Signaling and regulation in early life forms represent an essential aspect of cellular function and adaptation. The study of primitive signal transduction mechanisms provides insights into how the earliest cells would have responded to their environment. Regulatory networks in these early cells demonstrate the coordination required for survival and reproduction. Environmental sensing and adaptation showcase the remarkable ability of primordial organisms to thrive in diverse conditions. This exploration of early life's signaling and regulatory systems reveals the complexity present even in the most basic forms of life.
22.1. Primitive signal transduction mechanisms
Primitive signal transduction mechanisms represent the foundational systems that allowed early life forms to detect and respond to environmental stimuli. These mechanisms, though rudimentary compared to modern cellular signaling pathways, were essential for the survival and adaptation of primordial organisms. The study of these early signaling systems provides critical insights into the fundamental processes that enabled life to persist and evolve in diverse environments. At their core, primitive signal transduction mechanisms likely involved simple molecular interactions that could translate external stimuli into internal cellular responses. These may have included basic chemical reactions triggered by environmental factors such as pH changes, temperature fluctuations, or the presence of specific molecules. The ability to sense and respond to these stimuli would have been essential for early cells to maintain their internal equilibrium and adapt to changing conditions. One of the most fundamental aspects of these early signaling systems was likely the use of small molecules as messengers. These molecules could diffuse across primitive membranes or interact with rudimentary membrane-bound proteins to initiate cellular responses. Such interactions may have led to changes in membrane permeability, activation of simple enzymatic reactions, or alterations in the cell's metabolic state. The development of these primitive signaling mechanisms would have required a delicate balance of molecular specificity and flexibility. The systems needed to be specific enough to respond to relevant stimuli while remaining adaptable to a changing environment. This balance poses significant challenges to explanations relying solely on undirected processes, as the level of coordination and specificity observed even in these early systems suggests a degree of complexity that is difficult to account for through random events alone. The study of primitive signal transduction mechanisms continues to challenge our understanding of early life and the origins of cellular complexity. The intricate nature of even the most basic signaling systems raises questions about how such essential processes could have emerged without guided or pre-established pathways. As research in this field progresses, it becomes increasingly apparent that the origins of these fundamental cellular functions require explanations that go beyond current models of unguided evolutionary processes.
Key players in primitive signal transduction mechanisms:
1. Simple molecular sensors
2. Basic chemical messengers
3. Rudimentary membrane-bound proteins
4. Primordial enzymatic systems
5. Environmental stimuli (pH, temperature, specific molecules)
Unresolved Challenges in Primitive Signal Transduction Origins
1. Molecular Specificity and Flexibility
Primitive signaling systems needed to be both specific enough to respond to relevant stimuli and flexible enough to adapt to changing environments.
Conceptual Problem: Balanced Complexity
- The delicate balance between specificity and flexibility in early signaling systems is difficult to explain through undirected processes alone.
- It remains unclear how such finely tuned systems could emerge without a guiding mechanism to optimize their function.
2. Coordinated Emergence of Multiple Components
Effective signal transduction requires the simultaneous presence of sensors, messengers, and response mechanisms.
Conceptual Problem: Synchronized Development
- The interdependence of these components raises questions about how they could have co-emerged under naturalistic conditions.
- Explaining the coordinated appearance of multiple, interrelated molecular systems without invoking guided processes remains an open challenge.
3. Information Processing in Primitive Systems
Even basic signal transduction involves a form of information processing to convert external stimuli into appropriate cellular responses.
Conceptual Problem: Origin of Biological Information Processing
- The emergence of systems capable of processing environmental information and generating specific responses poses significant challenges to undirected explanations.
- It is unclear how such information-processing capabilities could arise spontaneously in early life forms.
4. Membrane Interaction and Signaling
Primitive signaling often involved interactions with or across early cell membranes.
Conceptual Problem: Membrane-Signal System Compatibility
- The development of signaling systems compatible with early membrane structures presents a chicken-and-egg problem.
- Explaining how these systems co-evolved with membrane structures without pre-existing coordination mechanisms remains unresolved.
5. Adaptive Responses to Environmental Stimuli
Early signaling systems needed to produce adaptive responses to a variety of environmental changes.
Conceptual Problem: Origin of Adaptive Mechanisms
- The ability of primitive cells to generate beneficial responses to environmental stimuli suggests a level of pre-existing "knowledge" about what constitutes an adaptive response.
- Accounting for the origin of this implicit knowledge through unguided processes presents a significant challenge.
These unresolved challenges highlight the complexity inherent even in primitive signal transduction mechanisms. The level of coordination, specificity, and adaptability observed in these early systems raises significant questions about their origins. Current naturalistic explanations struggle to adequately account for the emergence of such sophisticated molecular machinery without invoking guided or pre-established processes.
22.2. Regulatory networks in early cells
Regulatory networks in early cells represent a fundamental aspect of primordial life, enabling basic organisms to maintain homeostasis and respond to environmental changes. These networks, though simpler than those found in modern cells, were essential for coordinating cellular processes and ensuring the survival of early life forms. The study of these primitive regulatory systems provides valuable insights into the foundational mechanisms that allowed life to persist and diversify. Early cellular regulatory networks likely consisted of interconnected molecular components that worked together to control gene expression, metabolic pathways, and cellular responses to external stimuli. These networks may have involved simple feedback loops, where the products of certain reactions influenced the activity of enzymes or the expression of genes. Such basic regulatory mechanisms would have been crucial for maintaining optimal concentrations of vital molecules and adapting to fluctuating environmental conditions. One of the key features of these early regulatory networks was probably their ability to integrate multiple signals and generate appropriate cellular responses. This integration would have allowed primitive cells to process information from various sources and make "decisions" about resource allocation, energy production, and cellular division. The development of such decision-making capabilities, even at a rudimentary level, represents a significant leap in cellular complexity. The emergence of these regulatory networks poses considerable challenges to explanations relying solely on undirected processes. The level of coordination and specificity required for even the simplest regulatory systems suggests a degree of complexity that is difficult to account for through random events alone. The interdependence of various network components and their ability to work in concert to achieve cellular goals raises questions about how such systems could have arisen without guided or pre-established pathways. As research in this field continues, it becomes increasingly clear that the origins of these fundamental cellular regulatory networks require explanations that go beyond current models of unguided evolutionary processes. The sophistication observed even in the most basic regulatory systems of early cells points to the need for a deeper understanding of how such essential biological machinery could have emerged.
