How to explain best the origin and emergence of the brain ?
http://reasonandscience.heavenforum.org/t2324-how-to-explain-best-the-origin-and-emergence-of-the-brain
An Intricate Cross-Section of the Brain Depicted With Thousands of Layers of Gold Leaf
http://www.thisiscolossal.com/2017/04/brain-depicted-with-gold-leaf/
Some naturalistic explanations:
THE EVOLUTIONARY LAYERS OF THE HUMAN BRAIN 1
The most efficient model for understanding the brain in terms of its evolutionary history is the famous triune brain theory developed by Paul MacLean. According to this theory, the following three distinct brains emerged successively in the course of evolution and now co-inhabit the human skull:
The reptilian brain, the oldest of the three, controls the body's vital functions such as heart rate, breathing, body temperature and balance. Our reptilian brain includes the main structures found in a reptile's brain: the brainstem and the cerebellum. The reptilian brain is reliable but tends to be somewhat rigid and compulsive.
The limbic brain emerged in the first mammals. It can record memories of behaviours that produced agreeable and disagreeable experiences, so it is responsible for what are called emotions in human beings. The main structures of the limbic brain are the hippocampus, the amygdala, and the hypothalamus. The limbic brain is the seat of the value judgments that we make, often unconsciously, that exert such a strong influence on our behaviour.
The neocortex first assumed importance in primates and culminated in the human brain with its two large cerebral hemispheres that play such a dominant role. These hemispheres have been responsible for the development of human language, abstract thought, imagination, and consciousness. The neocortex is flexible and has almost infinite learning abilities. The neocortex is also what has enabled human cultures to develop. These three parts of the brain do not operate independently of one another. They have established numerous interconnections through which they influence one another. The neural pathways from the limbic system to the cortex, for example, are especially well developed.
The model of the triune brain proposed by MacLean in 1970 is a useful piece of shorthand for the complex evolutionary history of the human brain. But the brain's combination of reptilian, paleomammalian and neomammalian structures is far more intricate than a mere set of nested Russian dolls.
Ever since the first mammals appeared more than 200 million years ago, the cerebral cortex has assumed greater and greater importance compared with the brain's other, older structures. Because these structures had proven their effectiveness for meeting certain fundamental needs, there was no reason for them to disappear. Instead, evolution favoured a process of building expansions and additions, rather than rebuilding everything from the bottom up.
Evolution of the human brain: when bigger is better 2
The human brain contains about 100 billion neurons, more than 100,000 km of interconnections, and has an estimated storage capacity of 1.25 × 10^12 bytes (Cherniak, 1990; Hofman, 2012). These impressive numbers have led to the idea that our cognitive capabilities are virtually without limit. The human brain, however, has evolved from a set of underlying structures that constrain its size, and the amount of information it can store and process. If the ability of an organism to process information about its environment is a driving force behind evolution, then the more information a system, such as the brain, receives, and the faster it can process this information, the more adequately it will be able to respond to environmental challenges and the better will be its chances of survival (Macphail and Bolhuis, 2001; Roth and Dicke, 2012; Hofman, 2014). The limit to any intelligent system therefore lies in its abilities to process and integrate large amounts of sensory information and to compare these signals with as many memory states as possible, and all that in a minimum of time. It implies that the functional capacity of a neuronal structure is inherently limited by its neural architecture and signal processing time (see e.g., Laughlin and Sejnowski, 2003; Buzsáki et al., 2013). The object of this review is to present current perspectives on primate brain evolution, especially in humans, and to examine some hypothetical organizing principles that underlie the brain's complex organization. Some of the design principles and operational modes that underlie the information processing capacity of the cerebral cortex in primates will be explored, and it will be argued that with the evolution of the human brain we have nearly reached the limits of biological intelligence.
The Selection of Neurons and Synapses 3
The most complex object yet discovered anywhere in the universe is the organ that fills the space between our ears. Although weighing only about 1300 to 1500 grams (three to four pounds), the human brain contains over 11 billion specialized nerve cells, or neurons, capable of receiving, processing, and relaying the electrochemical pulses on which all our sensations, actions, thoughts, and emotions depend. But it is not the sheer number of neurons alone that is most striking about the brain, but how they are organized and interconnected. And to understand how neurons communicate with each other we first must consider their typical structure.
Although there are many different types of neurons, almost all of them share certain common features. The cell body, or soma, contains the nucleus of the neuron, which in turn houses a complete set of the organism's genes. The nucleus is surrounded by cytoplasm, the chemical "soup" of the cell that contains the organelles essential to the neuron's functioning and metabolism. In these respects, neurons are similar to other cells throughout the body, except for the fact that unlike most other cells they rarely divide to reproduce new neurons.
The ways in which neurons are specialized to carry out their communicative function is made evident by closer examination of the appendages they sport, that is, their dendrites and axons. The dendrites can be likened to a bushy antenna system that receives signals from other neurons. When a dendrite is stimulated in a certain way, the neuron to which it is attached suddenly changes its electrical polarity and may fire, sending a signal out along its single axon where it may be picked up by the dendrites of other neurons.[3] Considering the small size of the neuron's body, the length of an axon can be considerable, up to several meters in the neck of the giraffe. Thus the firing of one neuron can influence the firing of another one a considerable distance away.
For one neuron to influence another, the two must be connected, and this is accomplished by junctions called synapses (figure 5.2). These synaptic junctions usually connect the axon of one neuron with the dendrites of another, a typical neuron in the cortex of the human brain having about 10,000 synapses. The synapses therefore constitute an exceedingly complex wiring system that surpasses by many orders of magnitude the complexity of even the most advanced supercomputers. It is this organization of connections both within the skull and to more distant sense organs and muscles that gives the brain its amazing abilities. Indeed, it is widely believed today by neuroscientists, psychologists, and even philosophers that all of the knowledge the human brain contains--from being able to walk to the ability to perform abstract scientific and mathematical reasoning--is a function of the connections existing among the neurons.
How this unfathomably complex organization allows us to perceive, behave, think, feel, and control our environment presents us with what may be the most striking puzzle of fit we have yet encountered. The puzzle actually has three aspects. First, we must consider how over millions of years the primitive nervous system of our early ancestors evolved into an organ that has made it possible for the human species to become the most adaptable and powerful organism on the planet--living, thriving, and modifying the environment (both intentionally and unintentionally) from the tropics to the polar regions, and perhaps soon in outer space and on other planets.[4] Second, we must understand how it is possible for the intricate structure of the brain to develop from a single fertilized egg cell. Finally, we must try to comprehend how the mature brain is able to continue to modify its own structure so that it can acquire new skills and information to continue surviving and reproducing in an unpredictable, ever-changing world. In this chapter we will consider the research and theories that are beginning to provide answers to these questions. Indeed, the 1990s has been referred to as "the decade of the brain," as scholars and scientists in fields from philosophy to molecular neurobiology focus their energies on understanding humankind's ultimate inner frontier.
The Evolution of the Brain
Neurons are quite distinct from other body cells in ways that make them suited to their specialized role of signal processing and communication, but it is not too difficult to see how they could have evolved from less specialized cells. All living cells are surrounded by a cell membrane that separates the special chemical composition of its interior from that of the external world. This difference in chemical composition results in a small electrical potential between the inside and outside of the cell, in much the same way that a voltage exists between the two sides of a battery. When a part of a cell's membrane is disturbed in a certain way, it loses its electrical potential, becoming depolarized at the site of the disturbance. This sudden change in electrical potential can itself be a disturbance, causing additional depolarizations along the membrane. In most cells, such depolarization would not spread far, certainly not to neighboring cells. But a few changes in the shape and arrangement of cells (in just the way that neurons are fashioned) permits depolarization to propagate quickly from one neuron to the next, and allows it to travel quickly as an electrochemical signal from one end of an animal to the other.
An example of a simple nervous system is provided by the jellyfish (or Medusa). The jellyfish's nervous system forms an undifferentiated network and serves primarily to coordinate the animal's swimming motions. Since the jellyfish's skirt must open and contract in a coordinated manner for the animal to move through the water, its nervous system serves as a simple communications network making it possible for all parts of the skirt to open repeatedly and then contract at the same time.
Worms are the simplest organisms to have a central nervous system, which includes a distinct brain that is connected to groups of neurons organized as nerve cords running along the length of its body. This more complicated nervous system allows worms to exhibit more complex forms of behavior. An anterior brain connected to a nerve cord is the basic design for all organisms with central nervous systems, from the earthworm on the hook to the human on the other end of the fishing rod. But although we can discern a separate brain in worms, it is not the case that the brain is the sole "commander" of the animal that the rest of the nervous system and body obeys. Indeed, even with its brain removed, worms are able to perform many types of behaviors, including locomotion, mating, burrowing, feeding, and even maze learning.[5]
As we move to insects we find increased complexity in all aspects of the brain and nervous system. So-called giant fiber systems (also found to some extent in worms and jellyfish) that allow rapid conduction of nerve impulses connect parts of the brain to specific muscles in legs or wings. Such connections permit the cockroach to dart away as soon as it senses the moving air preceding a quickly descending human foot. The brain itself is typically divided into three specialized segments, the protocerebrum, the deutocerebrum, and the tritocerebrum. In addition, insects possess a greater variety of sensory receptors than any other group of organisms, including vertebrates, that are sensitive to the odors, sounds, light patterns, texture, pressure, humidity, temperature, and chemical composition of their surroundings. The concentration of these sensory organs on the insect's head provides for rapid communication with the tiny yet capable brain located within.
Although minuscule by human standards, the range of abilities made possible by insect brains is impressive. These creatures show a remarkable variety of behaviors for locomotion, obtaining food, mating, and aiding the survival of their offspring. They can crawl, hop, swim, fly, burrow, and even walk on water. The female wasp hunts down a caterpillar, paralyzes it with her venom, and then lays its egg on the motionless prey so that her offspring will have a fresh and wholesome meal immediately after hatching. Leafcutter ants harvest leaves and bring them into their nest where they use them to cultivate indoor gardens of edible fungus. Honeybees live in social communities where there is a strict division of labor, and where foodgathering worker bees perform a special dance to communicate the location and richness of food sources to their hivemates. It is the evolution of their brains, together with the complementary evolution of their other body parts, that make insects the most abundant multicellular organisms on our planet.
