https://reasonandscience.catsboard.com/t1545-origin-and-evolution-of-photosynthesis
http://www.genome.jp/kegg/pathway/map/map00195.html
The existence in the same organism of cyanobacterias of two conflicting metabolic systems, oxygen evolving photosynthesis and oxygen-sensitive nitrogen fixation, is a puzzling paradox. Explanations are pure guesswork.
Researchers have long been puzzled as to how the cyanobacteria could make all that oxygen without poisoning themselves. To avoid their DNA getting wrecked by a hydroxyl radical that naturally occurs in the production of oxygen, the cyanobacteria would have had to evolve protective enzymes. But how could natural selection have led the cyanobacteria to evolve these enzymes if the need for them didn’t even exist yet? The explanations are fantasious at best
If there was a reduced atmosphere without oxygen some time back in the past ( which btw. there is no scientific evidence for, rather the oposit is the case ) then there would be no ozone layer, and if there was no ozone layer the ultraviolet radiation would penetrate the atmosphere and would destroy the amino acids as soon as they were formed. If the Cyanobacterias however would overcome that problem ( its supposed the bacterias in the early earth lived in the water, but that would draw other unsurmountable problems ), and evolve photosynthesis, they would have to evolve at the same time protective enzymes that prevented them oxygen to damage their DNA through hydroxyl radicals. So what evolutionary advantage would there be they to do this ?
Cyanobacteria are the prerequisite for complex life forms. They are said to exist already 3,5 bio years, and did not change morphologically. They do oxygenic photosynthesis, where the energy of light is used to split water molecules into oxygen, protons, and electrons. It occurs in two stages. In the first stage, light-dependent reactions or light reactions capture the energy of light and use it to make the energy-storage molecules ATP and NADPH. During the second stage, the light-independent reactions use these products to capture and reduce carbon dioxide.
They have ATP synthase nano-motors. How could ATP synthase “evolve” from something that needs ATP, manufactured by ATP synthase, to function? Absurd “chicken-egg” paradox!
In photosynthesis , 26 protein complexes and enzymes are required to go through the light and light independent reactions, a chemical process that transforms sunlight into chemical energy, to get glucose as end product , a metabolic intermediate for cell respiration. The protein complexes are uniquely used in photosynthesis. The pathway must go all the way through, and all steps are required, otherwise glucose is not produced. Also, in the oxygen evolving complex, which splits water into electrons, protons, and CO2, if the light-induced electron transfer reactions do not go all the five steps through, no oxygen, no protons and electrons are produced, no advanced life would be possible on earth. So, photosynthesis is a interdependent system, that could not have evolved, since all parts had to be in place right from the beginning. So it seems that photosynthesis falsifies the theory of evolution, where all small steps need to provide a survival advantage.
The American astronomer George Greenstein discusses this in The Symbiotic Universe, p 96:
Chlorophyll is the molecule that accomplishes photosynthesis... The mechanism of photosynthesis is initiated by the absorption of sunlight by a chlorophyll molecule. But in order for this to occur, the light must be of the right color. Light of the wrong color won't do the trick.
A good analogy is that of a television set. In order for the set to receive a given channel it must be tuned to that channel; tune it differently and the reception will not occur. It is the same with photosynthesis, the Sun functioning as the transmitter in the analogy and the chlorophyll molecule as the receiving TV set. If the molecule and the Sun are not tuned to each other-tuned in the sense of colour- photosynthesis will not occur. As it turns out, the sun's color is just right.
One might think that a certain adaptation has been at work here: the adaptation of plant life to the properties of sunlight. After all, if the Sun were a different temperature could not some other molecule, tuned to absorb light of a different colour, take the place of chlorophyll? Remarkably enough the answer is no, for within broad limits all molecules absorb light of similar colours. The absorption of light is accomplished by the excitation of electrons in molecules to higher energy states, and the same no matter what molecule you are discussing. Furthermore, light is composed of photons, packets of energy and photons of the wrong energy simply can not be absorbed… As things stand in reality, there is a good fit between the physics of stars and that of molecules. Failing this fit, however, life would have been impossible.
