Deuterium, and fine-tuning
JAMES STEIN Planck's Constant: The Number That Rules Technology, Reality, and Life OCTOBER 24, 2011
More fundamentally, the discovery of Planck’s constant advanced the realization that, when we probe the deepest levels of the structure of matter, we are no longer looking at “things” in the conventional meaning of the word. A “thing”—like a moving car—has a definite location and velocity; a car may be 30 miles south of Los Angeles heading east at 40 miles per hour. The concepts of location, velocity, and even existence itself blur at the atomic and subatomic level. Electrons do not exist in the sense that cars do, they are, bizarrely, everywhere at once, but much more likely to be in some places than in others. Reconciling the probabilistic subatomic world with the macroscopic everyday world is one of the great unsolved problems in physics. The fundamental nuclear reaction eventually leading to the explosion of a supernova is the fusion of four hydrogen atoms to produce a single atom of helium. In the process, approximately 0.7% of the mass is converted to energy via E=mc 2 .
This 0.7% is known as the efficiency of hydrogen fusion, and our understanding of it is one of the consequences of Planck’s investigations. It requires a great deal of heat to enable hydrogen to fuse to helium, and the hydrogen atoms in the sun are moving at different speeds, much like cars on a freeway move at different speeds. The slower-moving hydrogen atoms just bounce off each other; they are insufficiently hot to fuse. Higher speeds, though, mean higher temperatures, and there is a small fraction of hydrogen atoms moving at sufficiently high speeds to fuse to helium.
The 0.7% efficiency of hydrogen fusion is what is sometimes referred to as a “Goldilocks number.” Like the porridge that Goldilocks eventually ate, which was neither too hot nor too cold, but just right, the 0.7% efficiency of hydrogen fusion is “just right” to permit the emergence of life as we know it. The process of hydrogen fusion is an intricate high-speed, high-temperature ballet. The first step of this reaction produces deuterium, an isotope of hydrogen whose nucleus consists of one proton and one neutron. In this process, two protons slam into one another, causing one of the protons to shed its electrical charge and metamorphose into a neutron. If the efficiency of hydrogen fusion were as low as 0.6%, the neutron and proton would not bond to each other to form a deuterium atom. In this case, we’d still have stars—huge glowing balls of hydrogen—but no star stuff would ever form because the porridge would be too cold to create helium, the first step on the road to creating the elements necessary for life.
On the other hand, if hydrogen fusion had an efficiency of 0.8%, it would be much too easy for helium to form. The hydrogen in the stars would become helium so quickly that there wouldn’t be much hydrogen left to form the molecule most essential for life—water. Starstuff would be produced, but without water life as we know it would not exist. Maybe something else would take the place of water, and maybe life could evolve—but not ours.
Planck’s quantization of energy was an essential step on the road to the theory of quantum mechanics, which is critical to our understanding of stellar evolution. Science hasn’t filled in all the pieces of the puzzle of how life actually evolved, but quantum mechanics did begin to answer the question of how the pieces got there in the first place,
https://www.pbs.org/wgbh/nova/article/plancks-constant/
Leonard Susskind The Cosmic Landscape: String Theory and the Illusion of Intelligent Design 2006, page 178
The chain of nuclear reactions in stars that starts with the lightest elements and leads to iron is complicated. A couple of examples will illustrate the point. The most familiar example is the fusion reaction that begins with hydrogen and produces helium. Here is where the weak interactions (diagrams with W- and Z-bosons) come in. The first step is the collision of two protons. Many things can happen when two protons collide, but if you know the Feynman diagrams for the Standard Model, you can find one that ends up with a proton, a neutron, a positron, and a neutrino. The positron finds a wandering electron in the star, and together they self-destruct into photons that eventually become the star’s thermal energy (heat). The neutrino just zips away and disappears with almost the speed of light. That leaves one sticky proton and one sticky neutron that sticks together to form an isotope of hydrogen called deuterium. Next, a third proton strikes the deuterium nucleus and sticks to it. The nucleus with two protons and a neutron is a form of helium called helium-three (3He), but it’s not the stable kind of helium that we use to fill balloons. That stuff is called helium-four (4He). The story continues: two 3He nuclei collide. All together that means four protons and two neutrons. But they don’t all stick together. Two of the protons fly off and leave a nucleus with two protons and two neutrons. That’s an ordinary 4He nucleus. You don’t need to remember all that. Very few physicists do.