The brain becomes both much larger and still more complex as we move to vertebrates such as fish, amphibians, and reptiles. The spinal cord, now protected within the vertebrae of the backbone, has become primarily a servant of the brain, a busy two-way highway of communication with fibers segregated into descending motor pathways and ascending sensory ones. The brain itself is now composed of a series of swellings of the anterior end of the spinal cord (the brain stem), the three major ones making up the three major parts of the vertebrate brain: the hindbrain, midbrain, and forebrain. From the hindbrain sprouts a distinctive structure, the "cerebellum" (Latin for "little brain").
Among mammals, the brain keeps its three major components, but with two new structures. The neocerebellum ("new cerebellum") is added to the cerebellum, looking much like a fungal growth at the base of the brain, and the neocortex ("new cortex") grows out of the front of the forebrain. In most mammals, these new additions are not particularly large relative to the brain stem. In primates they are much larger, and in the human they are so large that the original brain stem is almost completely hidden by this large convoluted mass of grey neural matter. In keeping with this remarkable increase of neocerebellar and neocortical tissue, humans enjoy the largest ratio of brain weight to body weight of any of earth's creatures.
It is not possible to know exactly why the human brain evolved as it did, but consideration of the structural evolution of the brain and results of comparative research on human and nonhuman brains provides some useful clues. It is now believed that during the long evolution of our brain, nervous systems changed in four principal ways. First, they became increasingly centralized in architecture, evolving from a loose network of nerve cells (as in the jellyfish) to a spinal column and complex brain with impressive swellings at the hindbrain and forebrain. This increasingly centralized structure also became increasingly hierarchical. It appears that newer additions to the human brain took over control from the previous additions and in effect became their new masters. Accordingly, the initiation of voluntary behavior as well as the ability to plan, engage in conscious thought, and use language depend on neocortical structures. Indeed, the human neocortex can actually destroy itself if it wishes, as when a severely depressed individual uses a gun to put a bullet through his or her skull.
Second, there was a trend toward encephalization, that is, a concentration of neurons and sense organs at one end of the organism. By concentrating neural and sensory equipment in one general location, transmission time from sense organs to brain was minimized. Third, the size, number, and variety of elements of the brain increased. Finally, there was an increase in plasticity, that is, the brain's ability to modify itself as a result of experience to make memory and the learning of new perceptual and motor abilities possible.
One way of understanding the evolution of the human brain is to see it as the addition of higher and higher levels of control. We will see in chapter 8 that the function of animal and human behavior can be understood as the control of perceptions, with perceptions corresponding to important aspects of the environment. For a sexually reproducing organism to survive and leave progeny, it must be able to control many different types of perceptions, that is, sensed aspects of its environment. At a minimum, it must be able to find food, avoid enemies, and mate. But as life evolved, the environment of our ancestors became more complex due to increasing numbers of competing organisms. So it would have been of considerable advantage to be able to perceive and control increasingly complex aspects of this environment. The bacterium E. coli can control its sensing of food and toxins only in a primitive way; organisms with more complex brains are able to sense and control much more complex aspects of their surroundings.
This capacity for increased environmental control is nowhere more striking than in our species. Using the advanced perceptual-behavioral capacities of our brain together with our culturally evolved knowledge of science and technology, we can visit ocean floors, scale the highest peaks, and set foot on other worlds. (The role that language is believed to have had in the evolution of the human brain will be considered separately in chapter 11.) But can the most complex human abilities and mental capacities be explained by natural selection? Our brain has certainly not changed appreciably over the last couple of hundred years, and yet we can solve mathematical, scientific, technological, and artistic problems that did not even exist a hundred years ago. So how could natural selection be responsible for the striking abilities of today's scientists, engineers, and artists?
This is actually the same problem that troubled Alfred Russel Wallace, as mentioned in chapter 3. It will be recalled that Wallace, despite being an independent codiscoverer of natural selection, could not, for example, imagine how natural selection could account for Africans' ability to sing and perform European music, since nothing in their native environment could have selected for such an ability. Consequently, for him the brain could only be a creation provided to us by God. We now know that in his embrace of this providential explanation, Wallace failed to realize that natural selection can lead to new abilities unrelated to those that were originally selected.
To use an example from technological evolution, the first personal computers were used to perform financial calculations in the form of electronic spreadsheets. However, these same machines with the proper software could also be used for word processing, telecommunications, computer games, and many other purposes, even though they were not originally designed with these functions in mind. A classic example of this phenomenon of functional shift in biological evolution is the transformation of stubby appendages for thermoregulation in insects and birds into wings for flight.[6] In the same way, selection pressure was undoubtedly exerted on early hominids to become better hunters. The ability to understand the behavior of other animals and organize hunting expeditions must have been very important in the evolution of our species. And the increasingly complex and adapted brain thus selected would have made other skills possible, such as making tools and using language, traits that in turn could become targets for continued natural selection. This transformation of biological structures and behaviors from one use to another was given the unfortunate name of preadaptation by Darwin, unfortunate since it can too easily be misunderstood to imply that somehow evolution "knows" what structures will be useful for future descendants of the current organisms.
American evolutionary paleontologist Stephen Jay Gould provided a better term for this phenomenon--exaptation. He made a major contribution to our understanding of evolution by insisting that we distinguish adaptation, the evolutionary process through which adaptedly complex structures and behaviors are progressively fine-tuned by natural selection with no marked change in the structure's or behavior's function, from exaptation, through which structures and behaviors originally selected for one function become involved in another, possibly quite unrelated, function. Exaptation makes it difficult if not impossible to understand why our brain evolved as it did. Although the brain allows us to speak, sing, dance, laugh, design computers, and solve differential equations, these and other abilities may well be accidental side effects of its evolution. As Gould and his associate Vrba cautioned:
. . . current utility carries no automatic implication about historical origin. Most of what the brain now does to enhance our survival lies in the domain of exaptation--and does not allow us to make hypotheses about the selective paths of human history. How much of the evolutionary literature on human behavior would collapse if we incorporated the principle of exaptation into the core of our evolutionary thinking?[7]
But although we may never know the actual events and specific selection pressures responsible for our brain power, we have no scientific reason to believe that evolution could not have fashioned our brain through natural selection. The fact that living organisms today have nervous systems and brains ranging from quite simple to amazingly complex is compelling evidence that our brain evolved through forgotten ancestors in progressive stages from simple to complex. And somehow, as a part of this evolutionary process, that most remarkable and mystifying of all natural phenomena came into being--human consciousness.
The Development of the Brain
From this evolutionary perspective, one might be led to conclude that our brain in all its striking adapted complexity is an inherited legacy of biological evolution. That once evolved it is thereafter provided to each individual by good old natural selection, specified in all its fine detail in the genome and transmitted through the generations from parent to offspring.
This type of genetically providential thinking of course is selectionist from the viewpoint of biological evolution, but nonetheless providential at the level of the individual organism. It can be seen in the pioneering work on brain development and function of Roger Sperry for which he shared a Nobel prize in 1981. This research in the 1950s involved disturbing the normal location of nerve fibers in the developing brains of fish and rats. For example, nerve fibers that normally connect the top part of the fish's retina with the bottom part of the brain, called the optic tectum, were surgically removed and reconnected to the top part of the optic tectum. Despite this modification, the nerve grew back to its normal position in the brain. Similar experiments carried out by other researchers on rats indicated that fibers that innervate muscles also "knew" to which muscle they should be attached and made their proper connections despite surgical disturbances. This led Sperry to conclude that the connections of the nervous system are completely specified in the organism's genes. As his former student Michael Gazzaniga explains:
In the original Sperry view of the nervous system, brain and body developed under tight genetic control. The specificity was accomplished by the genes' setting up chemical gradients, which allowed for the point-to-point connections of the nervous system.[8]
But there is a vexing problem with the notion that the genome provides complete information for the construction of the nervous system of humans and other mammals. It is estimated that just the human neocortex alone has about 1015 (one followed by 15 zeros, or one thousand million million) synapses.[9] Since the human genome has only about 3.5 billion (3.5 x 109) bits of information (nucleotide base pairs), with 30% to 70% of these appearing silent,[10] some neural and molecular scientists have concluded that our genes simply do not have enough storage capacity to specify all of these connections, in addition to including information on the location and type of each neuron plus similar information for the rest of the body. The problem is not unlike trying to save a document made up of 100 million characters on a computer disk that can hold only 1.4 million characters. As Changeux noted:
Once a nerve cell has become differentiated it does not divide anymore. A single nucleus, with the same DNA, must serve an entire lifetime for the formation and maintenance of tens of thousands of synapses. It seems difficult to imagine a differential distribution of genetic material from a single nucleus to each of these tens of thousands of synapses unless we conjure up a mysterious "demon" who selectively channels this material to each synapse according to a preestablished code! The differential expression of genes cannot alone explain the extreme diversity and specificity of connections between neurons. [11]
Additional understanding of the relation between the genome and the nervous system can be gained by considering Daphnia magna. Commonly referred to as the water flea or daphnid, this small fresh-water crustacean is familiar to many aquarium owners since it is relished by tropical fish. But what makes the daphnid interesting for our current purposes is that when the female is isolated from males, she can most conveniently reproduce by the asexual process of parthenogenesis, giving birth to genetically identical clones. In addition, the daphnid has a relatively simple nervous system that facilitates its study. If its genome completely controlled the development of its nervous system, it should be the case that genetically identical daphnids should have structurally identical nervous systems. However, examination of daphnid eyes using the electron microscope reveals that although genetically identical clones all have the same number of neurons, considerable variation exists in the exact number of synapses and in the configurations of connections leading to and away from the cell body of each neuron, that is, the dendritic and axonal branches. As we move to more complex organisms, the variability of their nervous systems increases. This provides clear evidence that the structure and wiring of the nervous system are not the result of following a detailed construction program provided by the genes.
