Cytoskeletal control of early mammalian development
Hui Yi Grace Lim Cytoskeletal control of early mammalian development 29 April 2021 1
The cytoskeleton — comprising actin filaments, microtubules and intermediate filaments — serves instructive roles in regulating cell function and behaviour during development. The cytoskeleton allows cells to integrate internal and external signals to perform essential functions at the cellular and tissue levels. This filamentous network functions as a sensor of biochemical and mechanical changes in the external environment of cells and transmits that information internally to effect changes in gene expression and cellular behaviour. At the same time, regulating cytoskeletal organization and function can generate mechanical force and activate biochemical signalling pathways to modify the extracellular space. This ability of the cytoskeleton to coordinate cellular functions and integrate diverse signals is especially important in the context of development, where cells must communicate and respond to their environment to robustly pattern a tissue and eventually the entire organism . Therefore, studying how the cytoskeleton controls key processes in development, including cell fate specification and morphogenesis, is a major area of interest in cell and developmental biology. Eukaryotic cells contain three main classes of cytoskeletal filaments: actin filaments (also referred to as F-actin), microtubules and intermediate filaments. Although key structural and functional differences set these filament classes apart, all three share important features: (1) they are composed of repeated subunits; (2) they exhibit dynamic assembly and disassembly, regulated by a diverse group of filament-binding proteins; and (3) they are connected to the cell membrane at adhesion complexes, and to the cell nucleus via the linker of nucleoskeleton and cytoskeleton (LINC) complex . These features allow the cytoskeleton to undergo dramatic reorganization within a short window of time to cope with changing cellular requirements, and to respond rapidly to both internal and external signals via their links to the nucleus and cell cortex. As a result, the cytoskeleton has been implicated in virtually all cellular processes, including cell division , migration , polarization and adhesion . Importantly, extensive crosstalk occurs between the three cytoskeletal systems, mediated by linker proteins, regulation of common biochemical pathways or direct mechanical interactions. Therefore, investigating the roles of actin filaments, microtubules and intermediate filaments in development cannot be done in isolation, but must be explored in the wider context of an integrated network of cytoskeletal filaments. The preimplantation mouse embryo is a leading model of mammalian development due to its amenability to genetic manipulation, ex utero culture, high-resolution live imaging and experimental perturbations. Over the course of 3–4 days following fertilization, the single-cell zygote undergoes multiple rounds of divisions to form a 32-cell to 64-cell blastocyst, taking the form of an outer trophectoderm layer surrounding an inner cell mass (ICM) and a fluid-filled cavity. Trophectoderm cells ultimately contribute to extraembryonic tissues such as the placenta, whereas cells in the ICM form the fetus and primitive endoderm. This trophectoderm–ICM lineage segregation is the first cell fate specification event in mammalian development. Organizing the morphogenetic changes to sculpt the final structural form of the blastocyst, while concurrently regulating the molecular pathways that specify cell fates, requires precise mechanisms that ensure the fidelity of this process. The genetic and molecular underpinnings of embryogenesis have long been a subject of intense study, revealing key insights into the biochemical and mechanical pathways that guide this developmental process. In addition, early mammalian development relies on cytoskeletal components, both in their regulation of signalling pathways and in the mechanical properties they confer that can influence cell shape and cavity formation. A thorough exploration of cytoskeletal roles in early embryos is therefore necessary, and these findings need to be integrated with our understanding of the genetic and molecular players during development to build a cohesive picture of early embryogenesis. Here we review the roles of the three major cytoskeletal filament systems during the initial stages of mammalian embryonic life. We summarize the current understanding of cytoskeletal functions driving key morphogenetic events in the preimplantation mouse embryo, organized into four broad sections in the order of their occurrence during development: compaction and polarization, inner cell versus outer cell allocation, fate specification and blastocyst formation. In each section, we highlight crosstalk between the cytoskeletal systems and discuss novel cytoskeletal structures that are thus far unique to the early embryo and could not have necessarily been predicted a priori from studies of non-mammalian embryos or mammalian cells grown in vitro.