The harmony between stellar and molecular physics that Greenstein refers to is a harmony too extraordinary ever to be explained by chance. There was only one chance in 1025 of the Sun's providing just the right kind of light necessary for us and that there should be molecules in our world that are capable of using that light. This perfect harmony is unquestionably proof of Creation.
http://reasonandscience.heavenforum.org/t1546-chlorophyll-biosynthesis-pathway
http://www.uncommondescent.com/intelligent-design/is-photosynthesis-irreducibly-complex/
Robert Blankenship, professor of biochemistry at Arizona State University
“The process of photosynthesis is a very complex set of interdependent metabolic pathways. How it could have evolved is a bit mysterious.”
Chlorophyll biosynthesis is a complex pathway with 17 highly specific steps, of which eigth last steps are used by specific enzymes uniquely in this pathway.
The pathway must go all the way through, otherwise chlorophyill is not synthesized.
Therefore, the Chlorophyill biosynthesis pathway is irreducible complex.
Evolution of photosynthetic pathways
Well, what i see, is a lot of guesswork, like probably, indicates,speculated, presumed, may explain, suggests, supposes, seem to have,
These two pathways, with the same effect on RuBisCO, evolved a number of times independently – indeed, C4 alone arose 62 times in 18 different plant families.
Well, if the evolution of just one time would be a unsurmountable problem, imagine 62 times...... why and how would it do so ??!!
So not much more than vague and superficial speculation is provided.
http://www.ps-19.org/Crea06EcoSys/index.html
According to an analysis of the cyanobacterial genome (Hasselkorn and Johnston (PNAS)) the earliest cyanobacteria already had the light & Calvin processes for photosynthesis in place. These are two very complex and subtly linked processes and involve many specialized molecules working together. These are such complex biological processes, that the complexity and early appearance on earth seems to indicate planning and design.
Biologists universally (as far as I am aware) point to the complexity and the similarity of photosynthesis among all species to imply that the process evolved only once in earth's history[FOOTNOTE: for example, Schopf p. ???; other references] -- the chance events that had to occur for photosynthesis to arise even once by natural processes are vanishingly low probability, so that assuming the same system would arise more than once defies even an evolutionist's credulity.
Evolution of photosynthesis
The biochemical capacity to use water as the source for electrons in photosynthesis evolved once, in a common ancestor of extant cyanobacteria. The geological record indicates that this transforming event took place early in Earth's history, at least 2450–2320 million years ago (Ma), and, it is speculated, much earlier.[2][3] Available evidence from geobiological studies of Archean (>2500 Ma) sedimentary rocks indicates that life existed 3500 Ma, but the question of when oxygenic photosynthesis evolved is still unanswered.
http://dippost.com/2014/03/22/wolf-ekkehard-lonnig-complex-systems-in-biology-overwhelmingly-point-to-an-intelligent-origin-of-living-beings/
Photosynthesis is a mind-blowing phenomenon that is another example of irreducible complexity which also points to an intelligent design: “In my mutation experiments I detected hundreds of totally white seedlings where usually one individual mutation alone, most often at different sides in different plant families, stopped the entire system from functioning at all (reminds, of course of Behe’s definition of “the removal of any one of the parts causes the system to effectively cease functioning”.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2949000/
The evolutionary origin of the oxygen evolving center has long been a mystery. Several sources have been suggested, but so far no convincing evidence has been found to resolve this issue
http://www.life.illinois.edu/govindjee/Part3/31_John_Olson.pdf
http://www.ncbi.nlm.nih.gov/pubmed/23258841
http:/Timeline_of_evolutionary_history_of_life
for the last 3.6 billion years, simple cells (prokaryotes);
for the last 3.4 billion years, cyanobacteria performing photosynthesis;
When did oxygenic photosynthesis evolve?
Abstract
The atmosphere has apparently been oxygenated since the ‘Great Oxidation Event’ ca 2.4 Ga ago, but when the photosynthetic oxygen production began is debatable. However, geological and geochemical evidence from older sedimentary rocks indicates that oxygenic photosynthesis evolved well before this oxygenation event. Fluid-inclusion oils in ca 2.45 Ga sandstones contain hydrocarbon biomarkers evidently sourced from similarly ancient kerogen, preserved without subsequent contamination, and derived from organisms producing and requiring molecular oxygen. Mo and Re abundances and sulphur isotope systematics of slightly older (2.5 Ga) kerogenous shales record a transient pulse of atmospheric oxygen. As early as ca 2.7 Ga, stromatolites and biomarkers from evaporative lake sediments deficient in exogenous reducing power strongly imply that oxygen-producing cyanobacteria had already evolved. Even at ca 3.2 Ga, thick and widespread kerogenous shales are consistent with aerobic photoautrophic marine plankton, and U–Pb data from ca 3.8 Ga metasediments suggest that this metabolism could have arisen by the start of the geological record. Hence, the hypothesis that oxygenic photosynthesis evolved well before the atmosphere became permanently oxygenated seems well supported.