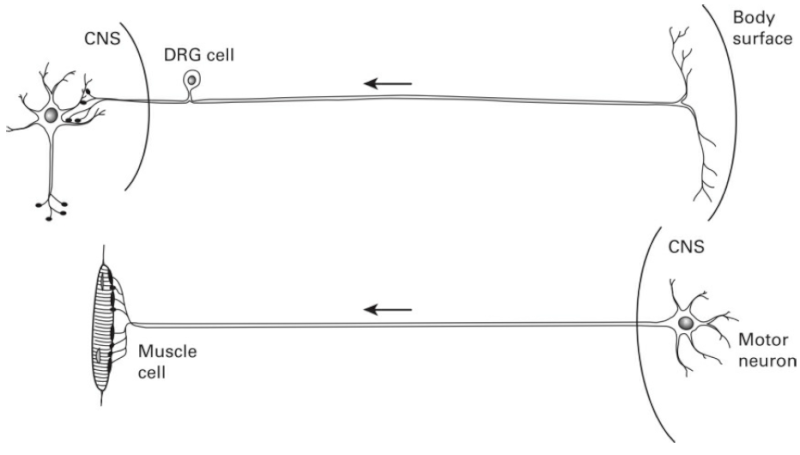
How then is the brain able to achieve the very specific and adapted wiring required to function in so many remarkable ways? For example, how does a motor neuron know to which particular muscle fiber it should connect? How is a sensory neuron in the visual system able to join itself to the correct cell in the visual cortex located in the occipital lobe of the brain? If this detailed neuron-to-neuron connection information is not provided by the genes, whence does it come?
The first clues to solving this puzzle go back to 1906 when it was observed that in embryonic nerve tissue, some neurons did not stain well and appeared to be degenerating and dying.[12] Since it had been assumed that in a developing embryo, nerve cells should be increasing in number and not dying off, this finding was somewhat surprising. But nerve cell death in the developing nervous system has since been observed repeatedly. The extent to which it occurs was dramatically demonstrated by Viktor Hamburger. He found that in a certain area of the spinal cord of the chicken embryo over 20,000 neurons were present, but that in the adult chicken only about 12,000, or 60%, of these cells remained.[13] Much of this neuronal death occurs during the early days of the embryo's existence. Nerve cells continue to expire thereafter, albeit at a slower pace.
A particularly striking example of neuronal elimination in development involves the death of an entire group of brain cells:
Most frequently, neuron death affects only some of the neurons in a given category. However, in one case . . . a whole category of cells dies. These particular neurons of layer I, the most superficial layer of the cerebral cortex, characteristically have axons and dendrites oriented parallel to the cortical surface rather than perpendicular to it, like the pyramidal cells. These cells were first observed in the human fetus but have since been found in other mammals. Purely and simply, they disappear in the adult.Changeux (1985, p. 217).[14]
But the death of obviously useless brain cells cannot account for the specific connections that are achieved by the remaining neurons. For example, the visual cortex of cats and monkeys has what are called ocular dominance columns within a specific region known as cortical layer 4. In any one column of this brain area in the adult animal we find only axons that are connected to the right eye, while in the neighboring column are located only axons with signals originating from the left eye. So not only must the axons find their way to a specific region of the brain, which can be quite far from where their cell bodies are located, they must also find a specific address within a certain neighborhood.
The ability of axons to connect to the appropriate regions of the brain during development has been studied in careful detail since the beginning of this century. Axons grow in the brain like the stem of a plant. At the end of the growing axon is found a growth cone which was described by Spanish neuroscientist Ramón y Cajal in 1909 as "a sort of club or battering ram, possessing an exquisite chemical sensitivity, rapid amoeboid movements, and a certain driving force that permits it to push aside, or cross, obstacles in its way . . . until it reaches its destination."[15] Although the exact mechanisms by which this is accomplished are still unknown, it appears that the growth cone is sensitive to certain chemicals along its path that are released by its target region. In this way visual system axons originating in the lateral geniculate nucleus find their way to cortical layer 4 in the occipital lobe of the brain in much the same way that a police bloodhound is able to sniff out the escaped prisoner hiding in an Illinois cornfield.
But although these growth cones lead their axons to the proper region of the brain (or muscle in the case of motor neurons), they cannot lead them to the precise target addresses. For a particular growth cone, it appears that any cell of a particular type will serve as a target. Indeed, in the newborn cat, ocular columns receive axons from both eyes, not just from one or the other, as in the adult brain. For this final and important fine-tuning to be achieved (on which stereoscopic vision depends), many of the original terminal connections of the axon must be eliminated. In the case of vision, all axonal connections from the wrong eye are eliminated, and those from the correct eye are retained. In the case of motor systems that initially have many-to-many connections between motor neurons of the spinal column and muscle fibers (that is, many motor neuron axons connected to same muscle fiber, and many muscle fibers connected to the same axon), the mature animal possesses a much more finely ordered system with each muscle fiber enervated by one and only one motor neuron. The mammalian nervous system changes from birth to maturity from a degenerate system having many redundant and diffuse connections, to a much more finely tuned system that makes both adaptedly complex behaviors and perceptions (such as stereoscopic vision) possible.
So now the question naturally arises, how does the nervous system know which connections to retain and which to eliminate? The work of David Hubel and Torsten Wiesel in the 1970s (both of whom shared a Nobel prize with Sperry in 1981) provided the first clue. They conducted their ground-breaking experiments by closing the lid of one eye of newborn cats, and found that even one week without sight altered the connections of the eyes to layer 4 of the occipital cortex. Axons carrying nervous signals from the closed eye made fewer connections with the cortex, whereas axons from the open eye made many more connections than was normal. This suggested that visual system axons compete for space in the visual cortex, with the result of the competition dependent on the amount and type of sensory stimulation carried by the axons. Subsequent research by others using drugs to block the firing of visual system neurons, as well as artificial stimulation of these neurons, showed that it is not neural activity per se that results in the selective elimination of synapses, but rather that only certain types of neural activity result in the retention of certain synapses, while all others are eventually eliminated.
In a sense, then, cells that fire together wire together. The timing of the action-potential activity is critical in determining which synaptic connections are strengthened and which are weakened and eliminated. Under normal circumstances, vision itself acts to correlate the activity of neighboring retinal ganglion cells, because the cells receive inputs from the same parts of the visual world.[16]
The dependence of the development of the visual system on sensory stimulation would seem to indicate that the fine-tuning of its connections would have to wait until the birth of the animal when it is delivered from the comforting warm darkness of the womb to the cold light of day. However, recent evidence suggests that this fine-tuning actually begins to take place in utero. Prenatal development appears to depend on spontaneous firing of retinal cells that do not depend on light stimulation from the external world. Similar endogenous patterns of activity may also exist in the spinal cord, and may refine the synaptic connections of motor systems as well.[17]
Nonetheless, interactive postnatal experience of the external world is required for normal development of senses and nervous systems in mammals. Cats who have one eye sewn shut at birth lose all ability to see with this eye when it is opened several months later. The same applies to humans. Before the widespread use of antibiotics, eye infections left many newborn infants with cloudy lenses and corneas that caused functional blindness, even though their retinas and visual nervous systems were normal at the time of birth. Years later a number of these individuals underwent operations to replace their cloudy lenses and corneas with clear ones, but it was too late. Contrary to initial expectations, none of these people was able to see after the transplant.[18]It was simply not known at the time that early visual experience was essential to the normal maturation of the brain's visual circuitry. Similarly, some children are born with a wandering eye that does not fixate the same part of the visual field as the normal eye, and other children have one eye that is seriously nearsighted or farsighted; in both cases, the retina of the abnormal eye must be provided with clear visual stimulation, usually by age four years, or it will become functionally blind since its connections to the brain's vision centers will be eliminated in favor of the normal eye.
We thus see that the normal development of the brain depends on a critical interaction between genetic inheritance and environmental experience. The genome provides the general structure of the central nervous system, and nervous system activity and sensory stimulation provide the means by which the system is fine-tuned and made operational. But this fine-tuning does not depend on adding new components and connections in the way that a radio is assembled in a factory, but rather it is achieved by eliminating much of what was originally present. It is as if the radio arrived on the assembly line with twice as many electrical components and connections as necessary to work. If such an overconnected radio were plugged in and turned on, nothing but silence, static, or a hum would be heard from its speaker. However, careful removal of unnecessary components and judicious snipping of redundant wires would leave just those components and connections that result in a functioning radio. This snipping is analogous to the elimination of synapses in the human brain as part of its normal development.
The process by which brain connections change over time as maturing animals interact with their environments has been studied in detail by psychologist William Greenough of the University of Illinois at Urbana-Champaign. Using sophisticated techniques for determining the numbers and densities of neurons and synapses in specific regions of the rat's brain, he and his associates found that during the first months of the rat's life a rapid spurt in the growth of synapses occurs regardless of the amount or type of sensory experience.[19] This period of synaptic "blooming" is followed by a sharp decline in the number of synapses. That is, an elimination or "pruning" of synapses then takes place based on the activity and sensory stimulation of the brain, and ultimately results in the configuration of connections characteristic of the mature rat's brain. Greenough refers to this initial blooming and pruning of synapses as "experience-expectant" learning, since the initial synaptic overproduction appears to be relatively independent of the animal's experiences. It is as though the brain is expecting important things to be happening during the first weeks and months of life, and is prepared for these experiences with an overabundance of synapses, only a fraction of which, however, will be selectively retained.