Compaction and polarization
During preimplantation development, the first prominent change in cell morphology occurs when cells of the embryo, typically referred to as blastomeres, undergo compaction at the 8-cell stage16.
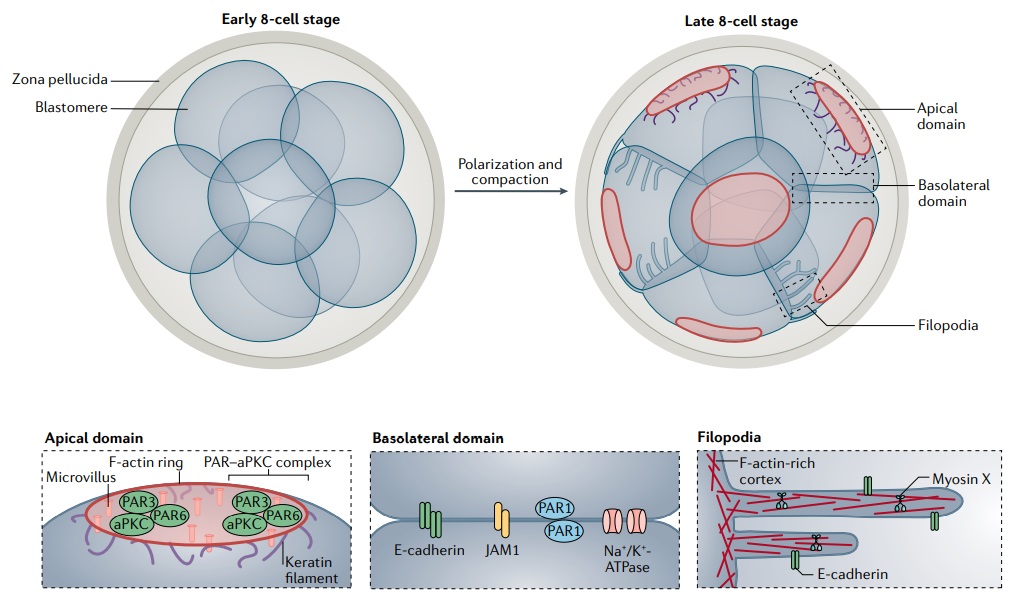
Key structures formed by the cytoskeleton during embryo compaction and polarization.
At the 8-cell stage, blastomeres flatten against their neighbours and increase cell–cell contacts to form a compacted morula. At the same time, the embryo acquires apical–basal polarity via the enrichment of F-actin, microvilli and PAR–aPKC proteins in the apical cortex, and the localization of E-cadherin, JAM1, PAR1 and Na+ /K+ -ATPase to the basolateral membranes. Keratin filaments also start to assemble in a subset of cells, localizing to the apical cortex via interactions with the F-actin-rich apical domain. In parallel, some cells in the embryo extend actin-based filopodial protrusions over the apical surfaces of their neighbours, which exert forces that draw these adjacent cells closer to one another to facilitate compaction. Therefore, by the 8-cell stage, the major components of the cytoskeleton acquire a distinct polarized organization, and some, including keratin filaments and filopodia, additionally display prominent cell-to-cell variability.
