Neurons, remarkable evidence of design
Neurons have long processes, generate action potentials, receive input from other neurons (or, if they are sensory neurons, from appropriate stimuli), and provide output to other cells (for example, neurons, muscles, glands) via synapses. 1
A neuron, or nerve cell is an electrically excitable cell that processes and transmits information through electrical and chemical signals. These signals between neurons occur via synapses, specialized connections with other cells. Neurons can connect to each other to form neural networks. Neurons are the core components of the brain and spinal cord of the central nervous system (CNS), and of the ganglia of the peripheral nervous system (PNS). Specialized types of neurons include: sensory neurons which respond to touch, sound, light and all other stimuli affecting the cells of the sensory organs that then send signals to the spinal cord and brain, motor neurons that receive signals from the brain and spinal cord to cause muscle contractions and affect glandular outputs, and interneurons which connect neurons to other neurons within the same region of the brain, or spinal cord in neural networks.
A typical neuron consists of a cell body (soma), dendrites, and an axon. The term neurite is used to describe either a dendrite or an axon, particularly in its undifferentiated stage. Dendrites are thin structures that arise from the cell body, often extending for hundreds of micrometres and branching multiple times, giving rise to a complex "dendritic tree". An axon (also called a nerve fiber when myelinated) is a special cellular extension (process) that arises from the cell body at a site called the axon hillock and travels for a distance, as far as 1 meter in humans or even more in other species. Nerve fibers are often bundled into fascicles, and in the peripheral nervous system, bundles of fascicles make up nerves (like strands of wire make up cables). The cell body of a neuron frequently gives rise to multiple dendrites, but never to more than one axon, although the axon may branch hundreds of times before it terminates. At the majority of synapses, signals are sent from the axon of one neuron to a dendrite of another. There are, however, many exceptions to these rules: neurons that lack dendrites, neurons that have no axon, synapses that connect an axon to another axon or a dendrite to another dendrite, etc.
All neurons are electrically excitable, maintaining voltage gradients across their membranes by means of metabolically driven ion pumps, which combine with ion channels embedded in the membrane to generate intracellular-versus-extracellular concentration differences of ions such as sodium, potassium,chloride, and calcium. Changes in the cross-membrane voltage can alter the function of voltage-dependent ion channels. If the voltage changes by a large enough amount, an all-or-none electrochemical pulse called an action potential is generated, which travels rapidly along the cell's axon, and activates synaptic connections with other cells when it arrives.
Neurons do not undergo cell division. In most cases, neurons are generated by special types of stem cells. Astrocytes are star-shaped glial cells that have also been observed to turn into neurons by virtue of the stem cell characteristic pluripotency. In humans, neurogenesis largely ceases during adulthood; but in two brain areas, the hippocampus and olfactory bulb, there is strong evidence for generation of substantial numbers of new neurons.12
Although neurons are very diverse and there are exceptions to nearly every rule, it is convenient to begin with a schematic description of the structure and function of a "typical" neuron. A typical neuron is divided into three parts: the soma or cell body, dendrites, and axon. The soma is usually compact; the axon and dendrites are filaments that extrude from it. Dendrites typically branch profusely, getting thinner with each branching, and extending their farthest branches a few hundred micrometers from the soma. The axon leaves the soma at a swelling called the axon hillock, and can extend for great distances, giving rise to hundreds of branches. Unlike dendrites, an axon usually maintains the same diameter as it extends. The soma may give rise to numerous dendrites, but never to more than one axon. Synaptic signals from other neurons are received by the soma and dendrites; signals to other neurons are transmitted by the axon. A typical synapse, then, is a contact between the axon of one neuron and a dendrite or soma of another. Synaptic signals may be excitatory or inhibitory. If the net excitation received by a neuron over a short period of time is large enough, the neuron generates a brief pulse called an action potential, which originates at the soma and propagates rapidly along the axon, activating synapses onto other neurons as it goes. This is called saltatory conduction.
Many neurons fit the foregoing schema in every respect, but there are also exceptions to most parts of it. There are no neurons that lack a soma, but there are neurons that lack dendrites, and others that lack an axon. Furthermore, in addition to the typical axodendritic and axosomatic synapses, there are axoaxonic (axon-to-axon) and dendrodendritic (dendrite-to-dendrite) synapses.
The key to neural function is the synaptic signaling process, which is partly electrical and partly chemical. The electrical aspect depends on properties of the neuron's membrane. Like all animal cells, the cell body of every neuron is enclosed by a plasma membrane, a bilayer of lipid molecules with many types of protein structures embedded in it. A lipid bilayer is a powerful electrical insulator, but in neurons, many of the protein structures embedded in the membrane are electrically active. These include ion channels that permit electrically charged ions to flow across the membrane, and ion pumps that actively transport ions from one side of the membrane to the other. Most ion channels are permeable only to specific types of ions. Some ion channels are voltage gated, meaning that they can be switched between open and closed states by altering the voltage difference across the membrane. Others are chemically gated, meaning that they can be switched between open and closed states by interactions with chemicals that diffuse through the extracellular fluid. The interactions between ion channels and ion pumps produce a voltage difference across the membrane, typically a bit less than 1/10 of a volt at baseline. This voltage has two functions: first, it provides a power source for an assortment of voltage-dependent protein machinery that is embedded in the membrane; second, it provides a basis for electrical signal transmission between different parts of the membrane.
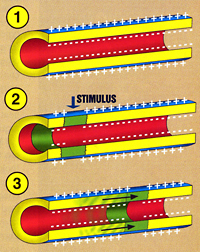
Neurons communicate by chemical and electrical synapses in a process known as neurotransmission, also called synaptic transmission. The fundamental process that triggers the release of neurotransmitters is the action potential, a propagating electrical signal that is generated by exploiting the electrically excitable membrane of the neuron. This is also known as a wave of depolarization.