https://3lib.net/book/2472017/1d5be1
Fred C. Adams On the Habitability of Universes without Stable Deuterium 30 Mar 2017
In both stars and in the early universe, the production of deuterium is the first step on the way to producing heavier nuclei. If the strong force were slightly weaker, then deuterium would not be stable, and many authors have noted that nuclesynthesis would be compromised so that helium production could not proceed through standard reaction chains. If the strong force were sufficiently weaker, then deuterium would not have a bound state. In a universe with no deuterium, the usual stepping stone on the pathway to heavy elements would not be available. Many authors have speculated that the absence of stable deuterium would lead to a universe with no heavy elements at all, and hence a lifeless universe
https://arxiv.org/pdf/1612.04741.pdf
John D. Barrow The Anthropic Cosmological Principle 1986 page 295
The existence of deuterium and the non-existence of the diproton therefore hinge precariously on the precise strength of the nuclear force. If the strong interaction were a little stronger the diproton would be a stable bound state with catastrophic consequences—all the hydrogen in the Universe would have been burnt to He 2 during the early stages of the Big Bang and no hydrogen compounds or long-lived stable stars would exist today. If the di-proton existed we would not! Also, if the nuclear force was a little weaker the deuteron would be unbound with other adverse consequences for the nucleosynthesis of biological elements because a key link in the chain of nucleosynthesis would be removed
https://3lib.net/book/1131892/9a639b
LUKE A. BARNES: The Cosmic Revolutionary’s Handbook (Or: How to Beat the Big Bang) 2020
An important principle is at work here: if you want to make something in the universe, whether a proton or a lead nucleus, you’ll need to wait until things cool off enough for it to survive the ambient temperature. Our focus here is on the elements, so we ask: when is the universe cool enough for nuclei to survive? For most of the periodic table, this would be between 1 and 10 seconds after the big bang. But we’ve got a problem: most of the periodic table doesn’t exist yet. We have to make small nuclei before we can make big ones. And the smallest stable nucleus is that of our old friend deuterium: one proton and one neutron. Why is this a problem? Because deuterium is more fragile than most other elements: it takes about four times less energy to break a deuterium nucleus than any other. So, we have to wait a whole minute before the universe is cool enough that appreciable quantities of deuterium can build up. This delay is known as the deuterium bottleneck. But now the universe can finally make some progress! Protons join with neutrons to form deuterium. Because there are more protons than neutrons, they don’t all pair off in this way – there are left-over protons. After this, and very quickly, deuterium and more protons combine to form helium-3 (two protons and a neutron) and then these combine into ordinary helium (known as helium-4, two protons and two neutrons). It seems that the universe is on its way to making a whole host of elements, including the carbon and oxygen essential to you and me.
Luke A. Barnes the Deuteron in Stars: Anthropic Limits on Fundamental Constants 6 Jul 2017
Stellar nucleosynthesis proceeds via the deuteron (D), but only a small change in the fundamental constants of nature is required to unbind it. The most definitive boundary in parameter space that divides probably life-permitting universes from probably life-prohibiting ones is between a bound and unbound deuteron. Due to neutrino losses, balls of gas will undergo rapid cooling or stabilization by electron degeneracy pressure before they can form a stable, nuclear burning star. By contrast, the transition to endothermic pp and pep reactions, and the resulting beta-decay instability of the deuteron, do not seem to represent catastrophic problems for life.
https://arxiv.org/pdf/1703.07161.pdf
The Binding of the Deuteron
Whilst the deuteron itself is not important for life, its significance lies in the fact that the formation of all the elements beyond hydrogen proceeds via deuterium. If the deuteron were not stable at least for a few seconds the chemical elements would not exist in the universe. Decreasing the strength of the strong nuclear coupling constant (gs) by about 15% would result in the deuteron being unbound. Conversely, an increase in gs of ~10% would be sufficient to cause the diproton to be stable, and an increase in gs of only ~5% is sufficient to stabilize the dineutron. If gs were reduced by more than ~15%, and hence the deuteron did not exist, the implications for the universe would be catastrophic. The lack of deuterium would remove the pathway for the formation of heavier elements. Thus, whilst heavier nuclei might still be stable, there would be no means of producing them. Hence, we conclude that, as regards di-nucleon stability, gs exhibits a single-sided fine-tuning.
http://www.rickbradford.co.uk/CCC9b_SensitivityofDeuteronStabilitytoNuclearForce.pdf
helium-3 (deuterium + one more proton) and helium-4 (helium-3 + another neutron).6 As we’ve seen, these processes depend on the temperature of the Universe. The expansion and cooling of the Universe is controlled by the force of gravity. In particular, gravity dictates how much time elapses between the time when the Universe is too cool to make more neutrons and the time when neutrons are bound in nuclei. If this time is more than about 15 minutes, then the neutron stocks will be depleted. It shouldn’t be surprising, then, that changing the strengths of the fundamental forces can have a dramatic effect on the nuclear reactions in the very early Universe.
Ethan Siegel How The Big Bang Failed To Set The Universe Up For The Emergence Of Life Aug 11, 2021
If you imagine starting off the Universe in a very hot, dense, and uniform state, but one that’s expanding very rapidly, the laws of physics themselves will paint a remarkable picture of what’s to come.