Initially rounded blastomeres increase cell–cell contact with their neighbours and flatten their apical surfaces, producing cells with a clear apical and basal domain. Concurrently, blastomeres polarize along their radial axis, accumulating members of the PAR–aPKC complex, including PAR3, PAR6 and PKCζ on their apical surface, and E-cadherin, PAR1 and JAM1 along their basal membranes(Fig. above). This association between compaction and polarization remains incompletely understood as it is difficult to perturb the two processes independently. For example, premature activation of PKC22,23 and blocking the intercellular engagement of E-cadherin molecules are two perturbations widely used to accelerate or interfere with embryo compaction, but these methods also influence apical–basal cell polarization. Isolated blastomeres lacking E-cadherin or cellular contacts remain capable of polarizing to form an apical domain-like structure on one end of the cell, suggesting that polarization could occur cell autonomously. This indicates that compaction is not an absolute prerequisite for polarization, but it remains open to what extent compaction facilitates the polarization process by mediating the formation of cell–cell interactions to establish the apical and basal domains of the embryo. The cytoskeleton is a primary regulator of cell shape due to its intrinsic mechanical properties allowing it to resist deformation. At the same time, cytoskeletal contractility can generate forces on the surface of the cell to influence cell morphology. During the early stages of embryogenesis, changes in cell and embryo morphology are accompanied by rearrangements in the cytoskeleton. Hence, these early compaction and polarization events have been tightly connected to the cytoskeleton and its dynamics.
Compaction and polarization are associated with cytoskeletal rearrangements.
At the time of compaction, a marked redistribution of microtubules occurs, from an even cortical localization in early blastomeres to a pronounced asymmetry following compaction, accumulating within the apical region of compacted blastomeres. At the same time, an opposite asymmetry in a stabler pool of acetylated microtubules is set up. Like microtubules, actin filaments are also enriched cortically during compaction, coupled with an apical reorganization of finger-like protrusions composed of linear arrays of actin filaments known as microvilli. This restriction of microvilli to the apical domain is likely controlled by spatial regulation of the phosphorylation of ezrin — a protein acting as a linker between the plasma membrane and the actin cytoskeleton — as shown in epithelial cells, although the precise roles of microvilli in the embryo remain unknown.
Following this initial apical accumulation of actin, keratin intermediate filaments also become enriched at the apical membrane (Fig. above). Disrupting the apically polarized pool of actin filaments in turn blocks the apical localization of keratin filaments, resulting in a more homogeneous keratin filament distribution throughout these early blastomeres. Therefore, all three major cytoskeletal systems undergo extensive reorganization early in development, setting up the first apical–basal cell asymmetries polarizing the early embryo. Cytoskeleton-generated mechanical forces driving compaction. The morphological changes occurring during embryo compaction are regulated in part by actomyosin driven tensile forces generated at the apical cell cortex. These forces can be probed by micropipette aspiration of 8-cell mouse embryos, revealing a role for increasing surface tension in driving embryo compaction. This is regulated by a complex interplay of actin regulators that is still not well understood: inhibiting actin polymerization with cytochalasin D blocks embryo compaction, but decreasing levels of cofilin 1, an actin-binding protein that also blocks actin polymerization, accelerates the compaction process. One driver of forces generated during compaction is filopodia that form specifically during the 8-cell to 16-cell stage of development (Fig. above). Some cells extend these filopodia containing actin, E-cadherin and myosin X over the apical surface of their neighbouring cells during embryo compaction. These filopodia were under tension, and disrupting filopodia by laser ablation or downregulation of filopodial components, including E-cadherin and myosin X, was sufficient to block compaction, resulting in more segregated, rounder cells. Conversely, forcing premature formation of filopodia by myosin X overexpression accelerated compaction. Together, these results suggest that the extension of filopodia over the surface of neighbouring cells facilitates the cell shape changes underlying compaction. Consistent with the role of filopodia in promoting compaction, cells retract their filopodia just before division, when they decompact and become spherical in preparation for mitosis. Retraction of filopodia could serve as a trigger for mitosis, although a role for filopodia in regulating cell division in the mouse embryo has not yet been