot.

Parameter space of the masses of the up and down quark. The axes span ∼ 60 orders of magnitude.

The figure zooms in on a region of parameter space, showing boundaries of 9 independent life-permitting criteria
Each point on the graph corresponds to possible values for the masses of the up and down quarks (Mu , Md ). The masses are scaled by the Planck mass, Mpl , since Planck units are the most natural in cosmology. Each of the nine lines on the graph separate the regions corresponding to life-permitting and non-life-permitting universes for a specific criterion such as allowing for the existence of stable protons. In a universe capable of supporting life, all nine criteria must be met simultaneously, so the life-permitting region is the intersection of all nine life-permitting regions, marked in gray. That area corresponds to a minuscule proportion of all plausible values.
1. Above the blue line, there is only one stable element, which consists of a single particle ∆++. This element has the chemistry of helium, an inert, monatomic gas (above 4 K) with no known stable chemical compounds.
2. Above this red line, the deuteron is strongly unstable, decaying via the strong force. The first step in stellar nucleosynthesis in hydrogen-burning stars would fail.
3. Above the green curve, neutrons in nuclei decay, so that hydrogen is the only stable element.
4. Below this red curve, the diproton is stable15. Two protons can fuse to helium-2 via a very fast electromagnetic reaction, rather than the much slower, weak nuclear pp-chain.
5. Above this red line, the production of deuterium in stars absorbs energy rather than releasing it. Also, the deuterium is unstable to weak decay.
6. Below this red line, a proton in a nucleus can capture an orbiting electron and become a neutron. Thus, atoms are unstable.
7. Below the orange curve, isolated protons are unstable, leaving no hydrogen left over from the early universe to power long-lived stars and play a crucial role in organic chemistry.
8. Below this green curve, protons in nuclei decay, so that any atoms that formed would disintegrate into a cloud of neutrons.
9. Below this blue line, the only stable element consists of a single particle ∆−, which can combine with a positron to produce an element with the chemistry of hydrogen. A handful of chemical reactions are possible, with their most complex product being (an analogue of) H2.
Why Cosmic Fine-tuning Demands Explanation
Fine-tuning is necessary in order to form the protons and the neutrons; and of course electrons. And all of these bits and pieces have to mesh like a clock - or even a watch. You can't just assemble these bits and pieces in just any old way and expect things to work out. These processes have nothing to do with the emergence of atoms from the fundamental particles, forces and fields that form the bedrock of the atomic realm. It's all governed by the laws, principles and relationships of quantum mechanics, all of which had to come from somewhere or from someone or from something. For example, there's the Pauli Exclusion Principle which requires that not more than two electrons can occupy exactly the same 'orbit' and then only if they have differing quantum values, like spin-up and spin-down. This prevents all electrons being together like commuters crowded into a Tokyo subway carriage at rush hour. Then there's the energy levels that electrons are allowed to have while 'orbiting' around the nucleus. That can be in this level or that level or the next level but not at in-between levels. This prevents electrons from spiraling down and impacting the positively charged nucleus which, being negatively charged, electrons would otherwise want to do. Design and fine-tuning by any other name still appears to be design and fine-tuning.
Consider further that the partial (fractional) electrical charges on the up-quarks and the down-quarks had to arrange themselves just-so such that a proton is a unity of positive electric charge and a neutron is a unity of electric charge neutrality. Then, the positive electric charge on the proton has to balance just so (to an infinite number of decimal places, at least as close to infinity as one can actually measure and calculate) the negative electric charge of the electron. How can the electric charge of the electron be EXACTLY equal and opposite to that of the proton when they otherwise share nothing in common?
If hydrogen atoms couldn't link up with oxygen atoms there could be no water and no water implies no life could arise. The same applies to dozens of other essential molecules that life requires.
On the other hand, why isn't there a universal solvent or acid that disassembles molecules? Everything can be stored in at least one kind of container. That too seems to be essential for life as is the requirement that some things need to be in solution some of the time. An atom is literally 99.99% empty space. And the part which supposedly is matter, might be just pure energy. And as such, the whole universe is simply held together by Gods power and his word: information.