In the initial stages, every quantum of energy that exists will be so hot that it will be traveling at speeds indistinguishable from the speed of light, smashing into other quanta countless times per second due to the overwhelming densities.
When a collision occurs, there’s a substantial chance that any particle-antiparticle pair that can get created — restricted only by the quantum mechanical conservation laws that govern the Universe and the amount of energy available for particle creation from Einstein’s famous E = mc2 relation — will come into existence.
Similarly, whenever a particle-antiparticle pair happen to collide, there’s a substantial chance that they’ll annihilate back into photons.
So long as you have an initially hot, dense, expanding Universe filled with interacting quanta of energy, those quanta will populate the Universe with all the various types of particles and antiparticles that are permitted to exist.
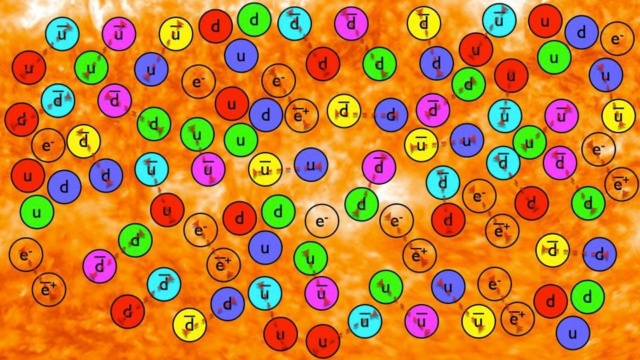
As matter and antimatter annihilate away in the early Universe, the leftover quarks and gluons cool to form stable protons and neutrons. Somehow, in the very early stages of the hot Big Bang, a slight imbalance of matter over antimatter was created, with the remainder annihilating away. Today, photons outnumber protons-and-neutrons by approximately 1.4 billion to one.
But what happens next? As the Universe expands, everything cools: massive particles lose kinetic energy while massless particles get redshifted to longer wavelengths. Early on, at very high energies, everything was in equilibrium: particles and antiparticles got created at the same rate they got annihilated. But as the Universe cools, the “forward” reaction rates, where you create new particles-and-antiparticles based on collisions, begin to occur less rapidly than the “backwards” reaction rates, where particles-and-antiparticles annihilate away back into massless particles, such as photons.
At very high energies, all of the known particles and antiparticles of the Standard Model are easy to create in large quantities. As the Universe cools, however, the more massive particles and antiparticles become more difficult to create, and they eventually annihilate away until there’s a negligible amount left. This winds up leading to a Universe filled with radiation, with just a tiny bit of leftover matter: protons, neutrons, and electrons, which somehow came to exist slightly more abundantly — about 1 extra matter particle per 1.4 billion photons — than antimatter. (How, exactly, that occurred is still an open area of research, and is known as the baryogenesis problem.)

A logarithmic scale showing the masses of the Standard Model's fermions: the quarks and leptons. Note the tininess of the neutrino masses. Data from the early Universe indicates that the sum of all three neutrino masses can be no greater than 0.17 eV. Meanwhile, in the early hot Big Bang stages, the heavier particles (and antiparticles) stop getting created earlier, while the lighter particles and antiparticles can continue to be created as long as there's enough available energy via Einstein's E=mc^2.
About 1 second after the Big Bang, the Universe is still very hot, with temperatures in the tens of billions of degrees: about ~1000 times hotter than in the center of our Sun. The Universe still has a little bit of antimatter left, because it’s still hot enough for electron-positron pairs to be created as quickly as they’re destroyed, and because neutrinos and antineutrinos are as equally copious as one another, and almost as copious as photons. The Universe is hot and dense enough for the remnant protons and neutrons to begin the process of nuclear fusion, building their way up the periodic table to create the heavy elements. If the Universe could do precisely this, then as soon as the Universe becomes cool enough to form neutral atoms and enough time passes so that the gravitational imperfections can attract enough matter to form stars and star systems, we’d have chances for life. The atoms necessary for life — the raw ingredients — can bind together into all sorts of molecular configurations all on their own, through natural, abiotic processes, just like we find today all throughout interstellar space. If we could begin building elements in these early stages of the hot Big Bang, the high temperatures and densities could permit not just fusion of hydrogen into helium, but helium into carbon, and so on into nitrogen, oxygen, and many of the heavier elements found all throughout the modern cosmos.