The soma is the body of the neuron. As it contains the nucleus, most protein synthesis occurs here. The nucleus can range from 3 to 18 micrometers in diameter.The dendrites of a neuron are cellular extensions with many branches. This overall shape and structure is referred to metaphorically as a dendritic tree. This is where the majority of input to the neuron occurs via the dendritic spine.The axon is a finer, cable-like projection that can extend tens, hundreds, or even tens of thousands of times the diameter of the soma in length. The axon carries nerve signals away from the soma (and also carries some types of information back to it). Many neurons have only one axon, but this axon may—and usually will—undergo extensive branching, enabling communication with many target cells. The part of the axon where it emerges from the soma is called the axon hillock. Besides being an anatomical structure, the axon hillock is also the part of the neuron that has the greatest density of voltage-dependent sodium channels. This makes it the most easily excited part of the neuron and the spike initiation zone for the axon: in electrophysiological terms it has the most negative action potential threshold. While the axon and axon hillock are generally involved in information outflow, this region can also receive input from other neurons.The axon terminal contains synapses, specialized structures whereneurotransmitter chemicals are released to communicate with target neurons.
The canonical view of the neuron attributes dedicated functions to its various anatomical components; however dendrites and axons often act in ways contrary to their so-called main function.
Axons and dendrites in the central nervous system are typically only about one micrometer thick, while some in the peripheral nervous system are much thicker. The soma is usually about 10–25 micrometers in diameter and often is not much larger than the cell nucleus it contains. The longest axon of a humanmotor neuron can be over a meter long, reaching from the base of the spine to the toes.
Sensory neurons can have axons that run from the toes to the posterior columnof the spinal cord, over 1.5 meters in adults. Giraffes have single axons several meters in length running along the entire length of their necks. Much of what is known about axonal function comes from studying the squid giant axon, an ideal experimental preparation because of its relatively immense size (0.5–1 millimeters thick, several centimeters long).
Fully differentiated neurons are permanently postmitotic;5 however, research starting around 2002 shows that additional neurons throughout the brain can originate from neural stem cells through the process of neurogenesis. These are found throughout the brain, but are particularly concentrated in thesubventricular zone and subgranular zone.6

Golgi-stained neurons in human hippocampal tissue
Numerous microscopic clumps called Nissl substance (or Nissl bodies) are seen when nerve cell bodies are stained with a basophilic ("base-loving") dye. These structures consist of rough endoplasmic reticulum and associated ribosomal RNA. Named after German psychiatrist and neuropathologist Franz Nissl (1860–1919), they are involved in protein synthesis and their prominence can be explained by the fact that nerve cells are very metabolically active. Basophilic dyes such as aniline or (weakly) haematoxylin 7 highlight negatively charged components, and so bind to the phosphate backbone of the ribosomal RNA.
The cell body of a neuron is supported by a complex mesh of structural proteins called neurofilaments, which are assembled into larger neurofibrils. Some neurons also contain pigment granules, such as neuromelanin (a brownish-black pigment that is byproduct of synthesis of catecholamines), and lipofuscin (a yellowish-brown pigment), both of which accumulate with age.8910 Other structural proteins that are important for neuronal function are actin and thetubulin of microtubules. Actin is predominately found at the tips of axons and dendrites during neuronal development.
There are different internal structural characteristics between axons and dendrites. Typical axons almost never contain ribosomes, except some in the initial segment. Dendrites contain granular endoplasmic reticulum or ribosomes, in diminishing amounts as the distance from the cell body increases.
Neurons exist in a number of different shapes and sizes and can be classified by their morphology and function.12 The anatomist Camillo Golgi grouped neurons into two types; type I with long axons used to move signals over long distances and type II with short axons, which can often be confused with dendrites. Type I cells can be further divided by where the cell body or soma is located. The basic morphology of type I neurons, represented by spinal motor neurons, consists of a cell body called the soma and a long thin axon covered by the myelin sheath. Around the cell body is a branching dendritic tree that receives signals from other neurons. The end of the axon has branching terminals (axon terminal) that release neurotransmitters into a gap called the synaptic cleft between the terminals and the dendrites of the next neuron.
Bipolar: axon and single dendrite on opposite ends of the soma.
Multipolar: two or more dendrites, separate from the axon:
Golgi I: neurons with long-projecting axonal processes; examples are pyramidal cells, Purkinje cells, and anterior horn cells.
Golgi II: neurons whose axonal process projects locally; the best example is the granule cell.
Anaxonic: where axon cannot be distinguished from dendrites.
Basket cells, interneurons that form a dense plexus of terminals around the soma of target cells, found in the cortex and cerebellum.
Betz cells, large motor neurons.
Lugaro cells, interneurons of the cerebellum.
Medium spiny neurons, most neurons in the corpus striatum.
Purkinje cells, huge neurons in the cerebellum, a type of Golgi I multipolar neuron.
Pyramidal cells, neurons with triangular soma, a type of Golgi I.
Renshaw cells, neurons with both ends linked to alpha motor neurons.
Unipolar brush cells, interneurons with unique dendrite ending in a brush-like tuft.
Granule cells, a type of Golgi II neuron.
Anterior horn cells, motoneurons located in the spinal cord.
Spindle cells, interneurons that connect widely separated areas of the brain
Functional classification
Afferent and efferent also refer generally to neurons that, respectively, bring information to or send information from the brain region.
The two most common neurotransmitters in the brain, glutamate and GABA, have actions that are largely consistent. Glutamate acts on several different types of receptors, and have effects that are excitatory at ionotropic receptorsand a modulatory effect at metabotropic receptors. Similarly GABA acts on several different types of receptors, but all of them have effects (in adult animals, at least) that are inhibitory. Because of this consistency, it is common for neuroscientists to simplify the terminology by referring to cells that release glutamate as "excitatory neurons", and cells that release GABA as "inhibitory neurons". Since over 90% of the neurons in the brain release either glutamate or GABA, these labels encompass the great majority of neurons. There are also other types of neurons that have consistent effects on their targets, for example "excitatory" motor neurons in the spinal cord that release acetylcholine, and "inhibitory" spinal neurons that release glycine.
