Aquaporins , amazing evidence of design
Aquaporins: Extraordinary filters, permeable to water, but impermeable to Ions
Because cells are mostly water (typically ~70% by weight), water movement across cell membranes is fundamentally important for life. Cells also contain a high concentration of solutes, including numerous negatively charged organic molecules that are confined inside the cell (the so-called fixed anions) and their accompanying cations that are required for charge balance. This creates an osmotic gradient, which mostly is balanced by an opposite osmotic gradient due to a high concentration of inorganic ions in the extracellular fluid. The small remaining osmotic force tends to “pull” water into the cell, causing it to swell until the forces are balanced. Because all biological membranes are moderately permeable to water, cell volume equilibrates in minutes or less in response to an osmotic gradient.
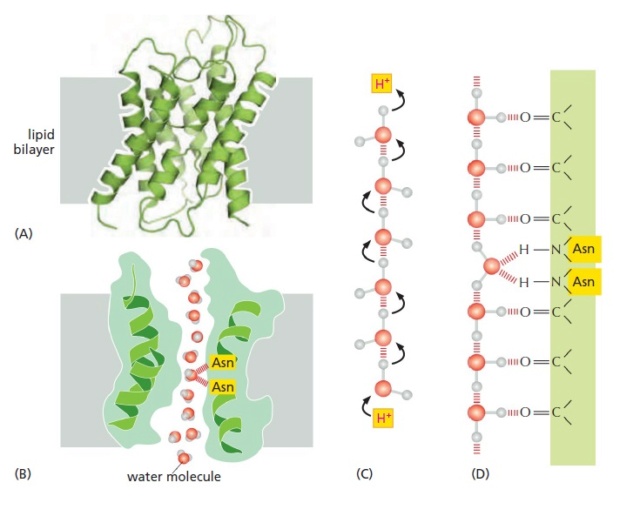
The structure of aquaporins.
(A) A ribbon diagram of an aquaporin monomer. In the membrane, aquaporins form tetramers, with each monomer containing an aqueous pore in its center (not shown). Each individual aquaporin channel passes about 109
water molecules per second.
(B) A longitudinal cross section through one aquaporin monomer, in the plane of the central pore. One face of the pore is lined with hydrophilic amino acids, which provide transient hydrogen bonds to watermolecules; these bonds help line up the transiting water molecules in a single row and orient them as they traverse the pore.
(C and D) A model explaining why aquaporins are impermeable to H+.
(C) In water, H+ diffuses extremely rapidly by being relayed from one water molecule to the next.
(D) Carbonyl groups (C=O) lining the hydrophilic face of the pore align water molecules, and two strategically placed asparagines in the center help tether a central water molecule such that both valences on its oxygen are occupied. This arrangement bipolarizes the entire line of water molecules, with each water molecule acting as a hydrogen-bond acceptor from its inner neighbor
For most animal cells, however, osmosis has only a minor role in regulating cell volume. This is because most of the cytoplasm is in a gel-like state and resists large changes in its volume in response to changes in osmolarity. In addition to the direct diffusion of water across the lipid bilayer, some prokaryotic and eukaryotic cells have water channels, or aquaporins, embedded in their plasma membrane to allow water to move more rapidly. Aquaporins are particularly abundant in animal cells that must transport water at high rates, such as the epithelial cells of the kidney or exocrine cells that must transport or secrete large volumes of fluids, respectively (Figure below).
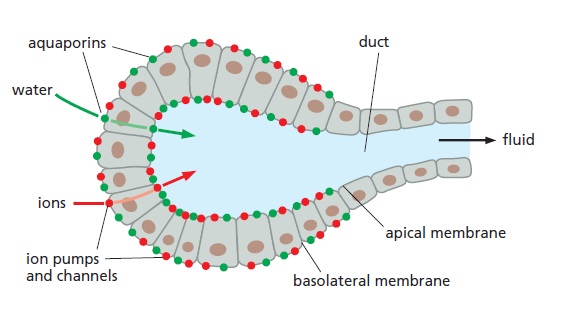
The role of aquaporins in fluid secretion.
