AXON ENSHEATHMENT AND MYELIN GROWTH - AMAZING EVIDENCE OF DESIGN
http://reasonandscience.heavenforum.org/t2586-axon-ensheathment-and-myelin-growth-amazing-evidence-of-design
Myelin Facilitation of Whole Brain Neuroplasticity 2
For decades, myelin has been understood as a simple insulator of axons that increases the speed of neuronal transmission. There was little known about oligodendrocytes—the cells that make myelin. Recently, vast complexity in the process of oligodendrocytes’ wrapping and compressing layers of myelin has been discovered. In fact, this three dimensional process is one of the most elaborate in nature. It involves signaling between the oligodendrocytes and neurons for many different stages of the process—determining where myelin will be built and where first contact occurs between the two cells; wrapping the layers and growing them along the axon; determining how many layers are wrapped and where the nodes will be; and the intricate compaction of the layers. The process, also, keeps lines of communication through the compacted regions and maintains the structure sometimes for decades. Also, stem cells making oligodendrocytes travel throughout the brain determining where the myelin will be built. All of this is connected with the needs of the neuron and astrocyte networks across the brain. It is vital for all learning and cognition.
Laying down myelin is far more complex than synapses and neuro muscular junctions. The immensely elaborate process of modeling, remodeling, and maintenance of myelin occurs all over the brain simultaneously. Neuroplasticity has been assumed to be a vast array of mechanisms related to synapses. Now, new research shows an entirely new dimension, where myelin is a vital part of neuroplasticity for all memory, not just motor memory. Myelin neuroplasticity is directed by electrical activity of the neuron and by elaborate communication with oligodendrocytes and many other cells. The stem cells that produce oligodendrocytes are, also, directed by conversations with epithelial cells in blood vessels. Oligodendrocytes are multi polar cells that produce fatty myelin sheaths and wrap them around the axons many times. This sheath greatly enhances the speed of the electrical signal along the axon. Without it, the electrical wave depends on movement from each small sodium and potassium pore to the next all along the membrane. With myelin, the electrical signal rapidly jumps between nodes with long stretches of insulated myelin.
As regions of the brain form myelin and the connections begin to operate, specific abilities coincide with the development. The gradual growing of myelin down the long motor neurons from the brain produce a stepwise increase in function in babies—first, stiffening neck, then arm movement, sitting up, standing up and walking. Later, speaking and cognitive abilities appear with myelinated brain circuits. It is only as young adults that the frontal lobes myelinate with the dawning of judgment and adult decision making.
Signals must arrive within milliseconds to be effective in complex circuits. Plasticity for synapses depends on simultaneous arrivals so that decisions can be appropriately made for the next axon firing in the postsynaptic neuron. The next signal is weakened if one contributing circuit arrives late and strengthened if early within a very narrow time scale. Myelin creates rapid speeds, but it must produce the exact level of speed needed for the neuroplasticity. In fact, speeds of myelinated axons vary widely in a scale of a thousand—a tenth of a yard per second to 200 yards per second.
As well as the number of wraps the the spacing of nodes, another consideration is different types of electrical signals. Some sensory signals are simply a singular spike of a certain intensity. In much of the brain however, there are circuits that provide timed pulsed signals at different frequencies for particular background tasks.
Timed pulses occur in circuits of the default mode network and, also, with attention, sleep, top down regulation of sensory input and perception, to name just a few. These need regulation of the velocities of signals at various rates. For example, brain wave oscillations, such as gamma waves can be altered by delays in the speed of the conduction by even a very small amount. When conduction is altered it can lead to brain disease related to white matter changes.
Regulation is always a informational task, and depends on the right pre-programming.
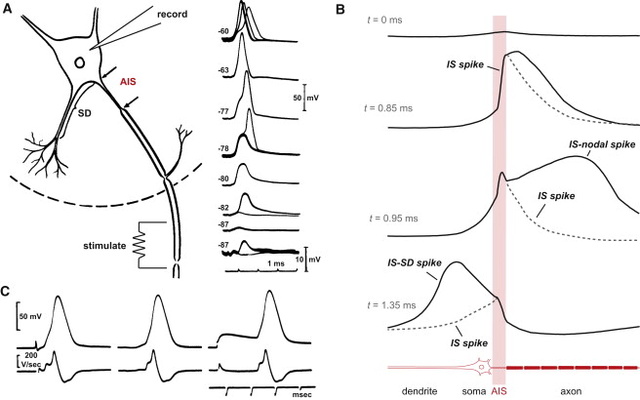
Even though changes cannot be well visualized in imaging studies, it is now known that alterations in white matter (axon bundles) occur related to the amount of electrical use of a nerve. Increased myelin is correlated with the amount of learning. Some changes occur very rapidly after only two hours of learning in humans and animals. The large white matter coming out of the hippocampus memory center is called the fornix, which was altered with 2 hours of learning. The first part of the axon (initial axon segment or AIS) near the cell body is unique in that only half of it is myelinated and therefore part of it is similar to half a node of Ranvier. The AIS is where the electrical spike is started with very complex ion channels that regulate the entire process. The AIS determines not just when the electrical current will start but how big it will be and its electrical “shape”. This determines exactly how much neurotransmitter will be discharged at the synapse, as well as the exact neurotransmitter to be sent. A very significant finding is that the AIS actually alters is size and position to regulate the complex information that is sent. Since the AIS is similar to a node of Ranvier, it is thought that similar alterations occur at each node with local stimulation. There is evidence of changes in the sizes of nodes and the length between them during neuroplasticity in the ear.