The distinction between excitatory and inhibitory neurotransmitters is not absolute, however. Rather, it depends on the class of chemical receptors present on the postsynaptic neuron. In principle, a single neuron, releasing a single neurotransmitter, can have excitatory effects on some targets, inhibitory effects on others, and modulatory effects on others still. For example, photoreceptor cells in the retina constantly release the neurotransmitter glutamate in the absence of light. So-called OFF bipolar cells are, like most neurons, excited by the released glutamate. However, neighboring target neurons called ON bipolar cells are instead inhibited by glutamate, because they lack the typical ionotropicglutamate receptors and instead express a class of inhibitory metabotropicglutamate receptors.13 When light is present, the photoreceptors cease releasing glutamate, which relieves the ON bipolar cells from inhibition, activating them; this simultaneously removes the excitation from the OFF bipolar cells, silencing them.
It is possible to identify the type of inhibitory effect a presynaptic neuron will have on a postsynaptic neuron, based on the proteins the presynaptic neuron expresses. Parvalbumin-expressing neurons typically dampen the output signal of the postsynaptic neuron in the visual cortex, whereas somatostatin-expressing neurons typically block dendritic inputs to the postsynaptic neuron.14
Tonic or regular spiking. Some neurons are typically constantly (or tonically) active. Example: interneurons in neurostriatum.
Phasic or bursting. Neurons that fire in bursts are called phasic
Fast spiking. Some neurons are notable for their high firing rates, for example some types of cortical inhibitory interneurons, cells in globus pallidus, retinal ganglion cells.1516
Classification by neurotransmitter production
Cholinergic neurons—acetylcholine. Acetylcholine is released from presynaptic neurons into the synaptic cleft. It acts as a ligand for both ligand-gated ion channels and metabotropic (GPCRs) muscarinic receptors.Nicotinic receptors, are pentameric ligand-gated ion channels composed of alpha and beta subunits that bind nicotine. Ligand binding opens the channel causing influx of Na+ depolarization and increases the probability of presynaptic neurotransmitter release. Acetylcholine is synthesized fromcholine and acetyl coenzyme A.
GABAergic neurons—gamma aminobutyric acid. GABA is one of two neuroinhibitors in the CNS, the other being Glycine. GABA has a homologous function to ACh, gating anion channels that allow Cl− ions to enter the post synaptic neuron. Cl− causes hyperpolarization within the neuron, decreasing the probability of an action potential firing as the voltage becomes more negative (recall that for an action potential to fire, a positive voltage threshold must be reached). GABA is synthesized from glutamate neurotransmitters by the enzyme glutamate decarboxylase.
Glutamatergic neurons—glutamate. Glutamate is one of two primary excitatory amino acid neurotransmitter, the other being Aspartate. Glutamate receptors are one of four categories, three of which are ligand-gated ion channels and one of which is a G-protein coupled receptor (often referred to as GPCR).
NMDA receptors are another cation channel that is more permeable to Ca2+. The function of NMDA receptors is dependant on Glycine receptor binding as a co-agonist within the channel pore. NMDA receptors do not function without both ligands present.
Metabotropic receptors, GPCRs modulate synaptic transmission and postsynaptic excitability.
Glutamate can cause excitotoxicity when blood flow to the brain is interrupted, resulting in brain damage. When blood flow is suppressed, glutamate is released from presynaptic neurons causing NMDA and AMPA receptor activation more so than would normally be the case outside of stress conditions, leading to elevated Ca2+ and Na+ entering the post synaptic neuron and cell damage. Glutamate is synthesized from the amino acid glutamine by the enzyme glutamate synthases
Dopaminergic neurons—dopamine. Dopamine is a neurotransmitter that acts on D1 type (D1 and D5) Gs coupled receptors, which increase cAMP and PKA, and D2 type (D2, D3, and D4) receptors, which activate Gi-coupled receptors that decrease cAMP and PKA. Dopamine is connected to mood and behavior, and modulates both pre and post synaptic neurotransmission. Loss of dopamine neurons in the substantia nigra has been linked to Parkinson's disease. Dopamine is synthesized from the amino acid tyrosine. Tyrosine is catalyzed into levadopa (or L-DOPA) by tyrosine hydroxlase, and levadopa is then converted into dopamine by amino acid decarboxylase
Serotonergic neurons—serotonin. Serotonin (5-Hydroxytryptamine, 5-HT) can act as excitatory or inhibitory. Of the four 5-HT receptor classes, 3 are GPCR and 1 is ligand gated cation channel. Serotonin is synthesized from tryptophan by tryptophan hydroxylase, and then further by aromatic acid decarboxylase. A lack of 5-HT at postsynaptic neurons has been linked to depression. Drugs that block the presynaptic serotonin transporter are used for treatment, such as Prozac and Zoloft
Connectivity
Neurons communicate with one another via synapses, where the axon terminal or en passant boutons (terminals located along the length of the axon) of one cell impinges upon another neuron's dendrite, soma or, less commonly, axon. Neurons such as Purkinje cells in the cerebellum can have over 1000 dendritic branches, making connections with tens of thousands of other cells; other neurons, such as the magnocellular neurons of the supraoptic nucleus, have only one or two dendrites, each of which receives thousands of synapses. Synapses can be excitatory or inhibitory and either increase or decrease activity in the target neuron. Some neurons also communicate via electrical synapses, which are direct, electrically conductive junctions between cells.[citation needed]
In a chemical synapse, the process of synaptic transmission is as follows: when an action potential reaches the axon terminal, it opens voltage-gated calcium channels, allowing calcium ions to enter the terminal. Calcium causes synaptic vesicles filled with neurotransmitter molecules to fuse with the membrane, releasing their contents into the synaptic cleft. The neurotransmitters diffuse across the synaptic cleft and activate receptors on the postsynaptic neuron. High cytosolic calcium in the axon terminal also triggers mitochondrial calcium uptake, which, in turn, activates mitochondrial energy metabolism to produce ATP to support continuous neurotransmission.1
The human brain has a huge number of synapses. Each of the 1011 (one hundred billion) neurons has on average 7,000 synaptic connections to other neurons. It has been estimated that the brain of a three-year-old child has about 1015synapses (1 quadrillion). This number declines with age, stabilizing by adulthood. Estimates vary for an adult, ranging from 1014 to 5 x 1014 synapses (100 to 500 trillion).