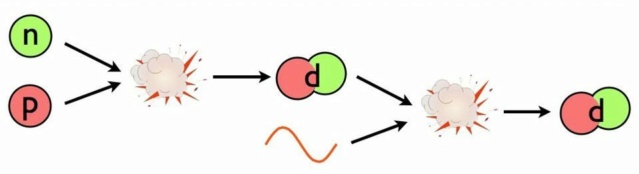
In a Universe loaded with neutrons and protons, it seems like building elements would be a cinch. All you have to do is start off with that first step: building deuterium, and the rest will follow from there. But making deuterium is easy; not destroying it is particularly hard. To avoid destruction, you have to wait until the Universe is cool enough so that there aren't sufficiently energetic photons around to destroy the deuterons.
This is the problem: deuterium. The Universe is full of protons and neutrons, and it’s hot and dense. Whenever a proton and neutron find one another, they’ll fuse into a deuteron, which is a heavy isotope of hydrogen, and is also more stable than a free proton and neutron separately; each time you form a deuteron from a proton and neutron, you liberate 2.2 million electron-volts of energy. (You can also form deuterium from nuclear reactions involving two protons, but the reaction rate is much lower than from a proton and a neutron.) So why, then, can’t you add protons or neutrons to each deuteron, building your way up to heavier isotopes and elements? The same hot, dense conditions lead to a “backwards” reaction that swamps the “forward” creation of deuterium by fusing protons with neutrons: the fact that enough photons, which outnumber protons and neutrons by more than a billion-to-one, have more than 2.2 million electron-volts of energy themselves. When they collide with a deuteron, which occurs far more frequently than a deuteron colliding with anything else made out of protons-and-neutrons, they immediately blast it apart. The inability of the cosmos to maintain deuterium in the early Universe for long enough periods to build up to heavier elements is the primary reason that the Big Bang can’t create the ingredients for life on its own.
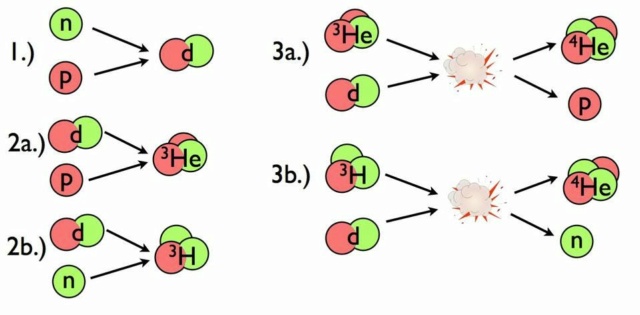
From beginning with just protons and neutrons, the Universe builds up helium-4 rapidly, with small but calculable amounts of deuterium, helium-3, and lithium-7 left over as well. In the aftermath of the first few minutes of the Big Bang, the Universe winds up being populated, in terms of normal matter, with over 99.99999% hydrogen and helium alone.
So, what can the Universe do? It’s compelled to wait until it’s expanded and cooled enough so that deuterium isn’t immediately blasted apart. But in the meanwhile, a whole slew of other things happen while we wait for the Universe to cool sufficiently. They include:
1. neutrinos and antineutrinos stop efficiently participating in interactions with other particles, also known as the freeze-out of the weak interactions,
2. electrons and positrons, like other species of matter and antimatter, annihilate away, leaving only the excess electrons, and the free neutrons, being unable to bind themselves up in heavier nuclei, begin to decay away into protons, electrons, and anti-electron neutrinos.
3. Finally, after a little more than about ~200 seconds, we can finally form deuterium without immediately blasting it apart. But at this point, it’s too late. The Universe has cooled but become much less dense: only about one-billionth the density found in the central core of our Sun. The deuterons can fuse with other protons, neutrons, and deuterons to build up copious amounts of helium, but that’s where the chain reaction ends.
With less energy per particle, with strong repulsive forces between the helium nuclei, and with every combination of:
helium-4 and a proton,
helium-4 and a neutron,
and helium-4 and helium-4,
being unstable, that’s pretty much the end of the line. The Universe, in the immediate aftermath of the Big Bang, is made of 99.99999%+ hydrogen and helium, exclusively.
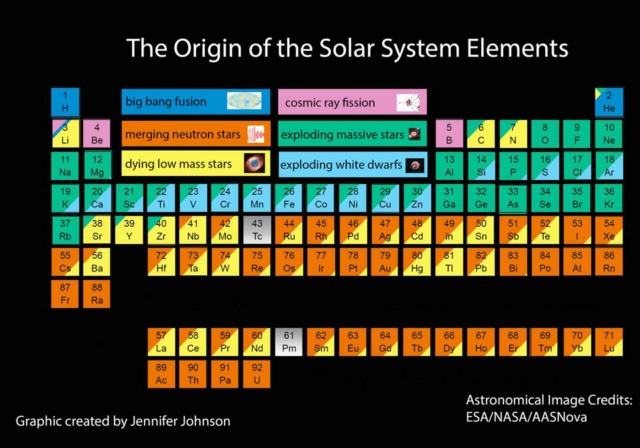
The most current, up-to-date image showing the primary origin of each of the elements that occur naturally in the periodic table. Neutron star mergers, white dwarf collisions, and core-collapse supernovae may allow us to climb even higher than this table shows. The Big Bang gives us almost all of the hydrogen and helium in the Universe, and almost none of everything else combined.