Key players in regulatory networks of early cells:
1. Simple genetic switches
2. Basic metabolic enzymes
3. Primitive transcription factors
4. Rudimentary signaling molecules
5. Early feedback mechanisms
Unresolved Challenges in Early Cellular Regulatory Network Origins
1. Coordinated Gene Regulation
Early regulatory networks needed to coordinate the expression of multiple genes to maintain cellular function.
Conceptual Problem: Origin of Coordinated Control
- The emergence of systems capable of regulating multiple genes in a coordinated manner is difficult to explain through undirected processes.
- It remains unclear how such sophisticated control mechanisms could arise spontaneously in early life forms.
2. Feedback Loop Development
Effective regulation often relies on feedback loops to maintain homeostasis and respond to changes.
Conceptual Problem: Emergence of Circular Causality
- The development of functional feedback loops requires the simultaneous presence of sensors, effectors, and regulatory elements.
- Explaining the coordinated emergence of these interdependent components without invoking guided processes remains a significant challenge.
3. Metabolic Pathway Regulation
Early cells needed mechanisms to regulate complex metabolic pathways efficiently.
Conceptual Problem: Origin of Pathway Control
- The ability to regulate multi-step metabolic pathways suggests a level of system-wide coordination that is difficult to account for through random processes.
- It is unclear how such sophisticated regulatory capabilities could emerge without pre-existing organizational principles.
4. Signal Integration and Decision-Making
Primitive regulatory networks had to integrate multiple signals and generate appropriate responses.
Conceptual Problem: Emergence of Cellular Decision-Making
- The development of systems capable of processing multiple inputs and producing coherent outputs poses significant challenges to undirected explanations.
- Accounting for the origin of this implicit decision-making capability through unguided processes remains unresolved.
5. Robustness and Adaptability
Early regulatory networks needed to be both stable enough to maintain cellular function and flexible enough to adapt to changing conditions.
Conceptual Problem: Balance of Stability and Flexibility
- The delicate balance between robustness and adaptability in early regulatory networks is difficult to explain through undirected processes alone.
- It remains unclear how such finely tuned systems could emerge without a guiding mechanism to optimize their function.
These unresolved challenges highlight the complexity inherent even in the most primitive cellular regulatory networks. The level of coordination, specificity, and adaptability observed in these early systems raises significant questions about their origins. Current naturalistic explanations struggle to adequately account for the emergence of such sophisticated regulatory machinery without invoking guided or pre-established processes.
22.3. Environmental sensing and adaptation
Environmental sensing and adaptation in early life forms represent essential capabilities that allowed primitive organisms to survive and thrive in diverse and changing conditions. These fundamental processes enabled early cells to detect environmental cues and respond appropriately, ensuring their survival and propagation. The study of these early adaptive mechanisms provides crucial insights into the basic principles that underlie all life. Primitive environmental sensing likely involved simple molecular interactions that could detect changes in factors such as temperature, pH, osmolarity, and the presence of specific chemicals. These rudimentary sensing mechanisms would have been directly linked to basic cellular responses, allowing early life forms to maintain internal stability and respond to external challenges. The ability to sense and adapt to environmental changes would have been critical for early cells to persist in the face of fluctuating conditions. One of the key aspects of early environmental adaptation was probably the development of stress response systems. These systems would have allowed primitive cells to cope with environmental stressors by altering their metabolism, modifying their membrane composition, or producing protective molecules. Such adaptive responses would have been essential for survival in the diverse and often harsh conditions of early Earth. The emergence of these sensing and adaptation mechanisms poses significant challenges to explanations relying solely on undirected processes. The level of coordination required between sensing molecules, signaling pathways, and cellular responses suggests a degree of complexity that is difficult to account for through random events alone. The ability of early cells to not only detect environmental changes but also to respond in ways that enhanced their survival raises questions about how such sophisticated systems could have arisen without guided or pre-established pathways. As research in this field progresses, it becomes increasingly apparent that the origins of these fundamental cellular capabilities require explanations that go beyond current models of unguided evolutionary processes. The remarkable efficiency and effectiveness observed even in the most basic environmental sensing and adaptation systems of early cells point to the need for a deeper understanding of how such essential biological machinery could have emerged.
Key players in environmental sensing and adaptation of early cells:
1. Simple molecular sensors
2. Basic stress response proteins
3. Primitive membrane adaptation mechanisms
4. Rudimentary osmoregulatory systems
5. Early metabolic adaptation pathways
Unresolved Challenges in Early Environmental Sensing and Adaptation Origins
1. Molecular Sensor Specificity
Early sensing mechanisms needed to be specific enough to detect relevant environmental changes.
Conceptual Problem: Origin of Molecular Recognition
- The development of sensors capable of recognizing specific environmental cues with sufficient accuracy is difficult to explain through undirected processes.
- It remains unclear how such precise molecular recognition capabilities could arise spontaneously in early life forms.
2. Coordinated Stress Responses
Effective adaptation required coordinated responses involving multiple cellular components.