The work of Greenough and his associates is limited to rats and monkeys, but their findings have much in common with those of Peter Huttenlocher of the University of Chicago who counted the synapses in specific regions of the brains of humans who died at various ages. Huttenlocher found that:
The increase in synaptic density plus expansion of total cortical volume leave no doubt that the postnatal period is one of very rapid synaptogenesis in human frontal cortex. By age 2 years, synaptic density is at its maximum, at about the same time when other components of cerebral cortex also cease growing and when total brain weight approaches that of the adult. Synaptic density declines subsequently, reaching by adolescence an adult value that is only about 60% of the maximum.[20]
This wealth of synapses is thought to be responsible for the striking plasticity of the immature brain that permits the learning of skills that can be learned only with much greater difficulty or not at all by the already pruned adult brain. We already saw how immature animals and children are unable to develop normal vision if they are not exposed to a sharply focused visual world during this period of brain development. It has also been repeatedly observed that although many adults initially may make quite rapid progress in learning a foreign language, young children appear to have an important advantage over adults in being able to master the sounds of languages. Canadian child language researchers Janet Werker and Richard Tees observed that children younger than one year appear able to distinguish between the speech sounds used by any human language. By age 12 months, however, they begin to lose the ability to discriminate between sound contrasts that are not used in the language they hear every day. So whereas all normal infants can distinguish between the two related but distinct sounds represented by the letter t in Hindi, those who hear only English quickly lose this ability, and Hindi-speaking children retain it.[21] The work of Werker and Tees therefore provides important human behavioral evidence that is consistent with the view that normal brain development involves the loss of synaptic connections, which results in the loss of certain skills as the brain approaches its adult form.
A sensitive period for the acquisition of a first language was demonstrated by the plight of Genie, an American girl who was brutally isolated from all normal human interaction until she was found at age 13 years, and who never subsequently developed normal language abilities.[22] There is striking evidence that the immature, overconnected brain is also better suited than a mature one to acquiring second languages and sign languages.[23]
Taken together, these findings paint a picture of the developing brain that contrasts sharply from the genetic providentialism favored by Sperry. Instead of the brain unfolding according to a genetically specified blueprint, we see instead a process of selection by which overly abundant neuronal connections are eliminated through a weeding-out process, leaving only those connections that permit the animal to interact successfully with its environment.
Learning and Memory: Rewiring the Brain
The mammalian brain appears most adaptive during the early postnatal period, and continues to adapt and learn from new experiences throughout its adult life. During the 1960s and 1970s a series of studies offered impressive evidence that rats grew thicker brains and new synapses when they were placed in complex and challenging environments. These findings were consistent with the then-popular belief that learning and memory in mature mammals (as opposed to the brain development of immature animals) were additive processes involving the formation of new synaptic connections or the strengthening of already existing ones. The influential Canadian psychologist Donald Hebb assumed that "the changed facilitation that constitutes learning" was the result of "the growth of synaptic knobs."[24] Similarly, Sir John C. Eccles, who shared a Nobel prize in 1963 for his research on the transmission of nerve impulses, believed that memory and learning involved "the growth . . . of bigger and better synapses."[25]
However, it was also suggested that more than just adding synapses was involved in learning. One of the first to propose that subtractive brain changes could be involved in adult learning and memory was J. Z. Young, who in 1964 posited that such learning could be the result of the elimination of neuronal connections.[26]Several years later J. S. Albus hypothesized that "pattern storage must be accomplished principally by weakening synaptic weights rather than by strengthening them,"[27] and Richard Dawkins speculated that the selective death of neurons could underlie the storage of memories.[28]
But how could a subtractive process of neuron elimination be involved in learning and memory? It is particularly difficult to understand how the learning of a new skill, such as riding a bicycle or speaking a foreign language, or acquisition of new memories, such as learning the words to a poem or song, could be made possible by loss of synapses. We saw in the development and maturation of the brain that synaptic connections that are rarely used are weakened or eliminated, whereas those in active neural pathways are retained and perhaps strengthened. This subtractive process makes sense when dealing with an overwired, immature brain that may have close to twice as many synapses as it will have as an adult. But how can it work for a mature adult brain that has already been substantially whittled down by synaptic pruning?
To illustrate this problem, imagine an adult Spaniard learning English. To do this, the Spaniard will have to learn to hear and produce certain sound distinctions that are not used in Spanish, such as the contrasts involved in ship versus sheep, sue versus zoo, and watch versus wash. The research of Werker and Tees would lead us to predict that the Spaniard would not initially be able to make these distinctions since they are not made in the language he has heard and spoken all his life. The synaptic connections necessary for making these discriminations were present when he was born, but we would expect them to have been promptly pruned away since they were not used in the language of his environment. It is therefore not clear how any further pruning of synapses would permit him to learn this aspect of the English language.
Instead, it seems more likely that a process involving the addition of new synapses, or at least reorganizing current ones, would be necessary for this learning to take place. But then we run into the equally thorny problem of understanding how the brain could ever know which new synapses to add or modify! Surely, some combination of synaptic changes should allow the Spaniard to learn English, since many adults learn English and other languages, and such learning must be the result of changes in the synaptic connections of the brain. But just which new combination of synapses will do the trick? At the very least it would appear that the brain would somehow have to try out a number of new combinations and select the best ones. But to select the best ones, a source of variation is necessary, perhaps not unlike the initial variation of synaptic connections present in the immature, overconnected brain.
A possible solution to this riddle was offered by French neurobiologist Jean-Pierre Changeux in 1983. In his book L'Homme Neuronal (published in English in 1985 as Neuronal Man), Changeux proposed a "Darwinism of the synapses"[29] to account for the development of the brain and the learning it undergoes within its cultural environment.
According to this scheme, culture makes its impression progressively. The 10,000 or so synapses per cortical neuron are not established immediately. On the contrary, they proliferate in successive waves from birth to puberty in man. With each wave, there is transient redundancy and selective stabilization. This causes a series of critical periods when activity exercises its regulatory effect.[30]
In effect, he was suggesting that all adaptive brain changes, or at least those occurring between birth and puberty in humans, involve the elimination of preexisting synapses, but that these preexisting synapses were not necessarily all present at the same time. From birth to puberty, Changeux hypothesized that waves of synaptic growth would occur, with subsequent experience serving to retain the useful ones and eliminate the useless and redundant ones. These waves of synaptic overproduction would provide the source of variation on which synaptic selection could operate. Such learning resulted in an absolute increase in synaptic growth and numbers over time. This growth was not constant, but was rather envisioned as analogous to repeatedly taking two steps forward--randomly adding new synapses--followed by one step backward--eliminating the useless ones just added.
Changeux provided no hard evidence for his hypothesis that synaptic variation in the form of overproduction would precede the elimination of synapses as part of the brain's restructuring to permit the learning of new skills and acquisition of new knowledge. But such evidence was found a few years after the publication of his book. William Greenough and his associates, whose work on the maturational development of the rat's brain was noted earlier, also conducted research on changes in the brain induced by placing adult rats in special, enriched environments. In one study this resulted in a 20% increase (roughly 2000) in the number of synapses per neuron in the upper layers of the visual cortex.[31] Later research showed that such dramatic increases in synapses were not restricted to the rat's visual cortex.[32]
These and other similar findings led Greenough's group to propose that the waves of synapse proliferation first described by Changeux could be elicited by the complex demands placed on the adult brain in a new, challenging environment. These researchers referred to this process as "experience-dependent" development since it depends on the environment triggering the formation of new synaptic growth on which the selective process can act.[33]
Greenough's conception of how the adult brain is able to learn new skills and form new memories offers an appealing solution to the problem concerning the additive and subtractive processes underlying the adult's brain adaptation to new environments. According to this theory, experience-dependent learning combines both additive and subtractive processes. The additive component involves the blooming of new synapses in response to the animal's attempt to control aspects of a new, complex environment. Although the brain does appear to know what part of itself has to be involved in this new synapse-construction project, it need not (indeed, could not) know which particular connections to make. By forming a large variety and number of new connections, the brain can select the combinations that work best, in the same way that the immature, developing brain retains useful connections from its initial oversupply of synapses. The long-term result is an overall addition to the number of synapses. But the actual selection process that fine-tunes the connections is a subtractive one in which the useful connections are selectively retained and less useful ones eliminated. Although clear evidence exists for synaptic increase in learning, as I write this we still have no such evidence in mature learning for an overproduction of synapses that are then pruned away. However, recent research has found evidence for an overproduction of dendrites in mature rats during readaptation of the brain after brain injury, which at least suggests that synaptic overproduction may be involved as well.[34] These findings fit very nicely with the subtractive synapse findings on brain maturation and provide a solution to the mystery of how the brain could know exactly which new synaptic connections to establish to enable it to acquire new knowledge, skills, and memories.
Although only a relatively small number of neuroscientists have opted for a selectionist approach to their research and theorizing, Changeux and Greenough and their associates are not the only ones whose research suggests that the adult brain develops and learns through a process of cumulative neural variation and selection. This theory has now been embraced and given additional support by several other leading neuroscientists. William Calvin refers to the brain as a "Darwin machine" that follows the plan "make lots of random variants by brute bashing about, then select the good ones."[35] Gerald Edelman, who shared a Nobel prize in 1972 for his research on the chemical structure of antibodies in the immune system, has contributed a remarkable outpouring of books describing aspects of his "neuronal group selection theory" of brain development and learning through a selectionist process he refers to as "neural Darwinism."[36]And noted psychologist and neuroscientist Michael Gazzaniga, best known for his ground-breaking research on humans with split brains, recently embraced a selectionist account of brain functioning and development.[37]
Current research is under way to determine whether unambiguous physical evidence can be found for the overproduction and elimination of newly formed synapses in the adult brain in response to environmental changes. Such a finding would place the brain alongside the immune system as another striking example of how cumulative variation and selection processes during the lifetime of an organism make it possible to adapt to complex, changing environments.
We have now seen that understanding both the adapted and adaptive complexity of the human brain involves finding answers to three questions: how did the brain originate as a biological organ?; how does it develop from a fertilized egg into a mature brain?; and how is it able when mature to rewire itself to learn from and adapt to changes in its environment?
Much more work must be done before we have detailed answers to these questions. But substantial progress has already been made as we move midway into the "decade of the brain." To a large extent this progress has consisted of rejecting providential and instructionist explanations for these puzzles of fit, and finding considerable evidence and reason in favor of selectionist explanations. The powerful process of cumulative blind variation and selection working over millions of years is not only the only reasonable theory for the biological evolution of the brain, but we find that it has surfaced again in a different but still recognizable form as an explanation for the brain's embryonic growth and continued development during its relatively brief lifetime.