Even though we’re talking about cosmic scales, it’s actually the laws that govern subatomic particles — nuclear and particle physics — that prevents the Universe from forming the heavy elements required for life in the early stages of the Big Bang. If the rules were a little bit different, like deuterium was more stable, there were much greater numbers of protons and neutrons, or there were fewer photons at high energies, nuclear fusion could have built up large quantities of heavy elements in the first few seconds of the Universe.
But the easily-destroyed nature of deuterium, combined with the enormous numbers of photons present in the early Universe, kills our dreams of having the necessary raw ingredients right at the beginning. Instead, it’s just hydrogen and helium, and we’ll have to wait stars to form before we build up any substantial quantities of anything heavier. The Big Bang was a great start to our Universe, but couldn’t set us up for life all on its own. For that, we need stars that enrich the interstellar medium with the heavier elements that all biochemical processes require. When it comes to your existence, the Big Bang absolutely isn’t enough to give rise to you. For that to occur, you can literally thankyour lucky the creator of stars: the ones that lived, died, and created the essential elements still inside you today.
https://www.forbes.com/sites/startswithabang/2021/08/11/how-the-big-bang-failed-to-set-the-universe-up-for-the-emergence-of-life/
JAMES STEIN Planck's Constant: The Number That Rules Technology, Reality, and Life OCTOBER 24, 2011
More fundamentally, the discovery of Planck’s constant advanced the realization that, when we probe the deepest levels of the structure of matter, we are no longer looking at “things” in the conventional meaning of the word. A “thing”—like a moving car—has a definite location and velocity; a car may be 30 miles south of Los Angeles heading east at 40 miles per hour. The concepts of location, velocity, and even existence itself blur at the atomic and subatomic level. Electrons do not exist in the sense that cars do, they are, bizarrely, everywhere at once, but much more likely to be in some places than in others. Reconciling the probabilistic subatomic world with the macroscopic everyday world is one of the great unsolved problems in physics. The fundamental nuclear reaction eventually leading to the explosion of a supernova is the fusion of four hydrogen atoms to produce a single atom of helium. In the process, approximately 0.7% of the mass is converted to energy via E=mc 2 .
This 0.7% is known as the efficiency of hydrogen fusion, and our understanding of it is one of the consequences of Planck’s investigations. It requires a great deal of heat to enable hydrogen to fuse to helium, and the hydrogen atoms in the sun are moving at different speeds, much like cars on a freeway move at different speeds. The slower-moving hydrogen atoms just bounce off each other; they are insufficiently hot to fuse. Higher speeds, though, mean higher temperatures, and there is a small fraction of hydrogen atoms moving at sufficiently high speeds to fuse to helium.
The 0.7% efficiency of hydrogen fusion is what is sometimes referred to as a “Goldilocks number.” Like the porridge that Goldilocks eventually ate, which was neither too hot nor too cold, but just right, the 0.7% efficiency of hydrogen fusion is “just right” to permit the emergence of life as we know it. The process of hydrogen fusion is an intricate high-speed, high-temperature ballet. The first step of this reaction produces deuterium, an isotope of hydrogen whose nucleus consists of one proton and one neutron. In this process, two protons slam into one another, causing one of the protons to shed its electrical charge and metamorphose into a neutron. If the efficiency of hydrogen fusion were as low as 0.6%, the neutron and proton would not bond to each other to form a deuterium atom. In this case, we’d still have stars—huge glowing balls of hydrogen—but no star stuff would ever form because the porridge would be too cold to create helium, the first step on the road to creating the elements necessary for life.
On the other hand, if hydrogen fusion had an efficiency of 0.8%, it would be much too easy for helium to form. The hydrogen in the stars would become helium so quickly that there wouldn’t be much hydrogen left to form the molecule most essential for life—water. Starstuff would be produced, but without water life as we know it would not exist. Maybe something else would take the place of water, and maybe life could evolve—but not ours.