Myelin Wrapping Process is Very Complex
Triggering oligodendrocytes to make myelin in special ways involves a very elaborate multi step process. Myelin must be produced by triggering elaborate timed genetic networks including correct placement of the first wrap of the sheath around the membrane of the axon. It cannot just be left there. The sheath has to, then, be serviced for a very long time, often decades. The oligodendrocyte sends out a hand like process to touch the axon membrane where a very unique junction region is created. Immunoglobulin on the neuron is necessary to trigger the oligodendrocyte’s activity. Very elaborate cascades produce a multi unit protein factory built out of scaffolding tubules, as well as receptors, to direct the unfolding of the complex myelin producing process. Signaling regulates the proteins needed to make myelin and the way they are placed in the membrane to hold it in place. Very complex cascades of molecules include kinase enzymes, integrin and molecules based on the lipid cholesterol. The original immunoglobulin is stimulated by particular frequencies from the neuron and is only essential for the first layer of the wrapping of the sheath.
Wrapping of the myelin will not occur without a special measurement of the size of the axon circumference including special curvature sensors. The axon has to be above a particular circumference for the process to start. The cellular process makes a spiral sheet in 3D. It is an amazing process where a length of 100 micrometers (10-6) with 10 spirals takes 6400 square micrometers of membrane. The wrapping process is very hard to understand and there are several competing models to grasp it, currently.A leading tongue crawls around the axon leaving a sheet of myelin. Additional wraps and lengthening of the total myelin happens at the same time. A process from oligodendrocytes connects to the myelin and more wraps occur there. The inner layers are smaller than the later layers. The tongues are pushed by actin. Later the inner layers grow and catch up to the covering layers. As the second wrap occurs, the bottom layer is growing bigger. Each layer attaches permanently in a particular placement near where the node of Ranvier will be forming small paranode attachment areas.
The myelin wrapping grows in two ways at once—more layers and the entire sheath gets longer at each level. When three layers are formed, a complex process of compaction starts at the outside layers. During compaction, special channels are created to allow transport of materials through the compacted areas and the growth zone. Compaction occurs both along the length of the axon and around the circular wrap at the same time. When compaction has eliminated most of the cytoplasm by pushing it out, a special protein binds the two membranes together as if in a tight junction between cells. All proteins with more than 30 amino acids are removed. This process is similar to a zipper.
Adhesions and compaction also occur between the extracellular membranes of two layers of wrap. Compaction is not fully understood, but it involves eliminating electrostatic repulsion and other forces. Many receptors and signals such as neureglin regulate the number of wraps, which determines the properties of the electrical signal for neuroplasticity. Another complication is that oligodendrocytes are in close communication with blood vessels. The brain has more types of cells than any organ in nature and they must travel to exact places over long distances. Oligodendrocytes migrate the most of all cells throughout life. It was recently found that stem cells to make oligodendrocytes travel along blood vessels. Elaborate signaling between the oligodendrocytes and the blood vessels define their routes.
New Myelin Code Adds to Brain Complexity 3
The common wisdom that myelin is just an insulator to make faster neuronal communication has been overthrown by recent research that finds much more complexity in how myelin is used by the brain. The old “neuron doctrine” (one of many useless dogmas) was that each neuron sends a signal to the next neuron, and so on, in computer like fashion. In fact, the process is vastly more complex and variable and the new myelin code adds to brain complexity in very important ways.Myelin patterns are different in each brain region, especially each of the six layers of the cortex—myelin appears to have its own very complex code. Un-myelinated axons are crucial for newly discovered types of communication between the naked axons and adjacent local immune and nervous system cells. Both myelin, interspersed patterns of myelin, and lack of myelin are essential for specific types of information transfer. Oligodendrocytes, also, make many decisions concerning the types of signals.
The neuron has many different methods of communication. In addition to the well-studied axon spikes triggering secretion of chemical neurotransmitters and the less studied electrical synapses and synchronous brain waves, there are now newly discovered sideways communication from the un-myelinated axons, nanotubes and vesicles. This can take the form of cytokine signaling to local immune cells and neurotransmitter signaling to other neurons, glia and immune cells. In addition, electrical information is transmitted in the form of local field potential with many contributing factors such as shape of cells, extracellular matrix and calcium spikes.
Myelin affects the way information is created and communicated. In layers II and III, there are different patterns of very long intermittent stretches without any myelin. Also, the critical initial segment that determines the type and strength of the signal was different in each region. In fact, many different regions of the brain have different amounts and distribution of myelin. The myelin in each region is so different from each other, and so different than what was previously assumed, it appears to be yet another brain code.
Initial Segment Is Important and Varied
Perhaps, most significantly, the initial region from the cell body to the start of myelination along the axon is very different in each of the cortical layers. This initial segment is considered to be very important for the propagation of the signal and can vary from 5um to 80 um in length. It is where the decision is made whether or not to send an electrical signal along the axon. The initial segment has a very complex set of ion channels and structures that help make the decision to spike. It, also, determines the specific shape of the electrical spike and the amount of neurotransmitter release. The length of this segment was longer in layers III and IV than in V and VI.
The initial segment, also, determines whether the spike can go backwards or not. Backward transmission determines aspects of neuroplasticity, such as in memory consolidation in the hipppocampus during deep sleep and during quiet reflection when awake.
Myelin, it now appears, also, plays a part in determining where synapses will occur. There are specific proteins in the myelin sheath that stop the budding of an axon. The oligodendrocyte and its myelin therefore determines the structure of the circuit.