Photosynthesis tree
Inferences Based on the Observed Branching Order
Earliest Branching Photosynthetic Bacteria
The Genome of Heliobacterium modesticaldum, a Phototrophic Representative of the FirmicutesContaining the Simplest Photosynthetic Apparatus 1
Firmicutes (Heliobacterium) are indicated to be earliest branching photosynthetic bacteria. The ancestral nature of this group is also supported by a number of other observations:
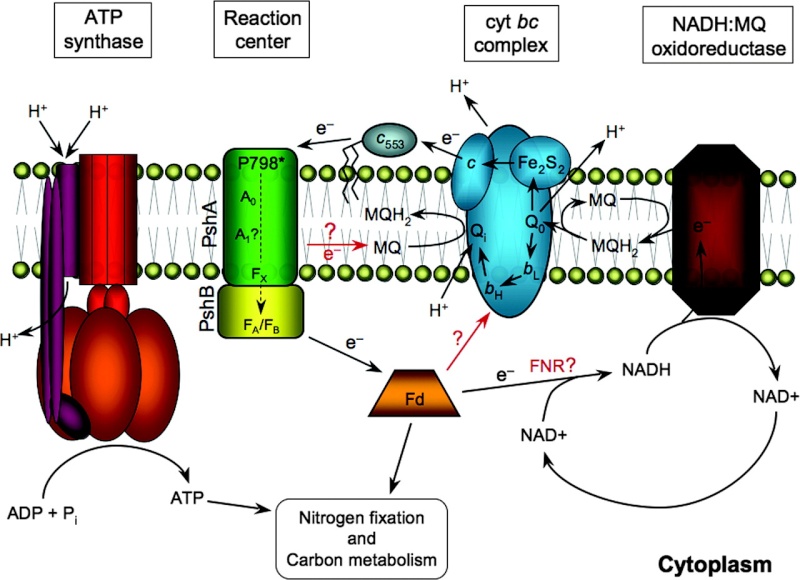
Diagram showing a putative pathway of electron transfer based on genetic components present in H. modesticaldum. Cyclic electron transfer has not been confirmed in heliobacteria. In addition, the reduction of NAD+ by cytoplasmic ferredoxin has not been confirmed, as a gene encoding FNR was not identified in the genome. Despite this, genes encoding all 14 subunits of NADH:quinone oxidoreductase (nuoA to nuoN) were putatively identified.
Unlike other photosynthetic bacteria, both antenna and reaction center activities are present within a single protein in Heliobacteria;
The reaction center complex in Heliobacteria (and also green sulfur bacteria) has a simpler homodimeric structure as opposed to being heterodimeric in other photosynthetic bacteria;
The RC in Heliobacteria contains a unique photosynthetic pigment Bchl g, which is indicated to be primitive in comparison to the pigments found in other photosynthetic organisms.
Of the different photosynthetic bacteria, only Heliobacteria are bounded by a single unit lipid membrane (monoderm cell structure), which is indicated to be an ancestral characteristic in comparison to the cells containing both an inner and outer cell membranes (Diderm cell structure).
2. The Second Photosynthetic Bacteria
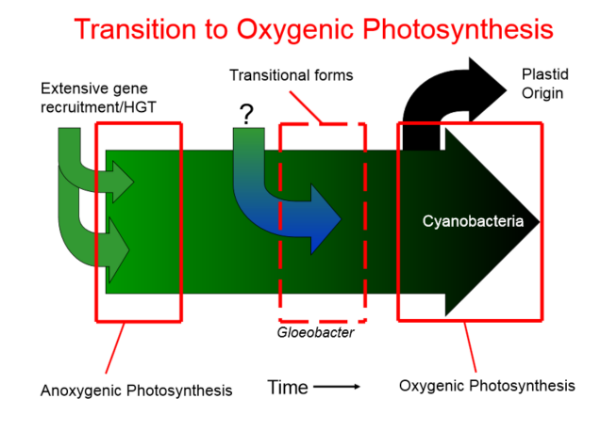
Following Heliobacteria, Chloroflexi are indicated to be the next group of photosynthetic organisms that branched off from the common ancestor. The branching of both Heliobacteria and Chloroflexi prior to Cyanobacteria provides evidence that both RC-1 and RC-2 had already evolved prior to the emergence of Cyanobacteria, which contain both of these reactions centers linked to each other.
