Air Breathing: Oxygen Homeostasis and the Transitions from Water to Land and Sky 1
http://reasonandscience.heavenforum.org/t2304-air-breathing-oxygen-homeostasis-and-the-transitions-from-water-to-land-and-sky
All gas exchangers share basic features, for example, thin blood-gas barrier, large interface, ventilatory regulation, and low cost of breathing.We focuses on the first step of the oxygen cascade—convection and diffusion in the gas-exchange organ—to provide an overview of the diversity of nature's “solutions” to the dilemma of acquiring enough but not too much oxygen from the environment.
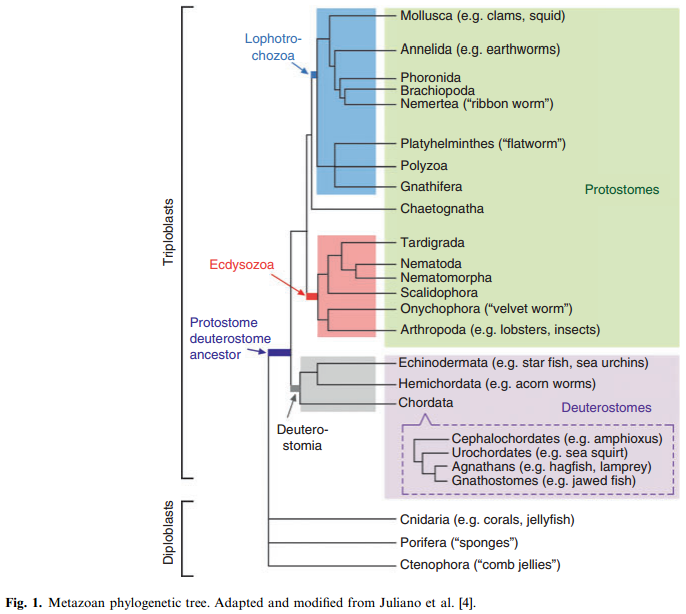
The origin of the Metazoa dates back to approximately 770–850 million years. The most primitive living phylum of animals is the Porifera (sponges), followed by Cnidaria (corals and jellyfish) and Ctenophora (comb jellies). Only two germ layers (endoderm and ectoderm) develop in these latter phyla. Hence, they are called diploblastic animals. Between 600 and 700 million years ago, a new body plan emerged that demonstrated bilateral symmetry and a third germ layer (mesoderm). These animals, referred to as triploblasts, gave rise to two separate lineages: the protostomes and deuterostomes. The deuterostome lineage gave rise to the chordates (including cephalochordates, urochordates, and vertebrates) as well as hemichordates (acorn worms) and echinoderms (e.g. sea urchins and starfish). Protostomes are further divided into two groups: the Ecdysozoans (including the arthropods and nematodes) and the Lophotrochozoans (including the mollusks and annelids). These various branch points mark important transitions in the development of vascular systems
Size matters Changes in size mandate changes in structural design. All unicellular and multicellular animals depend on diffusion to supply oxygen and nutrients, and to remove carbon dioxide. Diffusion, while energetically inexpensive, is a very slow process and works only over small distances (diffusion path < 1 mm). A change in body size (whether for a single cell or a multicellular organism) disproportionately changes the ratio of surface area to volume. Specifically, as a solid 3-dimensional body enlarges, its surface area increases in proportion to the radius squared (r 2 ), whereas its volume increases more rapidly (r 3 ). At some point, the cell will reach a size where its surface area cannot meet the needs of its volume. Single cells optimize their surface area-to-volume ratio by developing folded surfaces, or a flattened or thread-like shape. Another strategy to increase size is to incorporate many cells into a single organism. Simple multicellular organisms (diploblastic animals and some of the early triploblastic animals, such as flatworms) obtain oxygen by diffusion alone. They do so by minimizing metabolic demands, by assuming a body geometry that maximizes the surface area, by localizing most of their cells at the environment/body interface and/or by pumping external environmental water to their internal surfaces. However, these strategies have inherent design constraints that place an upper limit on body size. To achieve further 3-dimensional increases in size, it is necessary to employ internal transport and exchange systems (i.e. circulatory systems) to provide bulk flow delivery of substances (e.g. gases, nutrients, wastes) to and from each cell in the body.
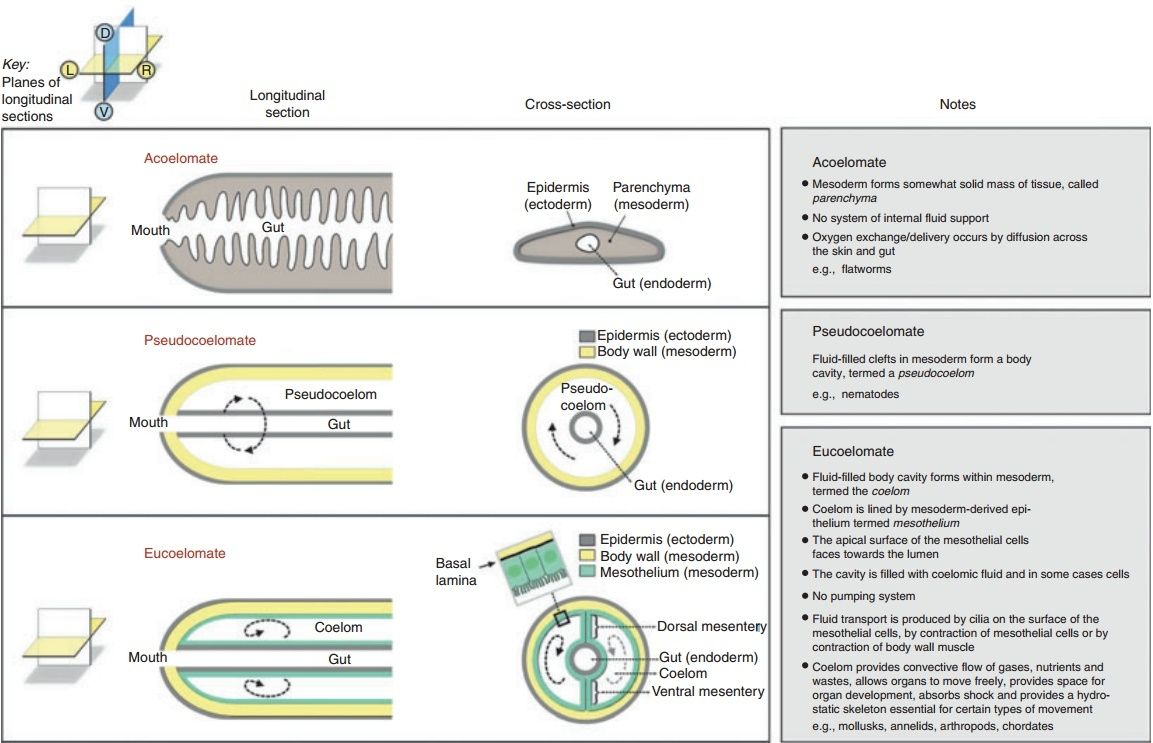
Coelomic circulatory systems Some small and flat triploblastic animals (e.g. flatworms) have no system of internal fluid support (i.e. they lack a coelom or blood vascular system). They are called acoelomates (Fig. 2).