Planck’s quantization of energy was an essential step on the road to the theory of quantum mechanics, which is critical to our understanding of stellar evolution. Science hasn’t filled in all the pieces of the puzzle of how life actually evolved, but quantum mechanics did begin to answer the question of how the pieces got there in the first place,
https://www.pbs.org/wgbh/nova/article/plancks-constant/
Leonard Susskind The Cosmic Landscape: String Theory and the Illusion of Intelligent Design 2006, page 178
The chain of nuclear reactions in stars that starts with the lightest elements and leads to iron is complicated. A couple of examples will illustrate the point. The most familiar example is the fusion reaction that begins with hydrogen and produces helium. Here is where the weak interactions (diagrams with W- and Z-bosons) come in. The first step is the collision of two protons. Many things can happen when two protons collide, but if you know the Feynman diagrams for the Standard Model, you can find one that ends up with a proton, a neutron, a positron, and a neutrino. The positron finds a wandering electron in the star, and together they self-destruct into photons that eventually become the star’s thermal energy (heat). The neutrino just zips away and disappears with almost the speed of light. That leaves one sticky proton and one sticky neutron that sticks together to form an isotope of hydrogen called deuterium. Next, a third proton strikes the deuterium nucleus and sticks to it. The nucleus with two protons and a neutron is a form of helium called helium-three (3He), but it’s not the stable kind of helium that we use to fill balloons. That stuff is called helium-four (4He). The story continues: two 3He nuclei collide. All together that means four protons and two neutrons. But they don’t all stick together. Two of the protons fly off and leave a nucleus with two protons and two neutrons. That’s an ordinary 4He nucleus. You don’t need to remember all that. Very few physicists do.
https://3lib.net/book/2472017/1d5be1
Fred C. Adams On the Habitability of Universes without Stable Deuterium 30 Mar 2017
In both stars and in the early universe, the production of deuterium is the first step on the way to producing heavier nuclei. If the strong force were slightly weaker, then deuterium would not be stable, and many authors have noted that nuclesynthesis would be compromised so that helium production could not proceed through standard reaction chains. If the strong force were sufficiently weaker, then deuterium would not have a bound state. In a universe with no deuterium, the usual stepping stone on the pathway to heavy elements would not be available. Many authors have speculated that the absence of stable deuterium would lead to a universe with no heavy elements at all, and hence a lifeless universe
https://arxiv.org/pdf/1612.04741.pdf
John D. Barrow The Anthropic Cosmological Principle 1986 page 295
The existence of deuterium and the non-existence of the diproton therefore hinge precariously on the precise strength of the nuclear force. If the strong interaction were a little stronger the diproton would be a stable bound state with catastrophic consequences—all the hydrogen in the Universe would have been burnt to He 2 during the early stages of the Big Bang and no hydrogen compounds or long-lived stable stars would exist today. If the di-proton existed we would not! Also, if the nuclear force was a little weaker the deuteron would be unbound with other adverse consequences for the nucleosynthesis of biological elements because a key link in the chain of nucleosynthesis would be removed
https://3lib.net/book/1131892/9a639b
LUKE A. BARNES: The Cosmic Revolutionary’s Handbook (Or: How to Beat the Big Bang) 2020
An important principle is at work here: if you want to make something in the universe, whether a proton or a lead nucleus, you’ll need to wait until things cool off enough for it to survive the ambient temperature. Our focus here is on the elements, so we ask: when is the universe cool enough for nuclei to survive? For most of the periodic table, this would be between 1 and 10 seconds after the big bang. But we’ve got a problem: most of the periodic table doesn’t exist yet. We have to make small nuclei before we can make big ones. And the smallest stable nucleus is that of our old friend deuterium: one proton and one neutron. Why is this a problem? Because deuterium is more fragile than most other elements: it takes about four times less energy to break a deuterium nucleus than any other. So, we have to wait a whole minute before the universe is cool enough that appreciable quantities of deuterium can build up. This delay is known as the deuterium bottleneck. But now the universe can finally make some progress! Protons join with neutrons to form deuterium. Because there are more protons than neutrons, they don’t all pair off in this way – there are left-over protons. After this, and very quickly, deuterium and more protons combine to form helium-3 (two protons and a neutron) and then these combine into ordinary helium (known as helium-4, two protons and two neutrons). It seems that the universe is on its way to making a whole host of elements, including the carbon and oxygen essential to you and me.
Luke A. Barnes the Deuteron in Stars: Anthropic Limits on Fundamental Constants 6 Jul 2017
Stellar nucleosynthesis proceeds via the deuteron (D), but only a small change in the fundamental constants of nature is required to unbind it. The most definitive boundary in parameter space that divides probably life-permitting universes from probably life-prohibiting ones is between a bound and unbound deuteron. Due to neutrino losses, balls of gas will undergo rapid cooling or stabilization by electron degeneracy pressure before they can form a stable, nuclear burning star. By contrast, the transition to endothermic pp and pep reactions, and the resulting beta-decay instability of the deuteron, do not seem to represent catastrophic problems for life.