reported. Alternatively, mitotic onset could regulate retraction of filopodia, given that mitotic cells undergo significant cytoskeletal reorganization. In other systems, filopodia have been shown to mediate the transport of signalling molecules such as Wnt in zebrafish embryos and SHH in chick embryos, both of which can act as morphogens to pattern the developing tissue. Future work investigating a potential role for filopodia in directional transport of signalling molecules between cells of the early mouse embryo could reveal parallel mechanisms between these systems. This would demonstrate how cytoskeletal structures can integrate cell shape changes with the regulation of the timing of cell division and lineage specification using biochemical cues. Beyond actin, it is plausible that more extensive cytoskeletal crosstalk between the three filament systems drives compaction in the 8-cell embryo. For example, the asymmetries in microtubule organization during compaction suggest that microtubules could interact with actin, via direct connections or by mediating intercellular trafficking to deliver proteins that differentially regulate actin organization to the apical membrane. This is in line with work demonstrating the importance of protein intracellular transport in driving compaction in the mouse embryo, along with a study showing transport of E-cadherin along microtubule bridges of the mouse embryo, although the latter has not been directly tested in the context of compaction. Moreover, inhibiting microtubule polymerization increased the rate of compaction in early 8-cell embryos. Another possibility for microtubule regulation of compaction is via cell shape changes, akin to the microtubule-driven flattening of differentiated mouse keratinocytes in the mature epidermis, for example. However, whether microtubules in the mouse embryo indeed have a mechanical role in regulating cell shape during compaction remains an important open question. Lastly, keratin filaments, too, anchor to the apical cell cortex following polarization, where they are known to stabilize actin filaments at later stages of development. It remains to be investigated whether the mechanical support conferred by keratin filaments is also involved in establishing a compacted embryo containing the first polarized cells in development.
Actin regulation of apical polarity.
The establishment of apical polarity within the developing mouse embryo has recently been dissected in detail, and involves a complex interplay of actin filaments, polarity proteins and upstream molecular regulators. This polarization occurs in two main steps: first, an initiation phase whereby phospholipase C (PLC)-driven RHOA activation at the apical cell cortex promotes the apical enrichment of actin filaments and myosin II activity, followed by a second, maturation phase that recruits polarity proteins, including PAR3, PAR6 and aPKC, to establish a mature apical domain51,52. Interestingly, this two-step mechanism of apical polarization was also recently found to occur within human embryos, similarly regulated by PLC activation, suggesting that this process could be evolutionarily conserved among early mammalian embryos53,54. During the latter maturation phase, actin remodelling, downstream of the activity of the transcription factors TFAP2C and TEAD4, was shown to promote cooperative recruitment of apical polarity proteins such as ezrin55. Indeed, double knockdown of TFAP2C and TEAD4 reduced levels of actin regulators, including the ARP2/3 complex component ARPC1B, and completely blocked embryo polarization at the 8-cell stage. In addition to cooperative recruitment, which is driven by a positive-feedback mechanism promoting increasing rates of ezrin binding to the apical membrane, RHOA-dependent lateral mobility of ezrin is also required to establish the size of the rounded apical patch of accumulated polarity proteins that forms across the contact-free surface of these early blastomeres. The extent of apical polarization is robustly controlled by the balance between these two opposing mechanisms: actin-mediated cooperative recruitment of polarity factors alone without RHOA activation would generate only small abnormal apical protrusions, whereas lateral mobility by itself cannot establish proper apical–basal polarization, resulting in excessive distribution of proteins across the cell surface. Although a detailed mechanism for how actin dynamics can function to recruit and organize the apical domain remains unknown, these studies highlight the central role of the cytoskeleton in establishing the first polarized cells in development.
Cytoskeleton in the regulation of cell division patterns.
Eight-cell-stage blastomeres can divide with different angles relative to the surface of the embryo or apical–basal cell axis. Many studies have often referred to divisions occurring along the surface of the embryo and producing two outer daughter cells as ‘symmetric’, whereas divisions perpendicular to the embryo surface producing an outer daughter cell and an inner daughter cell have been referred to as ‘asymmetric’ (Fig. a).