Conceptual Problem: Emergence of Systemic Responses
- The ability to mount coordinated, multi-component stress responses suggests a level of system-wide organization that is challenging to account for through random processes.
- Explaining the origin of these integrated adaptive responses without invoking guided mechanisms remains unresolved.
3. Rapid Response Mechanisms
Early cells needed to respond quickly to sudden environmental changes to ensure survival.
Conceptual Problem: Development of Timely Reactions
- The emergence of systems capable of rapidly detecting and responding to environmental shifts poses significant challenges to undirected explanations.
- It is unclear how such time-sensitive response mechanisms could evolve without pre-existing organizational principles.
4. Adaptive Membrane Modifications
Primitive cells had to modify their membranes in response to environmental stressors.
Conceptual Problem: Origin of Dynamic Membrane Adaptation
- The ability to dynamically alter membrane composition in response to environmental cues suggests a sophisticated level of cellular control.
- Accounting for the origin of this adaptive capability through unguided processes presents a significant challenge.
5. Metabolic Flexibility
Early life forms needed to adjust their metabolism in response to changing resource availability.
Conceptual Problem: Emergence of Metabolic Adaptability
- The development of systems capable of switching between different metabolic pathways based on environmental conditions is difficult to explain through undirected processes alone.
- It remains unclear how such flexible metabolic systems could emerge without a guiding mechanism to optimize their function.
These unresolved challenges highlight the complexity inherent even in the most primitive environmental sensing and adaptation mechanisms. The level of coordination, specificity, and adaptability observed in these early systems raises significant questions about their origins. Current naturalistic explanations struggle to adequately account for the emergence of such sophisticated biological machinery without invoking guided or pre-established processes.
22.4. Regulation and Signaling Proteins
Signaling pathways linked to phospholipid metabolism and turnover in bacteria involve a variety of components. The best-known signaling pathways associated with bacterial lipids pertain to the two-component regulatory systems, which enable bacteria to sense and respond to environmental stimuli. Some of these systems are linked to lipid metabolism, either directly or indirectly. Below, I'm listing some of the players in these signaling pathways related to lipid turnover and homeostasis:
Key Proteins Involved
Histidine Kinase (HK) (EC 2.7.13.3): Smallest known version: 350 amino acids (estimated, varies by species)
Histidine kinases are sensor proteins that autophosphorylate in response to external signals. They play a critical role in two-component signal transduction systems by transferring the phosphate group to a response regulator.
Response Regulator (RR) (EC 2.7.7.59): Smallest known version: 200 amino acids (estimated, varies by species)
Response regulators become phosphorylated by histidine kinases and typically act as transcription factors to effect changes in gene expression. They serve as fundamental elements in bacterial signal transduction, including pathways related to lipid metabolism and turnover.
Total number of proteins in the group: 2 . Total amino acid count for the smallest known versions: 550 (estimated)
Information on Metal Clusters or Cofactors
Histidine Kinase (HK) (EC 2.7.13.3): Requires ATP and Mg²⁺ for autophosphorylation.
Response Regulator (RR) (EC 2.7.7.59): Requires Mg²⁺ for phosphoryl transfer from the histidine kinase.
Two-component signaling systems, involving histidine kinases and response regulators, enable bacteria to sense and respond to environmental stimuli, including those related to lipid metabolism and turnover.
22.5. Cardiolipin Synthase in Bacterial Lipid Metabolism
Cardiolipin synthase (Cls) is a crucial enzyme in bacterial lipid metabolism, playing a pivotal role in the synthesis of cardiolipin, a unique phospholipid essential for various cellular functions. This enzyme is particularly important in the context of bacterial energy metabolism and membrane organization.
Cardiolipin Synthase (Cls) (EC 2.7.8.41)
- Function: Catalyzes the formation of cardiolipin from phosphatidylglycerol and CDP-diacylglycerol
- Smallest known version: Approximately 450 amino acids (varies by species)
- Importance: Plays a pivotal role in the electron transport chain and overall cellular energy metabolism
Cardiolipin synthase catalyzes the final step in cardiolipin biosynthesis. The reaction involves the condensation of two phosphatidylglycerol molecules or the condensation of phosphatidylglycerol with CDP-diacylglycerol. This reaction can be represented as:
2 Phosphatidylglycerol → Cardiolipin + Glycerol or Phosphatidylglycerol + CDP-diacylglycerol → Cardiolipin + CMP. The product, cardiolipin, is a dimeric phospholipid that plays crucial roles in bacterial physiology:
- Membrane Structure: Cardiolipin contributes to the curvature and stability of bacterial membranes, particularly at the poles and septum.
- Energy Metabolism: It is enriched in membranes involved in energy transduction, such as the inner mitochondrial membrane in eukaryotes and the plasma membrane in bacteria.
- Electron Transport Chain: Cardiolipin interacts with and stabilizes many of the protein complexes involved in the electron transport chain, thus playing a pivotal role in cellular energy production.
In early life forms, the ability to synthesize cardiolipin likely provided significant advantages:
- Enhanced membrane stability, particularly important in potentially harsh primordial environments.
- Improved efficiency of energy production, allowing for more complex cellular processes and structures.
- Facilitation of cell division processes through its role in membrane curvature at the septum.
While the regulation of cardiolipin synthase is not fully understood in all bacterial species, it is generally believed to be regulated in response to growth phase, environmental stresses, and metabolic demands. The maintenance of appropriate cardiolipin levels (cardiolipin homeostasis) is crucial for optimal bacterial growth and survival. Cardiolipin synthase represents a key enzyme in bacterial lipid metabolism, with implications reaching far beyond simple membrane composition. Its role in energy metabolism, particularly in the electron transport chain, underscores the intricate relationships between lipid synthesis, membrane structure, and energy production in bacterial cells. The presence of this enzyme in early life forms suggests that sophisticated lipid metabolism was a critical development in the evolution of cellular life, enabling the complex energy-dependent processes that characterize living systems.