Theevolution origin of complex nervous systems in vertebrates has been accompanied by, and probably dependent on, the acquisition of the myelin sheath. Although there has been substantial progress in our understanding of the factors that determine glial cell fate, much less is known about the cellular mechanisms that determine how the myelin sheath is extended and stabilized around axons. Functional integration of the vertebrate nervous system requires rapid nerve impulse conduction. This is achieved through myelin-forming glial cells — oligodendrocytes in the Central Nervous system CNS, and Schwann cells in the Peripheral Nervous System PNS. These cells wrap around axons so that the molecular machinery responsible for propagating action potentials is concentrated at regular, discontinuous sites along the axon. These are known as nodes of Ranvier. The presence of myelin as an internodal insulator ensures that membrane depolarization can only occur at the nodes. The result is rapid, saltatory (from the Latin saltare, to jump, or to dance) nerve conduction. The myelin sheath is one of the best-studied mammalians membranes, not least because of its vital function, and also owing to its abundance and the ease of isolation of enriched myelin fractions. Consequently, there is a vast literature on the biochemical and biophysical properties of this membrane in health and disease, and considerable detail has been amassed about the bio synthesis of its constituent lipids and proteins. Furthermore, and reflecting our growing understanding of how nervous systems develop in general, the embryonic origins and cell lineages of oligo dendrocytes and Schwann cells and their precursors have been more clearly defined over the past few years. These discoveries have revealed the involvement of a steadily increasing number of receptor signalling pathways and transcription factors in the differentiation of glial cells. Therefore, there has been, and continues to be, steady progress in our understanding of where myelin-forming glia come from and which molecules regulate their specification. In spite of this burgeoning knowledge, until recently surprisingly little was known about the molecular basis and dynamics of the cell–cell interactions that determine how the myelin sheath is extended and stabilized around axons in the first place. Progress has also been slow in understanding the mechanisms that allow nerves to continue growing in the postnatal animal. These are key questions for understanding nervous system function, as they relate directly to the role of myelin as an insulator of nerve fibres and to the way that myelin-forming glia participate in the assembly of nodes of Ranvier. These issues are of more than academic interest, as progress in revealing the mechanisms of repair and the essential role of myelin-forming glia in providing trophic support for axons will inevitably affect the development of therapies to arrest or even reverse the neurodegeneration that accompanies demyelinating diseases. 1
Selection of axons and initiation of contact
The reasons why some axons are myelinated and others are not are still baffling.
If a complex nervous system does not depend on myelin sheath, why did natural selection produce glial cells and myelination in the first place?
In general, a minimum calibre is required (~1 μm) before an axon can be myelinated. The physiological rationale for this threshold is that the saltatory mode of conduction would probably not confer significantly enhanced rates of nerve impulse transmission on small calibre fibres, but how axons of a minimum calibre are selected for myelination is still not understood. This is of particular relevance in the context of demyelinating diseases such as MULTIPLE SCLEROSIS, for which it has been proposed that changes in the expression of proteins on the axonal surface might make axons less susceptible to remyelination. Certain cell adhesion molecules, such as L1 and polysialylated NCAM (neural cell adhesion molecule), are known to be expressed on unmyelinated axons and to be downregulated during axonal myelination. However, whether or not the disappearance of these molecules is mechanistically connected to axon ensheathment is not clear. The L1-knockout mouse shows no obvious derangements in myelination, such as ensheathment of inappropriate axons . Other candidates for sensing axonally presented molecules on oligo dendrocytes and Schwann cells include the integrins and neuro fascin. Integrins seem to be most important for survival mechanisms in early oligodendrocytes and in Schwann cells their best-characterized role is in the radial sorting of unmyelinated axons. Laminins, the ligands for some integrins, also have an important role in the defasciculation of axons. Dystroglycan, the other laminin receptor on Schwann cells, is not vital for the early stages of myelination, and seems to be more important for the organization of the nodal microvilli. The neurofascin gene and its products do play an important part in later stages of myelination (see below), however, they do not seem to be necessary for initial ensheathment.
Myelin process extension around target axons
The function of myelin in the Central Nervous System CNS and Peripheral Nervous System PNS appears to be identical, but there are differences in the cell biology of myelination when oligodendrocytes are compared with Schwann cells. For example, the Schwann cell has an intimate association with the axon that it myelinates, and lines up along the axon to define a single internode, whereas oligodendrocytes extend several processes, each of which myelinates distinct internodes, often on different axons. Nevertheless, in both cases a myelinating process that is continuous with the plasma membrane must be assembled and extended. Current concepts of LIPID RAFTS, which propose the existence of microdomains in membranes, might help to explain how proteins and lipids are delivered to the growing membrane. However, such concepts might be less useful for understanding how the myelin macrodomain, with its distinct protein and lipid content, is stably segregated from the plasma membrane of the myelin-forming glial cell.The myelin membrane contains a high percentage of lipids compared with the plasma membranes of most eukaryotic cells.
Cholesterol is a major constituent, and mice with oligodendrocytes that lack the ability to synthesize cholesterol show a delay in myelination that seems to be at least partially compensated for by cholesterol uptake. There has been particular interest in determining the significance of the high galactolipid content of myelin. Mice with a disruption in the gene that encodes the biosynthetic enzyme UDP-galactose: ceramide galactosyl transferase (CGT) cannot
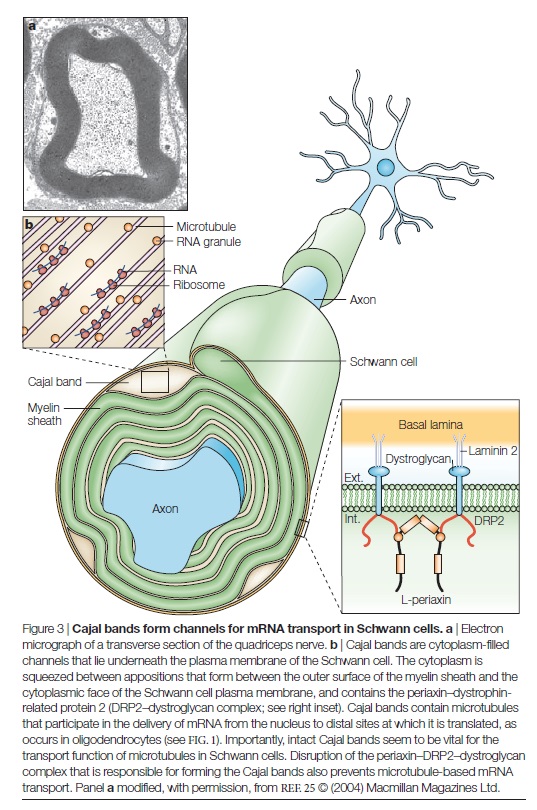
synthesize galactolipids, but seem to compensate by replacing these lipids with their gluco-analogues. Surprisingly, only the myelin of the CNS shows any resulting structural and functional defects, including disrupted PARANODAL AXOGLIAL JUNCTIONS and reduced nerve conduction velocities. In order to discriminate between the effects of the absence of galactolipids and their sulphated derivatives, mice that lack cerebroside sulphotransferase were generated. These mice show a two- to threefold enhancement in the number of terminally differentiated oligodendrocytes, both in culture and in vivo, and also have disrupted paranodes like their CGT-null counterparts. It would seem, therefore, that the effects on myelin domain structure seen in mice lacking CGT might result primarily from the absence of sulphatide. The precise function of both lipids remains to be determined. Lipids are probably targeted to the growing process as a consequence of their interactions with particular proteins. If this is so, then the question remains as to how proteins are segregated into nascent myelin. It seems likely that this occurs as a result of a combination of factors, such as the specific targeting of proteins during their biosynthesis, and the consolidation of cis associations between proteins within the myelin domain by trans adhesive associations during compaction and axon–glia interaction. The discovery that myelin basic protein (MBP) is synthesized in the growing myelin process was a major step forwards in our understanding of how proteins might be delivered to the myelin membrane. This was one of the first demonstrations of localized mRNA translation in a eukaryotic cell, and indicated that MBP is incorporated into the growing myelinating process at sites that are quite distant from the oligodendrocyte cell
body (FIG. 1).