3. Anoxygenic Photosynthesis vs Oxygenic Photosynthesis
The bacterial groups utilizing anoxygenic photosynthesis mode evolved much earlier than those capable of oxygenic photosynthesis. This is in accordance with the observation that change in atmosphere from anoxygenic to oxygenic occurred much later (between 1.5-2 billion year) after the evolution of earlier organisms. This observation indicates that the earlier prokaryotic fossils probably do not correspond to Cyanobacteria but some other groups of photosynthetic bacteria.
4. Later Branching Photosynthetic Bacteria
The later branching photosynthetic phyla which contain either one or both of these RCs could have acquired such genes from the earlier branching lineages by either direct descent or by means of lateral gene transfer.
5. Speculations About the Earliest Organism
The presence of photosynthetic ability in the earliest branching bacterial phylum indicates that photosynthesis evolved very early in evolution and it is possible that the earliest organism that evolved were photosynthetic.
Early photosynthetic systems, such as those from green and purple sulfur and green and purple nonsulfur bacteria, are thought to have been anoxygenic, using various molecules as electron donors. Green and purple sulfur bacteria are thought to have used hydrogen and sulfur as an electron donor.
http://www.fceqyn.unam.edu.ar/~celular/paginas/Articulos%20Biol%20Cel%202004/Annual%20Reviews/Review%20photosyntesis.pdf
The evolutionary path of type I
And type II reaction center apoproteins is still unresolved owing to the fact that a unified evolutionary tree cannot be generated for these divergent reaction center subunits.
http://www.javeriana.edu.co/Facultades/Ciencias/neurobioquimica/libros/metabolismo/metabolismo_archivos/Evolution%20of%20Photosynthesis.pdf
One of the main reasons for interest in Chloroflexus aurantiacus is in the study of the evolution of photosynthesis. As terrestrial mammals, we are most familiar with photosynthetic plants such as trees. However, photosynthetic eukaryotes are a relatively recent evolutionary development. Photosynthesis by eukaryotic organisms can be traced back to endosymbiotic events in which non-photosynthetic eukaryotes internalized photosynthetic organisms. The chloroplasts of trees still retain their own DNA as a molecular remnant that indicated their origin as photosynthetic bacteria.
The "respiration early" hypothesis
How did photosynthesis arise in bacteria? The answer to this question is complicated by the fact that there are several types of light-harvesting energy capture systems. Chloroflexus aurantiacus has been of interest in the search for origins of the so-called type II photosynthetic reaction center. One idea is that bacteria with respiratory electron transport evolved photosynthesis by coupling a light-harvesting energy capture system to the pre-existing respiratory electron transport chain. Thus, rare organisms like Chloroflexus aurantiacus that can survive using either respiration or photosynthesis are of interest in on-going attempts to trace the evolution of photosynthesis.
The first organisms were self-replicating iron-rich clays which fixed carbon dioxide into oxalic and other dicarboxylic acids
Self-organizing biochemical cycles
http://www.pnas.org/content/97/23/12503.full.pdf
One could summarize the work of Ferris and colleagues by saying that montmorillonite clays catalyze the oligomerization of nucleoside -phosphorimidazolides and modify the ratio of 2 phosphodiester bonds that are formed without greatly changing the regiospecificity of the reactions. The specificity of the formose reaction is not increased by catalysis on hydroxylapatite or other minerals (23). Similar conclusions can be drawn from the work of Arrhenius and his colleagues (27), who find that a number of inorganic layer hydroxides catalyze the dimerization of glycolaldehyde phosphate and modify the ratio of threose 2,4-bisphosphates to erythrose 2,4-bisphosphates in the products, but without greatly increasing the regiospecificity of the reaction.