Cells lining the ducts of exocrine glands (as found, for example, in the pancreas and liver, and in mammary, sweat, and salivary glands) secrete large volumes of body fluids. These cells are
organized into epithelial sheets in which their apical plasma membrane faces the lumen of the duct. Ion pumps and channels situated in the basolateral and apical plasma membrane move ions (mostly Na+ and Cl–) into the ductal lumen, creating an osmotic gradient between the surrounding tissue and the duct. Water molecules rapidly follow the osmotic gradient through aquaporins that are present in high concentrations in both the apical and basolateral membranes.
Aquaporins must solve a problem that is opposite to that facing ion channels. To avoid disrupting ion gradients across membranes, they have to allow the rapid passage of water molecules while completely blocking the passage of ions. The three-dimensional structure of an aquaporin reveals how it achieves this remarkable selectivity. The channels have a narrow pore that allows water molecules to traverse the membrane in single file, following the path of carbonyl oxygens that line one side of the pore (Figure A and B). Hydrophobic amino acids line the other side of the pore. The pore is too narrow for any hydrated ion to enter, and the energy cost of dehydrating an ion would be enormous because the hydrophobic wall of the pore cannot interact with a dehydrated ion to compensate for the loss of water. This design readily explains why the aquaporins cannot conduct K+ Na+, Ca2+, or Cl– ions. These channels are also impermeable to H+, which is mainly present in cells as H3O+. These hydronium ions diffuse through water extremely rapidly, using a molecular relay mechanism that requires the making and breaking of hydrogen bonds between adjacent water molecules (Figure C). Aquaporins contain two strategically placed asparagines, which bind to the oxygen atom of the central water molecule in the line of water molecules traversing the pore, imposing a bipolarity on the entire column of water molecules (Figure C and D). This makes it impossible for the “making and breaking” sequence of hydrogen bonds (shown in Figure C) to get past the central asparagine-bonded water molecule. Because both valences of this central oxygen are unavailable for hydrogen- bonding, the central water molecule cannot participate in an H+ relay, and the pore is therefore impermeable to H+.
Lipid bilayer membranes protect and accommodate life, the cell also needs channels to ferry essential materials in and out. If we had contracted out the job to a top nano-tech company employing all its powers of knowledge of engineering, we wouldn't get a better job done. These lipid bilayer membranes come with 3-D protein assemblies that work beautifully as selective channels. These channels select what needs to be let in and keep out what needs to be kept out. Membrane-associated proteins, membrane bioenergetics, and lipid bilayers work in an interdependent manner. A. Y. Mulkidjanian wrote that “the origin(s) of the membrane(s) and membrane proteins remain enigmatic.” Permitting water to get in and out from cells is essential to survive and thrive, and aquaporins do that job. However, water entry and exit must be carefully controlled if the cell. This need for control arises because water molecules are connected by hydrogen bonds, and its hydrogen-bonding network makes water function as a “proton wire” that carries protons (H+) down it, much as an electrical wire carries electrical current. But for metabolic reasons, all cells must keep their interiors electrically negative. Cells manage this with special membrane channels that control the transport of sodium (Na+) and potassium (K+) ions. If aquaporins were to let water enter the cell freely, the “proton wires” would allow positively charged hydrogen ions (H+) to overwhelm the cell’s efforts to remain electronegative. So a simple watergate isn’t enough. This engineering challenge is no easy one to solve. If the intrinsic properties of the H2O molecule to remove its proton-wire ability would change, this would muck up many of the other unusual and life-essential properties of H2O. Aquaporins in cell membranes not only let H2O into and out of the cell, but also keep out impurities such as undesirable ions and other harmful biomolecules, as well as the positively charged hydrogen ions (H+) that normally travel freely along H2O’s proton wires. So how is this intricate task accomplished? In the aquaporin water gates, asparagine amino acid is perfectly positioned, at the exact point of the passage of a single H2O molecule. Asparagine is a member of the amino acids that are important for building and shaping the structures of proteins, but in addition it possesses a side group able to establish two very strong and spatially oriented Hydrogen bonds with H2O molecules. The perfect 3-D alignment of this amino acid, perpendicular to the passage of the H2O proton wire, then can function as a true “molecular plier” to cut the H2O wire. Exactly at the moment it passes through the filter, H2O is twisted by asparagine. This exquisitely orchestrated maneuver, driven by stronger H-bonds, breaks the network of water’s H bonds, thereby cutting the H+ wire. With a broken H+ wire, H2O freely enters the cell while H+ is blocked at the door.