It is here, as Changeux remarked, that "the Darwinism of synapses replaces the Darwinism of genes."[38] To close the circle, it should be noted that a striking consequence of the joint effects of among-organism genetic and within-organism synaptic selection is the brain's understanding both itself and the process of selection that is responsible for its extraordinary abilities.
[1]Changeux (1985, p. 248; first emphasis added).
1) http://thebrain.mcgill.ca/flash/d/d_05/d_05_cr/d_05_cr_her/d_05_cr_her.html
2) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3973910/
3) http://faculty.education.illinois.edu/g-cziko/wm/05.html#Heading2
http://reasonandscience.heavenforum.org/t2324-how-to-explain-best-the-origin-and-emergence-of-the-brain
An Intricate Cross-Section of the Brain Depicted With Thousands of Layers of Gold Leaf
http://www.thisiscolossal.com/2017/04/brain-depicted-with-gold-leaf/
Some naturalistic explanations:
THE EVOLUTIONARY LAYERS OF THE HUMAN BRAIN 1
The most efficient model for understanding the brain in terms of its evolutionary history is the famous triune brain theory developed by Paul MacLean. According to this theory, the following three distinct brains emerged successively in the course of evolution and now co-inhabit the human skull:
The reptilian brain, the oldest of the three, controls the body's vital functions such as heart rate, breathing, body temperature and balance. Our reptilian brain includes the main structures found in a reptile's brain: the brainstem and the cerebellum. The reptilian brain is reliable but tends to be somewhat rigid and compulsive.
The limbic brain emerged in the first mammals. It can record memories of behaviours that produced agreeable and disagreeable experiences, so it is responsible for what are called emotions in human beings. The main structures of the limbic brain are the hippocampus, the amygdala, and the hypothalamus. The limbic brain is the seat of the value judgments that we make, often unconsciously, that exert such a strong influence on our behaviour.
The neocortex first assumed importance in primates and culminated in the human brain with its two large cerebral hemispheres that play such a dominant role. These hemispheres have been responsible for the development of human language, abstract thought, imagination, and consciousness. The neocortex is flexible and has almost infinite learning abilities. The neocortex is also what has enabled human cultures to develop. These three parts of the brain do not operate independently of one another. They have established numerous interconnections through which they influence one another. The neural pathways from the limbic system to the cortex, for example, are especially well developed.
The model of the triune brain proposed by MacLean in 1970 is a useful piece of shorthand for the complex evolutionary history of the human brain. But the brain's combination of reptilian, paleomammalian and neomammalian structures is far more intricate than a mere set of nested Russian dolls.
Ever since the first mammals appeared more than 200 million years ago, the cerebral cortex has assumed greater and greater importance compared with the brain's other, older structures. Because these structures had proven their effectiveness for meeting certain fundamental needs, there was no reason for them to disappear. Instead, evolution favoured a process of building expansions and additions, rather than rebuilding everything from the bottom up.
Evolution of the human brain: when bigger is better 2
The human brain contains about 100 billion neurons, more than 100,000 km of interconnections, and has an estimated storage capacity of 1.25 × 10^12 bytes (Cherniak, 1990; Hofman, 2012). These impressive numbers have led to the idea that our cognitive capabilities are virtually without limit. The human brain, however, has evolved from a set of underlying structures that constrain its size, and the amount of information it can store and process. If the ability of an organism to process information about its environment is a driving force behind evolution, then the more information a system, such as the brain, receives, and the faster it can process this information, the more adequately it will be able to respond to environmental challenges and the better will be its chances of survival (Macphail and Bolhuis, 2001; Roth and Dicke, 2012; Hofman, 2014). The limit to any intelligent system therefore lies in its abilities to process and integrate large amounts of sensory information and to compare these signals with as many memory states as possible, and all that in a minimum of time. It implies that the functional capacity of a neuronal structure is inherently limited by its neural architecture and signal processing time (see e.g., Laughlin and Sejnowski, 2003; Buzsáki et al., 2013). The object of this review is to present current perspectives on primate brain evolution, especially in humans, and to examine some hypothetical organizing principles that underlie the brain's complex organization. Some of the design principles and operational modes that underlie the information processing capacity of the cerebral cortex in primates will be explored, and it will be argued that with the evolution of the human brain we have nearly reached the limits of biological intelligence.
Brain Evolution and Development:
The Selection of Neurons and Synapses 3
Although there are many different types of neurons, almost all of them share certain common features. The cell body, or soma, contains the nucleus of the neuron, which in turn houses a complete set of the organism's genes. The nucleus is surrounded by cytoplasm, the chemical "soup" of the cell that contains the organelles essential to the neuron's functioning and metabolism. In these respects, neurons are similar to other cells throughout the body, except for the fact that unlike most other cells they rarely divide to reproduce new neurons.
The ways in which neurons are specialized to carry out their communicative function is made evident by closer examination of the appendages they sport, that is, their dendrites and axons. The dendrites can be likened to a bushy antenna system that receives signals from other neurons. When a dendrite is stimulated in a certain way, the neuron to which it is attached suddenly changes its electrical polarity and may fire, sending a signal out along its single axon where it may be picked up by the dendrites of other neurons.[3] Considering the small size of the neuron's body, the length of an axon can be considerable, up to several meters in the neck of the giraffe. Thus the firing of one neuron can influence the firing of another one a considerable distance away.
For one neuron to influence another, the two must be connected, and this is accomplished by junctions called synapses (figure 5.2). These synaptic junctions usually connect the axon of one neuron with the dendrites of another, a typical neuron in the cortex of the human brain having about 10,000 synapses. The synapses therefore constitute an exceedingly complex wiring system that surpasses by many orders of magnitude the complexity of even the most advanced supercomputers. It is this organization of connections both within the skull and to more distant sense organs and muscles that gives the brain its amazing abilities. Indeed, it is widely believed today by neuroscientists, psychologists, and even philosophers that all of the knowledge the human brain contains--from being able to walk to the ability to perform abstract scientific and mathematical reasoning--is a function of the connections existing among the neurons.
How this unfathomably complex organization allows us to perceive, behave, think, feel, and control our environment presents us with what may be the most striking puzzle of fit we have yet encountered. The puzzle actually has three aspects. First, we must consider how over millions of years the primitive nervous system of our early ancestors evolved into an organ that has made it possible for the human species to become the most adaptable and powerful organism on the planet--living, thriving, and modifying the environment (both intentionally and unintentionally) from the tropics to the polar regions, and perhaps soon in outer space and on other planets.[4] Second, we must understand how it is possible for the intricate structure of the brain to develop from a single fertilized egg cell. Finally, we must try to comprehend how the mature brain is able to continue to modify its own structure so that it can acquire new skills and information to continue surviving and reproducing in an unpredictable, ever-changing world. In this chapter we will consider the research and theories that are beginning to provide answers to these questions. Indeed, the 1990s has been referred to as "the decade of the brain," as scholars and scientists in fields from philosophy to molecular neurobiology focus their energies on understanding humankind's ultimate inner frontier.
The Evolution of the Brain
Neurons are quite distinct from other body cells in ways that make them suited to their specialized role of signal processing and communication, but it is not too difficult to see how they could have evolved from less specialized cells. All living cells are surrounded by a cell membrane that separates the special chemical composition of its interior from that of the external world. This difference in chemical composition results in a small electrical potential between the inside and outside of the cell, in much the same way that a voltage exists between the two sides of a battery. When a part of a cell's membrane is disturbed in a certain way, it loses its electrical potential, becoming depolarized at the site of the disturbance. This sudden change in electrical potential can itself be a disturbance, causing additional depolarizations along the membrane. In most cells, such depolarization would not spread far, certainly not to neighboring cells. But a few changes in the shape and arrangement of cells (in just the way that neurons are fashioned) permits depolarization to propagate quickly from one neuron to the next, and allows it to travel quickly as an electrochemical signal from one end of an animal to the other.
An example of a simple nervous system is provided by the jellyfish (or Medusa). The jellyfish's nervous system forms an undifferentiated network and serves primarily to coordinate the animal's swimming motions. Since the jellyfish's skirt must open and contract in a coordinated manner for the animal to move through the water, its nervous system serves as a simple communications network making it possible for all parts of the skirt to open repeatedly and then contract at the same time.
Worms are the simplest organisms to have a central nervous system, which includes a distinct brain that is connected to groups of neurons organized as nerve cords running along the length of its body. This more complicated nervous system allows worms to exhibit more complex forms of behavior. An anterior brain connected to a nerve cord is the basic design for all organisms with central nervous systems, from the earthworm on the hook to the human on the other end of the fishing rod. But although we can discern a separate brain in worms, it is not the case that the brain is the sole "commander" of the animal that the rest of the nervous system and body obeys. Indeed, even with its brain removed, worms are able to perform many types of behaviors, including locomotion, mating, burrowing, feeding, and even maze learning.[5]
As we move to insects we find increased complexity in all aspects of the brain and nervous system. So-called giant fiber systems (also found to some extent in worms and jellyfish) that allow rapid conduction of nerve impulses connect parts of the brain to specific muscles in legs or wings. Such connections permit the cockroach to dart away as soon as it senses the moving air preceding a quickly descending human foot. The brain itself is typically divided into three specialized segments, the protocerebrum, the deutocerebrum, and the tritocerebrum. In addition, insects possess a greater variety of sensory receptors than any other group of organisms, including vertebrates, that are sensitive to the odors, sounds, light patterns, texture, pressure, humidity, temperature, and chemical composition of their surroundings. The concentration of these sensory organs on the insect's head provides for rapid communication with the tiny yet capable brain located within.