Mechanisms for propagating action potential
A signal propagating down an axon to the cell body and dendrites of the next cell
In 1937, John Zachary Young suggested that the squid giant axon could be used to study neuronal electrical properties.19 Being larger than but similar in nature to human neurons, squid cells were easier to study. By inserting electrodes into the giant squid axons, accurate measurements were made of the membrane potential.
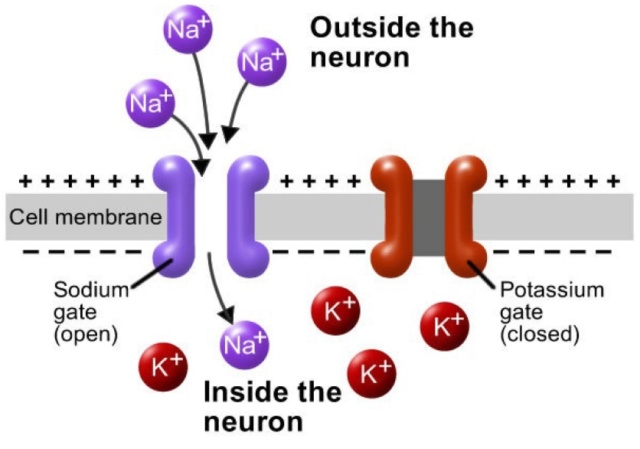
The cell membrane of the axon and soma contain voltage-gated ion channels that allow the neuron to generate and propagate an electrical signal (an action potential). These signals are generated and propagated by charge-carrying ionsincluding sodium (Na+), potassium (K+), chloride (Cl−), and calcium (Ca2+).
There are several stimuli that can activate a neuron leading to electrical activity, including pressure, stretch, chemical transmitters, and changes of the electric potential across the cell membrane.20 Stimuli cause specific ion-channels within the cell membrane to open, leading to a flow of ions through the cell membrane, changing the membrane potential.
Thin neurons and axons require less metabolic expense to produce and carry action potentials, but thicker axons convey impulses more rapidly. To minimize metabolic expense while maintaining rapid conduction, many neurons have insulating sheaths of myelin around their axons. The sheaths are formed by glial cells: oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system. The sheath enables action potentials to travel fasterthan in unmyelinated axons of the same diameter, whilst using less energy. The myelin sheath in peripheral nerves normally runs along the axon in sections about 1 mm long, punctuated by unsheathed nodes of Ranvier, which contain a high density of voltage-gated ion channels. Multiple sclerosis is a neurological disorder that results from demyelination of axons in the central nervous system.
Some neurons do not generate action potentials, but instead generate a graded electrical signal, which in turn causes graded neurotransmitter release. Suchnonspiking neurons tend to be sensory neurons or interneurons, because they cannot carry signals long distances.
There are a number of other receptor types that are called quickly adapting or phasic receptors, where firing decreases or stops with steady stimulus; examples include: skin when touched by an object causes the neurons to fire, but if the object maintains even pressure against the skin, the neurons stop firing. The neurons of the skin and muscles that are responsive to pressure and vibration have filtering accessory structures that aid their function.
The pacinian corpuscle is one such structure. It has concentric layers like an onion, which form around the axon terminal. When pressure is applied and the corpuscle is deformed, mechanical stimulus is transferred to the axon, which fires. If the pressure is steady, there is no more stimulus; thus, typically these neurons respond with a transient depolarization during the initial deformation and again when the pressure is removed, which causes the corpuscle to change shape again. Other types of adaptation are important in extending the function of a number of other neurons.23
The term neuron was coined by the German anatomist Heinrich Wilhelm Waldeyer. The neuron's place as the primary functional unit of the nervous system was first recognized in the early 20th century through the work of the Spanish anatomist Santiago Ramón y Cajal.24 Ramón y Cajal proposed that neurons were discrete cells that communicated with each other via specialized junctions, or spaces, between cells.24 This became known as the neuron doctrine, one of the central tenets of modern neuroscience.24 To observe the structure of individual neurons, Ramón y Cajal improved a silver staining process known as Golgi's method, which had been developed by his rival,Camillo Golgi.24 Cajal's improvement, which involved a technique he called "double impregnation", is still in use. The silver impregnation stains are an extremely useful method for neuroanatomical investigations because, for reasons unknown, it stains a very small percentage of cells in a tissue, so one is able to see the complete micro structure of individual neurons without much overlap from other cells in the densely packed brain.25
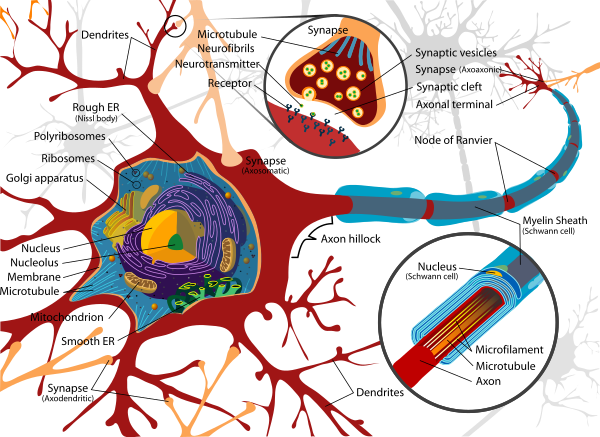
Diagram of a typical myelinated vertebrate motor neuron
1. http://sci-hub.tw/https://www.sciencedirect.com/science/article/pii/S0960982216304894
Neurons have long processes, generate action potentials, receive input from other neurons (or, if they are sensory neurons, from appropriate stimuli), and provide output to other cells (for example, neurons, muscles, glands) via synapses. 1
A neuron, or nerve cell is an electrically excitable cell that processes and transmits information through electrical and chemical signals. These signals between neurons occur via synapses, specialized connections with other cells. Neurons can connect to each other to form neural networks. Neurons are the core components of the brain and spinal cord of the central nervous system (CNS), and of the ganglia of the peripheral nervous system (PNS). Specialized types of neurons include: sensory neurons which respond to touch, sound, light and all other stimuli affecting the cells of the sensory organs that then send signals to the spinal cord and brain, motor neurons that receive signals from the brain and spinal cord to cause muscle contractions and affect glandular outputs, and interneurons which connect neurons to other neurons within the same region of the brain, or spinal cord in neural networks.