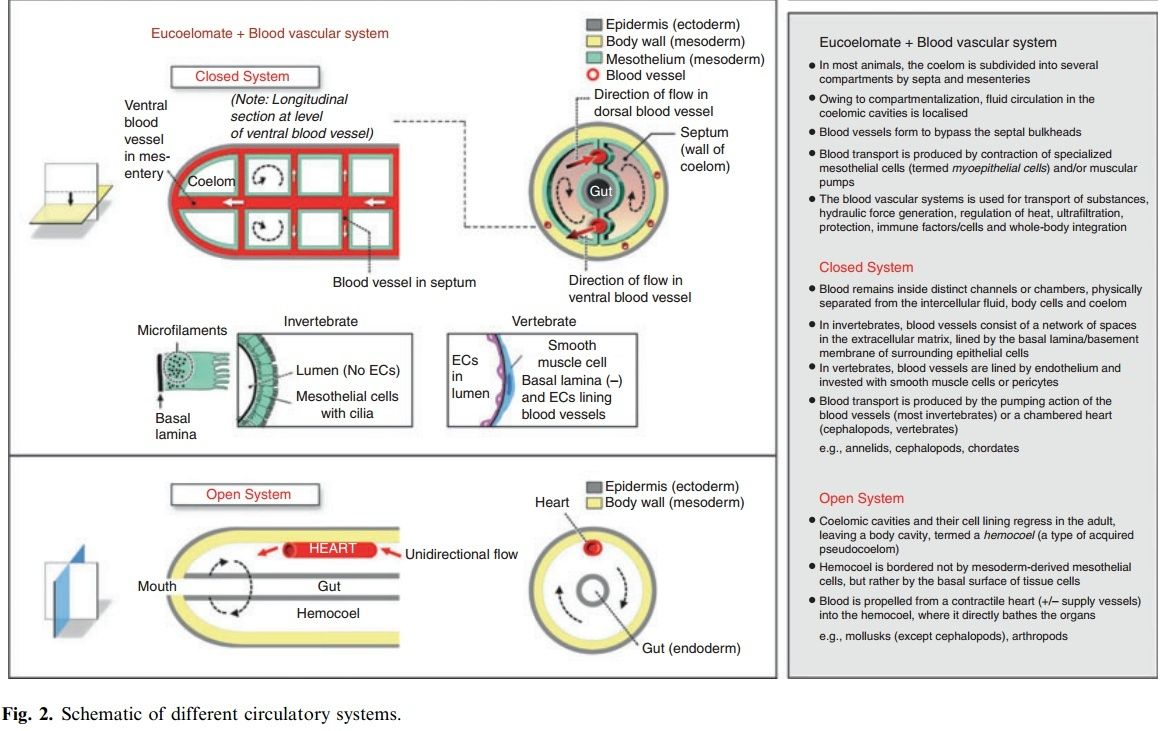
Blood vascular systems
The blood vascular system consists of blood-filled spaces (vessels, sinuses, hemocoels, and/or pumping organs) within the connective tissue compartment, which is continuous around and between all tissue layers in the body. In invertebrates, the spaces are lined only by matrix. Vertebrates have a secondary cell lining, termed endothelium. Fluid transport is produced by contraction of specialized mesothelial cells (myoepithelial cells) and/or muscular pumps.
The blood vascular system is used for:
1. the transport of substances (e.g. nutrients, oxygen, carbon dioxide)
2. hydraulic force generation (e.g. head–foot protrusion in mollusks and penile erection in vertebrates)
3. regulation of heat (e.g. via countercurrent flow)
4. ultrafiltration (e.g. in the kidney), defense (e.g. through delivery of clotting factors, immune factors/cells)
5. and whole-body integration (e.g. hormonal regulation)
Blood vascular systems follow one of two principal designs: open or closed. In keeping with their paraphyletic origins, invertebrates display diverse phenotypes ranging from open to closed systems. In contrast, all vertebrates have a closed cardiovascular system. The division between closed and open systems is not always clear-cut. Open circulatory systems occur in arthropods (e.g. insects and crustaceans) and non-cephalopod mollusks (e.g. clams, snails and slugs). In these animals, the mesoderm forms coelomic cavities during embryogenesis. However, the cavities and their cell lining regress in the adult. Some populations of mesodermal cells reaggregate and form the blood vessels (e.g. the dorsal vessel in insects). The system is considered open because the blood (called hemolymph) empties from a contractile heart and major supply vessels into the body cavity (termed a hemocoel), where it directly bathes the organs. In other words, the hemocoel is bordered not by mesoderm-derived mesothelial cells, but rather by the basal surface of the tissue cells themselves. Thus, once blood empties from the lumen of the distributing vessels, there is no distinction between hemolymph and interstitial fluid/extracellular fluid. Hemolymph returns to the heart either through ostia in the ventricle (arthropods) or via the atrium (mollusks). Compared with the closed circulation in lower invertebrates (e.g. annelids), the open circulation boasts a more efficient pump. Whereas annelids rely on peristaltic blood vessels to propel blood, animals with an open circulation have true hearts (containing muscle striations and Z-bands), which display automatic and synchronized beating. However, compared with more advanced closed systems (e.g. in cephalopods and vertebrates), open circulatory systems have a larger blood volume and lower flow rates/pressures. Indeed, the velocity and blood pressure drop abruptly once the blood leaves the heart and vessels and enters the hemocoel. As a final point of comparison, open systems provide flow to organs in series, such that tissues lying further downstream of the heart receive less oxygen compared with more proximal organs. By contrast, the capillary networks of closed circulatory systems are arranged in parallel allowing for equal (and regulated) distribution among tissues. In insects, the open circulation is not responsible for delivering oxygen. Oxygen delivery is carried out by an elaborate, highly branched tracheal system, which facilitates diffusion to each and every cell of the body. As a result, insects have a larger capacity for aerobic metabolism compared with other open circulation invertebrates. Closed circulatory systems occur in a wide variety of invertebrates including annelids, cephalopods (e.g. octopus and squid), and non-vertebrate chordates, as well as in vertebrates. In closed systems, the blood remains inside distinct channels or chambers, where it is physically separated from the intercellular fluid, body cells, and coelom. Closed systems consist of collecting and distributing vessels, usually with a central meeting site in a propulsive pump. Exchange with the interstitial fluid and body cells takes place in special areas such as capillary beds or plexi, where the walls are thin to optimize diffusion. The closed system of invertebrates consists of a network of spaces in the extracellular matrix between epithelial cells where the coelom contacts coelom (e.g. mesentery or septa) or where coelom contacts endoderm or ectoderm (Fig. 2). Thus, the wall of these vessels consists of basement membranes derived from different types of epithelia, and the vessels are outlined by the basal surfaces of these cells. In some cases (e.g. annelids), blood is propelled by the pumping action of the blood vessels, which in turn is mediated by the contraction of specialized, differentiated coelomic mesothelial cells (myoepithelial cells), or by muscular blood vessels that contract in peristaltic waves. In other cases (e.g. in cephalopods), chambered hearts have to promote fluid movement. In vertebrates, the closed vascular system consists of a series of closed vessels with an endothelial lining, invested with smooth muscle cells or pericytes. Vertebrate blood vessels, while contractile, do not propel blood. Rather, transport is mediated by a central muscular pump. Most vertebrates have a lymphatic system, which collects and recycles interstitial fluid back to the circulation. There are variations on the common vertebrate plan, many of which are related to different requirements of living in water or on land . fish have a single circulatory system, consisting of an undivided heart with a single atrium and single ventricle in series with oxygen-exchanging gills. By contrast, adult birds and mammals have a double circulatory system in which the heart is completely divided into right and left sides, resulting in separation of deoxygenated and oxygenated blood. The circulatory systems of lungfish, amphibians, and reptiles demonstrate a broad array of intermediate designs, each of which is characterized by partial separation of the air-breathing organ and the systemic circulation, and thus the potential for left–right and right–left shunts.