This concentration of MBP mRNA at distal sites has also been observed in Schwann cells, which indicates a common mechanism for process assembly in the CNS and PNS. Surprisingly, few other mRNAs have been shown to be similarly localized in either oligodendrocytes or Schwann cells, and those that have been identified are of unknown function. How might the mRNAs for myelin proteins be translocated to the growing process for local translation? A series of studies from the laboratory of E. Barbarese and J. H. Carson has firmly implicated the microtubule system in oligodendrocytes. Translocated mRNAs, such as MBP mRNA, contain an A2RE sequence element that binds the heteronuclear ribonucleo protein (hnRNP) A2 protein. Localization of this protein in oligodendrocytes is microtubule-dependent, and recent data show that a microtubule-associated protein, TOG2, is probably responsible for the association of hnRNP A2-positive mRNA granules with micro tubules during transport and/or localization. It seems likely that a similar system operates in Schwann cells, as transport of MBP mRNA from the nucleus to distal sites at the paranodal regions also requires intact microtubules. There is a growing appreciation that the transport of mRNAs in ribonucleoprotein granules from the nucleus to distal sites in polarized cells (such as glia and neurons) might add another layer of regulation to both the assembly and the local function of membrane domains. Recent work has indicated that the targeting and local translation of neuronal mRNAs might also require the association of short (~20 nucleotides long) microRNAs (miRNAs) with both mRNAs and polyribosomes in RNA granules. Base-pairing of these miRNAs to comple mentary sequences in target RNAs is believed to suppress translation until the granules reach their target sites — for example, dendritic synapses. How translation arrest is released is not yet clear. However, the fact that the complement of miRNAs changes as neurons mature indicates that these regulatory mechanisms might operate during development as well as in response to local synaptic activity. It will be of great interest to determine whether similar mechanisms operate in myelin-forming glia during both myelination and remyelination. In addition to microtubules, other components of the glial cytoskeleton, such as the microfilaments, are likely to have a role during process extension. An important step in determining these was the recent identification of Rho kinase (ROCK) as a key regulator of myelinating process morphology and extension in Schwann cells. ROCK regulates the actin–myosin mechanical transduction system by phosphorylating myosin light chains. ROCK itself is an effector of Rho, a well-known regulator of actin cytoskeleton dynamics in various cell types. In Schwann cells, ROCK inhibition does not influence proliferation or differentiation. However, in the absence of ROCK activity the single myelinating process of the Schwann cell splits to form many smaller internodes, each with their appropriate paranodes and nodes. As we discuss below when considering the mechanisms of Schwann cell elongation and the functional consequences of short internodes, splitting of the myelinated segments would be expected to have deleterious consequences for nerve conduction velocities. Nevertheless, these experiments do point to a role for actomyosin in ensuring that the internode comprises a single Schwann cell.
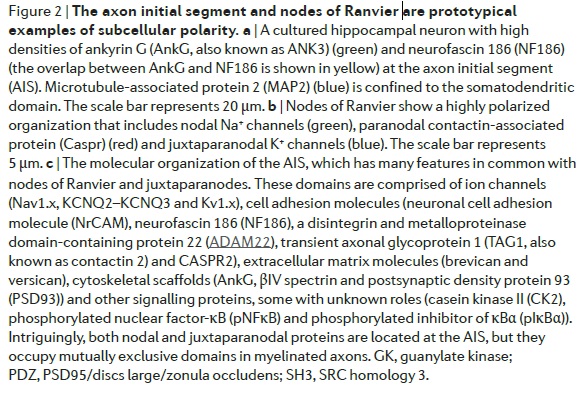
Ensheathment by myelin-forming glia and node formation are almost certainly inextricably linked (FIG. 2a)

Myelinating glia promote axonal survival
This is perhaps the most important issue if we wish to apply what we learn about axon–glia interactions to treating human demyelinating diseases. The disastrous consequences of demyelination on axon degeneration explains the serious long-term disabilities that patients experience in the CNS disease multiple sclerosis and in demyelinating diseases of the PNS such as CHARCOT MARIE TOOTH DISEASE. Myelin itself might not be a crucial factor, as in mice with oligodendrocytes that lacked a functional cyclic nucleotide phosphodiesterase 1 (Cnp1) gene, axonal degeneration could occur even after mild derangements to glial cells without any obvious effect on myelination. The fact that ensheathment of axons by myelin-forming glia has profound effects on the axon is clear from the fact that myelination establishes distinct domains in the axonal membrane where proteins associate in specific macromolecular assemblies, such as the sodium channel complex at the node of Ranvier. However, the influence of glial contact is not restricted to the axonal membrane. Myelinating Schwann cells not only affect the extent of neurofilament phosphorylation but also reduce the rates of slow axonal transport. Axonal calibre was also reduced by the absence of glial contact, presumably as a consequence of the reduced neurofilament phosphorylation. Similar effects of demyelination have been observed in the CNS, where reduced rates of fast axonal transport were also observed. Aberrant axonal transport can certainly lead to axonal degeneration, as has been shown in both animal models and human diseases in which mutations that affect the microtubule-based motors kinesin KIF5A, KIF1B and the dynein–dynactin complex cause axonal degeneration.