Montmorillonite-catalysed formation of RNA oligomers: the possible role of catalysis in the origins of life
J. P. Ferris (2006)
https://www.rpi.edu/dept/cogsci/yesterday/chem/chem_faculty/profiles/pdfs/ferris/Joshi_Homochiral_Chem_Com_2000.pdf
https://www.academia.edu/3742566/Photosynthesis_and_the_Origin_of_Life
PHOTOSYNTHESIS AND THE ORIGIN OF LIFE
HYMAN HARTMAN
IASB, 880 Spruce St., Berkeley, CA 94707, U.S.A.
(Received 30 August, 1996)
Abstract.
The origin and evolution of photosynthesis is considered to be the key to the origin of life. This eliminates the need for a soup as the synthesis of the bioorganics are to come from the fixation of carbon dioxide and nitrogen. No soup then no RNAworld or Protein world. Cyanobacteria have been formed by the horizontal transfer of green sulfur bacterial photoreaction center genesby means of a plasmid into a purple photosynthetic bacterium. The fixation of carbon dioxide isconsidered to have evolved from a reductive dicarboxylic acid cycle (Chloroflexus) which was thenfollowed by a reductive tricarboxylic acid cycle (Chlorobium) and finally by the reductive pentosephosphate cycle (Calvin cycle). The origin of life is considered to have occurred in a hot springon the outgassing early earth. The first organisms were self-replicating iron-rich clays which fixedcarbon dioxide into oxalic and other dicarboxylic acids. This system of replicating clays and theirmetabolic phenotype then evolved into the sulfide rich region of the hotspring acquiring the ability to fix nitrogen. Finally phosphate was incorporated into the evolving system which allowed the synthesis of nucleotides and phospholipids. If biosynthesis recapitulates biopoesis, then the synthesis of aminoacids preceded the synthesis of the purine and pyrimidine bases. Furthermore the polymerization of the amino acid thioesters into polypeptides preceded the directed polymerization of amino acidesters by polynucleotides. Thus the origin and evolution of the genetic code is a late development and records the takeover of the clay by RNA.
Introduction
The major premise which underlies the field of either the ‘RNA world’ or the ‘Proteinoid world’ is the prior existence of a soup of monomers consisting of sugars, pyrimidine and purine bases and amino acids. In the old conundrum asto which came first the chicken (protein) or the egg (RNA), the answer is the chicken soup (of monomers). The major premise of this paper is that the soup is a hypothetical construct.
In the absence of a soup the carbon entering the biosphere is in the form of carbon dioxide, the nitrogen is in the form of nitrogen gas, the hydrogen andoxygen enter the biosphere in the form of liquid water, the sulfur in the form of sulfide ion and the phosphate in the form of the phosphate ion.
The early atmosphere of the earth is considered to have been neutral (nitrogen and carbon dioxide). The problem arises of how oxygenic photosynthesis could have evolved under these conditions.There were abundant reducing agents such asferrous ion which made it unlikely that water would be used as an electron donor.It was thus suggested ‘that atmospheric hydrogen peroxide played a key role in inducing oxygenic photosynthesis because asperoxide increased in a localenvironment, organisms would not only be faced with a loss of reductant, but they would also be pressed to develop the biochemical apparatus (e.g., catalase) that would be ultimately be needed to protect against the products of oxygenic photosynthesis. This scenario allows for the early evolution of oxygen photosynthesis while global conditions were still anaerobic’ (McKay and Hartman, 1991).
Oxygenic photosynthesis developed in the cyanobacteria.The earliest bacterial fossils are found in the 3.5billion year old stromatolites of Western Australia. These fossil bacteria resemble cyanobacteria (Awramik, 1992).One possible conclusion from the ancient stromatolites and the bacterial fossils is that oxygenic photosynthesis was being carried out by the cyanobacteria 3.5billionyears ago.The cyanobacteria have two reaction centers; photosystem I and photosystem II. This is in contrast with the purple and the green photosynthetic bacteria which have only one reaction center. The purple bacteria have a pheophytin-quinone reaction center which is related to photosystem II. The green sulfur bacteria havea Fe-S reaction center which is related to photosystem I. ‘The simplest scenario giving rise to the linked photosystems found in oxygen-evolving organisms is that some sort of genetic fusion event took place between two bacteria, one with apheophytin-quinone reaction center and the other with an Fe-S reaction center.This produced a chimeric organism with two unlinked photosystems. Subsequently, the two photosystems were linked, and the oxygen evolving system added ’(Blankenship, 1992).