Who fine-tuned the atomic parameters? Why fine-tuning? Well fine-tuning implies that something(s) exist against all the odds. Fine-tuning requires a tuner. The universe started with the design and fine-tuned engineering of the humble atom
https://www.closertotruth.com/series/why-cosmic-fine-tuning-demands-explanation
Sean D. Pitman, MD: The Detection of Intelligent Design Simple Stuff Mindless Nature Cannot Do
The proton mass is 1836 times that of an electron. If this ratio were off even slightly, molecules would not form properly. It is also interesting to note that although protons are very different in size and mass, the charges are exactly the same in opposite degree. If this were not the case, again, molecules necessary to support complex life could not form. The same is true of the electromagnetic coupling constant between protons and electrons - it is very precisely balanced to support complex life.
http://www.detectingdesign.com/detectingdesign.html
John Prytz: Is The Atom An Example Of Cosmic Design And Fine-Tuning? Feb.7.2016
The electron, the proton, and the quark are all entities within the realm of particle hence quantum physics. All three carry an electrical charge. All three have mass. After those observations, things get interesting, or messy, depending on your point of view. The electric charge of the proton is exactly equal and the opposite of the electric charge on the electron, despite the proton being nearly 2000 times more massive. There’s no set-in-concrete theoretical reason why this should be so. It cannot be determined from first principles, only experimentally measured. An electron has a negative charge exactly equal and opposite to that of a proton. Note: the charge is exactly equal, even though the proton has a far greater mass than the electron (some 2000 times heavier in fact, not that there has to be of necessity any relationship between mass and charge).
Now that’s strange since the electron is a fundamental particle but the positively charged proton is a composite particle, made up of a trio of quarks (as is the neutron with no net charge). The proton has two quarks each with a positive 2/3rds charge (up quark) and one quark with a negative 1/3rd charge (down quark) for an overall balance of one positive charge. (The neutron, on the other hand, has one up quark with a positive 2/3rds charge and two down quarks each with a negative 1/3rd charge, for an overall balance of zero charge – neither positive nor negative.)
Now you might suggest that an electron might be a fusion of a trio of down quarks, each with a negative 1/3rd charge, except the electron, again, isn’t a composite particle, and the mass is all wrong for that scenario. If an electron were a composite of a trio of down quarks, each with a minus 1/3rd charge, the electron would be thirty times more massive than it is – not something particle physicists would fail to take notice of.
Further, the force particle that governs the electron is the photon; that which governs the quarks inside the proton and the neutron is the gluon, which further differentiates the two things – quarks and electrons. In any event, if you could have a composite particle of a trio of negative 1/3rd down quarks, if that were the case, and it is the case, and it’s called the Negative Delta, you’d also need a composite particle that’s the fusion of a trio of positive 2/3rds up quarks for an overall charge of plus two. To the best of my knowledge, there is only one such critter in the particle zoo and it’s called the Doubly Positive Delta. I’m sure you’ve never heard of these Delta particles, which goes to show how much bearing or impact they have on life, the Universe, and everything.
In case you were wondering, there would be an anti-quark of minus 2/3rds charge, and an anti-quark of a positive 1/3rd charge, to yield an antiproton and an anti-neutron. The anti-proton would of course have an equal and opposite charge to the anti-electron (which has a formal name – the position). So things are equally as mysterious in the realm of the anti-world.
https://www.scientificexploration.org/forum/is-the-atom-an-example-of-cosmic-design-and-fine-tuning?fbclid=IwAR25Qb-8HnDFbuvaKqSfJBuRTJ0QahExap_tg93SwZnHljdZcgBJMYpTDy0
Question: How do you get 1/3rd or 2/3rds of an electric charge in any event? Of course one could just multiply by three and that does away with the fractions, but that doesn’t resolve the larger issues, like for that matter, what exactly is electric charge and how does it come to be?
Is the Universe Fine Tuned for Life?
The Right Atoms
In order for life to be possible, sufficient quantities of essential elements must be available – which means atoms of various sizes must be able to form. For that to occur, other delicate balances must exist among the constants of physics – the strong and weak nuclear forces, gravity, and nuclear ground state energies.*
The strong nuclear force is the force which governs he degree to which protons and neutrons “stick together” in atomic nuclei. If this force was weaker that it is, protons and neutrons would not stick together. In that case only one element would exist in the universe – hydrogen (the hydrogen atom has only one proton and no neutrons in its nuclei). If this force were too strong, however, protons and neutrons would have such an affinity for each other that not one would remain alone. In such a universe, there would be no hydrogen – only heavy elements. And life chemistry is impossible without hydrogen.
How delicate is the balance for the strong nuclear force? If it were just 2% weaker, or .3% stronger than it actually is, life would be impossible – and not just our form of life. We are talking about any form of life, at any time, anywhere in the universe.
There is also the weak nuclear force – the force that, among other things, governs the rate of radioactive decay. If this force were much stronger than it is, all matter in the universe would quickly be converted into “heavy” elements. On the other hand, if it were much weaker, then all matter in the universe would remain in the form of just the lightest elements. To have the elements that are essential for life chemistry – carbon, oxygen, nitrogen, phosphorus, for example – these forces must be delicately balanced.