Concluding remarks and future perspectives
The association between myelin-forming glia and the axons that they ensheath is a remarkable example of cell–cell interaction[/b], not least because the paranodal axo–glial junction is by far the largest intercellular adhesion complex found in vertebrate biology. Glia influence the establishment of distinct axonal domains and the subsequent growth of both myelin and myelinated nerves. These processes are the result of a fascinating combination of reciprocal intercellular signalling and cell autonomous mechanisms.Although we now have a much clearer idea of the constituents of the paranodal and nodal domains, the key molecules that nucleate their assembly and how these molecules are targeted to these domains are still not clear. With respect to the node of Ranvier, the current assumption is that one or a few proteins in the axon pioneer the assembly of the node in response to either diffusible or membranebound proteins that originate from the ensheathing glial cells. Once these nodal proteins arrive at the nascent node they might act as anchors for the clustering of other proteins, including sodium channels themselves. Although the snow-plough model, by which the extending glial sheath pushes these pioneer axonal proteins to the nascent node, is intuitively attractive, it seems unlikely to be correct in a simple mechanical sense because physical interaction at the paranode between axonal and glial cell is not essential for sodium channel clustering, at least in the CNS. Clearly, identifying the signalling pathways that link glia to the trafficking of membrane proteins in neurons will be a key area for further study. Progress in these areas and in understanding how axons are selected for myelination will no doubt accelerate as the involvement of more candidate proteins is revealed, in addition to the neuregulins and neurotrophic factors. An important challenge will be to understand the molecular basis for the longer term effects of glial ensheathment on the cell biology of the axon. These include the molecular mechanisms through which glia promote axon survival and the factors (and reasons) that underlie the relatively late switch in sodium channel type at the nodes of Ranvier
1. http://www.nature.com.ololo.sci-hub.cc/nrn/journal/v6/n9/full/nrn1743.html
2. http://jonlieffmd.com/blog/myelin-facilitation-of-whole-brain-neuroplasticity
3. http://jonlieffmd.com/blog/new-myelin-code-adds-to-brain-complexity
http://reasonandscience.heavenforum.org/t2586-axon-ensheathment-and-myelin-growth-amazing-evidence-of-design
Myelin Facilitation of Whole Brain Neuroplasticity 2
For decades, myelin has been understood as a simple insulator of axons that increases the speed of neuronal transmission. There was little known about oligodendrocytes—the cells that make myelin. Recently, vast complexity in the process of oligodendrocytes’ wrapping and compressing layers of myelin has been discovered. In fact, this three dimensional process is one of the most elaborate in nature. It involves signaling between the oligodendrocytes and neurons for many different stages of the process—determining where myelin will be built and where first contact occurs between the two cells; wrapping the layers and growing them along the axon; determining how many layers are wrapped and where the nodes will be; and the intricate compaction of the layers. The process, also, keeps lines of communication through the compacted regions and maintains the structure sometimes for decades. Also, stem cells making oligodendrocytes travel throughout the brain determining where the myelin will be built. All of this is connected with the needs of the neuron and astrocyte networks across the brain. It is vital for all learning and cognition.
Laying down myelin is far more complex than synapses and neuro muscular junctions. The immensely elaborate process of modeling, remodeling, and maintenance of myelin occurs all over the brain simultaneously. Neuroplasticity has been assumed to be a vast array of mechanisms related to synapses. Now, new research shows an entirely new dimension, where myelin is a vital part of neuroplasticity for all memory, not just motor memory. Myelin neuroplasticity is directed by electrical activity of the neuron and by elaborate communication with oligodendrocytes and many other cells. The stem cells that produce oligodendrocytes are, also, directed by conversations with epithelial cells in blood vessels. Oligodendrocytes are multi polar cells that produce fatty myelin sheaths and wrap them around the axons many times. This sheath greatly enhances the speed of the electrical signal along the axon. Without it, the electrical wave depends on movement from each small sodium and potassium pore to the next all along the membrane. With myelin, the electrical signal rapidly jumps between nodes with long stretches of insulated myelin.
As regions of the brain form myelin and the connections begin to operate, specific abilities coincide with the development. The gradual growing of myelin down the long motor neurons from the brain produce a stepwise increase in function in babies—first, stiffening neck, then arm movement, sitting up, standing up and walking. Later, speaking and cognitive abilities appear with myelinated brain circuits. It is only as young adults that the frontal lobes myelinate with the dawning of judgment and adult decision making.
Signals must arrive within milliseconds to be effective in complex circuits. Plasticity for synapses depends on simultaneous arrivals so that decisions can be appropriately made for the next axon firing in the postsynaptic neuron. The next signal is weakened if one contributing circuit arrives late and strengthened if early within a very narrow time scale. Myelin creates rapid speeds, but it must produce the exact level of speed needed for the neuroplasticity. In fact, speeds of myelinated axons vary widely in a scale of a thousand—a tenth of a yard per second to 200 yards per second.
As well as the number of wraps the the spacing of nodes, another consideration is different types of electrical signals. Some sensory signals are simply a singular spike of a certain intensity. In much of the brain however, there are circuits that provide timed pulsed signals at different frequencies for particular background tasks.