The idea that cardiovascular systems are either closed or open is an oversimplification. For example, in many mollusks and crustaceans, blood is ejected from the heart into a highly ramified network of branching vessels before emptying into the hemocoel. This is illustrated in the lobster (see below)

Fig. 3. Blood vascular system of the lobster.
(A) Schematic of the blood vascular system of the lobster. a, artery.
(B) Open view of the dorsal thorax showing the single-chambered heart suspended in the pericardial sinus by alary ligaments.
(C) Electron micrograph of the heart showing a cardiomyocyte (cm) filled with cross sections of thick and thin microfibrils (consistent with myosin and actin) (*) and mitochondria. The inner surface of the heart chamber is covered by a thin basal lamina. There is no endocardial lining. A hemocyte (h) is shown in the heart chamber. 9 4,500
(D) A 1-lm transverse histological section of the dorsal abdominal artery stained with Giemsa.
(E) Electron micrograph of the dorsal abdominal artery shows the wall consisting of extracellular matrix composed of a dense meshwork of collagen fibrils. There is no endothelial lining. 912,900 (F) Photomicrograph of a swimmeret of a lobster that has been injected with Evans blue dye in the dorsal abdominal artery. Note the branching network of vessels ending in plumes of extravasated dye in the interstitial space. Panel A is adapted from McLaughlin [20].
and even more prominently in the sea snail, Haliotis, where the abdominal arteries end up in a complex meshwork of small capillary-like vessels.18 Moreover, studies in drosophila have shown that blood flows along preferred routes in the open hemocoel despite the absence of discrete return channels. Conversely, the closed system of vertebrates contains vascular beds, such as the sinusoids of the liver, spleen, and bone marrow, where there is direct contact between blood and the interstitial space. In hemochorial placentation (e.g. in primates), the maternal spiral arteries become openended, and blood is released into a placental labyrinth where it bathes the chorionic villi and is drained by the spiral veins.
Endothelium
Invertebrate vessels are always lined by extracellular matrix. Only vertebrates possess a true endothelial lining, defined as a layer of epithelial cells expressing basoapical polarity (with the apical surface facing the lumen),intercellular junctions, and anchoring to basement membrane. The blood vessels of some invertebrates, including cephalopods, annelids, and amphioxus have cells clinging to the luminal surface, internal to the basement membrane. These cells have sometimes been referred to as ‘endothelial cells’. However, they form an incomplete lining, they lack intercellular junctions typical of vertebrate endothelial cells, and they rarely appear attached to the underlying basal lamina. A more appropriate term for this cell type is an amoebocyte. It likely represents a type of circulating hemocyte.
Gas exchange
The circulatory system has co-evolved with gas exchange mechanisms.
Question: how do they know ??
Some invertebrates obtain their oxygen by simple diffusion through the skin. Integumentary gas exchange is characteristic of small soft-bodied animals with high surface/volume ratios. Gas exchange in the majority of marine and many freshwater invertebrates occurs via gills. Terrestrial invertebrates employ specialized invaginated structures, including book lungs (spiders) or trachea (insects). Many invertebrates have metal ioncontaining respiratory pigments to increase the oxygencarrying capacity of their blood. For example, hemoglobin is found in many annelids, as well as some crustaceans, insects, and mollusks, whereas hemocyanins are the most commonly occurring respiratory pigments in mollusks and arthropods. Invertebrate respiratory pigments are usually carried in solution in blood, but they are occasionally packaged in blood cells. Vertebrates breathe through their gills, skin, and/or lungs. All vertebrates with the exception of Antarctic icefishes (Channichthyidae) have hemoglobin, which is packaged in red blood cells.
Hagfish (Myxine glutinosa)
Modern vertebrates are classified into two major groups: the gnathostomes (jawed vertebrates) and the agnathans (jawless vertebrates). The agnathans are further classified into two groups, myxinoids (hagfish) and petromyzonids (lamprey). Most evidence suggests that the hagfish diverged before lamprey and is thus the oldest living vertebrate. Shared traits in hagfish and jawed vertebrates indicate that the trait was present in the common ancestor of all craniates. Hagfish have a closed circulation (Fig. 6A, Fig. S22).
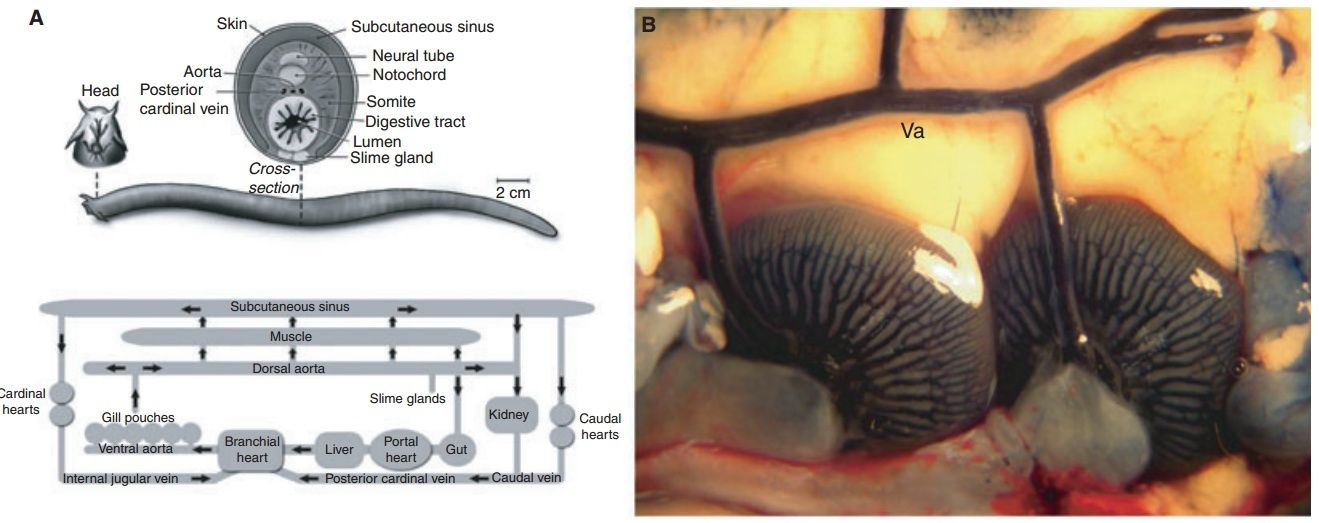
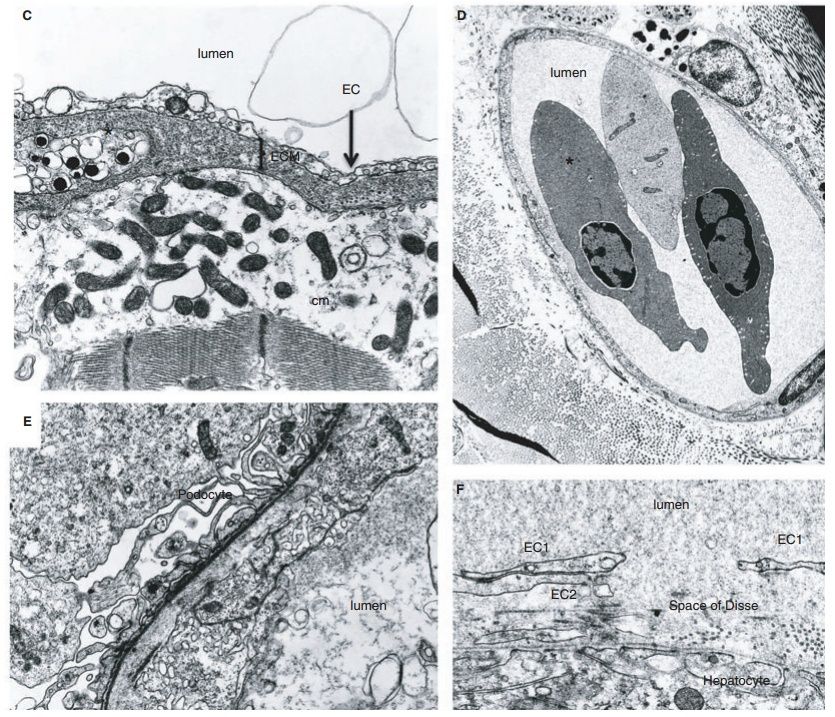
Fig. 6. Blood vascular system of the hagfish.