The strength of gravity is responsible for determining how hot the nuclear furnaces in the cores of stars will burn. If the force of gravity were any stronger, then stars would be so hot tht they would burn up too quickly and too erratically for life to form. In addition, a planet that is capable of sustaining life (such as earth) must be supported by a start that is stable, and long burning. On the other hand, if the gravitational force weaker than it is, stars would never become hot enough to ignite nuclear fusion.*
The Right Nucleons
The universe is also fine tuned to the extent that there is just enough nucleons (protons and neutrons) to form the elements essential for life. After the “big bang”, all of the galaxies and stars that make up the universe today were form from left over nucleons from this initial singularity. Turns out that if the initial excess of nucleons over anti-nucleons were any smaller, there would not be enough matter for galaxies, stars and the heavy elements to form. If the excess were any greater, galaxies would form, but they would condense to the point that none of them would fragment to form stars and planets.*
The Right Electrons
Not must the universe have just the right number of nucleons – a precise number of electrons must also exist. Unless the number of electrons is equivalent to the number of protons to an accuracy of one part in 10(37) or better, electromagnetic forces in the universe would have so overcome gravitational forces that galaxies, stars, and planets never would have formed. *
https://evidencetobelieve.com/fine-tuning-of-the-universe-2/
ANIL ANANTHASWAMYIs the Universe Fine-Tuned for Life? MARCH 7, 2012
Take, for instance, the neutron. It is 1.00137841870 times heavier than the proton, which is what allows it to decay into a proton, electron and neutrino—a process that determined the relative abundances of hydrogen and helium after the big bang and gave us a universe dominated by hydrogen. If the neutron-to-proton mass ratio were even slightly different, we would be living in a very different universe: one, perhaps, with far too much helium, in which stars would have burned out too quickly for life to evolve, or one in which protons decayed into neutrons rather than the other way around, leaving the universe without atoms. So, in fact, we wouldn’t be living here at all—we wouldn’t exist.
https://www.pbs.org/wgbh/nova/article/is-the-universe-fine-tuned-for-life/
Dr. Walter L. Bradley: Is There Scientific Evidence for the Existence of God? How the Recent Discoveries Support a Designed Universe 20 August 2010
The Rest Mass of Subatomic Particles - Key to Universe Rich in Elemental Diversity
Scientists have been surprised to discover the extraordinary tuning of the masses of the elementary particles to each other and to the forces in nature. Stephen Hawking has noted that the difference in the rest mass of the neutron and the rest mass of the proton must be approximately equal to twice the mass of the electron. The mass-energy of the proton is 938.28 MeV and the mass-energy of the neutron is 939.57 MeV. The mass-energy of the electron is 0.51 MeV, or approximately half of the difference in neutron and proton mass-energies, just as Hawking indicated it must be. If the mass-energy of the proton plus the mass-energy of the electron were not slightly smaller than the mass-energy of the neutron, then electrons would combine with protons to form neutrons, with all atomic structure collapsing, leaving an inhospitable world composed only of neutrons.
On the other hand, if this difference were larger, then neutrons would all decay into protons and electrons, leaving a world of pure hydrogen, since neutrons are necessary for protons to combine to build heavier nuclei and the associated elements. As things stand, the neutron is just heavy enough to ensure that the Big Bang would yield one neutron to every seven protons, allowing for an abundant supply of hydrogen for star fuel and enough neutrons to build up the heavier elements in the universe.
The Nuclear Weak Coupling Force - Tuned to Give an Ideal Balance Between Hydrogen (as Fuel for Sun) and Heavier Elements as Building Blocks for Life
The weak force governs certain interactions at the subatomic or nuclear level. If the weak force coupling constant were slightly larger, neutrons would decay more rapidly, reducing the production of deuterons, and thus of helium and elements with heavier nuclei. On the other hand, if the weak force coupling constant were slightly weaker, the Big Bang would have burned almost all of the hydrogen into helium, with the ultimate outcome being a universe with little or no hydrogen and many heavier elements instead. This would leave no long-lived stars and no hydrogen-containing compounds, especially water. In 1991, Breuer noted that the appropriate mix of hydrogen and helium to provide hydrogen-containing compounds, long-term stars, and heavier elements is approximately 75 percent hydrogen and 25 percent helium, which is just what we find in our universe.{32}
This is obviously only an illustrative--but not exhaustive--list of cosmic "coincidences." Clearly, the four forces in nature and the universal constants must be very carefully calibrated or scaled to provide a universe that satisfies the key requirements for life that we enumerated in our initial "needs statement": for example, elemental diversity, an abundance of oxygen and carbon, and a long-term energy source (our sun) that is precisely matched to the bonding strength of organic molecules, with minimal absorption by water or Earth's terrestrial atmosphere.