Cytoskeletal heterogeneities between cells that promote inner cell versus outer cell segregation.
a | From the 8-cell stage, cells can divide with various angles, resulting in more spatially symmetric divisions that allocate two daughter cells to the outer surface of the embryo, or more asymmetric divisions that segregate daughters into outer and inner positions. The grey shapes represent the rest of the cells of the embryo.
b | Some cell divisions during the transition from the 8-cell stage to the 16-cell stage display tilted angles and can be followed by the internalization of one of the daughter cells to form the inner cell mass.
c | Cytoskeletal differences between sister cells that favour inner segregation events versus outer segregation events. Inheritance of keratin filaments stabilizes the F-actin-rich apical cell cortex and hinders cell internalization. By contrast, cells that do not inherit keratin filaments do not seem to repolarize their apical cell cortex and undergo apical constriction to form the inner cell mass, a process dependent on myosin II-mediated cortical tension. In parallel, the microtubule bridge connecting the two sister cells is retained after division, and it promotes increased transport of RAB11A-positive vesicles carrying E-cadherin to the cell membrane of the internalizing cell. This is essential to establish adhesion with basolateral neighbours as the cell internalizes and acquires a larger basolateral contact region compared with the sister cell remaining in the outer part of the embryo.
A third category of ‘intermediate’ divisions has also been identified as divisions displaying tilted angles, whereby two outer daughter cells are initially generated, but one of them gradually internalizes via apical constriction (Fig. b above). It remains debated whether the orientation of these divisions occurs stochastically or whether it is prepatterned by heterogeneities in the molecular or cytoskeletal organization of the parental cells before division. One possible mechanism regulating division orientation is cell shape. It has long been postulated that cell shape can orient the mitotic spindle, a phenomenon described by Hertwig’s long-axis rule whereby cells divide along their long axis (or line of tension). This could explain the bias towards symmetric divisions occurring within the 16-cell embryo, where outer cells acquire a more elongated morphology extending across the surface of the embryo. This is also consistent with higher levels of myosin II phosphorylation and likely also of cortical tension at the apical surface of the 16-cell-stage embryo when compared with the preceding stage. However, cells in the 8-cell embryo display a diversity of division angles, despite having similar cellular morphologies. This suggests that the early mouse embryo could use distinct mechanisms during the divisions occurring from the 8-cell stage to the 16-cell stage. In various cellular and organismal contexts, the cytoskeleton plays a major role in the control of division orientation. For example, asymmetric division within the Caenorhabditis elegans zygote involves pulling forces exerted by the cell cortex on astral microtubules growing from centrosomes, mediated by cortical force regulators that position the mitotic spindle within the dividing cell. By contrast, the mechanisms controlling division orientation in the early mouse embryo are less well understood. The mouse embryo does not have functional centrosomes serving as the main microtubule organizing centres (MTOCs) until blastocyst stages, and is thought to lack clear astral microtubule arrays.
Therefore, division patterns could instead involve actin-driven mechanisms as seen in the mouse oocyte, which also lacks centrosomes. During meiosis I, the meiotic spindle migrates from the centre of the oocyte, where the spindle is formed, to the periphery of the cell to generate the polar body and produce a mature oocyte. This spindle positioning is controlled by a formin 2-dependent dense cytoplasmic F-actin meshwork that encases the mitotic spindle, exerting pulling forces via myosin II at the actin-rich cell cortex. It was recently shown that a cytoplasmic F-actin meshwork is also present in all cells of the early mouse embryo, yet it remains unknown whether spindle positioning in the embryo is similarly regulated by this F-actin meshwork, and how it may influence division patterns and inner cell–outer cell allocation. Some studies point to an important role of apical polarity proteins in positioning the mitotic spindle to regulate division orientation during development. This is the case in asymmetric cell divisions in a number of non-mammalian systems, including Drosophila melanogaster neuroblasts and the C. elegans zygote. Given that apical–basal polarization is already established at the 8-cell stage in the mouse embryo, it has been suggested that the apical domain could control spindle orientation also in these early mammalian blastomeres by accumulating MTOC components at the apical pole.