(A) Schematic of the blood vascular system of hagfish. Top, Transverse section through midregion shows several features that are typical of other vertebrates, including the arrangement of myomeres, neural tube, and aorta. Features that are unique to hagfish include the large subcutaneous sinus between skin and skeletal muscle, the retention of the notochord in the adult, and the presence of slime glands on the ventrolateral surface. Bottom, schematic of hagfish circulation. The gills are in series with the systemic circulation. Blood enters the subcutaneous sinus via skeletal muscle capillaries and reenters the systemic circulation via accessory hearts (the caudal and cardinal hearts). The portal heart pumps blood from the intestinal vasculature into the systemic heart via the common portal vein.
(B) Photomicrograph of ventral aorta and two (of a total of 12) gills in an animal that has been injected with Evans blue dye through the heart.
(C) Electron micrograph of the heart shows electron-lucent endothelial cells (EC) overlying a thick extracellular matrix containing a chromaffin-like cell, and a cardiomyocyte (cm) with electron-lucent cytoplasm, and well-preserved mitochondria and muscle filaments.
(D) Electron micrograph of the dermis shows a microvessel in cross-section containing two nucleated red blood cells. The blood vessel is lined by a continuous layer of endothelial cells. A melanocyte is seen on the left side of the vessel. 92,500.
(E) Electron micrograph of a kidney glomerulus shows podocyte foot processes abutting a well-formed basal lamina. On the other side of the basal lamina is an endothelial cell (EC) facing the lumen of a glomerular capillary. The endothelial cell contains many vesicles, vacuoles and tubular structures. 99,100.
(F) Electron micrograph of a liver sinusoid shows a large gap in a single endothelial cell (E1, double-headed arrow), well-preserved attachments between two endothelial cells (EC1 and EC2), and a continuum of proteinaceous material from the lumen to extravascular space (Space of Disse). EC2 is a second endothelial cell. 932,400. Panel A is reprinted with permission from Cheruvu et al. [48]. Panels C, D and F are reprinted with permission from Yano et al.
Similar to the case in other fishes, the branchial (i.e. gill) circulation is found in series with the systemic circulation. The gills are unique among vertebrates in that they are internalized and organized as pouches, typically six on each side in M. glutinosa (Fig. 6B). The hagfish circulation also possesses a series of blood sinuses, which are in direct communication with systemic vessels . The most prominent of these is the large subcutaneous vascular sinus located between skeletal muscle and the skin, stretching from the tentacles of the snout to the caudal fin fold. Hagfish can hold up to 30% of their blood volume within the sinus system. It is believed that the caudal and cardinal ‘hearts’ (composed of skeletal muscle) function to reintroduce sinus blood into the systemic circulation. The hagfish heart exhibits a Frank–Starling mechanism. Thus, blood that is redistributed from sinus to central compartments may serve to increase preload and cardiac output. Hagfish maintain the lowest arterial blood pressures (dorsal aorta blood pressure 5.8–9.8 mmHg) and highest relative blood volumes (18%) of any vertebrate. Cardiac output in M. glutinosa is 8–9 mL min1 kg1, which approaches the values seen in some teleosts. Similar to other fish, hagfish have a double-chambered heart containing a single atrium and a single ventricle. The heart consists of an avascular mesh of muscle cells (a so-called spongy heart). There is no coronary circulation. Rather, deoxygenated blood in the ventricle circulates through spaces (sinusoidal channels or lacunae) in the myocardial wall. Unlike other vertebrates, the hagfish heart lacks cardioregulatory nerves. The heart rate ranges from 20 to 30 beats per minute. Hagfish possess a second cardiomyocyte-containing chambered heart, the portal heart, which beats asynchronously with the systemic heart, as it propels blood from the gut to the liver via the common portal vein . We and others have previously shown that hagfish blood vessels are lined by a single layer of endothelial cells. All endothelial cells have vesicles along both the blood and tissue fronts of the cell. The lateral borders of endothelial cells are typical for those seen in mammals. Occasional Weibel–Palade bodies are observed, suggesting that hagfish express von Willebrand factor. Similar to other vertebrates, endothelial phenotypes vary from one vascular bed to another. For example, endothelial cells lining the heart sinuses are attenuated, electron lucent, and contain many vesicular structures (Fig. 6C, Fig. S23). Dermal microvessels display a continuous endothelium, with lateral plasma membrane borders containing specializations typical of other vertebrates (Fig. 6D, Fig. S24). The kidney glomerulus contains endothelial cells with few fenestrae, and many vesicle and vacuoles of varying sizes (Fig. 6E, Fig. S25). As a final example, ultrastructural studies of the liver demonstrate a discontinuous endothelium with many gaps (Fig. 6F, Fig. S26). In addition to vascular bed-specific differences in ultrastructure, the endothelium of hagfish also demonstrates molecular and functional heterogeneity. For example, lectin staining of various tissues revealed significant differences in lectin binding to the endothelium. Moreover, in intravital microscopy studies of the dermal microvasculature, histamine was shown to induce neutrophil adhesion in capillaries and postcapillary venules, but not arterioles. As a final example, arteries from the mesentery and skeletal muscle demonstrated site-specific mechanisms of endothelium-dependent vasomotor relaxation. In summary, our findings in hagfish confirm that the closed system of even the most basal vertebrate is lined by endothelium and that phenotypic heterogeneity of the endothelium is a conserved property of this cell type.
Evolutionary implications
When approaching the evolutionary origins of the blood vascular system and the endothelium, we must consider three important questions.
First, when did these systems evolve?
Second, why did they evolve? In other words, what survival advantage do these structures confer at the level of species?
inally, how did the blood vessels and their endothelial linings develop ontogenetically (as evolutionary novelties) in the first place?
In this section, we will consider each question in turn
When did the blood vascular system and endothelium first evolve?
As we survey the present landscape of body plans, we find a wide variety of blood vascular systems (Fig. 7).