Timed pulses occur in circuits of the default mode network and, also, with attention, sleep, top down regulation of sensory input and perception, to name just a few. These need regulation of the velocities of signals at various rates. For example, brain wave oscillations, such as gamma waves can be altered by delays in the speed of the conduction by even a very small amount. When conduction is altered it can lead to brain disease related to white matter changes.
Regulation is always a informational task, and depends on the right pre-programming.
Even though changes cannot be well visualized in imaging studies, it is now known that alterations in white matter (axon bundles) occur related to the amount of electrical use of a nerve. Increased myelin is correlated with the amount of learning. Some changes occur very rapidly after only two hours of learning in humans and animals. The large white matter coming out of the hippocampus memory center is called the fornix, which was altered with 2 hours of learning. The first part of the axon (initial axon segment or AIS) near the cell body is unique in that only half of it is myelinated and therefore part of it is similar to half a node of Ranvier. The AIS is where the electrical spike is started with very complex ion channels that regulate the entire process. The AIS determines not just when the electrical current will start but how big it will be and its electrical “shape”. This determines exactly how much neurotransmitter will be discharged at the synapse, as well as the exact neurotransmitter to be sent. A very significant finding is that the AIS actually alters is size and position to regulate the complex information that is sent. Since the AIS is similar to a node of Ranvier, it is thought that similar alterations occur at each node with local stimulation. There is evidence of changes in the sizes of nodes and the length between them during neuroplasticity in the ear.
Myelin Wrapping Process is Very Complex
Triggering oligodendrocytes to make myelin in special ways involves a very elaborate multi step process. Myelin must be produced by triggering elaborate timed genetic networks including correct placement of the first wrap of the sheath around the membrane of the axon. It cannot just be left there. The sheath has to, then, be serviced for a very long time, often decades. The oligodendrocyte sends out a hand like process to touch the axon membrane where a very unique junction region is created. Immunoglobulin on the neuron is necessary to trigger the oligodendrocyte’s activity. Very elaborate cascades produce a multi unit protein factory built out of scaffolding tubules, as well as receptors, to direct the unfolding of the complex myelin producing process. Signaling regulates the proteins needed to make myelin and the way they are placed in the membrane to hold it in place. Very complex cascades of molecules include kinase enzymes, integrin and molecules based on the lipid cholesterol. The original immunoglobulin is stimulated by particular frequencies from the neuron and is only essential for the first layer of the wrapping of the sheath.
Wrapping of the myelin will not occur without a special measurement of the size of the axon circumference including special curvature sensors. The axon has to be above a particular circumference for the process to start. The cellular process makes a spiral sheet in 3D. It is an amazing process where a length of 100 micrometers (10-6) with 10 spirals takes 6400 square micrometers of membrane. The wrapping process is very hard to understand and there are several competing models to grasp it, currently.A leading tongue crawls around the axon leaving a sheet of myelin. Additional wraps and lengthening of the total myelin happens at the same time. A process from oligodendrocytes connects to the myelin and more wraps occur there. The inner layers are smaller than the later layers. The tongues are pushed by actin. Later the inner layers grow and catch up to the covering layers. As the second wrap occurs, the bottom layer is growing bigger. Each layer attaches permanently in a particular placement near where the node of Ranvier will be forming small paranode attachment areas.
The myelin wrapping grows in two ways at once—more layers and the entire sheath gets longer at each level. When three layers are formed, a complex process of compaction starts at the outside layers. During compaction, special channels are created to allow transport of materials through the compacted areas and the growth zone. Compaction occurs both along the length of the axon and around the circular wrap at the same time. When compaction has eliminated most of the cytoplasm by pushing it out, a special protein binds the two membranes together as if in a tight junction between cells. All proteins with more than 30 amino acids are removed. This process is similar to a zipper.
Adhesions and compaction also occur between the extracellular membranes of two layers of wrap. Compaction is not fully understood, but it involves eliminating electrostatic repulsion and other forces. Many receptors and signals such as neureglin regulate the number of wraps, which determines the properties of the electrical signal for neuroplasticity. Another complication is that oligodendrocytes are in close communication with blood vessels. The brain has more types of cells than any organ in nature and they must travel to exact places over long distances. Oligodendrocytes migrate the most of all cells throughout life. It was recently found that stem cells to make oligodendrocytes travel along blood vessels. Elaborate signaling between the oligodendrocytes and the blood vessels define their routes.
New Myelin Code Adds to Brain Complexity 3
The common wisdom that myelin is just an insulator to make faster neuronal communication has been overthrown by recent research that finds much more complexity in how myelin is used by the brain. The old “neuron doctrine” (one of many useless dogmas) was that each neuron sends a signal to the next neuron, and so on, in computer like fashion. In fact, the process is vastly more complex and variable and the new myelin code adds to brain complexity in very important ways.Myelin patterns are different in each brain region, especially each of the six layers of the cortex—myelin appears to have its own very complex code. Un-myelinated axons are crucial for newly discovered types of communication between the naked axons and adjacent local immune and nervous system cells. Both myelin, interspersed patterns of myelin, and lack of myelin are essential for specific types of information transfer. Oligodendrocytes, also, make many decisions concerning the types of signals.
The neuron has many different methods of communication. In addition to the well-studied axon spikes triggering secretion of chemical neurotransmitters and the less studied electrical synapses and synchronous brain waves, there are now newly discovered sideways communication from the un-myelinated axons, nanotubes and vesicles. This can take the form of cytokine signaling to local immune cells and neurotransmitter signaling to other neurons, glia and immune cells. In addition, electrical information is transmitted in the form of local field potential with many contributing factors such as shape of cells, extracellular matrix and calcium spikes.
Myelin affects the way information is created and communicated. In layers II and III, there are different patterns of very long intermittent stretches without any myelin. Also, the critical initial segment that determines the type and strength of the signal was different in each region. In fact, many different regions of the brain have different amounts and distribution of myelin. The myelin in each region is so different from each other, and so different than what was previously assumed, it appears to be yet another brain code.