Although minuscule by human standards, the range of abilities made possible by insect brains is impressive. These creatures show a remarkable variety of behaviors for locomotion, obtaining food, mating, and aiding the survival of their offspring. They can crawl, hop, swim, fly, burrow, and even walk on water. The female wasp hunts down a caterpillar, paralyzes it with her venom, and then lays its egg on the motionless prey so that her offspring will have a fresh and wholesome meal immediately after hatching. Leafcutter ants harvest leaves and bring them into their nest where they use them to cultivate indoor gardens of edible fungus. Honeybees live in social communities where there is a strict division of labor, and where foodgathering worker bees perform a special dance to communicate the location and richness of food sources to their hivemates. It is the evolution of their brains, together with the complementary evolution of their other body parts, that make insects the most abundant multicellular organisms on our planet.
The brain becomes both much larger and still more complex as we move to vertebrates such as fish, amphibians, and reptiles. The spinal cord, now protected within the vertebrae of the backbone, has become primarily a servant of the brain, a busy two-way highway of communication with fibers segregated into descending motor pathways and ascending sensory ones. The brain itself is now composed of a series of swellings of the anterior end of the spinal cord (the brain stem), the three major ones making up the three major parts of the vertebrate brain: the hindbrain, midbrain, and forebrain. From the hindbrain sprouts a distinctive structure, the "cerebellum" (Latin for "little brain").
Among mammals, the brain keeps its three major components, but with two new structures. The neocerebellum ("new cerebellum") is added to the cerebellum, looking much like a fungal growth at the base of the brain, and the neocortex ("new cortex") grows out of the front of the forebrain. In most mammals, these new additions are not particularly large relative to the brain stem. In primates they are much larger, and in the human they are so large that the original brain stem is almost completely hidden by this large convoluted mass of grey neural matter. In keeping with this remarkable increase of neocerebellar and neocortical tissue, humans enjoy the largest ratio of brain weight to body weight of any of earth's creatures.
It is not possible to know exactly why the human brain evolved as it did, but consideration of the structural evolution of the brain and results of comparative research on human and nonhuman brains provides some useful clues. It is now believed that during the long evolution of our brain, nervous systems changed in four principal ways. First, they became increasingly centralized in architecture, evolving from a loose network of nerve cells (as in the jellyfish) to a spinal column and complex brain with impressive swellings at the hindbrain and forebrain. This increasingly centralized structure also became increasingly hierarchical. It appears that newer additions to the human brain took over control from the previous additions and in effect became their new masters. Accordingly, the initiation of voluntary behavior as well as the ability to plan, engage in conscious thought, and use language depend on neocortical structures. Indeed, the human neocortex can actually destroy itself if it wishes, as when a severely depressed individual uses a gun to put a bullet through his or her skull.
Second, there was a trend toward encephalization, that is, a concentration of neurons and sense organs at one end of the organism. By concentrating neural and sensory equipment in one general location, transmission time from sense organs to brain was minimized. Third, the size, number, and variety of elements of the brain increased. Finally, there was an increase in plasticity, that is, the brain's ability to modify itself as a result of experience to make memory and the learning of new perceptual and motor abilities possible.
One way of understanding the evolution of the human brain is to see it as the addition of higher and higher levels of control. We will see in chapter 8 that the function of animal and human behavior can be understood as the control of perceptions, with perceptions corresponding to important aspects of the environment. For a sexually reproducing organism to survive and leave progeny, it must be able to control many different types of perceptions, that is, sensed aspects of its environment. At a minimum, it must be able to find food, avoid enemies, and mate. But as life evolved, the environment of our ancestors became more complex due to increasing numbers of competing organisms. So it would have been of considerable advantage to be able to perceive and control increasingly complex aspects of this environment. The bacterium E. coli can control its sensing of food and toxins only in a primitive way; organisms with more complex brains are able to sense and control much more complex aspects of their surroundings.
This capacity for increased environmental control is nowhere more striking than in our species. Using the advanced perceptual-behavioral capacities of our brain together with our culturally evolved knowledge of science and technology, we can visit ocean floors, scale the highest peaks, and set foot on other worlds. (The role that language is believed to have had in the evolution of the human brain will be considered separately in chapter 11.) But can the most complex human abilities and mental capacities be explained by natural selection? Our brain has certainly not changed appreciably over the last couple of hundred years, and yet we can solve mathematical, scientific, technological, and artistic problems that did not even exist a hundred years ago. So how could natural selection be responsible for the striking abilities of today's scientists, engineers, and artists?
This is actually the same problem that troubled Alfred Russel Wallace, as mentioned in chapter 3. It will be recalled that Wallace, despite being an independent codiscoverer of natural selection, could not, for example, imagine how natural selection could account for Africans' ability to sing and perform European music, since nothing in their native environment could have selected for such an ability. Consequently, for him the brain could only be a creation provided to us by God. We now know that in his embrace of this providential explanation, Wallace failed to realize that natural selection can lead to new abilities unrelated to those that were originally selected.
To use an example from technological evolution, the first personal computers were used to perform financial calculations in the form of electronic spreadsheets. However, these same machines with the proper software could also be used for word processing, telecommunications, computer games, and many other purposes, even though they were not originally designed with these functions in mind. A classic example of this phenomenon of functional shift in biological evolution is the transformation of stubby appendages for thermoregulation in insects and birds into wings for flight.[6] In the same way, selection pressure was undoubtedly exerted on early hominids to become better hunters. The ability to understand the behavior of other animals and organize hunting expeditions must have been very important in the evolution of our species. And the increasingly complex and adapted brain thus selected would have made other skills possible, such as making tools and using language, traits that in turn could become targets for continued natural selection. This transformation of biological structures and behaviors from one use to another was given the unfortunate name of preadaptation by Darwin, unfortunate since it can too easily be misunderstood to imply that somehow evolution "knows" what structures will be useful for future descendants of the current organisms.
American evolutionary paleontologist Stephen Jay Gould provided a better term for this phenomenon--exaptation. He made a major contribution to our understanding of evolution by insisting that we distinguish adaptation, the evolutionary process through which adaptedly complex structures and behaviors are progressively fine-tuned by natural selection with no marked change in the structure's or behavior's function, from exaptation, through which structures and behaviors originally selected for one function become involved in another, possibly quite unrelated, function. Exaptation makes it difficult if not impossible to understand why our brain evolved as it did. Although the brain allows us to speak, sing, dance, laugh, design computers, and solve differential equations, these and other abilities may well be accidental side effects of its evolution. As Gould and his associate Vrba cautioned:
. . . current utility carries no automatic implication about historical origin. Most of what the brain now does to enhance our survival lies in the domain of exaptation--and does not allow us to make hypotheses about the selective paths of human history. How much of the evolutionary literature on human behavior would collapse if we incorporated the principle of exaptation into the core of our evolutionary thinking?[7]
But although we may never know the actual events and specific selection pressures responsible for our brain power, we have no scientific reason to believe that evolution could not have fashioned our brain through natural selection. The fact that living organisms today have nervous systems and brains ranging from quite simple to amazingly complex is compelling evidence that our brain evolved through forgotten ancestors in progressive stages from simple to complex. And somehow, as a part of this evolutionary process, that most remarkable and mystifying of all natural phenomena came into being--human consciousness.
The Development of the Brain
From this evolutionary perspective, one might be led to conclude that our brain in all its striking adapted complexity is an inherited legacy of biological evolution. That once evolved it is thereafter provided to each individual by good old natural selection, specified in all its fine detail in the genome and transmitted through the generations from parent to offspring.
This type of genetically providential thinking of course is selectionist from the viewpoint of biological evolution, but nonetheless providential at the level of the individual organism. It can be seen in the pioneering work on brain development and function of Roger Sperry for which he shared a Nobel prize in 1981. This research in the 1950s involved disturbing the normal location of nerve fibers in the developing brains of fish and rats. For example, nerve fibers that normally connect the top part of the fish's retina with the bottom part of the brain, called the optic tectum, were surgically removed and reconnected to the top part of the optic tectum. Despite this modification, the nerve grew back to its normal position in the brain. Similar experiments carried out by other researchers on rats indicated that fibers that innervate muscles also "knew" to which muscle they should be attached and made their proper connections despite surgical disturbances. This led Sperry to conclude that the connections of the nervous system are completely specified in the organism's genes. As his former student Michael Gazzaniga explains:
In the original Sperry view of the nervous system, brain and body developed under tight genetic control. The specificity was accomplished by the genes' setting up chemical gradients, which allowed for the point-to-point connections of the nervous system.[8]
But there is a vexing problem with the notion that the genome provides complete information for the construction of the nervous system of humans and other mammals. It is estimated that just the human neocortex alone has about 1015 (one followed by 15 zeros, or one thousand million million) synapses.[9] Since the human genome has only about 3.5 billion (3.5 x 109) bits of information (nucleotide base pairs), with 30% to 70% of these appearing silent,[10] some neural and molecular scientists have concluded that our genes simply do not have enough storage capacity to specify all of these connections, in addition to including information on the location and type of each neuron plus similar information for the rest of the body. The problem is not unlike trying to save a document made up of 100 million characters on a computer disk that can hold only 1.4 million characters. As Changeux noted:
Once a nerve cell has become differentiated it does not divide anymore. A single nucleus, with the same DNA, must serve an entire lifetime for the formation and maintenance of tens of thousands of synapses. It seems difficult to imagine a differential distribution of genetic material from a single nucleus to each of these tens of thousands of synapses unless we conjure up a mysterious "demon" who selectively channels this material to each synapse according to a preestablished code! The differential expression of genes cannot alone explain the extreme diversity and specificity of connections between neurons. [11]
Additional understanding of the relation between the genome and the nervous system can be gained by considering Daphnia magna. Commonly referred to as the water flea or daphnid, this small fresh-water crustacean is familiar to many aquarium owners since it is relished by tropical fish. But what makes the daphnid interesting for our current purposes is that when the female is isolated from males, she can most conveniently reproduce by the asexual process of parthenogenesis, giving birth to genetically identical clones. In addition, the daphnid has a relatively simple nervous system that facilitates its study. If its genome completely controlled the development of its nervous system, it should be the case that genetically identical daphnids should have structurally identical nervous systems. However, examination of daphnid eyes using the electron microscope reveals that although genetically identical clones all have the same number of neurons, considerable variation exists in the exact number of synapses and in the configurations of connections leading to and away from the cell body of each neuron, that is, the dendritic and axonal branches. As we move to more complex organisms, the variability of their nervous systems increases. This provides clear evidence that the structure and wiring of the nervous system are not the result of following a detailed construction program provided by the genes.