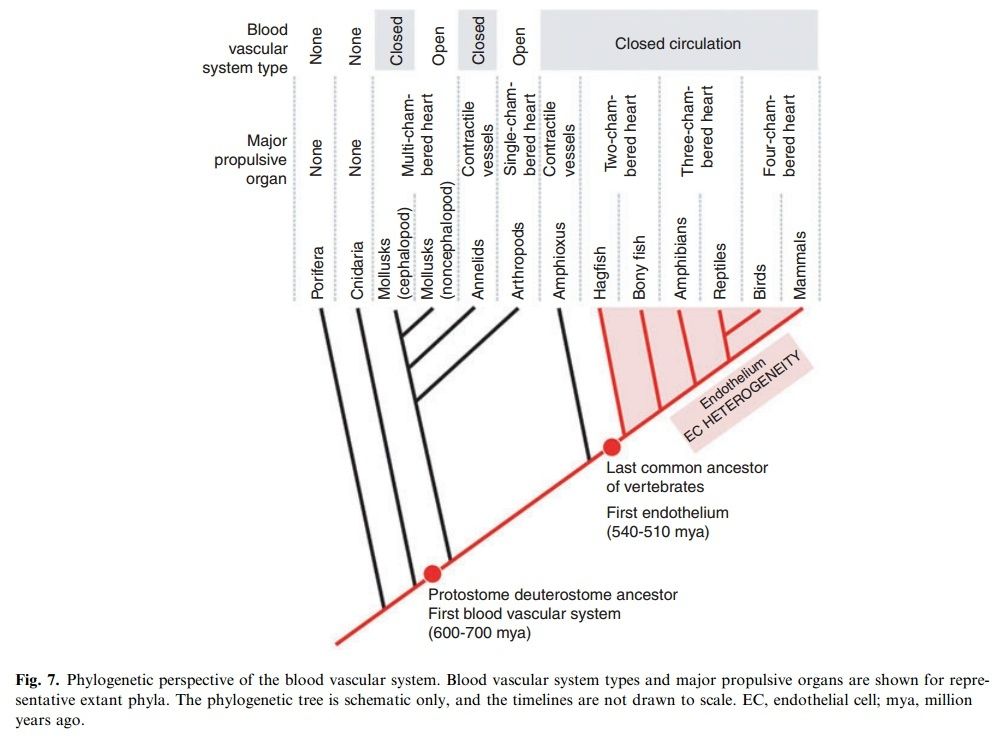
Some are closed, others are open. Some use hearts to propel blood, whereas others employ pulsatile blood vessels. Only a minority are lined by endothelium. When and how did all of these diverse structures evolve? The answer is likely through a combination of homology and convergence.
Likely ? Why is it likely ?
The last common ancestor of vertebrates and annelids, or of vertebrates and mollusks was the ancestor of the protostome–deuterostome ancestor, which lived between 600 and 700 million years ago . Although the fossil record is scarce, it is widely believed that this precursor animal was a segmented bilaterian (triploblastic coelomate) . If we are to accept that the blood vascular system evolved as a means to bypass the bulkheads of a segmented animal , then the first such system likely arose during this time. Flow would have been mediated by peristaltic vessels, perhaps like those described in the annelid. Blood probably percolated through spaces in the extracellular matrix, and thus, the system was by definition closed, albeit primitive. This scenario supports homology of all blood vascular systems. Over the past 600–700 million years of evolution, the blood vascular system has undergone significant modifications, in response to selective pressures experienced by individual phyla. In some cases, the blood vascular system has regressed (e.g. in flatworms and nematodes). In other cases, the primitive system transitioned to an open circulation.
This does not answer how and why it evolved in the first place.
The open system reverted back to a closed design in an ancestral cephalopod. This is an example of convergent evolution, whereby an analogous structure (the closed circulation in cephalopods) arose independently of the closed circulation in other invertebrates (e.g. worms) and in vertebrates. Finally, the endothelium is present in all vertebrates, yet distinctly absent in invertebrates. Although cells appear to cling to the inner surface of some invertebrate closed vessels, these lack the classical morphological features of endothelial cells. Therefore, the endothelium appeared in an ancestral vertebrate following the divergence from the urochordates and cephalochordates between 540 and 510 million years ago.
This is further a just so story. How is this backed up by evidence ?
Our findings in hagfish indicate that endothelial heterogeneity appeared during the same narrow window of time.
Why did the blood vascular system and endothelium evolve?
Circulatory systems most certainly evolved to overcome the time and distance constraints of diffusion, thus permitting increased body size and metabolic rates, as well as increased levels of integration and organization in Metazoa.
Why should that have been a evolutionary necessity ? The most diverse phyla still today are insects and different animals of small size. That is hardly a credible and plausible explanation, but rather a just so story to fit the paradigm of evolution.
Although the primitive coelom provided bulk flow delivery of substances, these structures never developed an efficient pumping mechanism. More importantly, they became increasingly localized to distinct compartments in the body. Ruppert and Carle hypothesized that the blood vascular system evolved as a means to provide bulk fluid transport along the entire length of a segmented animal, in effect bypassing the septal bulkheads. The earliest design may have involved the formation of spaces in between the basal laminae of endodermal and coelomic epithelia, allowing for improved distribution of nutrients absorbed by the intestine. Myoepithelial differentiation of the visceral layer of coelomic epithelium would have provided the necessary pumping action to drive nutrients toward the viscera. Subsequent vascularization
of the skin would have facilitated gas exchange at the body surface.
There is no consistent explanation provided why evolutionary pressure would have favoured that process. Its just asserted that this is what most probably happened.
The movement of fluid in blood vascular systems was originally mediated by the peristaltic motion of certain blood vessels, similar to what occurs in the earthworm and amphioxus. However, peristaltic pumps lack effective coordination between the fluid that is entering the contractile region and the fluid that is leaving it. Despite some tweaks to the peristaltic design, such as the evolution of one-way valves and partial coordination in contractions, the loss of fluid energy associated with backflow, distension of wall segments ahead of the stream, and pump reversals would have constrained body size and metabolic activity. This would have provided a selective pressure for the appearance of true hearts, in which inflow and outflow are tightly coupled via multiple chambers, efficient electrical connection between myocytes (hence, simultaneous contraction) and one-way valves. If the closed circulatory system was the ancestral condition, then why did the open circulatory system evolve in the precursors of mollusks and arthropods? One explanation for this change in the circulatory blueprint is that the rigid exoskeleton of these animals, which evolved for protection against predation and physical injury, eliminated the need for a coelomic hydrostatic skeleton . The subsequent regression of the coelom removed barriers to bulk transport and therefore the need for a closed system of vessels. At the same time, the exoskeleton impaired any contribution of body wall musculature to the movement of blood. This may have provided selective pressure for the evolution of a well-developed muscular heart, which is a characteristic feature of most animals with an open circulation.
Wow. Thats as superficial as it can get. Best example of pseudo-scientific , superficial claims, which avoid to go into the details, and explan, how this process really happened, and what molecular mechanism was involved. They just assert and say , it is most probably what happened, and thats it. At this point, i see no reasons to analise the paper further, as the picture is clear, no serious inspection and analysis of molecular evolutionary mechanisms are made.
1)http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3926130/
http://reasonandscience.heavenforum.org/t2304-air-breathing-oxygen-homeostasis-and-the-transitions-from-water-to-land-and-sky
All gas exchangers share basic features, for example, thin blood-gas barrier, large interface, ventilatory regulation, and low cost of breathing.We focuses on the first step of the oxygen cascade—convection and diffusion in the gas-exchange organ—to provide an overview of the diversity of nature's “solutions” to the dilemma of acquiring enough but not too much oxygen from the environment.