22.6. PhoR-PhoB Two-Component System in Bacterial Phosphate Regulation and signaling
The Pho regulon is a crucial regulatory system in bacteria that controls phosphate uptake, metabolism, and homeostasis. This system allows bacteria to sense and adapt to changes in environmental phosphate levels, particularly in phosphate-limited conditions. While the specific PhoR-PhoB system may not have been present in the earliest life forms, the ability to sense and respond to environmental nutrient levels was likely a fundamental feature of early cellular life. The PhoR-PhoB two-component system plays a pivotal role in bacterial adaptation to phosphate scarcity, illustrating the sophisticated regulatory mechanisms that have evolved in modern bacteria.
Key Components of the PhoR-PhoB System:
1. PhoR (EC 2.7.1.63)
- Function: Histidine kinase that senses phosphate levels
- Role: Part of the Pho regulon, involved in phosphate sensing and adaptation to phosphate scarcity
- Smallest known version: Approximately 430 amino acids (varies by species)
- Significance: Represents a specialized sensor for an essential nutrient (phosphate), highlighting the importance of phosphate in cellular processes
2. PhoB (EC 2.7.7.59)
- Function: Response regulator in the Pho regulon
- Role: Works with PhoR to regulate genes associated with phosphate uptake and utilization
- Smallest known version: Approximately 220 amino acids (varies by species)
- Significance: Illustrates the coupling of environmental sensing to gene regulation, a principle that likely evolved from simpler regulatory systems in early life
3. PhoU
- Function: Negative regulator of the Pho regulon
- Role: Modulates the activity of the PhoR-PhoB system
- Smallest known version: Approximately 240 amino acids (varies by species)
- Significance: Demonstrates the complexity of bacterial regulatory systems, with multiple layers of control
The PhoR-PhoB system consists of 3 key components. The total number of amino acids for the smallest known versions of these proteins is approximately 890.
Information on metal clusters or cofactors:
PhoR (EC 2.7.1.63): Requires ATP and Mg²⁺ for its kinase activity. The metal ion is crucial for phosphoryl transfer.
PhoB (EC 2.7.7.59): Does not typically require metal cofactors but is phosphorylated by PhoR on a conserved aspartate residue.
PhoU: May interact with metal ions (e.g., Zn²⁺) as part of its regulatory function, but this is not universally established across all species. The PhoR-PhoB two-component system represents a sophisticated mechanism for nutrient sensing and regulation that has evolved in modern bacteria. While this specific system may not have been present in the earliest life forms, the fundamental principles it embodies - environmental sensing, signal transduction, and gene regulation - were likely crucial for the survival and evolution of early cellular life. The importance of phosphate in this regulatory system underscores the central role of phosphate in cellular metabolism and information storage (DNA/RNA), a feature that was likely present from the very beginnings of life. The use of ATP and metal ions (particularly Mg²⁺) in the functioning of this system highlights the deep integration of energy metabolism and inorganic cofactors in cellular processes, aspects that were probably fundamental to even the most primitive forms of life.
22.7. Metabolites Involved in Bacterial Signaling
Bacteria utilize various small molecule metabolites as signaling molecules to regulate their physiological responses to environmental changes. These signaling metabolites play crucial roles in adapting bacterial metabolism, gene expression, and behavior to different conditions. While the specific molecules like (p)ppGpp and cyclic-di-GMP may not have been present in the earliest life forms, the use of small molecules for signaling and regulation was likely a fundamental feature of early cellular life. Two important signaling metabolites in modern bacteria are (p)ppGpp and cyclic-di-GMP.
Key Signaling Metabolites:
1. (p)ppGpp
- Function: Alarmone involved in the stringent response
- Role: Regulates bacterial metabolism during nutrient limitation
- Chemical structure: Guanosine tetraphosphate (ppGpp) or guanosine pentaphosphate (pppGpp)
- Significance: Represents a rapid response mechanism to stress, potentially evolving from early metabolic regulatory systems
2. Cyclic-di-GMP
- Function: Secondary messenger in bacteria
- Role: Regulates various cellular processes including biofilm formation, motility, and virulence
- Chemical structure: Cyclic diguanylate monophosphate
- Significance: Illustrates the use of cyclic nucleotides in bacterial signaling, a principle that may have roots in early cellular regulation
3. cAMP (cyclic adenosine monophosphate)
- Function: Secondary messenger in various cellular processes
- Role: Regulates carbon metabolism, virulence, and other cellular functions
- Chemical structure: Cyclic adenosine monophosphate
- Significance: One of the most universal signaling molecules, potentially present in early forms of life
Enzymes involved in the metabolism of these signaling molecules:
1. RelA/SpoT (EC 2.7.6.5): Smallest known: ~700 amino acids (varies among species)
- Function: Synthesis and hydrolysis of (p)ppGpp
- Substrates: ATP, GTP or GDP
2. Diguanylate cyclase (EC 2.7.7.65): Smallest known: ~170 amino acids (GGDEF domain)
- Function: Synthesis of cyclic-di-GMP
- Substrate: GTP
3. Phosphodiesterase (EC 3.1.4.52): Smallest known: ~180 amino acids (EAL domain)
- Function: Degradation of cyclic-di-GMP
- Substrate: Cyclic-di-GMP
The signaling metabolite enzyme group consists of 3 key enzymes. The total number of amino acids for the smallest known versions of these enzymes is approximately 1050.