Without the fully set-up of this mechanism, there would be no life. That raises the question: How did this mechanism come to be if it is an all or nothing business? The problem is ingeniously solved. How could it have been solved by unguided mindless fortuitous accidental events? The problem is, natural selection can only go to work once a viable, self-reproducing cell exists, and it can only progress if each stage in the proposed evolutionary process of construction can somehow be preserved and passed along. Yet nothing gets preserved and passed along if the first proto-cells die a swift death for lack of a fully functioning cell membrane, able to accomplish the many essential tasks outlined above (among many others). No multi-task cell membrane, no life. The discovery of this marvel of molecular ingenuity earned the 2003 Nobel Prize in Chemistry, “for the discovery of water channels” and “for structural and mechanistic studies of ion channels.” But if Nobel-caliber intelligence was required to figure out how this existing engineering marvel works, what was required to invent it in the first place? A hypothetical primitive membrane with a partly evolved aquaporin, one that allowed water in but hadn’t yet evolved the ability to block the entry of H+, would have no chance of survival. Such a cell, surrounded by the many enemies of a primordial ocean or “warm little pond,” would quickly die. No survival. No reproduction. The fully functioning H2O-only gates (no H+ allowed) are a “must” for any type of cell, from the most sophisticated to the hypothetical most “rudimentary,” .
These highly selective and exquisitely engineered gates need to be there from the very beginning. No H+-free water, no life. And the proton-wire challenge is just one of the problems. An only partly evolved water gate with holes either too small or too big would either block water altogether or allow other contaminant molecules to enter the cell and destroy it. A successful water gate poses an “all or nothing” challenge for life. Foresee the need for these exquisitely precise water gates and somehow engineer them for just-in-time delivery, or the grand start-up called life quickly goes bust. And what’s true of the water gates is true of many other aspects of the cell membrane. If we are guided only by the evidence, this complex and multifaceted engineering marvel appears well out of reach of random fortuna. Another type of cause appears necessary, one that can foresee and engineer a cell membrane in all its marvelous sophistication, for just-in-time delivery. And indeed, multi-faceted solutions of this sort, ones that anticipate problems that otherwise would stop any potential evolutionary development in its tracks, are evident throughout life.
Aquaporins: Extraordinary filters, permeable to water, but impermeable to Ions
Because cells are mostly water (typically ~70% by weight), water movement across cell membranes is fundamentally important for life. Cells also contain a high concentration of solutes, including numerous negatively charged organic molecules that are confined inside the cell (the so-called fixed anions) and their accompanying cations that are required for charge balance. This creates an osmotic gradient, which mostly is balanced by an opposite osmotic gradient due to a high concentration of inorganic ions in the extracellular fluid. The small remaining osmotic force tends to “pull” water into the cell, causing it to swell until the forces are balanced. Because all biological membranes are moderately permeable to water, cell volume equilibrates in minutes or less in response to an osmotic gradient.

The structure of aquaporins.
(A) A ribbon diagram of an aquaporin monomer. In the membrane, aquaporins form tetramers, with each monomer containing an aqueous pore in its center (not shown). Each individual aquaporin channel passes about 109
water molecules per second.
(B) A longitudinal cross section through one aquaporin monomer, in the plane of the central pore. One face of the pore is lined with hydrophilic amino acids, which provide transient hydrogen bonds to watermolecules; these bonds help line up the transiting water molecules in a single row and orient them as they traverse the pore.