The origin of the Metazoa dates back to approximately 770–850 million years. The most primitive living phylum of animals is the Porifera (sponges), followed by Cnidaria (corals and jellyfish) and Ctenophora (comb jellies). Only two germ layers (endoderm and ectoderm) develop in these latter phyla. Hence, they are called diploblastic animals. Between 600 and 700 million years ago, a new body plan emerged that demonstrated bilateral symmetry and a third germ layer (mesoderm). These animals, referred to as triploblasts, gave rise to two separate lineages: the protostomes and deuterostomes. The deuterostome lineage gave rise to the chordates (including cephalochordates, urochordates, and vertebrates) as well as hemichordates (acorn worms) and echinoderms (e.g. sea urchins and starfish). Protostomes are further divided into two groups: the Ecdysozoans (including the arthropods and nematodes) and the Lophotrochozoans (including the mollusks and annelids). These various branch points mark important transitions in the development of vascular systems

Size matters Changes in size mandate changes in structural design. All unicellular and multicellular animals depend on diffusion to supply oxygen and nutrients, and to remove carbon dioxide. Diffusion, while energetically inexpensive, is a very slow process and works only over small distances (diffusion path < 1 mm). A change in body size (whether for a single cell or a multicellular organism) disproportionately changes the ratio of surface area to volume. Specifically, as a solid 3-dimensional body enlarges, its surface area increases in proportion to the radius squared (r 2 ), whereas its volume increases more rapidly (r 3 ). At some point, the cell will reach a size where its surface area cannot meet the needs of its volume. Single cells optimize their surface area-to-volume ratio by developing folded surfaces, or a flattened or thread-like shape. Another strategy to increase size is to incorporate many cells into a single organism. Simple multicellular organisms (diploblastic animals and some of the early triploblastic animals, such as flatworms) obtain oxygen by diffusion alone. They do so by minimizing metabolic demands, by assuming a body geometry that maximizes the surface area, by localizing most of their cells at the environment/body interface and/or by pumping external environmental water to their internal surfaces. However, these strategies have inherent design constraints that place an upper limit on body size. To achieve further 3-dimensional increases in size, it is necessary to employ internal transport and exchange systems (i.e. circulatory systems) to provide bulk flow delivery of substances (e.g. gases, nutrients, wastes) to and from each cell in the body.
Coelomic circulatory systems Some small and flat triploblastic animals (e.g. flatworms) have no system of internal fluid support (i.e. they lack a coelom or blood vascular system). They are called acoelomates (Fig. 2).


Blood vascular systems
The blood vascular system consists of blood-filled spaces (vessels, sinuses, hemocoels, and/or pumping organs) within the connective tissue compartment, which is continuous around and between all tissue layers in the body. In invertebrates, the spaces are lined only by matrix. Vertebrates have a secondary cell lining, termed endothelium. Fluid transport is produced by contraction of specialized mesothelial cells (myoepithelial cells) and/or muscular pumps.
The blood vascular system is used for:
1. the transport of substances (e.g. nutrients, oxygen, carbon dioxide)
2. hydraulic force generation (e.g. head–foot protrusion in mollusks and penile erection in vertebrates)
3. regulation of heat (e.g. via countercurrent flow)
4. ultrafiltration (e.g. in the kidney), defense (e.g. through delivery of clotting factors, immune factors/cells)
5. and whole-body integration (e.g. hormonal regulation)
Blood vascular systems follow one of two principal designs: open or closed. In keeping with their paraphyletic origins, invertebrates display diverse phenotypes ranging from open to closed systems. In contrast, all vertebrates have a closed cardiovascular system. The division between closed and open systems is not always clear-cut. Open circulatory systems occur in arthropods (e.g. insects and crustaceans) and non-cephalopod mollusks (e.g. clams, snails and slugs). In these animals, the mesoderm forms coelomic cavities during embryogenesis. However, the cavities and their cell lining regress in the adult. Some populations of mesodermal cells reaggregate and form the blood vessels (e.g. the dorsal vessel in insects). The system is considered open because the blood (called hemolymph) empties from a contractile heart and major supply vessels into the body cavity (termed a hemocoel), where it directly bathes the organs. In other words, the hemocoel is bordered not by mesoderm-derived mesothelial cells, but rather by the basal surface of the tissue cells themselves. Thus, once blood empties from the lumen of the distributing vessels, there is no distinction between hemolymph and interstitial fluid/extracellular fluid. Hemolymph returns to the heart either through ostia in the ventricle (arthropods) or via the atrium (mollusks). Compared with the closed circulation in lower invertebrates (e.g. annelids), the open circulation boasts a more efficient pump. Whereas annelids rely on peristaltic blood vessels to propel blood, animals with an open circulation have true hearts (containing muscle striations and Z-bands), which display automatic and synchronized beating. However, compared with more advanced closed systems (e.g. in cephalopods and vertebrates), open circulatory systems have a larger blood volume and lower flow rates/pressures. Indeed, the velocity and blood pressure drop abruptly once the blood leaves the heart and vessels and enters the hemocoel. As a final point of comparison, open systems provide flow to organs in series, such that tissues lying further downstream of the heart receive less oxygen compared with more proximal organs. By contrast, the capillary networks of closed circulatory systems are arranged in parallel allowing for equal (and regulated) distribution among tissues. In insects, the open circulation is not responsible for delivering oxygen. Oxygen delivery is carried out by an elaborate, highly branched tracheal system, which facilitates diffusion to each and every cell of the body. As a result, insects have a larger capacity for aerobic metabolism compared with other open circulation invertebrates. Closed circulatory systems occur in a wide variety of invertebrates including annelids, cephalopods (e.g. octopus and squid), and non-vertebrate chordates, as well as in vertebrates. In closed systems, the blood remains inside distinct channels or chambers, where it is physically separated from the intercellular fluid, body cells, and coelom. Closed systems consist of collecting and distributing vessels, usually with a central meeting site in a propulsive pump. Exchange with the interstitial fluid and body cells takes place in special areas such as capillary beds or plexi, where the walls are thin to optimize diffusion. The closed system of invertebrates consists of a network of spaces in the extracellular matrix between epithelial cells where the coelom contacts coelom (e.g. mesentery or septa) or where coelom contacts endoderm or ectoderm (Fig. 2). Thus, the wall of these vessels consists of basement membranes derived from different types of epithelia, and the vessels are outlined by the basal surfaces of these cells. In some cases (e.g. annelids), blood is propelled by the pumping action of the blood vessels, which in turn is mediated by the contraction of specialized, differentiated coelomic mesothelial cells (myoepithelial cells), or by muscular blood vessels that contract in peristaltic waves. In other cases (e.g. in cephalopods), chambered hearts have to promote fluid movement. In vertebrates, the closed vascular system consists of a series of closed vessels with an endothelial lining, invested with smooth muscle cells or pericytes. Vertebrate blood vessels, while contractile, do not propel blood. Rather, transport is mediated by a central muscular pump. Most vertebrates have a lymphatic system, which collects and recycles interstitial fluid back to the circulation. There are variations on the common vertebrate plan, many of which are related to different requirements of living in water or on land . fish have a single circulatory system, consisting of an undivided heart with a single atrium and single ventricle in series with oxygen-exchanging gills. By contrast, adult birds and mammals have a double circulatory system in which the heart is completely divided into right and left sides, resulting in separation of deoxygenated and oxygenated blood. The circulatory systems of lungfish, amphibians, and reptiles demonstrate a broad array of intermediate designs, each of which is characterized by partial separation of the air-breathing organ and the systemic circulation, and thus the potential for left–right and right–left shunts.