What is the origin of plastids ?
http://www.evolutionnews.org/2012/03/seeking_the_lin057151.html
Price et al. look at plastids that were supposedly derived from cyanobacteria. These plastids all contain certain features that are similar to cyanobacteria, and those organisms that contain them are therefore thought to be along the same phylogenetic lineage. They include Glaucophyta, Rhodophyta, and green algae, along with their plant descendants.
These three lineages are postulated to form the monophyletic group Plantae (or Archaeplastida), a hypothesis that suggests the primary cyanobacterial endosymbiosis occurred exclusively in their single common ancestor.
Unfortunately, as the authors point out, phylogenies based on other features, such as genetics, do not support the cyanobacteria- and plastid-derived phylogeny.
http://www.plantcell.org/content/25/1/4.abstract
The idea of an endosymbiotic origin of plastids has become incontrovertible, but many important aspects of plastid origins remain obscured in the mists of more than a billion years of evolutionary history
Endosymbiosis is now a well substantiated theory that explains how cells gained their great complexity and was made famous most recently by the work of the late biologist Lynn Margulis, best known for her theory on the origin of eukaryotic organelles.
In a paper "Cyanophora paradoxa genome elucidates origin of photosynthesis in algae and plants" that appeared this week in the journal Science, an international team led by evolutionary biologist and Rutgers University professor Debashish Bhattacharya has shed light on the early events leading to photosynthesis, the result of the sequencing of 70 million base pair nuclear genome of the one-celled alga Cyanophora.
Thinking about the evolution of photosynthesis
There are two schools of thought concerning the origin of reaction
centers and photosynthesis. One school pictures the evolution of
reaction centers beginning in the prebiotic phase
while the other school sees reaction centers evolving later from cytochrome b
in bacteria. Two models have been put forth for the subsequent evolution
of reaction centers in proteobacteria, green filamentous (non-sulfur) bacteria,
cyanobacteria, heliobacteria and green sulfur bacteria. In the selective loss model the most recent common ancestor
of all subsequent photosynthetic systems is postulated to have contained both RC1 and RC2. The evolution of
reaction centers in proteobacteria and green filamentous bacteria resulted from the loss of RC1, while the evolution
of reaction centers in heliobacteria and green sulfur bacteria resulted from the loss of RC2. Both RC1 and RC2
were retained in the cyanobacteria. In the fusion model the most recent common ancestor is postulated to have
given rise to two lines, one containing RC1 and the other containing RC2. The RC1 line gave rise to the reaction
centers of heliobacteria and green sulfur bacteria, and the RC2 line led to the reaction centers of proteobacteria
and green filamentous bacteria. The two reaction centers of cyanobacteria were the result of a genetic fusion of
an organism containing RC1 and an organism containing RC2. The evolutionary histories of the various classes
of antenna/light-harvesting complexes appear to be completely independent. The transition from anoxygenic to
oxygenic photosynthesis took place when the cyanobacteria learned how to use water as an electron donor for
carbon dioxide reduction. Before that time hydrogen peroxide may have served as a transitional donor, and before
that, ferrous iron may have been the original source of reducing power.
This hypothesis answers the problem of how
biosynthetic pathways to complex products could have
evolved by random mutation. The pathway is built
forward with each step fulfilling a useful function,
eventually to be replaced by the next step selected for
improved utility. Olson and Pierson (1987a, b) used the Granick
hypothesis to construct a hypothetical evolutionary
history of RCs from the biosynthetic pathways of Chl
a and BChla . They proposed that protochlorophyll
a might have functioned in a primitive RC at some time
before Chla existed, and that protoporphyrin IX and
Mg protoporphyrin IX might have served as RC pig-
ments before protochlorophyll a existed. In agreement
with Mauzerall (1978), Olson (1970, 1981) had previ-
ously interpreted the biosynthetic pathway of BChl
a to indicate that Chla had appeared before BChl a
in evolutionary history.
The nature of the ancient reductase that predated the
gene duplication is not known, but typically ancient
enzymes are both less specific and less efficient than
modern enzymes (Benner 2002).
Reaction centers
The evolution of photosynthesis begins with the evo-
lution of photochemical reaction centers (RCs). Two
schools of thought exist concerning the origin of
RCs and photosynthesis. The first school pictures the
evolution of RCs/photosynthesis beginning in the pre-
biotic phase (e.g., Mauzerall 1992; Hartman 1998)
whereas the second school sees RCs and photosyn-
thesis evolving much later from anaerobic respiration
in Bacteria (eubacteria) containing DNA, electron-
transport proteins, and ATP synthase (e.g., Meyer et al.