(C and D) A model explaining why aquaporins are impermeable to H+.
(C) In water, H+ diffuses extremely rapidly by being relayed from one water molecule to the next.
(D) Carbonyl groups (C=O) lining the hydrophilic face of the pore align water molecules, and two strategically placed asparagines in the center help tether a central water molecule such that both valences on its oxygen are occupied. This arrangement bipolarizes the entire line of water molecules, with each water molecule acting as a hydrogen-bond acceptor from its inner neighbor
For most animal cells, however, osmosis has only a minor role in regulating cell volume. This is because most of the cytoplasm is in a gel-like state and resists large changes in its volume in response to changes in osmolarity. In addition to the direct diffusion of water across the lipid bilayer, some prokaryotic and eukaryotic cells have water channels, or aquaporins, embedded in their plasma membrane to allow water to move more rapidly. Aquaporins are particularly abundant in animal cells that must transport water at high rates, such as the epithelial cells of the kidney or exocrine cells that must transport or secrete large volumes of fluids, respectively (Figure below).

The role of aquaporins in fluid secretion.
Cells lining the ducts of exocrine glands (as found, for example, in the pancreas and liver, and in mammary, sweat, and salivary glands) secrete large volumes of body fluids. These cells are
organized into epithelial sheets in which their apical plasma membrane faces the lumen of the duct. Ion pumps and channels situated in the basolateral and apical plasma membrane move ions (mostly Na+ and Cl–) into the ductal lumen, creating an osmotic gradient between the surrounding tissue and the duct. Water molecules rapidly follow the osmotic gradient through aquaporins that are present in high concentrations in both the apical and basolateral membranes.
Aquaporins must solve a problem that is opposite to that facing ion channels. To avoid disrupting ion gradients across membranes, they have to allow the rapid passage of water molecules while completely blocking the passage of ions. The three-dimensional structure of an aquaporin reveals how it achieves this remarkable selectivity. The channels have a narrow pore that allows water molecules to traverse the membrane in single file, following the path of carbonyl oxygens that line one side of the pore (Figure A and B). Hydrophobic amino acids line the other side of the pore. The pore is too narrow for any hydrated ion to enter, and the energy cost of dehydrating an ion would be enormous because the hydrophobic wall of the pore cannot interact with a dehydrated ion to compensate for the loss of water. This design readily explains why the aquaporins cannot conduct K+ Na+, Ca2+, or Cl– ions. These channels are also impermeable to H+, which is mainly present in cells as H3O+. These hydronium ions diffuse through water extremely rapidly, using a molecular relay mechanism that requires the making and breaking of hydrogen bonds between adjacent water molecules (Figure C). Aquaporins contain two strategically placed asparagines, which bind to the oxygen atom of the central water molecule in the line of water molecules traversing the pore, imposing a bipolarity on the entire column of water molecules (Figure C and D). This makes it impossible for the “making and breaking” sequence of hydrogen bonds (shown in Figure C) to get past the central asparagine-bonded water molecule. Because both valences of this central oxygen are unavailable for hydrogen- bonding, the central water molecule cannot participate in an H+ relay, and the pore is therefore impermeable to H+.