Initial Segment Is Important and Varied
Perhaps, most significantly, the initial region from the cell body to the start of myelination along the axon is very different in each of the cortical layers. This initial segment is considered to be very important for the propagation of the signal and can vary from 5um to 80 um in length. It is where the decision is made whether or not to send an electrical signal along the axon. The initial segment has a very complex set of ion channels and structures that help make the decision to spike. It, also, determines the specific shape of the electrical spike and the amount of neurotransmitter release. The length of this segment was longer in layers III and IV than in V and VI.
The initial segment, also, determines whether the spike can go backwards or not. Backward transmission determines aspects of neuroplasticity, such as in memory consolidation in the hipppocampus during deep sleep and during quiet reflection when awake.
Myelin, it now appears, also, plays a part in determining where synapses will occur. There are specific proteins in the myelin sheath that stop the budding of an axon. The oligodendrocyte and its myelin therefore determines the structure of the circuit.
The
Selection of axons and initiation of contact
The reasons why some axons are myelinated and others are not are still baffling.
If a complex nervous system does not depend on myelin sheath, why did natural selection produce glial cells and myelination in the first place?
In general, a minimum calibre is required (~1 μm) before an axon can be myelinated. The physiological rationale for this threshold is that the saltatory mode of conduction would probably not confer significantly enhanced rates of nerve impulse transmission on small calibre fibres, but how axons of a minimum calibre are selected for myelination is still not understood. This is of particular relevance in the context of demyelinating diseases such as MULTIPLE SCLEROSIS, for which it has been proposed that changes in the expression of proteins on the axonal surface might make axons less susceptible to remyelination. Certain cell adhesion molecules, such as L1 and polysialylated NCAM (neural cell adhesion molecule), are known to be expressed on unmyelinated axons and to be downregulated during axonal myelination. However, whether or not the disappearance of these molecules is mechanistically connected to axon ensheathment is not clear. The L1-knockout mouse shows no obvious derangements in myelination, such as ensheathment of inappropriate axons . Other candidates for sensing axonally presented molecules on oligo dendrocytes and Schwann cells include the integrins and neuro fascin. Integrins seem to be most important for survival mechanisms in early oligodendrocytes and in Schwann cells their best-characterized role is in the radial sorting of unmyelinated axons. Laminins, the ligands for some integrins, also have an important role in the defasciculation of axons. Dystroglycan, the other laminin receptor on Schwann cells, is not vital for the early stages of myelination, and seems to be more important for the organization of the nodal microvilli. The neurofascin gene and its products do play an important part in later stages of myelination (see below), however, they do not seem to be necessary for initial ensheathment.
Myelin process extension around target axons
The function of myelin in the Central Nervous System CNS and Peripheral Nervous System PNS appears to be identical, but there are differences in the cell biology of myelination when oligodendrocytes are compared with Schwann cells. For example, the Schwann cell has an intimate association with the axon that it myelinates, and lines up along the axon to define a single internode, whereas oligodendrocytes extend several processes, each of which myelinates distinct internodes, often on different axons. Nevertheless, in both cases a myelinating process that is continuous with the plasma membrane must be assembled and extended. Current concepts of LIPID RAFTS, which propose the existence of microdomains in membranes, might help to explain how proteins and lipids are delivered to the growing membrane. However, such concepts might be less useful for understanding how the myelin macrodomain, with its distinct protein and lipid content, is stably segregated from the plasma membrane of the myelin-forming glial cell.The myelin membrane contains a high percentage of lipids compared with the plasma membranes of most eukaryotic cells.
Cholesterol is a major constituent, and mice with oligodendrocytes that lack the ability to synthesize cholesterol show a delay in myelination that seems to be at least partially compensated for by cholesterol uptake. There has been particular interest in determining the significance of the high galactolipid content of myelin. Mice with a disruption in the gene that encodes the biosynthetic enzyme UDP-galactose: ceramide galactosyl transferase (CGT) cannot
synthesize galactolipids, but seem to compensate by replacing these lipids with their gluco-analogues. Surprisingly, only the myelin of the CNS shows any resulting structural and functional defects, including disrupted PARANODAL AXOGLIAL JUNCTIONS and reduced nerve conduction velocities. In order to discriminate between the effects of the absence of galactolipids and their sulphated derivatives, mice that lack cerebroside sulphotransferase were generated. These mice show a two- to threefold enhancement in the number of terminally differentiated oligodendrocytes, both in culture and in vivo, and also have disrupted paranodes like their CGT-null counterparts. It would seem, therefore, that the effects on myelin domain structure seen in mice lacking CGT might result primarily from the absence of sulphatide. The precise function of both lipids remains to be determined. Lipids are probably targeted to the growing process as a consequence of their interactions with particular proteins. If this is so, then the question remains as to how proteins are segregated into nascent myelin. It seems likely that this occurs as a result of a combination of factors, such as the specific targeting of proteins during their biosynthesis, and the consolidation of cis associations between proteins within the myelin domain by trans adhesive associations during compaction and axon–glia interaction. The discovery that myelin basic protein (MBP) is synthesized in the growing myelin process was a major step forwards in our understanding of how proteins might be delivered to the myelin membrane. This was one of the first demonstrations of localized mRNA translation in a eukaryotic cell, and indicated that MBP is incorporated into the growing myelinating process at sites that are quite distant from the oligodendrocyte cell
body (FIG. 1).