This could potentially be mediated by mechanical coupling between F-actin and microtubules at the mitotic spindle, as previously documented in Xenopus laevis embryos. In addition, the organization and regulation of the microtubule network during interphase could also influence division orientation. In support of this possibility, one study demonstrated that microtubule-dependent positioning of nuclei in 8-cell blastomeres is predictive of their subsequent division orientation. Mechanistically, it was shown that the expression of the apical domain protein aPKC regulates dynein-mediated versus kinesin-mediated pulling forces on the nucleus to position it apically or basally, thereby favouring symmetric or asymmetric divisions, respectively. Thus, the polarized apical domain of the 8-cell embryo could regulate spindle orientation via a number of mechanisms. Alternatively or in parallel, nuclear positioning and cell division orientation could be influenced by a recently identified non-centrosomal MTOC that regulates microtubule organization during interphase. In most dividing cells, the thin cytokinetic bridges formed at the end of cell division and connecting the daughter cells are abscised. However, the cells of the preimplantation mouse embryo retain their cytokinetic bridge and do not abscise it following cytokinesis. Instead, they maintain this structure throughout most of interphase and convert it into a non-centrosomal MTOC. Some of the microtubule bundles originating from this bridge may be in direct contact with the cell nucleus and help to position the nucleus within the cell. Interestingly, live-imaging experiments showed that the disassembly of this bridge occurs just before cell division and in a sequential manner: it starts with the depolymerization of microtubules of the bridge of one sister cell, which enters mitosis first, and is then followed by the depolymerization of the bridge of the neighbouring cell, which enters mitosis with a delay as compared with its sister. Thus, it is plausible that the temporal pattern of bridge disassembly could provide a mechanism to influence the division pattern or establish differences between sister cells. Overall, given that the mouse embryo is a unique acentrosomal system, further investigation into cytoskeletal regulation of division orientation will likely yield novel insights into how actin and microtubules, both individually and in combination, interact with the mitotic spindle apparatus to generate the diversity of spindle angles and division patterns during early development.
The interplay of cytoskeletal components in driving apical constriction.
Inner cells can also be allocated by cell internalization, following intermediate or tilted divisions (Fig. b above). This process is driven by apical constriction, characterized by a reduction in apical cell area of constricting cells that results in an altered wedge-shaped cell geometry. On a larger scale, the coordinated regulation of apical constriction within a continuous tissue ultimately results in the acquisition of higher-order tissue structures, such as three-dimensional tubes or furrows. Therefore, apical constriction is a key mechanism utilized in many developmental contexts to guide tissue morphogenesis, ranging from tissue invagination in the D. melanogaster mesoderm to extrusion of apoptotic cells. Apical constriction is primarily driven by a conserved set of molecular components, including actomyosin and the upstream regulators RhoGEF, RHOA and ROCK. Nevertheless, apical constriction mechanisms display key differences in actomyosin localization, organization and contractile behaviour across the divergent model systems. During D. melanogaster ventral furrow formation, myosin II accumulates as a medio-apical pool together with the actin cytoskeleton, linked to adherens junction complexes at the cell surface. This medio-apical actomyosin network draws the opposing adherens junctions closer together during apical constriction via periodic cycles of contraction and stabilization in a ratchet-like manner. By contrast, neural tube closure in chick embryos is driven by a different pool of myosin II localized to cell junctions. Here, contractile actomyosin cables around the apical domain constrict the apical cell surface to drive apical constriction and tissue folding. In the early mouse embryo, apical constriction events occur heterogeneously in only a few cells that internalize to form the ICM, resulting in changes in cell position without grossly affecting embryo architecture (Fig. c above). Myosin II accumulates both along cell junctions and at the apical cortex of internalizing cells, increasing tensile forces relative to neighbouring cells. At the same time, cortical tension