Lipid bilayer membranes protect and accommodate life, the cell also needs channels to ferry essential materials in and out. If we had contracted out the job to a top nano-tech company employing all its powers of knowledge of engineering, we wouldn't get a better job done. These lipid bilayer membranes come with 3-D protein assemblies that work beautifully as selective channels. These channels select what needs to be let in and keep out what needs to be kept out. Membrane-associated proteins, membrane bioenergetics, and lipid bilayers work in an interdependent manner. A. Y. Mulkidjanian wrote that “the origin(s) of the membrane(s) and membrane proteins remain enigmatic.” Permitting water to get in and out from cells is essential to survive and thrive, and aquaporins do that job. However, water entry and exit must be carefully controlled if the cell. This need for control arises because water molecules are connected by hydrogen bonds, and its hydrogen-bonding network makes water function as a “proton wire” that carries protons (H+) down it, much as an electrical wire carries electrical current. But for metabolic reasons, all cells must keep their interiors electrically negative. Cells manage this with special membrane channels that control the transport of sodium (Na+) and potassium (K+) ions. If aquaporins were to let water enter the cell freely, the “proton wires” would allow positively charged hydrogen ions (H+) to overwhelm the cell’s efforts to remain electronegative. So a simple watergate isn’t enough. This engineering challenge is no easy one to solve. If the intrinsic properties of the H2O molecule to remove its proton-wire ability would change, this would muck up many of the other unusual and life-essential properties of H2O. Aquaporins in cell membranes not only let H2O into and out of the cell, but also keep out impurities such as undesirable ions and other harmful biomolecules, as well as the positively charged hydrogen ions (H+) that normally travel freely along H2O’s proton wires. So how is this intricate task accomplished? In the aquaporin water gates, asparagine amino acid is perfectly positioned, at the exact point of the passage of a single H2O molecule. Asparagine is a member of the amino acids that are important for building and shaping the structures of proteins, but in addition it possesses a side group able to establish two very strong and spatially oriented Hydrogen bonds with H2O molecules. The perfect 3-D alignment of this amino acid, perpendicular to the passage of the H2O proton wire, then can function as a true “molecular plier” to cut the H2O wire. Exactly at the moment it passes through the filter, H2O is twisted by asparagine. This exquisitely orchestrated maneuver, driven by stronger H-bonds, breaks the network of water’s H bonds, thereby cutting the H+ wire. With a broken H+ wire, H2O freely enters the cell while H+ is blocked at the door.
Without the fully set-up of this mechanism, there would be no life. That raises the question: How did this mechanism come to be if it is an all or nothing business? The problem is ingeniously solved. How could it have been solved by unguided mindless fortuitous accidental events? The problem is, natural selection can only go to work once a viable, self-reproducing cell exists, and it can only progress if each stage in the proposed evolutionary process of construction can somehow be preserved and passed along. Yet nothing gets preserved and passed along if the first proto-cells die a swift death for lack of a fully functioning cell membrane, able to accomplish the many essential tasks outlined above (among many others). No multi-task cell membrane, no life. The discovery of this marvel of molecular ingenuity earned the 2003 Nobel Prize in Chemistry, “for the discovery of water channels” and “for structural and mechanistic studies of ion channels.” But if Nobel-caliber intelligence was required to figure out how this existing engineering marvel works, what was required to invent it in the first place? A hypothetical primitive membrane with a partly evolved aquaporin, one that allowed water in but hadn’t yet evolved the ability to block the entry of H+, would have no chance of survival. Such a cell, surrounded by the many enemies of a primordial ocean or “warm little pond,” would quickly die. No survival. No reproduction. The fully functioning H2O-only gates (no H+ allowed) are a “must” for any type of cell, from the most sophisticated to the hypothetical most “rudimentary,” .
These highly selective and exquisitely engineered gates need to be there from the very beginning. No H+-free water, no life. And the proton-wire challenge is just one of the problems. An only partly evolved water gate with holes either too small or too big would either block water altogether or allow other contaminant molecules to enter the cell and destroy it. A successful water gate poses an “all or nothing” challenge for life. Foresee the need for these exquisitely precise water gates and somehow engineer them for just-in-time delivery, or the grand start-up called life quickly goes bust. And what’s true of the water gates is true of many other aspects of the cell membrane. If we are guided only by the evidence, this complex and multifaceted engineering marvel appears well out of reach of random fortuna. Another type of cause appears necessary, one that can foresee and engineer a cell membrane in all its marvelous sophistication, for just-in-time delivery. And indeed, multi-faceted solutions of this sort, ones that anticipate problems that otherwise would stop any potential evolutionary development in its tracks, are evident throughout life.
Last edited by Admin on Sat Aug 15, 2020 10:18 am; edited 3 times in total