How then is the brain able to achieve the very specific and adapted wiring required to function in so many remarkable ways? For example, how does a motor neuron know to which particular muscle fiber it should connect? How is a sensory neuron in the visual system able to join itself to the correct cell in the visual cortex located in the occipital lobe of the brain? If this detailed neuron-to-neuron connection information is not provided by the genes, whence does it come?
The first clues to solving this puzzle go back to 1906 when it was observed that in embryonic nerve tissue, some neurons did not stain well and appeared to be degenerating and dying.[12] Since it had been assumed that in a developing embryo, nerve cells should be increasing in number and not dying off, this finding was somewhat surprising. But nerve cell death in the developing nervous system has since been observed repeatedly. The extent to which it occurs was dramatically demonstrated by Viktor Hamburger. He found that in a certain area of the spinal cord of the chicken embryo over 20,000 neurons were present, but that in the adult chicken only about 12,000, or 60%, of these cells remained.[13] Much of this neuronal death occurs during the early days of the embryo's existence. Nerve cells continue to expire thereafter, albeit at a slower pace.
A particularly striking example of neuronal elimination in development involves the death of an entire group of brain cells:
Most frequently, neuron death affects only some of the neurons in a given category. However, in one case . . . a whole category of cells dies. These particular neurons of layer I, the most superficial layer of the cerebral cortex, characteristically have axons and dendrites oriented parallel to the cortical surface rather than perpendicular to it, like the pyramidal cells. These cells were first observed in the human fetus but have since been found in other mammals. Purely and simply, they disappear in the adult.Changeux (1985, p. 217).[14]
But the death of obviously useless brain cells cannot account for the specific connections that are achieved by the remaining neurons. For example, the visual cortex of cats and monkeys has what are called ocular dominance columns within a specific region known as cortical layer 4. In any one column of this brain area in the adult animal we find only axons that are connected to the right eye, while in the neighboring column are located only axons with signals originating from the left eye. So not only must the axons find their way to a specific region of the brain, which can be quite far from where their cell bodies are located, they must also find a specific address within a certain neighborhood.
The ability of axons to connect to the appropriate regions of the brain during development has been studied in careful detail since the beginning of this century. Axons grow in the brain like the stem of a plant. At the end of the growing axon is found a growth cone which was described by Spanish neuroscientist Ramón y Cajal in 1909 as "a sort of club or battering ram, possessing an exquisite chemical sensitivity, rapid amoeboid movements, and a certain driving force that permits it to push aside, or cross, obstacles in its way . . . until it reaches its destination."[15] Although the exact mechanisms by which this is accomplished are still unknown, it appears that the growth cone is sensitive to certain chemicals along its path that are released by its target region. In this way visual system axons originating in the lateral geniculate nucleus find their way to cortical layer 4 in the occipital lobe of the brain in much the same way that a police bloodhound is able to sniff out the escaped prisoner hiding in an Illinois cornfield.
But although these growth cones lead their axons to the proper region of the brain (or muscle in the case of motor neurons), they cannot lead them to the precise target addresses. For a particular growth cone, it appears that any cell of a particular type will serve as a target. Indeed, in the newborn cat, ocular columns receive axons from both eyes, not just from one or the other, as in the adult brain. For this final and important fine-tuning to be achieved (on which stereoscopic vision depends), many of the original terminal connections of the axon must be eliminated. In the case of vision, all axonal connections from the wrong eye are eliminated, and those from the correct eye are retained. In the case of motor systems that initially have many-to-many connections between motor neurons of the spinal column and muscle fibers (that is, many motor neuron axons connected to same muscle fiber, and many muscle fibers connected to the same axon), the mature animal possesses a much more finely ordered system with each muscle fiber enervated by one and only one motor neuron. The mammalian nervous system changes from birth to maturity from a degenerate system having many redundant and diffuse connections, to a much more finely tuned system that makes both adaptedly complex behaviors and perceptions (such as stereoscopic vision) possible.
So now the question naturally arises, how does the nervous system know which connections to retain and which to eliminate? The work of David Hubel and Torsten Wiesel in the 1970s (both of whom shared a Nobel prize with Sperry in 1981) provided the first clue. They conducted their ground-breaking experiments by closing the lid of one eye of newborn cats, and found that even one week without sight altered the connections of the eyes to layer 4 of the occipital cortex. Axons carrying nervous signals from the closed eye made fewer connections with the cortex, whereas axons from the open eye made many more connections than was normal. This suggested that visual system axons compete for space in the visual cortex, with the result of the competition dependent on the amount and type of sensory stimulation carried by the axons. Subsequent research by others using drugs to block the firing of visual system neurons, as well as artificial stimulation of these neurons, showed that it is not neural activity per se that results in the selective elimination of synapses, but rather that only certain types of neural activity result in the retention of certain synapses, while all others are eventually eliminated.
In a sense, then, cells that fire together wire together. The timing of the action-potential activity is critical in determining which synaptic connections are strengthened and which are weakened and eliminated. Under normal circumstances, vision itself acts to correlate the activity of neighboring retinal ganglion cells, because the cells receive inputs from the same parts of the visual world.[16]
The dependence of the development of the visual system on sensory stimulation would seem to indicate that the fine-tuning of its connections would have to wait until the birth of the animal when it is delivered from the comforting warm darkness of the womb to the cold light of day. However, recent evidence suggests that this fine-tuning actually begins to take place in utero. Prenatal development appears to depend on spontaneous firing of retinal cells that do not depend on light stimulation from the external world. Similar endogenous patterns of activity may also exist in the spinal cord, and may refine the synaptic connections of motor systems as well.[17]
Nonetheless, interactive postnatal experience of the external world is required for normal development of senses and nervous systems in mammals. Cats who have one eye sewn shut at birth lose all ability to see with this eye when it is opened several months later. The same applies to humans. Before the widespread use of antibiotics, eye infections left many newborn infants with cloudy lenses and corneas that caused functional blindness, even though their retinas and visual nervous systems were normal at the time of birth. Years later a number of these individuals underwent operations to replace their cloudy lenses and corneas with clear ones, but it was too late. Contrary to initial expectations, none of these people was able to see after the transplant.[18]It was simply not known at the time that early visual experience was essential to the normal maturation of the brain's visual circuitry. Similarly, some children are born with a wandering eye that does not fixate the same part of the visual field as the normal eye, and other children have one eye that is seriously nearsighted or farsighted; in both cases, the retina of the abnormal eye must be provided with clear visual stimulation, usually by age four years, or it will become functionally blind since its connections to the brain's vision centers will be eliminated in favor of the normal eye.
We thus see that the normal development of the brain depends on a critical interaction between genetic inheritance and environmental experience. The genome provides the general structure of the central nervous system, and nervous system activity and sensory stimulation provide the means by which the system is fine-tuned and made operational. But this fine-tuning does not depend on adding new components and connections in the way that a radio is assembled in a factory, but rather it is achieved by eliminating much of what was originally present. It is as if the radio arrived on the assembly line with twice as many electrical components and connections as necessary to work. If such an overconnected radio were plugged in and turned on, nothing but silence, static, or a hum would be heard from its speaker. However, careful removal of unnecessary components and judicious snipping of redundant wires would leave just those components and connections that result in a functioning radio. This snipping is analogous to the elimination of synapses in the human brain as part of its normal development.
The process by which brain connections change over time as maturing animals interact with their environments has been studied in detail by psychologist William Greenough of the University of Illinois at Urbana-Champaign. Using sophisticated techniques for determining the numbers and densities of neurons and synapses in specific regions of the rat's brain, he and his associates found that during the first months of the rat's life a rapid spurt in the growth of synapses occurs regardless of the amount or type of sensory experience.[19] This period of synaptic "blooming" is followed by a sharp decline in the number of synapses. That is, an elimination or "pruning" of synapses then takes place based on the activity and sensory stimulation of the brain, and ultimately results in the configuration of connections characteristic of the mature rat's brain. Greenough refers to this initial blooming and pruning of synapses as "experience-expectant" learning, since the initial synaptic overproduction appears to be relatively independent of the animal's experiences. It is as though the brain is expecting important things to be happening during the first weeks and months of life, and is prepared for these experiences with an overabundance of synapses, only a fraction of which, however, will be selectively retained.
The work of Greenough and his associates is limited to rats and monkeys, but their findings have much in common with those of Peter Huttenlocher of the University of Chicago who counted the synapses in specific regions of the brains of humans who died at various ages. Huttenlocher found that:
The increase in synaptic density plus expansion of total cortical volume leave no doubt that the postnatal period is one of very rapid synaptogenesis in human frontal cortex. By age 2 years, synaptic density is at its maximum, at about the same time when other components of cerebral cortex also cease growing and when total brain weight approaches that of the adult. Synaptic density declines subsequently, reaching by adolescence an adult value that is only about 60% of the maximum.[20]
This wealth of synapses is thought to be responsible for the striking plasticity of the immature brain that permits the learning of skills that can be learned only with much greater difficulty or not at all by the already pruned adult brain. We already saw how immature animals and children are unable to develop normal vision if they are not exposed to a sharply focused visual world during this period of brain development. It has also been repeatedly observed that although many adults initially may make quite rapid progress in learning a foreign language, young children appear to have an important advantage over adults in being able to master the sounds of languages. Canadian child language researchers Janet Werker and Richard Tees observed that children younger than one year appear able to distinguish between the speech sounds used by any human language. By age 12 months, however, they begin to lose the ability to discriminate between sound contrasts that are not used in the language they hear every day. So whereas all normal infants can distinguish between the two related but distinct sounds represented by the letter t in Hindi, those who hear only English quickly lose this ability, and Hindi-speaking children retain it.[21] The work of Werker and Tees therefore provides important human behavioral evidence that is consistent with the view that normal brain development involves the loss of synaptic connections, which results in the loss of certain skills as the brain approaches its adult form.