1996; Nitschke et al. 1998). The first school envi-
sions the evolution of photosynthesis and the origin
of life to be tightly intertwined; the second school
sees the origin of photosynthesis coming much later
than the appearance of the common ancestor of all
life. According to this view photosynthesis arose in
the Bacteria after they had separated from the Archaea
(archaebacteria).
378
Figure 2.
Hypothetical history of reaction center evolution based on protein homology.
See text for details. (Reprinted with permission of Data
Trace Publishing Company. Chemtracts Biochem Mol Biol 12: 468–482,1999.)
all disagree with Olson and Pierson (1987a,
b) in one fundamental aspect. They propose that RC1
and RC2 evolved independently in different organ-
isms, and then were brought together in one organism
by genetic fusion to produce the cyanobacterial line
of photosynthesis with two photosystems in series.
Mathis (1990) proposed an evolutionary tree in which
genes for RC1 were transferred from a ‘heliobac-
terium’ to the genome of a ‘purple bacterium’ already
possessing genes for RC2. The resulting fusion be-
came the forerunner of the cyanobacteria. Blankenship
(1992) proposed that the ancestral RC evolved in two
directions simultaneously as shown in Figure 4. One
direction led to the evolution of RC2, and the other
direction led to RC1.
Antennas
LH1 and LH2 antenna complexes
Since both peptides are similar to helix D of subunit L
(bacterial RC2), the presumed ancient single helix or
homodimeric antenna complex may have been derived
from helix D of an ancient subunit L (Mulkidjian and
Junge 1997).
Phycobilisomes
Cyanobacteria and red algae contain supramolecu-
lar light-harvesting complexes called phycobilisomes,
that are attached to the stromal side of the photo-
synthetic membrane (Blankenship 2002). These com-
plexes can transfer excitation energy to the core
complex (CP43, CP47 and RC2) with more than
95% efficiency. The phycobilisomes consist of related
pairs of α and β phycobiliproteins (allophycocyanin,
phycocyanin and sometimes phycoerythrocyanin and
phycoerythrin). The phycobiliproteins are pigment
proteins that have evolved by multiple gene duplica-
tions and divergences from an ancestral form that
could form short rods (Grossman et al. 1995).
The most widespread source of reducing power in
late Archean and early Proterozoic (2.9–1.6 Ga) sea-
water was ferrous iron (0.1–1.0 mM) (Walker 1983),
and several authors (Olson 1978; Cohen 1984; Olson
and Pierson 1987a; Pierson and Olson 1989) proposed
that ferrous iron may have been an early electron
donor to PS II. The common ancestor of proteobacteria
and cyanobacteria might well have used Fe(OH)
+ as the principal electron donor for CO 2
fixation (Widdle et al. 1993; Ehrenreich and Widdle 1994; Heising
and Schink 1998). Because contemporary green sul-
fur bacteria utilize Fe(OH) +
rather poorly (Heising et al. 1999), one of us (Olson 2001) proposed that
the driving force for the evolution of RC2 in addi-
tion to RC1 was the necessity to utilize Fe(OH)
+ effectively for CO 2 fixation in the absence of reduced
sulfur compounds. However, some contemporary pro-
teobacteria can utilize Fe(OH) + for CO 2 fixation with
RC2 alone (Widdle et al. 1993; Ehrenreich and Widdle
1994).
Concluding remarks
This paper traces the history of thinking on how
photosynthesis originated and developed, from primit-
ive cells through anoxygenic photosynthetic bacteria,
through cyanobacteria and eventually to chloroplasts.
It is now clear that earlier attempts to reconstruct ‘the
path’ of evolutionary development were doomed to
failure. There have been many such paths that different
parts of the photosynthetic apparatus have followed,
which have not been the same in either of two classes
of organisms or even in different parts of the same
organism. Thus there is not one linear path that was
followed. The much more complex and non-linear
history that has taken place is challenging and inter-
esting to unravel, and will undoubtedly keep scientists
occupied for at least another 30 years
Captured, the moment photosynthesis changed the world
BILLIONS of years ago, a tiny cyanobacterium cracked open a water molecule - and let loose a poison that wrought death and destruction on an epic scale. The microbe had just perfected photosynthesis, ( amazing, how did it figure out that little trick ? ) a process that freed the oxygen trapped inside water and killed early Earth's anaerobic inhabitants.