Information on metal clusters or cofactors:
RelA/SpoT (EC 2.7.6.5): Requires Mg²⁺ or Mn²⁺ for catalytic activity.
Diguanylate cyclase (EC 2.7.7.65): Often contains a metal-binding site, typically for Mg²⁺ or Mn²⁺, which is essential for catalysis.
Phosphodiesterase (EC 3.1.4.52): Many require divalent metal ions (e.g., Mg²⁺, Mn²⁺, or Ca²⁺) for catalytic activity.
The use of small molecule metabolites as signaling molecules represents a fundamental aspect of cellular regulation that likely has its roots in early life forms. While the specific molecules like (p)ppGpp and cyclic-di-GMP are sophisticated signals found in modern bacteria, the principle of using metabolic intermediates or derivatives for signaling may have been present in primitive cells. The reliance on nucleotide-based signaling molecules suggests a deep connection between metabolism and regulation in cellular systems. The requirement for metal cofactors in the enzymes involved in synthesizing and degrading these signaling molecules highlights the crucial role of inorganic elements in early biochemical processes, a feature that was likely present in the earliest forms of life.
22.8. Quorum Sensing in Bacterial Communication
Quorum sensing is a sophisticated cell-to-cell communication system used by bacteria to coordinate their behavior based on population density. This process involves the production, release, and detection of small signaling molecules called autoinducers. While quorum sensing as we know it today may not have been present in the earliest life forms, the ability to respond to environmental cues and coordinate cellular behavior was likely crucial for the survival and evolution of early microorganisms. The basic principles underlying quorum sensing may have evolved from simpler chemical signaling mechanisms in primitive cellular communities.
Key Components of Quorum Sensing:
1. N-acyl homoserine lactones (AHLs)
- Function: Autoinducer molecules in Gram-negative bacteria
- Role: Mediate intraspecies communication
- Chemical structure: Lactone ring with an acyl side chain of varying length
- Significance: AHLs represent a class of signaling molecules that allow bacteria to communicate within their own species, potentially evolving from simpler metabolic byproducts that gained signaling functions
2. Autoinducer-2 (AI-2)
- Function: Universal autoinducer for interspecies communication
- Role: Regulates diverse bacterial behaviors across species
- Chemical structure: Furanosyl borate diester (in Vibrio species)
- Significance: AI-2 is considered a more universal signal, potentially representing an evolutionarily older form of bacterial communication that spans across different species
3. Oligopeptides
- Function: Autoinducer molecules in Gram-positive bacteria
- Role: Mediate species-specific quorum sensing
- Chemical structure: Short peptides, often cyclized or modified
- Significance: Peptide-based signaling may have evolved from early peptide synthesis mechanisms, representing an alternative to small molecule-based communication
Enzymes involved in quorum sensing:
1. LuxI-type synthases (EC 2.7.13.3): Smallest known: ~190 amino acids (varies among species)
- Function: Synthesis of AHL molecules
- Substrate: S-adenosylmethionine and acyl-acyl carrier protein
2. LuxS (EC 5.3.1.2): Smallest known: ~160 amino acids (Escherichia coli)
- Function: Synthesis of AI-2 precursor
- Substrate: S-ribosylhomocysteine
The quorum sensing component group consists of 2 key enzymes. The total number of amino acids for the smallest known versions of these enzymes is approximately 350.
Information on metal clusters or cofactors:
LuxI-type synthases (EC 2.7.13.3): Do not typically require metal cofactors but use S-adenosylmethionine as a substrate.
LuxS (EC 5.3.1.2): Contains a Fe²⁺ ion at its active site, which is crucial for its catalytic activity.
While the complex quorum sensing systems observed in modern bacteria may not have been present in the earliest life forms, the underlying principles of chemical signaling and coordinated behavior were likely fundamental to the survival and evolution of early microbial communities. The diversity of signaling molecules (AHLs, AI-2, oligopeptides) and the enzymes involved in their synthesis suggest that these communication systems evolved from simpler metabolic processes. The presence of metal cofactors in some quorum sensing enzymes, such as LuxS, highlights the importance of inorganic elements in early biochemical processes. These basic signaling mechanisms may have provided a foundation for the development of more complex cellular communication systems as life evolved.
22.9. Response Regulators and Kinases in Quorum Sensing
Quorum sensing systems in bacteria often utilize two-component signal transduction pathways to detect and respond to autoinducer molecules. These pathways typically involve sensor kinases and response regulators that work together to translate extracellular signals into changes in gene expression. While these sophisticated systems may not have been present in the earliest life forms, they represent fundamental mechanisms of cellular signaling that likely evolved from simpler precursors. The LuxPQ-LuxU-LuxO system in Vibrio species is a well-studied example of such a pathway, playing a crucial role in AI-2-mediated quorum sensing.
Key Components of the LuxPQ-LuxU-LuxO System:
1. LuxQ (EC 2.7.13.3): Smallest known: ~850 amino acids (Vibrio harveyi)
- Function: Sensor histidine kinase
- Role: Detects AI-2 and initiates signal transduction
- Structure: Membrane-bound protein with periplasmic sensor domain and cytoplasmic kinase domain
- Significance: Acts as the initial sensor in the AI-2 quorum sensing pathway, translating extracellular signals into intracellular responses
2. LuxU (EC 2.7.13.3): Smallest known: ~110 amino acids (Vibrio harveyi)
- Function: Phosphotransfer protein
- Role: Transfers phosphate from LuxQ to LuxO
- Structure: Small cytoplasmic protein with a conserved histidine residue
- Significance: Serves as an intermediate in the phosphorelay system, allowing for additional regulation points in the signaling pathway
3. LuxO (EC 2.7.13.3): Smallest known: ~450 amino acids (Vibrio harveyi)
- Function: Response regulator
- Role: Regulates gene expression in response to AI-2 levels
- Structure: Cytoplasmic protein with receiver domain and DNA-binding output domain
- Significance: Acts as the final effector in the pathway, directly modulating gene expression based on quorum sensing signals
The LuxPQ-LuxU-LuxO system consists of 3 key components. The total number of amino acids for the smallest known versions of these proteins is approximately 1410.