in cells adjacent to internalizing cells is also important for apical constriction, as wild-type blastomeres with myosin II-knockdown neighbours fail to constrict. This indicates that all cells need to have a minimal level of cortical tension to exert forces against each other to support inner cell–outer cell segregation. However, how these tension anisotropies are first established within the embryo to drive cell internalization is an open question. It has been suggested that the apical domain of outer cells could play a role in blocking actomyosin contractility, thus preventing their internalization. However, not all 16-cell-stage outer cells re-establish an apical domain immediately after cell division, and there remain temporal differences in the timing of reassembly of the apical domain that cannot fully explain the internalization patterns visualized in the embryo. Differences in keratin filament assembly between blastomeres have been shown to correlate with cell internalization. Cells assembling a keratin filament network before their neighbours do not undergo apical constriction, thereby remaining in the outer layer of the embryo. In other systems, keratins are known to confer cellular stiffness and promote overall tissue integrity. It is thus likely that keratin filaments in the early mammalian embryo may also contribute to cell stiffness to prevent cell internalization. Additionally, keratins may regulate the dynamics of the F-actin cortex, as these two cytoskeletal components have been found to interact in other systems, and also in the mammalian embryo. Apart from apical cytoskeletal changes, apical constriction also depends on cell–cell adhesion along basolateral junctions, as cell internalization is associated with cells acquiring a more extensive basolateral membrane area. Cadherin–cadherin interactions are necessary to couple cells for internalization and ICM establishment, in line with E-cadherin inhibition by the DECMA-1 blocking antibody resulting in apical constriction failures and fewer cells being allocated to the ICM56. This phenomenon is also observed in embryos with disrupted microtubule bridges, the microtubule-based connections between sister cells that are retained after cytokinesis and persist throughout interphase without abscission. Downregulation of the minus-end microtubule-binding protein CAMSAP3, which stabilizes microtubule ends, results in impaired cell internalization and ICM formation. This is consistent with the role of the bridges in supporting cell adhesion, as their microtubule bundles have been shown to facilitate the transport of vesicular structures labelled by RAB11A that carry E-cadherin to the plasma membrane (Fig. c above). Together, actomyosin-driven apical constriction, keratin regulation of cell stiffness and microtubule-regulated E-cadherin transport exemplify how the three major cytoskeletal components could act in a coordinate manner to bias inner cell versus outer cell segregation during early mammalian development.
1. https://pubmed.ncbi.nlm.nih.gov/33927361/
Hui Yi Grace Lim Cytoskeletal control of early mammalian development 29 April 2021 1
The cytoskeleton — comprising actin filaments, microtubules and intermediate filaments — serves instructive roles in regulating cell function and behaviour during development. The cytoskeleton allows cells to integrate internal and external signals to perform essential functions at the cellular and tissue levels. This filamentous network functions as a sensor of biochemical and mechanical changes in the external environment of cells and transmits that information internally to effect changes in gene expression and cellular behaviour. At the same time, regulating cytoskeletal organization and function can generate mechanical force and activate biochemical signalling pathways to modify the extracellular space. This ability of the cytoskeleton to coordinate cellular functions and integrate diverse signals is especially important in the context of development, where cells must communicate and respond to their environment to robustly pattern a tissue and eventually the entire organism . Therefore, studying how the cytoskeleton controls key processes in development, including cell fate specification and morphogenesis, is a major area of interest in cell and developmental biology. Eukaryotic cells contain three main classes of cytoskeletal filaments: actin filaments (also referred to as F-actin), microtubules and intermediate filaments. Although key structural and functional differences set these filament classes apart, all three share important features: (1) they are composed of repeated subunits; (2) they exhibit dynamic assembly and disassembly, regulated by a diverse group of filament-binding