A typical neuron consists of a cell body (soma), dendrites, and an axon. The term neurite is used to describe either a dendrite or an axon, particularly in its undifferentiated stage. Dendrites are thin structures that arise from the cell body, often extending for hundreds of micrometres and branching multiple times, giving rise to a complex "dendritic tree". An axon (also called a nerve fiber when myelinated) is a special cellular extension (process) that arises from the cell body at a site called the axon hillock and travels for a distance, as far as 1 meter in humans or even more in other species. Nerve fibers are often bundled into fascicles, and in the peripheral nervous system, bundles of fascicles make up nerves (like strands of wire make up cables). The cell body of a neuron frequently gives rise to multiple dendrites, but never to more than one axon, although the axon may branch hundreds of times before it terminates. At the majority of synapses, signals are sent from the axon of one neuron to a dendrite of another. There are, however, many exceptions to these rules: neurons that lack dendrites, neurons that have no axon, synapses that connect an axon to another axon or a dendrite to another dendrite, etc.
All neurons are electrically excitable, maintaining voltage gradients across their membranes by means of metabolically driven ion pumps, which combine with ion channels embedded in the membrane to generate intracellular-versus-extracellular concentration differences of ions such as sodium, potassium,chloride, and calcium. Changes in the cross-membrane voltage can alter the function of voltage-dependent ion channels. If the voltage changes by a large enough amount, an all-or-none electrochemical pulse called an action potential is generated, which travels rapidly along the cell's axon, and activates synaptic connections with other cells when it arrives.
Neurons do not undergo cell division. In most cases, neurons are generated by special types of stem cells. Astrocytes are star-shaped glial cells that have also been observed to turn into neurons by virtue of the stem cell characteristic pluripotency. In humans, neurogenesis largely ceases during adulthood; but in two brain areas, the hippocampus and olfactory bulb, there is strong evidence for generation of substantial numbers of new neurons.12
Overview
A neuron is a specialized type of cell found in the bodies of all eumetozoans. Only sponges and a few other simpler animals lack neurons. The features that define a neuron are electrical excitability and the presence of synapses, which are complex membrane junctions that transmit signals to other cells. The body's neurons, plus the glial cells that give them structural and metabolic support, together constitute the nervous system. In vertebrates, the majority of neurons belong to the central nervous system, but some reside in peripheral ganglia, and many sensory neurons are situated in sensory organs such as the retina and cochlea.Although neurons are very diverse and there are exceptions to nearly every rule, it is convenient to begin with a schematic description of the structure and function of a "typical" neuron. A typical neuron is divided into three parts: the soma or cell body, dendrites, and axon. The soma is usually compact; the axon and dendrites are filaments that extrude from it. Dendrites typically branch profusely, getting thinner with each branching, and extending their farthest branches a few hundred micrometers from the soma. The axon leaves the soma at a swelling called the axon hillock, and can extend for great distances, giving rise to hundreds of branches. Unlike dendrites, an axon usually maintains the same diameter as it extends. The soma may give rise to numerous dendrites, but never to more than one axon. Synaptic signals from other neurons are received by the soma and dendrites; signals to other neurons are transmitted by the axon. A typical synapse, then, is a contact between the axon of one neuron and a dendrite or soma of another. Synaptic signals may be excitatory or inhibitory. If the net excitation received by a neuron over a short period of time is large enough, the neuron generates a brief pulse called an action potential, which originates at the soma and propagates rapidly along the axon, activating synapses onto other neurons as it goes. This is called saltatory conduction.
Many neurons fit the foregoing schema in every respect, but there are also exceptions to most parts of it. There are no neurons that lack a soma, but there are neurons that lack dendrites, and others that lack an axon. Furthermore, in addition to the typical axodendritic and axosomatic synapses, there are axoaxonic (axon-to-axon) and dendrodendritic (dendrite-to-dendrite) synapses.
The key to neural function is the synaptic signaling process, which is partly electrical and partly chemical. The electrical aspect depends on properties of the neuron's membrane. Like all animal cells, the cell body of every neuron is enclosed by a plasma membrane, a bilayer of lipid molecules with many types of protein structures embedded in it. A lipid bilayer is a powerful electrical insulator, but in neurons, many of the protein structures embedded in the membrane are electrically active. These include ion channels that permit electrically charged ions to flow across the membrane, and ion pumps that actively transport ions from one side of the membrane to the other. Most ion channels are permeable only to specific types of ions. Some ion channels are voltage gated, meaning that they can be switched between open and closed states by altering the voltage difference across the membrane. Others are chemically gated, meaning that they can be switched between open and closed states by interactions with chemicals that diffuse through the extracellular fluid. The interactions between ion channels and ion pumps produce a voltage difference across the membrane, typically a bit less than 1/10 of a volt at baseline. This voltage has two functions: first, it provides a power source for an assortment of voltage-dependent protein machinery that is embedded in the membrane; second, it provides a basis for electrical signal transmission between different parts of the membrane.
Neurons communicate by chemical and electrical synapses in a process known as neurotransmission, also called synaptic transmission. The fundamental process that triggers the release of neurotransmitters is the action potential, a propagating electrical signal that is generated by exploiting the electrically excitable membrane of the neuron. This is also known as a wave of depolarization.
Anatomy and histology
Neurons are highly specialized for the processing and transmission of cellular signals. Given their diversity of functions performed in different parts of the nervous system, there is, as expected, a wide variety in their shape, size, and electrochemical properties. For instance, the soma of a neuron can vary from 4 to 100 micrometers in diameter.3The soma is the body of the neuron. As it contains the nucleus, most protein synthesis occurs here. The nucleus can range from 3 to 18 micrometers in diameter.The dendrites of a neuron are cellular extensions with many branches. This overall shape and structure is referred to metaphorically as a dendritic tree. This is where the majority of input to the neuron occurs via the dendritic spine.The axon is a finer, cable-like projection that can extend tens, hundreds, or even tens of thousands of times the diameter of the soma in length. The axon carries nerve signals away from the soma (and also carries some types of information back to it). Many neurons have only one axon, but this axon may—and usually will—undergo extensive branching, enabling communication with many target cells. The part of the axon where it emerges from the soma is called the axon hillock. Besides being an anatomical structure, the axon hillock is also the part of the neuron that has the greatest density of voltage-dependent sodium channels. This makes it the most easily excited part of the neuron and the spike initiation zone for the axon: in electrophysiological terms it has the most negative action potential threshold. While the axon and axon hillock are generally involved in information outflow, this region can also receive input from other neurons.The axon terminal contains synapses, specialized structures whereneurotransmitter chemicals are released to communicate with target neurons.