Information on metal clusters or cofactors:
LuxQ (EC 2.7.13.3): Requires ATP for its kinase activity. May also require metal ions (e.g., Mg²⁺) for structural stability and catalytic function.
LuxU (EC 2.7.13.3): Does not require specific cofactors but is phosphorylated on a conserved histidine residue.
LuxO (EC 2.7.13.3): Requires ATP for its function as a σ⁵⁴-dependent transcriptional activator. May also require metal ions for structural integrity.
The LuxPQ-LuxU-LuxO system exemplifies the sophisticated signal transduction mechanisms used in bacterial quorum sensing. This phosphorelay system enables bacteria to precisely sense and respond to changes in population density, coordinating group behaviors crucial for survival and virulence. Understanding these pathways is essential for developing strategies to manipulate quorum sensing in various applications, from controlling bacterial infections to engineering beneficial microbial behaviors in biotechnology and environmental management.
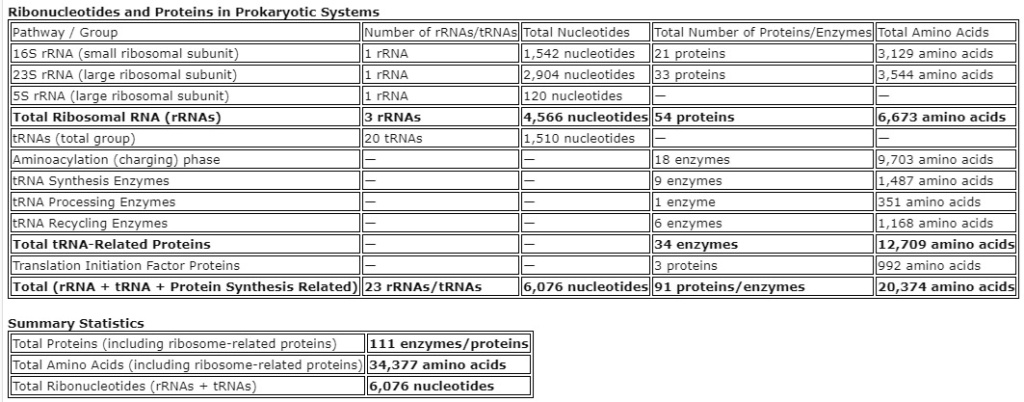
22.10. Ribosomal Signaling Pathways
The GTPase-Dependent Signaling Pathways: GTPases like EF-Tu and EF-G are crucial in ribosome function, facilitating various stages of translation, including tRNA selection and translocation. These molecules are essential in prokaryotic ribosomes. Early GTPase-like mechanisms would have been vital for facilitating accurate and efficient translation, ensuring correct tRNA-mRNA codon matching and error-free ribosome movement along the mRNA strand.
The Stress Response Pathways: Prokaryotes have stress response mechanisms that modulate ribosome function under different environmental conditions. Primitive stress response pathways would have been important for adapting ribosome activity in response to environmental changes or cellular stress, ensuring continued protein synthesis under suboptimal conditions.
The Ribozyme Activity: Ribozymes (RNA molecules with catalytic activity) likely played a crucial role in early protein synthesis before the evolution of protein-based enzymes. In early ribosomes, ribozymes may have facilitated critical reactions in protein synthesis, compensating for the absence of protein-based enzymes. The peptidyl transferase center of the ribosome, which is largely RNA-based, is a potential remnant of this early ribozyme activity.
Unresolved Challenges in Signaling Pathways and Early Ribosome Function
1. GTPase-Dependent Signaling Pathways
The complexity and specificity of GTPases like EF-Tu and EF-G in ribosome function present significant challenges for explaining their origin through unguided processes. These molecules play crucial roles in various stages of translation, including tRNA selection and translocation.
Conceptual problems:
- Molecular Precision: GTPases require highly specific structures to interact correctly with ribosomes, tRNAs, and mRNAs. The origin of this precision without a guided process remains unexplained.
- Functional Interdependence: GTPases and ribosomes must function together seamlessly. The co-emergence of these intricate, interrelated systems poses a significant explanatory challenge.
- Energy Coupling: GTPases harness energy from GTP hydrolysis to drive conformational changes. The origin of this sophisticated energy coupling mechanism is difficult to account for without invoking guidance.
Open questions:
- How could the complex structures of GTPases, capable of specific interactions with ribosomes, emerge spontaneously?
- What mechanisms could explain the simultaneous emergence of functionally interdependent GTPases and ribosomal components?
- How did the precise GTP binding and hydrolysis mechanisms, crucial for GTPase function, originate?
2. Stress Response Pathways
Prokaryotic stress response mechanisms that modulate ribosome function under varying environmental conditions present intricate regulatory systems challenging to explain through unguided processes.
Conceptual problems:
- Sensor Complexity: Stress response systems require sophisticated molecular sensors to detect environmental changes. The spontaneous emergence of such precise detection mechanisms is difficult to explain.
- Signal Transduction: These pathways involve complex cascades of molecular interactions to transmit signals from sensors to effectors. The origin of these intricate signaling networks poses significant challenges to unguided explanations.
- Regulatory Precision: Stress responses must be finely tuned to maintain cellular homeostasis. The emergence of such precise regulatory control without guidance is problematic.