John Wheeler, formerly Professor of Physics at Princeton, in discussing these observations asks:
Is man an unimportant bit of dust on an unimportant planet in an unimportant galaxy somewhere in the vastness of space? No! The necessity to produce life lies at the center of the universe's whole machinery and design.....Slight variations in physical laws such as gravity or electromagnetism would make life impossible
https://web.archive.org/web/20110805203154/http://www.leaderu.com/real/ri9403/evidence.html#ref21
John D. Barrow The Anthropic Cosmological Principle 1986 page 295
Remarkably, it transpires that the gross properties of all atomic and molecular systems are controlled by only two dimension-less physical parameters the fine structure constant,(1/137: It is the "coupling constant" or measure of the strength of the electromagnetic force that governs how electrically charged elementary particles (e.g., electron, muon) and light (photons) interact. ), and the electron to proton mass ratio, where the protons is 1836 heavier than the electron. No physical theory has yet been able to explain the numerical values of these two pure numbers that determine, to within an order of magnitude or so, all the qualitative features of bound states of the electromagnetic interaction. The difference between simple order of magnitude estimates for physical parameters involving powers of alpha and beta and the exact calculations of the quantum theory (which agree remarkably with observation) consists only of geometrical factors like 4pi/3 and integral quantum numbers. We shall see also that the particular values of alpha and beta are responsible for various 'coincidences' of Nature on which the possibility of our own existence is contingent. The first physicist to stress the all-encompassing role of alpha and beta in determining the inevitable structure of atomic systems seems to have been Max Born. In 1935 he delivered a lecture to the Indian Scientific Association entitled 'The Mysterious Number 137' which highlighted the importance of the fine structure constant in atomic physics. Electrons moving in orbits of quantized angular momentum about a central nucleus. The centripedal force required to sustain the rotational motion is supplied by the electromagnetic attraction between the positively charged nucleus and the negatively charged electron). The hydrogen atom can be modelled by two particles: a nuclear proton bound by the Coulomb force to an orbiting electron.
Our universe is determined by the fact that only the choice mN / mc = 1837 guarantees that there are long chain molecules of the right kinds and size as to make biological phenomena possible. It could be for instance that the slightest variation in these parameters would change critically the size and length of the rings in the DNA helix as to invalidate its typical way of replicating itself. In this sense we could say that mN / me = 1837 just because we are here.
The Exclusion Principle plays a key role in Nature. Aside from guaranteeing the stability of matter and the 'large' size of atomic and molecular structures, it creates the shell structure of atomic electrons. These electronic heirarchies are responsible for the existence and enormous diversity of chemical properties. One could imagine a world in which the Exclusion Principle did not exist or one in which electrons were bosons but it would be a world of compact, superdense bodies with little scope for complex structures or living organisms and any two molecules that encountered one another would release huge quantities of binding energy.
There is an aspect of physics that is essential for the existence and stability of atomic systems—quantization. In 1913 Niels Bohr proposed the radical revision of the naive atomic models that imagined the electrons to orbit a nucleus in the manner of a mini solar system. The quantization principle he used restricted the energy of the orbital electrons to certain discrete values: multiples of a universal energy quantum fixed by Planck's constant. In the non-quantum atom, electrons can possess all possible energies. They can reside at any orbital radius so long as their velocity is sufficient to establish an equilibrium between centrifugal and Coulomb forces. All atoms would be different under these circumstances and, worse still, the continuous buffeting of electrons by photons and other particles would cause a steady change in electron orbit (and hence chemistry). The quantum principle avoids this: if one electron is added to a proton there is only one orbital radius available to it in quantum theory and consequently all hydrogen atoms are identical. This could not be the case in a non-quantum theory. Likewise, tiny environmental perturbations do not upset the structure of the atom because an entire quantum of energy must be added before the electron orbital is altered. Thus, despite its traditional reputation as the harbinger of chance and indeterminism, quantum theory is the basis for the fidelity and large-scale stability of Nature.
https://3lib.net/book/1131892/9a639b

1. https://www.scientificexploration.org/forum/is-the-atom-an-example-of-cosmic-design-and-fine-tuning?fbclid=IwAR25Qb-8HnDFbuvaKqSfJBuRTJ0QahExap_tg93SwZnHljdZcgBJMYpTDy0
3. https://aeon.co/essays/cosmopsychism-explains-why-the-universe-is-fine-tuned-for-life
4. http://www.detectingdesign.com/detectingdesign.html
More links:
https://www.tapatalk.com/groups/vixra/electric-charge-an-example-of-fine-tuning-t121.html
Thayer Watkins What holds the nucleus of an atom together?
https://www.sjsu.edu/faculty/watkins/nucleus.htm
Last edited by Otangelo on Mon Aug 01, 2022 5:50 pm; edited 31 times in total