The canonical view of the neuron attributes dedicated functions to its various anatomical components; however dendrites and axons often act in ways contrary to their so-called main function.
Axons and dendrites in the central nervous system are typically only about one micrometer thick, while some in the peripheral nervous system are much thicker. The soma is usually about 10–25 micrometers in diameter and often is not much larger than the cell nucleus it contains. The longest axon of a humanmotor neuron can be over a meter long, reaching from the base of the spine to the toes.
Sensory neurons can have axons that run from the toes to the posterior columnof the spinal cord, over 1.5 meters in adults. Giraffes have single axons several meters in length running along the entire length of their necks. Much of what is known about axonal function comes from studying the squid giant axon, an ideal experimental preparation because of its relatively immense size (0.5–1 millimeters thick, several centimeters long).
Fully differentiated neurons are permanently postmitotic;5 however, research starting around 2002 shows that additional neurons throughout the brain can originate from neural stem cells through the process of neurogenesis. These are found throughout the brain, but are particularly concentrated in thesubventricular zone and subgranular zone.6
Histology and internal structure

Golgi-stained neurons in human hippocampal tissue
Numerous microscopic clumps called Nissl substance (or Nissl bodies) are seen when nerve cell bodies are stained with a basophilic ("base-loving") dye. These structures consist of rough endoplasmic reticulum and associated ribosomal RNA. Named after German psychiatrist and neuropathologist Franz Nissl (1860–1919), they are involved in protein synthesis and their prominence can be explained by the fact that nerve cells are very metabolically active. Basophilic dyes such as aniline or (weakly) haematoxylin 7 highlight negatively charged components, and so bind to the phosphate backbone of the ribosomal RNA.
The cell body of a neuron is supported by a complex mesh of structural proteins called neurofilaments, which are assembled into larger neurofibrils. Some neurons also contain pigment granules, such as neuromelanin (a brownish-black pigment that is byproduct of synthesis of catecholamines), and lipofuscin (a yellowish-brown pigment), both of which accumulate with age.8910 Other structural proteins that are important for neuronal function are actin and thetubulin of microtubules. Actin is predominately found at the tips of axons and dendrites during neuronal development.
There are different internal structural characteristics between axons and dendrites. Typical axons almost never contain ribosomes, except some in the initial segment. Dendrites contain granular endoplasmic reticulum or ribosomes, in diminishing amounts as the distance from the cell body increases.
Neurons exist in a number of different shapes and sizes and can be classified by their morphology and function.12 The anatomist Camillo Golgi grouped neurons into two types; type I with long axons used to move signals over long distances and type II with short axons, which can often be confused with dendrites. Type I cells can be further divided by where the cell body or soma is located. The basic morphology of type I neurons, represented by spinal motor neurons, consists of a cell body called the soma and a long thin axon covered by the myelin sheath. Around the cell body is a branching dendritic tree that receives signals from other neurons. The end of the axon has branching terminals (axon terminal) that release neurotransmitters into a gap called the synaptic cleft between the terminals and the dendrites of the next neuron.
Structural classification
Polarity
Most neurons can be anatomically characterized as:Unipolar or pseudounipolar: dendrite and axon emerging from same process.Bipolar: axon and single dendrite on opposite ends of the soma.
Multipolar: two or more dendrites, separate from the axon:
Golgi I: neurons with long-projecting axonal processes; examples are pyramidal cells, Purkinje cells, and anterior horn cells.
Golgi II: neurons whose axonal process projects locally; the best example is the granule cell.
Anaxonic: where axon cannot be distinguished from dendrites.
Other
Furthermore, some unique neuronal types can be identified according to their location in the nervous system and distinct shape. Some examples are:Basket cells, interneurons that form a dense plexus of terminals around the soma of target cells, found in the cortex and cerebellum.
Betz cells, large motor neurons.
Lugaro cells, interneurons of the cerebellum.
Medium spiny neurons, most neurons in the corpus striatum.
Purkinje cells, huge neurons in the cerebellum, a type of Golgi I multipolar neuron.
Pyramidal cells, neurons with triangular soma, a type of Golgi I.
Renshaw cells, neurons with both ends linked to alpha motor neurons.
Unipolar brush cells, interneurons with unique dendrite ending in a brush-like tuft.
Granule cells, a type of Golgi II neuron.
Anterior horn cells, motoneurons located in the spinal cord.
Spindle cells, interneurons that connect widely separated areas of the brain
Functional classification
Direction
Afferent neurons convey information from tissues and organs into the central nervous system and are sometimes also called sensory neurons.
Efferent neurons transmit signals from the central nervous system to the effector cells and are sometimes called motor neurons.
Interneurons connect neurons within specific regions of the central nervous system.
Afferent and efferent also refer generally to neurons that, respectively, bring information to or send information from the brain region.
Action on other neurons
A neuron affects other neurons by releasing a neurotransmitter that binds tochemical receptors. The effect upon the postsynaptic neuron is determined not by the presynaptic neuron or by the neurotransmitter, but by the type of receptor that is activated. A neurotransmitter can be thought of as a key, and a receptor as a lock: the same type of key can here be used to open many different types of locks. Receptors can be classified broadly as excitatory (causing an increase in firing rate), inhibitory (causing a decrease in firing rate), ormodulatory (causing long-lasting effects not directly related to firing rate).The two most common neurotransmitters in the brain, glutamate and GABA, have actions that are largely consistent. Glutamate acts on several different types of receptors, and have effects that are excitatory at ionotropic receptorsand a modulatory effect at metabotropic receptors. Similarly GABA acts on several different types of receptors, but all of them have effects (in adult animals, at least) that are inhibitory. Because of this consistency, it is common for neuroscientists to simplify the terminology by referring to cells that release glutamate as "excitatory neurons", and cells that release GABA as "inhibitory neurons". Since over 90% of the neurons in the brain release either glutamate or GABA, these labels encompass the great majority of neurons. There are also other types of neurons that have consistent effects on their targets, for example "excitatory" motor neurons in the spinal cord that release acetylcholine, and "inhibitory" spinal neurons that release glycine.