This concentration of MBP mRNA at distal sites has also been observed in Schwann cells, which indicates a common mechanism for process assembly in the CNS and PNS. Surprisingly, few other mRNAs have been shown to be similarly localized in either oligodendrocytes or Schwann cells, and those that have been identified are of unknown function. How might the mRNAs for myelin proteins be translocated to the growing process for local translation? A series of studies from the laboratory of E. Barbarese and J. H. Carson has firmly implicated the microtubule system in oligodendrocytes. Translocated mRNAs, such as MBP mRNA, contain an A2RE sequence element that binds the heteronuclear ribonucleo protein (hnRNP) A2 protein. Localization of this protein in oligodendrocytes is microtubule-dependent, and recent data show that a microtubule-associated protein, TOG2, is probably responsible for the association of hnRNP A2-positive mRNA granules with micro tubules during transport and/or localization. It seems likely that a similar system operates in Schwann cells, as transport of MBP mRNA from the nucleus to distal sites at the paranodal regions also requires intact microtubules. There is a growing appreciation that the transport of mRNAs in ribonucleoprotein granules from the nucleus to distal sites in polarized cells (such as glia and neurons) might add another layer of regulation to both the assembly and the local function of membrane domains. Recent work has indicated that the targeting and local translation of neuronal mRNAs might also require the association of short (~20 nucleotides long) microRNAs (miRNAs) with both mRNAs and polyribosomes in RNA granules. Base-pairing of these miRNAs to comple mentary sequences in target RNAs is believed to suppress translation until the granules reach their target sites — for example, dendritic synapses. How translation arrest is released is not yet clear. However, the fact that the complement of miRNAs changes as neurons mature indicates that these regulatory mechanisms might operate during development as well as in response to local synaptic activity. It will be of great interest to determine whether similar mechanisms operate in myelin-forming glia during both myelination and remyelination. In addition to microtubules, other components of the glial cytoskeleton, such as the microfilaments, are likely to have a role during process extension. An important step in determining these was the recent identification of Rho kinase (ROCK) as a key regulator of myelinating process morphology and extension in Schwann cells. ROCK regulates the actin–myosin mechanical transduction system by phosphorylating myosin light chains. ROCK itself is an effector of Rho, a well-known regulator of actin cytoskeleton dynamics in various cell types. In Schwann cells, ROCK inhibition does not influence proliferation or differentiation. However, in the absence of ROCK activity the single myelinating process of the Schwann cell splits to form many smaller internodes, each with their appropriate paranodes and nodes. As we discuss below when considering the mechanisms of Schwann cell elongation and the functional consequences of short internodes, splitting of the myelinated segments would be expected to have deleterious consequences for nerve conduction velocities. Nevertheless, these experiments do point to a role for actomyosin in ensuring that the internode comprises a single Schwann cell.
Ensheathment by myelin-forming glia and node formation are almost certainly inextricably linked (FIG. 2a)

Myelinating glia promote axonal survival
This is perhaps the most important issue if we wish to apply what we learn about axon–glia interactions to treating human demyelinating diseases. The disastrous consequences of demyelination on axon degeneration explains the serious long-term disabilities that patients experience in the CNS disease multiple sclerosis and in demyelinating diseases of the PNS such as CHARCOT MARIE TOOTH DISEASE. Myelin itself might not be a crucial factor, as in mice with oligodendrocytes that lacked a functional cyclic nucleotide phosphodiesterase 1 (Cnp1) gene, axonal degeneration could occur even after mild derangements to glial cells without any obvious effect on myelination. The fact that ensheathment of axons by myelin-forming glia has profound effects on the axon is clear from the fact that myelination establishes distinct domains in the axonal membrane where proteins associate in specific macromolecular assemblies, such as the sodium channel complex at the node of Ranvier. However, the influence of glial contact is not restricted to the axonal membrane. Myelinating Schwann cells not only affect the extent of neurofilament phosphorylation but also reduce the rates of slow axonal transport. Axonal calibre was also reduced by the absence of glial contact, presumably as a consequence of the reduced neurofilament phosphorylation. Similar effects of demyelination have been observed in the CNS, where reduced rates of fast axonal transport were also observed. Aberrant axonal transport can certainly lead to axonal degeneration, as has been shown in both animal models and human diseases in which mutations that affect the microtubule-based motors kinesin KIF5A, KIF1B and the dynein–dynactin complex cause axonal degeneration.

Concluding remarks and future perspectives
The association between myelin-forming glia and the axons that they ensheath is a remarkable example of cell–cell interaction[/b], not least because the paranodal axo–glial junction is by far the largest intercellular adhesion complex found in vertebrate biology. Glia influence the establishment of distinct axonal domains and the subsequent growth of both myelin and myelinated nerves. These processes are the result of a fascinating combination of reciprocal intercellular signalling and cell autonomous mechanisms.Although we now have a much clearer idea of the constituents of the paranodal and nodal domains, the key molecules that nucleate their assembly and how these molecules are targeted to these domains are still not clear. With respect to the node of Ranvier, the current assumption is that one or a few proteins in the axon pioneer the assembly of the node in response to either diffusible or membranebound proteins that originate from the ensheathing glial cells. Once these nodal proteins arrive at the nascent node they might act as anchors for the clustering of other proteins, including sodium channels themselves. Although the snow-plough model, by which the extending glial sheath pushes these pioneer axonal proteins to the nascent node, is intuitively attractive, it seems unlikely to be correct in a simple mechanical sense because physical interaction at the paranode between axonal and glial cell is not essential for sodium channel clustering, at least in the CNS. Clearly, identifying the signalling pathways that link glia to the trafficking of membrane proteins in neurons will be a key area for further study. Progress in these areas and in understanding how axons are selected for myelination will no doubt accelerate as the involvement of more candidate proteins is revealed, in addition to the neuregulins and neurotrophic factors. An important challenge will be to understand the molecular basis for the longer term effects of glial ensheathment on the cell biology of the axon. These include the molecular mechanisms through which glia promote axon survival and the factors (and reasons) that underlie the relatively late switch in sodium channel type at the nodes of Ranvier
1. http://www.nature.com.ololo.sci-hub.cc/nrn/journal/v6/n9/full/nrn1743.html
2. http://jonlieffmd.com/blog/myelin-facilitation-of-whole-brain-neuroplasticity
3. http://jonlieffmd.com/blog/new-myelin-code-adds-to-brain-complexity