Now, for the first time, geologists have found evidence of the crucial evolutionary stage just before cyanobacteria split water. The find offers a unique snapshot of the moment that made the modern world. With the advent of photosynthesis came an atmosphere dominated by oxygen and, ultimately, the diversity of life forms that we know today.
"This was the biggest change that ever occurred in the biosphere," says Kevin Redding at Arizona State University in Tempe. "The extinction caused by oxygen was probably the largest ever seen, but at the same time animal life wouldn't be possible without oxygen."
Photosynthesis uses light and a source of electrons to generate energy and power an organism. In the world as we know it, that source of electrons is water, with oxygen the waste product. But there are no signs that oxygen was being formed when photosynthesis first appeared around 3.4 billion years ago, so early photosynthesisers ( how did they come to be ? thats finally what we would like to know ) probably ( oh, so they don't know for sure ) scavenged electrons by splitting other molecules like hydrogen sulphide instead.
That had changed by about 2.4 billion years ago, when deposits of oxidised minerals tell us that oxygen was beginning to accumulate in the atmosphere. Photosynthesis as we know it had evolved.
( how do we know it evolved ?? )
To help work out how this happened, Woodward Fischer at the California Institute of Technology in Pasadena and his colleagues studied South African rocks that formed just before the 2.4-billion-year mark. Their analysis shows that although the rocks formed in the anoxic conditions that had prevailed since Earth's formation, all of the manganese in the rock was deposited in an oxidised form.
In the absence of atmospheric oxygen, manganese needs some sort of catalyst to help it oxidise - it won't react without a bit of help. The best explanation, say Fischer's team, is that a photosynthetic organism was using manganese as an electron source. That left unstable manganese ions behind, which reacted with water to form the oxides. Fischer presented the findings at the American Geophysical Union's conference in San Francisco on 6 December.
Every researcher contacted by New Scientist has hailed the significance of the study, in part because the evidence exactly matches what evolutionary theories have predicted.
A close look at today's plants and algae shows that manganese oxidation is still a vital part of photosynthesis. Within their photosynthetic structures are manganese-rich crystals that provide the electrons to drive photosynthesis. The crystals then snaffle electrons from passing water molecules to restore their deficit. It is this electron raid that cracks open water molecules and generates the oxygen we breathe.
This complicated process must have had simpler roots. In 2007, John Allen at Queen Mary, University of London, and William Martin at the University of Düsseldorf, Germany, suggested one scenario (Nature, doi.org/bs65kb).
They believe that modern photosynthesis was born when early cyanobacteria by chance floated into a watery environment rich in manganese, and quickly adapted to take advantage of the new source of electrons.
They adapted ?? How did that happen ?
Later, because manganese is a relatively scarce resource that can't be tapped indefinitely, the cyanobacteria evolved a different strategy. They incorporated manganese directly into their photosynthetic structures ( i have still not seen a explanation of how photosynthesis evolved in them )
and used it as a rechargeable battery: draining it of its electrons, but allowing its supplies to be replenished by stealing electrons from another, more plentiful source - water.
What Fischer's team has found is evidence of the initial step in this process: an anoxic environment rich in manganese that has been stripped of electrons and left in an oxidised state, almost certainly by primitive cyanobacteria. "There had to be some intermediate step in the evolutionary process," says Redding.
"This is big news," says Martin. He adds that we can expect publications in the near future that provide more evidence compatible with the theory. "But this somewhat more direct geochemical evidence is really exciting."
SO , THAT ARE ALL UNSUPPORTED CLAIMS, AD HOC EXPLANATIONS WITHOUT EXPERIMENTAL EVIDENCE WHATSOEVER, AND NEIThER DETAILED EXPLANATIONS HOW THE EVOLUTIONARY PROCESS OF PHOTOSYNTHESIS HAPPENED....
What was a simpler version of oxygen-producing photosynthesis, and how did it evolve to what we have today? What came first, the assembly factors of PSII, or PSII? the repair mechanism of D1 subunit, or the D1 subunit? And how was the assembly process and sequence, and repair sequence programmed? trial and error?
1) http://jb.asm.org/content/190/13/4687.full
Last edited by Admin on Sun Apr 29, 2018 3:04 pm; edited 22 times in total