The idea that cardiovascular systems are either closed or open is an oversimplification. For example, in many mollusks and crustaceans, blood is ejected from the heart into a highly ramified network of branching vessels before emptying into the hemocoel. This is illustrated in the lobster (see below)

Fig. 3. Blood vascular system of the lobster.
(A) Schematic of the blood vascular system of the lobster. a, artery.
(B) Open view of the dorsal thorax showing the single-chambered heart suspended in the pericardial sinus by alary ligaments.
(C) Electron micrograph of the heart showing a cardiomyocyte (cm) filled with cross sections of thick and thin microfibrils (consistent with myosin and actin) (*) and mitochondria. The inner surface of the heart chamber is covered by a thin basal lamina. There is no endocardial lining. A hemocyte (h) is shown in the heart chamber. 9 4,500
(D) A 1-lm transverse histological section of the dorsal abdominal artery stained with Giemsa.
(E) Electron micrograph of the dorsal abdominal artery shows the wall consisting of extracellular matrix composed of a dense meshwork of collagen fibrils. There is no endothelial lining. 912,900 (F) Photomicrograph of a swimmeret of a lobster that has been injected with Evans blue dye in the dorsal abdominal artery. Note the branching network of vessels ending in plumes of extravasated dye in the interstitial space. Panel A is adapted from McLaughlin [20].
and even more prominently in the sea snail, Haliotis, where the abdominal arteries end up in a complex meshwork of small capillary-like vessels.18 Moreover, studies in drosophila have shown that blood flows along preferred routes in the open hemocoel despite the absence of discrete return channels. Conversely, the closed system of vertebrates contains vascular beds, such as the sinusoids of the liver, spleen, and bone marrow, where there is direct contact between blood and the interstitial space. In hemochorial placentation (e.g. in primates), the maternal spiral arteries become openended, and blood is released into a placental labyrinth where it bathes the chorionic villi and is drained by the spiral veins.
Endothelium
Invertebrate vessels are always lined by extracellular matrix. Only vertebrates possess a true endothelial lining, defined as a layer of epithelial cells expressing basoapical polarity (with the apical surface facing the lumen),intercellular junctions, and anchoring to basement membrane. The blood vessels of some invertebrates, including cephalopods, annelids, and amphioxus have cells clinging to the luminal surface, internal to the basement membrane. These cells have sometimes been referred to as ‘endothelial cells’. However, they form an incomplete lining, they lack intercellular junctions typical of vertebrate endothelial cells, and they rarely appear attached to the underlying basal lamina. A more appropriate term for this cell type is an amoebocyte. It likely represents a type of circulating hemocyte.
Gas exchange
The circulatory system has co-evolved with gas exchange mechanisms.
Question: how do they know ??
Some invertebrates obtain their oxygen by simple diffusion through the skin. Integumentary gas exchange is characteristic of small soft-bodied animals with high surface/volume ratios. Gas exchange in the majority of marine and many freshwater invertebrates occurs via gills. Terrestrial invertebrates employ specialized invaginated structures, including book lungs (spiders) or trachea (insects). Many invertebrates have metal ioncontaining respiratory pigments to increase the oxygencarrying capacity of their blood. For example, hemoglobin is found in many annelids, as well as some crustaceans, insects, and mollusks, whereas hemocyanins are the most commonly occurring respiratory pigments in mollusks and arthropods. Invertebrate respiratory pigments are usually carried in solution in blood, but they are occasionally packaged in blood cells. Vertebrates breathe through their gills, skin, and/or lungs. All vertebrates with the exception of Antarctic icefishes (Channichthyidae) have hemoglobin, which is packaged in red blood cells.
Hagfish (Myxine glutinosa)
Modern vertebrates are classified into two major groups: the gnathostomes (jawed vertebrates) and the agnathans (jawless vertebrates). The agnathans are further classified into two groups, myxinoids (hagfish) and petromyzonids (lamprey). Most evidence suggests that the hagfish diverged before lamprey and is thus the oldest living vertebrate. Shared traits in hagfish and jawed vertebrates indicate that the trait was present in the common ancestor of all craniates. Hagfish have a closed circulation (Fig. 6A, Fig. S22).


Fig. 6. Blood vascular system of the hagfish.
(A) Schematic of the blood vascular system of hagfish. Top, Transverse section through midregion shows several features that are typical of other vertebrates, including the arrangement of myomeres, neural tube, and aorta. Features that are unique to hagfish include the large subcutaneous sinus between skin and skeletal muscle, the retention of the notochord in the adult, and the presence of slime glands on the ventrolateral surface. Bottom, schematic of hagfish circulation. The gills are in series with the systemic circulation. Blood enters the subcutaneous sinus via skeletal muscle capillaries and reenters the systemic circulation via accessory hearts (the caudal and cardinal hearts). The portal heart pumps blood from the intestinal vasculature into the systemic heart via the common portal vein.
(B) Photomicrograph of ventral aorta and two (of a total of 12) gills in an animal that has been injected with Evans blue dye through the heart.
(C) Electron micrograph of the heart shows electron-lucent endothelial cells (EC) overlying a thick extracellular matrix containing a chromaffin-like cell, and a cardiomyocyte (cm) with electron-lucent cytoplasm, and well-preserved mitochondria and muscle filaments.
(D) Electron micrograph of the dermis shows a microvessel in cross-section containing two nucleated red blood cells. The blood vessel is lined by a continuous layer of endothelial cells. A melanocyte is seen on the left side of the vessel. 92,500.
(E) Electron micrograph of a kidney glomerulus shows podocyte foot processes abutting a well-formed basal lamina. On the other side of the basal lamina is an endothelial cell (EC) facing the lumen of a glomerular capillary. The endothelial cell contains many vesicles, vacuoles and tubular structures. 99,100.
(F) Electron micrograph of a liver sinusoid shows a large gap in a single endothelial cell (E1, double-headed arrow), well-preserved attachments between two endothelial cells (EC1 and EC2), and a continuum of proteinaceous material from the lumen to extravascular space (Space of Disse). EC2 is a second endothelial cell. 932,400. Panel A is reprinted with permission from Cheruvu et al. [48]. Panels C, D and F are reprinted with permission from Yano et al.