https://arxiv.org/pdf/1703.07161.pdf
The Binding of the Deuteron
Whilst the deuteron itself is not important for life, its significance lies in the fact that the formation of all the elements beyond hydrogen proceeds via deuterium. If the deuteron were not stable at least for a few seconds the chemical elements would not exist in the universe. Decreasing the strength of the strong nuclear coupling constant (gs) by about 15% would result in the deuteron being unbound. Conversely, an increase in gs of ~10% would be sufficient to cause the diproton to be stable, and an increase in gs of only ~5% is sufficient to stabilize the dineutron. If gs were reduced by more than ~15%, and hence the deuteron did not exist, the implications for the universe would be catastrophic. The lack of deuterium would remove the pathway for the formation of heavier elements. Thus, whilst heavier nuclei might still be stable, there would be no means of producing them. Hence, we conclude that, as regards di-nucleon stability, gs exhibits a single-sided fine-tuning.
http://www.rickbradford.co.uk/CCC9b_SensitivityofDeuteronStabilitytoNuclearForce.pdf
helium-3 (deuterium + one more proton) and helium-4 (helium-3 + another neutron).6 As we’ve seen, these processes depend on the temperature of the Universe. The expansion and cooling of the Universe is controlled by the force of gravity. In particular, gravity dictates how much time elapses between the time when the Universe is too cool to make more neutrons and the time when neutrons are bound in nuclei. If this time is more than about 15 minutes, then the neutron stocks will be depleted. It shouldn’t be surprising, then, that changing the strengths of the fundamental forces can have a dramatic effect on the nuclear reactions in the very early Universe.
Ethan Siegel How The Big Bang Failed To Set The Universe Up For The Emergence Of Life Aug 11, 2021
If you imagine starting off the Universe in a very hot, dense, and uniform state, but one that’s expanding very rapidly, the laws of physics themselves will paint a remarkable picture of what’s to come.
In the initial stages, every quantum of energy that exists will be so hot that it will be traveling at speeds indistinguishable from the speed of light, smashing into other quanta countless times per second due to the overwhelming densities.
When a collision occurs, there’s a substantial chance that any particle-antiparticle pair that can get created — restricted only by the quantum mechanical conservation laws that govern the Universe and the amount of energy available for particle creation from Einstein’s famous E = mc2 relation — will come into existence.
Similarly, whenever a particle-antiparticle pair happen to collide, there’s a substantial chance that they’ll annihilate back into photons.
So long as you have an initially hot, dense, expanding Universe filled with interacting quanta of energy, those quanta will populate the Universe with all the various types of particles and antiparticles that are permitted to exist.

As matter and antimatter annihilate away in the early Universe, the leftover quarks and gluons cool to form stable protons and neutrons. Somehow, in the very early stages of the hot Big Bang, a slight imbalance of matter over antimatter was created, with the remainder annihilating away. Today, photons outnumber protons-and-neutrons by approximately 1.4 billion to one.
But what happens next? As the Universe expands, everything cools: massive particles lose kinetic energy while massless particles get redshifted to longer wavelengths. Early on, at very high energies, everything was in equilibrium: particles and antiparticles got created at the same rate they got annihilated. But as the Universe cools, the “forward” reaction rates, where you create new particles-and-antiparticles based on collisions, begin to occur less rapidly than the “backwards” reaction rates, where particles-and-antiparticles annihilate away back into massless particles, such as photons.
At very high energies, all of the known particles and antiparticles of the Standard Model are easy to create in large quantities. As the Universe cools, however, the more massive particles and antiparticles become more difficult to create, and they eventually annihilate away until there’s a negligible amount left. This winds up leading to a Universe filled with radiation, with just a tiny bit of leftover matter: protons, neutrons, and electrons, which somehow came to exist slightly more abundantly — about 1 extra matter particle per 1.4 billion photons — than antimatter. (How, exactly, that occurred is still an open area of research, and is known as the baryogenesis problem.)

A logarithmic scale showing the masses of the Standard Model's fermions: the quarks and leptons. Note the tininess of the neutrino masses. Data from the early Universe indicates that the sum of all three neutrino masses can be no greater than 0.17 eV. Meanwhile, in the early hot Big Bang stages, the heavier particles (and antiparticles) stop getting created earlier, while the lighter particles and antiparticles can continue to be created as long as there's enough available energy via Einstein's E=mc^2.
About 1 second after the Big Bang, the Universe is still very hot, with temperatures in the tens of billions of degrees: about ~1000 times hotter than in the center of our Sun. The Universe still has a little bit of antimatter left, because it’s still hot enough for electron-positron pairs to be created as quickly as they’re destroyed, and because neutrinos and antineutrinos are as equally copious as one another, and almost as copious as photons. The Universe is hot and dense enough for the remnant protons and neutrons to begin the process of nuclear fusion, building their way up the periodic table to create the heavy elements. If the Universe could do precisely this, then as soon as the Universe becomes cool enough to form neutral atoms and enough time passes so that the gravitational imperfections can attract enough matter to form stars and star systems, we’d have chances for life. The atoms necessary for life — the raw ingredients — can bind together into all sorts of molecular configurations all on their own, through natural, abiotic processes, just like we find today all throughout interstellar space. If we could begin building elements in these early stages of the hot Big Bang, the high temperatures and densities could permit not just fusion of hydrogen into helium, but helium into carbon, and so on into nitrogen, oxygen, and many of the heavier elements found all throughout the modern cosmos.