Open questions:
- How could complex molecular sensors, capable of detecting specific environmental stressors, emerge spontaneously?
- What mechanisms could explain the origin of intricate signal transduction cascades linking sensors to ribosomal modulation?
- How did the fine-tuned regulatory mechanisms, crucial for appropriate stress responses, come into existence without guided processes?
3. Ribozyme Activity
The concept of ribozymes playing a crucial role in early protein synthesis before the emergence of protein-based enzymes presents several challenges and open questions.
Conceptual problems:
- Catalytic Efficiency: RNA's catalytic capabilities are generally less efficient than those of protein enzymes. Explaining how early ribozymes could have catalyzed reactions efficiently enough to support life is challenging.
- Structural Stability: RNA molecules are less stable than proteins, particularly in early Earth conditions. The maintenance of functional ribozyme structures in a prebiotic environment is difficult to account for.
- Sequence Specificity: Functional ribozymes require specific sequences. The spontaneous emergence of these sequences in a prebiotic soup lacks a clear explanatory mechanism.
Open questions:
- How could ribozymes with sufficient catalytic efficiency to support early life have emerged spontaneously?
- What mechanisms could have stabilized functional ribozyme structures in the harsh conditions of early Earth?
- How did the specific sequences required for functional ribozymes arise in a prebiotic environment?
- What processes could explain the transition from ribozyme-based catalysis to the protein-dominated enzymatic systems observed in modern cells?
4. Peptidyl Transferase Center (PTC)
The RNA-based peptidyl transferase center of the ribosome, potentially a remnant of early ribozyme activity, presents unique challenges in explaining its origin.
Conceptual problems:
- Structural Complexity: The PTC has a highly specific structure crucial for its function. Explaining the spontaneous emergence of this complex RNA structure is problematic.
- Catalytic Precision: The PTC catalyzes peptide bond formation with high specificity and efficiency. The origin of this precise catalytic ability in an RNA structure without guidance is challenging to explain.
- Functional Integration: The PTC must work in concert with other ribosomal components and factors. The co-emergence of these interdependent elements poses significant explanatory challenges.
Open questions:
- How could the complex, specific structure of the PTC have emerged spontaneously?
- What mechanisms could explain the origin of the PTC's precise catalytic abilities?
- How did the PTC co-emerge and integrate functionally with other ribosomal components and factors?
- What processes could account for the transition from a purely RNA-based PTC to the RNA-protein hybrid structure observed in modern ribosomes?
In conclusion, the origin of these intricate signaling pathways and ribosomal functions presents significant challenges to unguided explanations. The complexity, specificity, and interdependence of these systems raise profound questions about their emergence. Further research is needed to address these open questions and challenges, potentially leading to new insights into the fundamental nature of these crucial biological processes.
1.6.2. The interdependence and integrated complexity of the Ribosomal Codes Necessary for Life to start[/b]
In the earliest stages of life, the emergence of functional ribosomes was essential for cellular processes, particularly protein synthesis. Several interdependent signaling pathways and molecular codes form a complex network ensuring proper ribosome function. This integrated system was crucial for the origin of life.
1. The Genetic Code:
- Operates with: The tRNA Code, The Translation Code
- Signaling Pathways: GTPase-Dependent Signaling Pathways
- Description: Crucial for translating mRNA into proteins. It works with the RNA Code for rRNA production, the tRNA Code for accurate translation, and the Translation Code to regulate protein synthesis. GTPase-dependent pathways ensure translation accuracy.
2. The Protein Folding Code:
- Operates with: The Protein Phosphorylation Code
- Signaling Pathways: The Ubiquitin-Proteasome System, The Autophagy Pathways
- Description: Ensures proper folding of newly synthesized proteins, including those critical for ribosomal function. Interacts with the tRNA Code for correct protein folding and the Protein Phosphorylation Code to modulate protein activity. The Ubiquitin-Proteasome System and Autophagy Pathways manage misfolded proteins and ribosomal component recycling.
3. The Ribozyme Activity:
- Operates with:The Ribosomal Code
- Description: Crucial in early ribosomes before protein-based enzymes emerged. Catalyzed critical reactions for protein synthesis, including peptide bond formation in the ribosome's peptidyl transferase center. Worked with the RNA Code for rRNA production and the Ribosomal Code for proper ribosome assembly.
4. The tRNA Code:
- Operates with: The Genetic Code, The Ribosomal Code
- Signaling Pathways: GTPase-Dependent Signaling Pathways
- Description: Essential for translating genetic information into proteins. Ensures correct amino acid delivery to ribosomes based on mRNA codon sequences. Operates with the Genetic Code for accurate translation and the Ribosomal Code for protein synthesis. GTPase-dependent pathways control tRNA accuracy and efficiency during translation.
These ribosomal codes and signaling pathways are deeply interconnected, forming an integrated system enabling protein synthesis, proper folding, and ribosome assembly. This intricate cooperation was essential for early cells to synthesize proteins, maintain ribosome integrity, and adapt to varying environmental conditions. Understanding the interplay of these codes provides crucial insights into the complexity required for the origin of life.
Key Implications:
1. The simultaneous presence and coordination of these complex systems raise significant questions about their origin and development.
2. Each code represents a sophisticated molecular system, highlighting the intricate nature of even the most basic cellular processes.
3. The importance of ribozyme activity lends support to the RNA world hypothesis, suggesting a crucial role for RNA in early life processes.
4. The interdependence of these codes suggests that they may have co-emerged, posing challenges for step-wise explanations of life's origin.