Similar to the case in other fishes, the branchial (i.e. gill) circulation is found in series with the systemic circulation. The gills are unique among vertebrates in that they are internalized and organized as pouches, typically six on each side in M. glutinosa (Fig. 6B). The hagfish circulation also possesses a series of blood sinuses, which are in direct communication with systemic vessels . The most prominent of these is the large subcutaneous vascular sinus located between skeletal muscle and the skin, stretching from the tentacles of the snout to the caudal fin fold. Hagfish can hold up to 30% of their blood volume within the sinus system. It is believed that the caudal and cardinal ‘hearts’ (composed of skeletal muscle) function to reintroduce sinus blood into the systemic circulation. The hagfish heart exhibits a Frank–Starling mechanism. Thus, blood that is redistributed from sinus to central compartments may serve to increase preload and cardiac output. Hagfish maintain the lowest arterial blood pressures (dorsal aorta blood pressure 5.8–9.8 mmHg) and highest relative blood volumes (18%) of any vertebrate. Cardiac output in M. glutinosa is 8–9 mL min1 kg1, which approaches the values seen in some teleosts. Similar to other fish, hagfish have a double-chambered heart containing a single atrium and a single ventricle. The heart consists of an avascular mesh of muscle cells (a so-called spongy heart). There is no coronary circulation. Rather, deoxygenated blood in the ventricle circulates through spaces (sinusoidal channels or lacunae) in the myocardial wall. Unlike other vertebrates, the hagfish heart lacks cardioregulatory nerves. The heart rate ranges from 20 to 30 beats per minute. Hagfish possess a second cardiomyocyte-containing chambered heart, the portal heart, which beats asynchronously with the systemic heart, as it propels blood from the gut to the liver via the common portal vein . We and others have previously shown that hagfish blood vessels are lined by a single layer of endothelial cells. All endothelial cells have vesicles along both the blood and tissue fronts of the cell. The lateral borders of endothelial cells are typical for those seen in mammals. Occasional Weibel–Palade bodies are observed, suggesting that hagfish express von Willebrand factor. Similar to other vertebrates, endothelial phenotypes vary from one vascular bed to another. For example, endothelial cells lining the heart sinuses are attenuated, electron lucent, and contain many vesicular structures (Fig. 6C, Fig. S23). Dermal microvessels display a continuous endothelium, with lateral plasma membrane borders containing specializations typical of other vertebrates (Fig. 6D, Fig. S24). The kidney glomerulus contains endothelial cells with few fenestrae, and many vesicle and vacuoles of varying sizes (Fig. 6E, Fig. S25). As a final example, ultrastructural studies of the liver demonstrate a discontinuous endothelium with many gaps (Fig. 6F, Fig. S26). In addition to vascular bed-specific differences in ultrastructure, the endothelium of hagfish also demonstrates molecular and functional heterogeneity. For example, lectin staining of various tissues revealed significant differences in lectin binding to the endothelium. Moreover, in intravital microscopy studies of the dermal microvasculature, histamine was shown to induce neutrophil adhesion in capillaries and postcapillary venules, but not arterioles. As a final example, arteries from the mesentery and skeletal muscle demonstrated site-specific mechanisms of endothelium-dependent vasomotor relaxation. In summary, our findings in hagfish confirm that the closed system of even the most basal vertebrate is lined by endothelium and that phenotypic heterogeneity of the endothelium is a conserved property of this cell type.
Evolutionary implications
When approaching the evolutionary origins of the blood vascular system and the endothelium, we must consider three important questions.
First, when did these systems evolve?
Second, why did they evolve? In other words, what survival advantage do these structures confer at the level of species?
inally, how did the blood vessels and their endothelial linings develop ontogenetically (as evolutionary novelties) in the first place?
In this section, we will consider each question in turn
When did the blood vascular system and endothelium first evolve?
As we survey the present landscape of body plans, we find a wide variety of blood vascular systems (Fig. 7).

Some are closed, others are open. Some use hearts to propel blood, whereas others employ pulsatile blood vessels. Only a minority are lined by endothelium. When and how did all of these diverse structures evolve? The answer is likely through a combination of homology and convergence.
Likely ? Why is it likely ?
The last common ancestor of vertebrates and annelids, or of vertebrates and mollusks was the ancestor of the protostome–deuterostome ancestor, which lived between 600 and 700 million years ago . Although the fossil record is scarce, it is widely believed that this precursor animal was a segmented bilaterian (triploblastic coelomate) . If we are to accept that the blood vascular system evolved as a means to bypass the bulkheads of a segmented animal , then the first such system likely arose during this time. Flow would have been mediated by peristaltic vessels, perhaps like those described in the annelid. Blood probably percolated through spaces in the extracellular matrix, and thus, the system was by definition closed, albeit primitive. This scenario supports homology of all blood vascular systems. Over the past 600–700 million years of evolution, the blood vascular system has undergone significant modifications, in response to selective pressures experienced by individual phyla. In some cases, the blood vascular system has regressed (e.g. in flatworms and nematodes). In other cases, the primitive system transitioned to an open circulation.
This does not answer how and why it evolved in the first place.
The open system reverted back to a closed design in an ancestral cephalopod. This is an example of convergent evolution, whereby an analogous structure (the closed circulation in cephalopods) arose independently of the closed circulation in other invertebrates (e.g. worms) and in vertebrates. Finally, the endothelium is present in all vertebrates, yet distinctly absent in invertebrates. Although cells appear to cling to the inner surface of some invertebrate closed vessels, these lack the classical morphological features of endothelial cells. Therefore, the endothelium appeared in an ancestral vertebrate following the divergence from the urochordates and cephalochordates between 540 and 510 million years ago.
This is further a just so story. How is this backed up by evidence ?
Our findings in hagfish indicate that endothelial heterogeneity appeared during the same narrow window of time.
Why did the blood vascular system and endothelium evolve?
Circulatory systems most certainly evolved to overcome the time and distance constraints of diffusion, thus permitting increased body size and metabolic rates, as well as increased levels of integration and organization in Metazoa.
Why should that have been a evolutionary necessity ? The most diverse phyla still today are insects and different animals of small size. That is hardly a credible and plausible explanation, but rather a just so story to fit the paradigm of evolution.
Although the primitive coelom provided bulk flow delivery of substances, these structures never developed an efficient pumping mechanism. More importantly, they became increasingly localized to distinct compartments in the body. Ruppert and Carle hypothesized that the blood vascular system evolved as a means to provide bulk fluid transport along the entire length of a segmented animal, in effect bypassing the septal bulkheads. The earliest design may have involved the formation of spaces in between the basal laminae of endodermal and coelomic epithelia, allowing for improved distribution of nutrients absorbed by the intestine. Myoepithelial differentiation of the visceral layer of coelomic epithelium would have provided the necessary pumping action to drive nutrients toward the viscera. Subsequent vascularization
of the skin would have facilitated gas exchange at the body surface.
There is no consistent explanation provided why evolutionary pressure would have favoured that process. Its just asserted that this is what most probably happened.
The movement of fluid in blood vascular systems was originally mediated by the peristaltic motion of certain blood vessels, similar to what occurs in the earthworm and amphioxus. However, peristaltic pumps lack effective coordination between the fluid that is entering the contractile region and the fluid that is leaving it. Despite some tweaks to the peristaltic design, such as the evolution of one-way valves and partial coordination in contractions, the loss of fluid energy associated with backflow, distension of wall segments ahead of the stream, and pump reversals would have constrained body size and metabolic activity. This would have provided a selective pressure for the appearance of true hearts, in which inflow and outflow are tightly coupled via multiple chambers, efficient electrical connection between myocytes (hence, simultaneous contraction) and one-way valves. If the closed circulatory system was the ancestral condition, then why did the open circulatory system evolve in the precursors of mollusks and arthropods? One explanation for this change in the circulatory blueprint is that the rigid exoskeleton of these animals, which evolved for protection against predation and physical injury, eliminated the need for a coelomic hydrostatic skeleton . The subsequent regression of the coelom removed barriers to bulk transport and therefore the need for a closed system of vessels. At the same time, the exoskeleton impaired any contribution of body wall musculature to the movement of blood. This may have provided selective pressure for the evolution of a well-developed muscular heart, which is a characteristic feature of most animals with an open circulation.
Wow. Thats as superficial as it can get. Best example of pseudo-scientific , superficial claims, which avoid to go into the details, and explan, how this process really happened, and what molecular mechanism was involved. They just assert and say , it is most probably what happened, and thats it. At this point, i see no reasons to analise the paper further, as the picture is clear, no serious inspection and analysis of molecular evolutionary mechanisms are made.
1)http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3926130/