In a Universe loaded with neutrons and protons, it seems like building elements would be a cinch. All you have to do is start off with that first step: building deuterium, and the rest will follow from there. But making deuterium is easy; not destroying it is particularly hard. To avoid destruction, you have to wait until the Universe is cool enough so that there aren't sufficiently energetic photons around to destroy the deuterons.
This is the problem: deuterium. The Universe is full of protons and neutrons, and it’s hot and dense. Whenever a proton and neutron find one another, they’ll fuse into a deuteron, which is a heavy isotope of hydrogen, and is also more stable than a free proton and neutron separately; each time you form a deuteron from a proton and neutron, you liberate 2.2 million electron-volts of energy. (You can also form deuterium from nuclear reactions involving two protons, but the reaction rate is much lower than from a proton and a neutron.) So why, then, can’t you add protons or neutrons to each deuteron, building your way up to heavier isotopes and elements? The same hot, dense conditions lead to a “backwards” reaction that swamps the “forward” creation of deuterium by fusing protons with neutrons: the fact that enough photons, which outnumber protons and neutrons by more than a billion-to-one, have more than 2.2 million electron-volts of energy themselves. When they collide with a deuteron, which occurs far more frequently than a deuteron colliding with anything else made out of protons-and-neutrons, they immediately blast it apart. The inability of the cosmos to maintain deuterium in the early Universe for long enough periods to build up to heavier elements is the primary reason that the Big Bang can’t create the ingredients for life on its own.

From beginning with just protons and neutrons, the Universe builds up helium-4 rapidly, with small but calculable amounts of deuterium, helium-3, and lithium-7 left over as well. In the aftermath of the first few minutes of the Big Bang, the Universe winds up being populated, in terms of normal matter, with over 99.99999% hydrogen and helium alone.
So, what can the Universe do? It’s compelled to wait until it’s expanded and cooled enough so that deuterium isn’t immediately blasted apart. But in the meanwhile, a whole slew of other things happen while we wait for the Universe to cool sufficiently. They include:
1. neutrinos and antineutrinos stop efficiently participating in interactions with other particles, also known as the freeze-out of the weak interactions,
2. electrons and positrons, like other species of matter and antimatter, annihilate away, leaving only the excess electrons, and the free neutrons, being unable to bind themselves up in heavier nuclei, begin to decay away into protons, electrons, and anti-electron neutrinos.
3. Finally, after a little more than about ~200 seconds, we can finally form deuterium without immediately blasting it apart. But at this point, it’s too late. The Universe has cooled but become much less dense: only about one-billionth the density found in the central core of our Sun. The deuterons can fuse with other protons, neutrons, and deuterons to build up copious amounts of helium, but that’s where the chain reaction ends.
With less energy per particle, with strong repulsive forces between the helium nuclei, and with every combination of:
helium-4 and a proton,
helium-4 and a neutron,
and helium-4 and helium-4,
being unstable, that’s pretty much the end of the line. The Universe, in the immediate aftermath of the Big Bang, is made of 99.99999%+ hydrogen and helium, exclusively.

The most current, up-to-date image showing the primary origin of each of the elements that occur naturally in the periodic table. Neutron star mergers, white dwarf collisions, and core-collapse supernovae may allow us to climb even higher than this table shows. The Big Bang gives us almost all of the hydrogen and helium in the Universe, and almost none of everything else combined.
Even though we’re talking about cosmic scales, it’s actually the laws that govern subatomic particles — nuclear and particle physics — that prevents the Universe from forming the heavy elements required for life in the early stages of the Big Bang. If the rules were a little bit different, like deuterium was more stable, there were much greater numbers of protons and neutrons, or there were fewer photons at high energies, nuclear fusion could have built up large quantities of heavy elements in the first few seconds of the Universe.
But the easily-destroyed nature of deuterium, combined with the enormous numbers of photons present in the early Universe, kills our dreams of having the necessary raw ingredients right at the beginning. Instead, it’s just hydrogen and helium, and we’ll have to wait stars to form before we build up any substantial quantities of anything heavier. The Big Bang was a great start to our Universe, but couldn’t set us up for life all on its own. For that, we need stars that enrich the interstellar medium with the heavier elements that all biochemical processes require. When it comes to your existence, the Big Bang absolutely isn’t enough to give rise to you. For that to occur, you can literally thank
https://www.forbes.com/sites/startswithabang/2021/08/11/how-the-big-bang-failed-to-set-the-universe-up-for-the-emergence-of-life/