The distinction between excitatory and inhibitory neurotransmitters is not absolute, however. Rather, it depends on the class of chemical receptors present on the postsynaptic neuron. In principle, a single neuron, releasing a single neurotransmitter, can have excitatory effects on some targets, inhibitory effects on others, and modulatory effects on others still. For example, photoreceptor cells in the retina constantly release the neurotransmitter glutamate in the absence of light. So-called OFF bipolar cells are, like most neurons, excited by the released glutamate. However, neighboring target neurons called ON bipolar cells are instead inhibited by glutamate, because they lack the typical ionotropicglutamate receptors and instead express a class of inhibitory metabotropicglutamate receptors.13 When light is present, the photoreceptors cease releasing glutamate, which relieves the ON bipolar cells from inhibition, activating them; this simultaneously removes the excitation from the OFF bipolar cells, silencing them.
It is possible to identify the type of inhibitory effect a presynaptic neuron will have on a postsynaptic neuron, based on the proteins the presynaptic neuron expresses. Parvalbumin-expressing neurons typically dampen the output signal of the postsynaptic neuron in the visual cortex, whereas somatostatin-expressing neurons typically block dendritic inputs to the postsynaptic neuron.14
Discharge patterns
Neurons can be classified according to their electrophysiological characteristics:Tonic or regular spiking. Some neurons are typically constantly (or tonically) active. Example: interneurons in neurostriatum.
Phasic or bursting. Neurons that fire in bursts are called phasic
Fast spiking. Some neurons are notable for their high firing rates, for example some types of cortical inhibitory interneurons, cells in globus pallidus, retinal ganglion cells.1516
Classification by neurotransmitter production
Cholinergic neurons—acetylcholine. Acetylcholine is released from presynaptic neurons into the synaptic cleft. It acts as a ligand for both ligand-gated ion channels and metabotropic (GPCRs) muscarinic receptors.Nicotinic receptors, are pentameric ligand-gated ion channels composed of alpha and beta subunits that bind nicotine. Ligand binding opens the channel causing influx of Na+ depolarization and increases the probability of presynaptic neurotransmitter release. Acetylcholine is synthesized fromcholine and acetyl coenzyme A.
GABAergic neurons—gamma aminobutyric acid. GABA is one of two neuroinhibitors in the CNS, the other being Glycine. GABA has a homologous function to ACh, gating anion channels that allow Cl− ions to enter the post synaptic neuron. Cl− causes hyperpolarization within the neuron, decreasing the probability of an action potential firing as the voltage becomes more negative (recall that for an action potential to fire, a positive voltage threshold must be reached). GABA is synthesized from glutamate neurotransmitters by the enzyme glutamate decarboxylase.
Glutamatergic neurons—glutamate. Glutamate is one of two primary excitatory amino acid neurotransmitter, the other being Aspartate. Glutamate receptors are one of four categories, three of which are ligand-gated ion channels and one of which is a G-protein coupled receptor (often referred to as GPCR).
NMDA receptors are another cation channel that is more permeable to Ca2+. The function of NMDA receptors is dependant on Glycine receptor binding as a co-agonist within the channel pore. NMDA receptors do not function without both ligands present.
Metabotropic receptors, GPCRs modulate synaptic transmission and postsynaptic excitability.
Glutamate can cause excitotoxicity when blood flow to the brain is interrupted, resulting in brain damage. When blood flow is suppressed, glutamate is released from presynaptic neurons causing NMDA and AMPA receptor activation more so than would normally be the case outside of stress conditions, leading to elevated Ca2+ and Na+ entering the post synaptic neuron and cell damage. Glutamate is synthesized from the amino acid glutamine by the enzyme glutamate synthases
Dopaminergic neurons—dopamine. Dopamine is a neurotransmitter that acts on D1 type (D1 and D5) Gs coupled receptors, which increase cAMP and PKA, and D2 type (D2, D3, and D4) receptors, which activate Gi-coupled receptors that decrease cAMP and PKA. Dopamine is connected to mood and behavior, and modulates both pre and post synaptic neurotransmission. Loss of dopamine neurons in the substantia nigra has been linked to Parkinson's disease. Dopamine is synthesized from the amino acid tyrosine. Tyrosine is catalyzed into levadopa (or L-DOPA) by tyrosine hydroxlase, and levadopa is then converted into dopamine by amino acid decarboxylase
Serotonergic neurons—serotonin. Serotonin (5-Hydroxytryptamine, 5-HT) can act as excitatory or inhibitory. Of the four 5-HT receptor classes, 3 are GPCR and 1 is ligand gated cation channel. Serotonin is synthesized from tryptophan by tryptophan hydroxylase, and then further by aromatic acid decarboxylase. A lack of 5-HT at postsynaptic neurons has been linked to depression. Drugs that block the presynaptic serotonin transporter are used for treatment, such as Prozac and Zoloft
Connectivity
Neurons communicate with one another via synapses, where the axon terminal or en passant boutons (terminals located along the length of the axon) of one cell impinges upon another neuron's dendrite, soma or, less commonly, axon. Neurons such as Purkinje cells in the cerebellum can have over 1000 dendritic branches, making connections with tens of thousands of other cells; other neurons, such as the magnocellular neurons of the supraoptic nucleus, have only one or two dendrites, each of which receives thousands of synapses. Synapses can be excitatory or inhibitory and either increase or decrease activity in the target neuron. Some neurons also communicate via electrical synapses, which are direct, electrically conductive junctions between cells.[citation needed]
In a chemical synapse, the process of synaptic transmission is as follows: when an action potential reaches the axon terminal, it opens voltage-gated calcium channels, allowing calcium ions to enter the terminal. Calcium causes synaptic vesicles filled with neurotransmitter molecules to fuse with the membrane, releasing their contents into the synaptic cleft. The neurotransmitters diffuse across the synaptic cleft and activate receptors on the postsynaptic neuron. High cytosolic calcium in the axon terminal also triggers mitochondrial calcium uptake, which, in turn, activates mitochondrial energy metabolism to produce ATP to support continuous neurotransmission.1
The human brain has a huge number of synapses. Each of the 1011 (one hundred billion) neurons has on average 7,000 synaptic connections to other neurons. It has been estimated that the brain of a three-year-old child has about 1015synapses (1 quadrillion). This number declines with age, stabilizing by adulthood. Estimates vary for an adult, ranging from 1014 to 5 x 1014 synapses (100 to 500 trillion).