Unresolved Challenges in the Integrated Complexity of Ribosomal Codes Necessary for Life to Start
1. The Genetic Code and Its Early Functionality
The genetic code, responsible for translating mRNA into proteins, is deeply integrated with other molecular codes and signaling pathways. For life to emerge, the genetic code had to function flawlessly in concert with the RNA Code, the tRNA Code, and the Translation Code. In early cells, this intricate system of codes would have had to coemerge fully operational, as any malfunction in translation would lead to defective proteins, hindering cell viability.
Conceptual problem: Immediate Functional Integrity
- How could the genetic code emerge fully integrated with the other molecular codes without prior guidance or error correction?
- The simultaneous operation of multiple interdependent codes in protein synthesis presents a major challenge for explanations based on spontaneous, unguided origins.
2. Protein Folding Code and Molecular Accuracy
Correct protein folding is critical for proper cellular function. The protein folding code operates alongside the tRNA Code and Protein Phosphorylation Code to ensure that newly synthesized proteins assume the correct three-dimensional structures. Early ribosomes would have needed accurate protein folding mechanisms to avoid the accumulation of misfolded or nonfunctional proteins.
Conceptual problem: Ensuring Folding Accuracy
- How did early cells ensure correct protein folding without advanced molecular chaperones or the sophisticated systems found in modern cells?
- The integrated complexity between the protein folding code and other systems suggests an immediate, functional protein synthesis mechanism was required from the start.
3. RNA Code and Ribosomal Assembly
The RNA code governs the synthesis and processing of rRNA, which is crucial for ribosome assembly and function. Without proper rRNA, ribosomes would not form correctly, preventing effective protein synthesis. For early ribosomes to function, the RNA Code had to interact seamlessly with the genetic and ribosomal codes, ensuring proper rRNA structure and integration.
Conceptual problem: Early Ribosome Assembly
- How did the RNA code emerge and integrate with the ribosomal machinery, without the guiding processes seen in more advanced cells?
- The high level of coordination needed for rRNA production and processing challenges unguided origin explanations.
4. tRNA Code and Translation Fidelity
The tRNA code ensures the accurate matching of tRNA molecules with mRNA codons during protein synthesis. The interaction between the tRNA Code and the Genetic Code was crucial for early translation, as errors would result in dysfunctional proteins. This interdependence highlights the need for an error-minimizing mechanism in early life forms.
Conceptual problem: Translation Accuracy
- How did the tRNA Code develop the precision needed to accurately translate mRNA sequences in early cells, without established error-correction systems?
- The emergence of this code poses a challenge for unguided scenarios, as even small translation errors could be catastrophic.
5. DNA Repair/Damage Codes and Genetic Stability
Genetic stability is essential for producing functional ribosomal components and accurate mRNA. DNA repair and damage codes would have been vital to prevent the degradation of genetic material. Without these codes, early cells would have been vulnerable to errors in DNA replication and transcription, threatening their survival.
Conceptual problem: Early DNA Integrity
- How did early cells protect genetic material from damage and ensure the integrity of ribosomal and other protein-producing genes?
- The requirement for sophisticated DNA repair mechanisms introduces another layer of complexity that needs addressing in unguided origin scenarios.
6. The Ribosomal Code and Integrated Functionality
The ribosomal code encompasses the functions of ribosomal RNA (rRNA) and ribosomal proteins, ensuring the proper assembly and operation of the ribosome. It integrates closely with the genetic, RNA, and tRNA codes, all of which are essential for accurate protein synthesis. Any disruption in these interactions would compromise the entire system.
Conceptual problem: Coordinated Emergence of Ribosome Functionality
- How did ribosomal components coemerge and function correctly without prior coordination mechanisms?
- The interdependent nature of ribosomal assembly and function challenges the notion of an unguided origin.
7. Transcription Factor Binding Code and Gene Expression Regulation
Regulation of gene expression is critical for the production of ribosomal components and other essential proteins. Early cells would have needed a precise transcription factor binding code to ensure that genes involved in protein synthesis were expressed at the right times. In modern cells, this is a highly regulated process, dependent on numerous factors.
Conceptual problem: Early Gene Expression Control
- How did early cells regulate the expression of genes related to ribosome production and protein synthesis without advanced regulatory systems?
- The complexity of gene regulation presents another hurdle for models suggesting spontaneous origins.
8. The Protein Phosphorylation Code and Ribosomal Function Regulation
Phosphorylation plays a critical role in regulating protein function, including ribosomal proteins. The protein phosphorylation code interacts with the ribosomal code and protein folding code, ensuring that ribosomal components are functional and responsive to cellular signals. This code would have been necessary to modulate ribosome activity in response to the cell's needs.
Conceptual problem: Phosphorylation-Based Regulation
- How did early cells develop phosphorylation-based regulatory mechanisms without a pre-existing system to control protein activity?
- The need for a fully functional regulatory system in early life further complicates unguided origin models.
9. Membrane Code and Ribosomal Localization
The membrane code relates to the assembly and function of cellular membranes, including the localization and transport of ribosomal components. Ribosomes had to be properly localized within the cell to ensure efficient protein synthesis. Membrane integrity and functionality were critical for early cellular operations, making this code essential.
Conceptual problem: Membrane and Ribosome Coordination
- How did early cells ensure the correct localization and transport of ribosomal components without advanced cellular machinery?
- The need for a functioning membrane code alongside ribosomal activity introduces additional complexity that challenges unguided origin explanations.
The integrated complexity and interdependence of these molecular codes raise numerous unresolved questions about how life could have emerged in a natural, unguided process. Each code relies on the functionality of others, making it difficult to conceive how they could have coemerged without a coordinated system. Addressing these challenges requires a reevaluation of current models and an exploration of alternative explanations for the origin of life.
Last edited by Otangelo on Sat Oct 12, 2024 4:27 pm; edited 2 times in total