A sensitive period for the acquisition of a first language was demonstrated by the plight of Genie, an American girl who was brutally isolated from all normal human interaction until she was found at age 13 years, and who never subsequently developed normal language abilities.[22] There is striking evidence that the immature, overconnected brain is also better suited than a mature one to acquiring second languages and sign languages.[23]
Taken together, these findings paint a picture of the developing brain that contrasts sharply from the genetic providentialism favored by Sperry. Instead of the brain unfolding according to a genetically specified blueprint, we see instead a process of selection by which overly abundant neuronal connections are eliminated through a weeding-out process, leaving only those connections that permit the animal to interact successfully with its environment.
Learning and Memory: Rewiring the Brain
The mammalian brain appears most adaptive during the early postnatal period, and continues to adapt and learn from new experiences throughout its adult life. During the 1960s and 1970s a series of studies offered impressive evidence that rats grew thicker brains and new synapses when they were placed in complex and challenging environments. These findings were consistent with the then-popular belief that learning and memory in mature mammals (as opposed to the brain development of immature animals) were additive processes involving the formation of new synaptic connections or the strengthening of already existing ones. The influential Canadian psychologist Donald Hebb assumed that "the changed facilitation that constitutes learning" was the result of "the growth of synaptic knobs."[24] Similarly, Sir John C. Eccles, who shared a Nobel prize in 1963 for his research on the transmission of nerve impulses, believed that memory and learning involved "the growth . . . of bigger and better synapses."[25]
However, it was also suggested that more than just adding synapses was involved in learning. One of the first to propose that subtractive brain changes could be involved in adult learning and memory was J. Z. Young, who in 1964 posited that such learning could be the result of the elimination of neuronal connections.[26]Several years later J. S. Albus hypothesized that "pattern storage must be accomplished principally by weakening synaptic weights rather than by strengthening them,"[27] and Richard Dawkins speculated that the selective death of neurons could underlie the storage of memories.[28]
But how could a subtractive process of neuron elimination be involved in learning and memory? It is particularly difficult to understand how the learning of a new skill, such as riding a bicycle or speaking a foreign language, or acquisition of new memories, such as learning the words to a poem or song, could be made possible by loss of synapses. We saw in the development and maturation of the brain that synaptic connections that are rarely used are weakened or eliminated, whereas those in active neural pathways are retained and perhaps strengthened. This subtractive process makes sense when dealing with an overwired, immature brain that may have close to twice as many synapses as it will have as an adult. But how can it work for a mature adult brain that has already been substantially whittled down by synaptic pruning?
To illustrate this problem, imagine an adult Spaniard learning English. To do this, the Spaniard will have to learn to hear and produce certain sound distinctions that are not used in Spanish, such as the contrasts involved in ship versus sheep, sue versus zoo, and watch versus wash. The research of Werker and Tees would lead us to predict that the Spaniard would not initially be able to make these distinctions since they are not made in the language he has heard and spoken all his life. The synaptic connections necessary for making these discriminations were present when he was born, but we would expect them to have been promptly pruned away since they were not used in the language of his environment. It is therefore not clear how any further pruning of synapses would permit him to learn this aspect of the English language.
Instead, it seems more likely that a process involving the addition of new synapses, or at least reorganizing current ones, would be necessary for this learning to take place. But then we run into the equally thorny problem of understanding how the brain could ever know which new synapses to add or modify! Surely, some combination of synaptic changes should allow the Spaniard to learn English, since many adults learn English and other languages, and such learning must be the result of changes in the synaptic connections of the brain. But just which new combination of synapses will do the trick? At the very least it would appear that the brain would somehow have to try out a number of new combinations and select the best ones. But to select the best ones, a source of variation is necessary, perhaps not unlike the initial variation of synaptic connections present in the immature, overconnected brain.
A possible solution to this riddle was offered by French neurobiologist Jean-Pierre Changeux in 1983. In his book L'Homme Neuronal (published in English in 1985 as Neuronal Man), Changeux proposed a "Darwinism of the synapses"[29] to account for the development of the brain and the learning it undergoes within its cultural environment.
According to this scheme, culture makes its impression progressively. The 10,000 or so synapses per cortical neuron are not established immediately. On the contrary, they proliferate in successive waves from birth to puberty in man. With each wave, there is transient redundancy and selective stabilization. This causes a series of critical periods when activity exercises its regulatory effect.[30]
In effect, he was suggesting that all adaptive brain changes, or at least those occurring between birth and puberty in humans, involve the elimination of preexisting synapses, but that these preexisting synapses were not necessarily all present at the same time. From birth to puberty, Changeux hypothesized that waves of synaptic growth would occur, with subsequent experience serving to retain the useful ones and eliminate the useless and redundant ones. These waves of synaptic overproduction would provide the source of variation on which synaptic selection could operate. Such learning resulted in an absolute increase in synaptic growth and numbers over time. This growth was not constant, but was rather envisioned as analogous to repeatedly taking two steps forward--randomly adding new synapses--followed by one step backward--eliminating the useless ones just added.
Changeux provided no hard evidence for his hypothesis that synaptic variation in the form of overproduction would precede the elimination of synapses as part of the brain's restructuring to permit the learning of new skills and acquisition of new knowledge. But such evidence was found a few years after the publication of his book. William Greenough and his associates, whose work on the maturational development of the rat's brain was noted earlier, also conducted research on changes in the brain induced by placing adult rats in special, enriched environments. In one study this resulted in a 20% increase (roughly 2000) in the number of synapses per neuron in the upper layers of the visual cortex.[31] Later research showed that such dramatic increases in synapses were not restricted to the rat's visual cortex.[32]
These and other similar findings led Greenough's group to propose that the waves of synapse proliferation first described by Changeux could be elicited by the complex demands placed on the adult brain in a new, challenging environment. These researchers referred to this process as "experience-dependent" development since it depends on the environment triggering the formation of new synaptic growth on which the selective process can act.[33]
Greenough's conception of how the adult brain is able to learn new skills and form new memories offers an appealing solution to the problem concerning the additive and subtractive processes underlying the adult's brain adaptation to new environments. According to this theory, experience-dependent learning combines both additive and subtractive processes. The additive component involves the blooming of new synapses in response to the animal's attempt to control aspects of a new, complex environment. Although the brain does appear to know what part of itself has to be involved in this new synapse-construction project, it need not (indeed, could not) know which particular connections to make. By forming a large variety and number of new connections, the brain can select the combinations that work best, in the same way that the immature, developing brain retains useful connections from its initial oversupply of synapses. The long-term result is an overall addition to the number of synapses. But the actual selection process that fine-tunes the connections is a subtractive one in which the useful connections are selectively retained and less useful ones eliminated. Although clear evidence exists for synaptic increase in learning, as I write this we still have no such evidence in mature learning for an overproduction of synapses that are then pruned away. However, recent research has found evidence for an overproduction of dendrites in mature rats during readaptation of the brain after brain injury, which at least suggests that synaptic overproduction may be involved as well.[34] These findings fit very nicely with the subtractive synapse findings on brain maturation and provide a solution to the mystery of how the brain could know exactly which new synaptic connections to establish to enable it to acquire new knowledge, skills, and memories.
Although only a relatively small number of neuroscientists have opted for a selectionist approach to their research and theorizing, Changeux and Greenough and their associates are not the only ones whose research suggests that the adult brain develops and learns through a process of cumulative neural variation and selection. This theory has now been embraced and given additional support by several other leading neuroscientists. William Calvin refers to the brain as a "Darwin machine" that follows the plan "make lots of random variants by brute bashing about, then select the good ones."[35] Gerald Edelman, who shared a Nobel prize in 1972 for his research on the chemical structure of antibodies in the immune system, has contributed a remarkable outpouring of books describing aspects of his "neuronal group selection theory" of brain development and learning through a selectionist process he refers to as "neural Darwinism."[36]And noted psychologist and neuroscientist Michael Gazzaniga, best known for his ground-breaking research on humans with split brains, recently embraced a selectionist account of brain functioning and development.[37]
Current research is under way to determine whether unambiguous physical evidence can be found for the overproduction and elimination of newly formed synapses in the adult brain in response to environmental changes. Such a finding would place the brain alongside the immune system as another striking example of how cumulative variation and selection processes during the lifetime of an organism make it possible to adapt to complex, changing environments.
We have now seen that understanding both the adapted and adaptive complexity of the human brain involves finding answers to three questions: how did the brain originate as a biological organ?; how does it develop from a fertilized egg into a mature brain?; and how is it able when mature to rewire itself to learn from and adapt to changes in its environment?
Much more work must be done before we have detailed answers to these questions. But substantial progress has already been made as we move midway into the "decade of the brain." To a large extent this progress has consisted of rejecting providential and instructionist explanations for these puzzles of fit, and finding considerable evidence and reason in favor of selectionist explanations. The powerful process of cumulative blind variation and selection working over millions of years is not only the only reasonable theory for the biological evolution of the brain, but we find that it has surfaced again in a different but still recognizable form as an explanation for the brain's embryonic growth and continued development during its relatively brief lifetime.
It is here, as Changeux remarked, that "the Darwinism of synapses replaces the Darwinism of genes."[38] To close the circle, it should be noted that a striking consequence of the joint effects of among-organism genetic and within-organism synaptic selection is the brain's understanding both itself and the process of selection that is responsible for its extraordinary abilities.
[1]Changeux (1985, p. 248; first emphasis added).
1) http://thebrain.mcgill.ca/flash/d/d_05/d_05_cr/d_05_cr_her/d_05_cr_her.html
2) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3973910/
3) http://faculty.education.illinois.edu/g-cziko/wm/05.html#Heading2
Last edited by Admin on Fri Apr 28, 2017 6:19 am; edited 1 time in total