Mechanisms for propagating action potential
A signal propagating down an axon to the cell body and dendrites of the next cell
In 1937, John Zachary Young suggested that the squid giant axon could be used to study neuronal electrical properties.19 Being larger than but similar in nature to human neurons, squid cells were easier to study. By inserting electrodes into the giant squid axons, accurate measurements were made of the membrane potential.
The cell membrane of the axon and soma contain voltage-gated ion channels that allow the neuron to generate and propagate an electrical signal (an action potential). These signals are generated and propagated by charge-carrying ionsincluding sodium (Na+), potassium (K+), chloride (Cl−), and calcium (Ca2+).
There are several stimuli that can activate a neuron leading to electrical activity, including pressure, stretch, chemical transmitters, and changes of the electric potential across the cell membrane.20 Stimuli cause specific ion-channels within the cell membrane to open, leading to a flow of ions through the cell membrane, changing the membrane potential.
Thin neurons and axons require less metabolic expense to produce and carry action potentials, but thicker axons convey impulses more rapidly. To minimize metabolic expense while maintaining rapid conduction, many neurons have insulating sheaths of myelin around their axons. The sheaths are formed by glial cells: oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system. The sheath enables action potentials to travel fasterthan in unmyelinated axons of the same diameter, whilst using less energy. The myelin sheath in peripheral nerves normally runs along the axon in sections about 1 mm long, punctuated by unsheathed nodes of Ranvier, which contain a high density of voltage-gated ion channels. Multiple sclerosis is a neurological disorder that results from demyelination of axons in the central nervous system.
Some neurons do not generate action potentials, but instead generate a graded electrical signal, which in turn causes graded neurotransmitter release. Suchnonspiking neurons tend to be sensory neurons or interneurons, because they cannot carry signals long distances.
Neural coding
Neural coding is concerned with how sensory and other information is represented in the brain by neurons. The main goal of studying neural coding is to characterize the relationship between the stimulus and the individual orensemble neuronal responses, and the relationships amongst the electrical activities of the neurons within the ensemble.21 It is thought that neurons can encode both digital and analog information.22All-or-none principle
The conduction of nerve impulses is an example of an all-or-none response. In other words, if a neuron responds at all, then it must respond completely. Greater intensity of stimulation does not produce a stronger signal but can produce a higher frequency of firing. There are different types of receptor response to stimulus, slowly adapting or tonic receptors respond to steady stimulus and produce a steady rate of firing. These tonic receptors most often respond to increased intensity of stimulus by increasing their firing frequency, usually as a power function of stimulus plotted against impulses per second. This can be likened to an intrinsic property of light where to get greater intensity of a specific frequency (color) there have to be more photons, as the photons can't become "stronger" for a specific frequency.There are a number of other receptor types that are called quickly adapting or phasic receptors, where firing decreases or stops with steady stimulus; examples include: skin when touched by an object causes the neurons to fire, but if the object maintains even pressure against the skin, the neurons stop firing. The neurons of the skin and muscles that are responsive to pressure and vibration have filtering accessory structures that aid their function.
The pacinian corpuscle is one such structure. It has concentric layers like an onion, which form around the axon terminal. When pressure is applied and the corpuscle is deformed, mechanical stimulus is transferred to the axon, which fires. If the pressure is steady, there is no more stimulus; thus, typically these neurons respond with a transient depolarization during the initial deformation and again when the pressure is removed, which causes the corpuscle to change shape again. Other types of adaptation are important in extending the function of a number of other neurons.23
History
Further information: History of neuroscienceThe term neuron was coined by the German anatomist Heinrich Wilhelm Waldeyer. The neuron's place as the primary functional unit of the nervous system was first recognized in the early 20th century through the work of the Spanish anatomist Santiago Ramón y Cajal.24 Ramón y Cajal proposed that neurons were discrete cells that communicated with each other via specialized junctions, or spaces, between cells.24 This became known as the neuron doctrine, one of the central tenets of modern neuroscience.24 To observe the structure of individual neurons, Ramón y Cajal improved a silver staining process known as Golgi's method, which had been developed by his rival,Camillo Golgi.24 Cajal's improvement, which involved a technique he called "double impregnation", is still in use. The silver impregnation stains are an extremely useful method for neuroanatomical investigations because, for reasons unknown, it stains a very small percentage of cells in a tissue, so one is able to see the complete micro structure of individual neurons without much overlap from other cells in the densely packed brain.25
Neurons in the brain
The number of neurons in the brain varies dramatically from species to species.32 One estimate (published in 1988) puts the human brain at about 100 billion (10^11) neurons and 100 trillion (10^14) synapses.32 A lower estimate (published in 2009) is 86 billion neurons, of which 16.3 billion are in the cerebral cortex, and 69 billion in the cerebellum.33 By contrast, the nematode wormCaenorhabditis elegans has just 302 neurons, making it an ideal experimental subject as scientists have been able to map all of the organism's neurons. The fruit fly Drosophila melanogaster, a common subject in biological experiments, has around 100,000 neurons and exhibits many complex behaviors. Many properties of neurons, from the type of neurotransmitters used to ion channel composition, are maintained across species, allowing scientists to study processes occurring in more complex organisms in much simpler experimental systems.
Diagram of a typical myelinated vertebrate motor neuron
1. http://sci-hub.tw/https://www.sciencedirect.com/science/article/pii/S0960982216304894
Last edited by Admin on Mon Feb 11, 2019 3:44 pm; edited 4 times in total