proteins; and (3) they are connected to the cell membrane at adhesion complexes, and to the cell nucleus via the linker of nucleoskeleton and cytoskeleton (LINC) complex . These features allow the cytoskeleton to undergo dramatic reorganization within a short window of time to cope with changing cellular requirements, and to respond rapidly to both internal and external signals via their links to the nucleus and cell cortex. As a result, the cytoskeleton has been implicated in virtually all cellular processes, including cell division , migration , polarization and adhesion . Importantly, extensive crosstalk occurs between the three cytoskeletal systems, mediated by linker proteins, regulation of common biochemical pathways or direct mechanical interactions. Therefore, investigating the roles of actin filaments, microtubules and intermediate filaments in development cannot be done in isolation, but must be explored in the wider context of an integrated network of cytoskeletal filaments. The preimplantation mouse embryo is a leading model of mammalian development due to its amenability to genetic manipulation, ex utero culture, high-resolution live imaging and experimental perturbations. Over the course of 3–4 days following fertilization, the single-cell zygote undergoes multiple rounds of divisions to form a 32-cell to 64-cell blastocyst, taking the form of an outer trophectoderm layer surrounding an inner cell mass (ICM) and a fluid-filled cavity. Trophectoderm cells ultimately contribute to extraembryonic tissues such as the placenta, whereas cells in the ICM form the fetus and primitive endoderm. This trophectoderm–ICM lineage segregation is the first cell fate specification event in mammalian development. Organizing the morphogenetic changes to sculpt the final structural form of the blastocyst, while concurrently regulating the molecular pathways that specify cell fates, requires precise mechanisms that ensure the fidelity of this process. The genetic and molecular underpinnings of embryogenesis have long been a subject of intense study, revealing key insights into the biochemical and mechanical pathways that guide this developmental process. In addition, early mammalian development relies on cytoskeletal components, both in their regulation of signalling pathways and in the mechanical properties they confer that can influence cell shape and cavity formation. A thorough exploration of cytoskeletal roles in early embryos is therefore necessary, and these findings need to be integrated with our understanding of the genetic and molecular players during development to build a cohesive picture of early embryogenesis. Here we review the roles of the three major cytoskeletal filament systems during the initial stages of mammalian embryonic life. We summarize the current understanding of cytoskeletal functions driving key morphogenetic events in the preimplantation mouse embryo, organized into four broad sections in the order of their occurrence during development: compaction and polarization, inner cell versus outer cell allocation, fate specification and blastocyst formation. In each section, we highlight crosstalk between the cytoskeletal systems and discuss novel cytoskeletal structures that are thus far unique to the early embryo and could not have necessarily been predicted a priori from studies of non-mammalian embryos or mammalian cells grown in vitro.
Compaction and polarization
During preimplantation development, the first prominent change in cell morphology occurs when cells of the embryo, typically referred to as blastomeres, undergo compaction at the 8-cell stage16.

Key structures formed by the cytoskeleton during embryo compaction and polarization.
At the 8-cell stage, blastomeres flatten against their neighbours and increase cell–cell contacts to form a compacted morula. At the same time, the embryo acquires apical–basal polarity via the enrichment of F-actin, microvilli and PAR–aPKC proteins in the apical cortex, and the localization of E-cadherin, JAM1, PAR1 and Na+ /K+ -ATPase to the basolateral membranes. Keratin filaments also start to assemble in a subset of cells, localizing to the apical cortex via interactions with the F-actin-rich apical domain. In parallel, some cells in the embryo extend actin-based filopodial protrusions over the apical surfaces of their neighbours, which exert forces that draw these adjacent cells closer to one another to facilitate compaction. Therefore, by the 8-cell stage, the major components of the cytoskeleton acquire a distinct polarized organization, and some, including keratin filaments and filopodia, additionally display prominent cell-to-cell variability.
Initially rounded blastomeres increase cell–cell contact with their neighbours and flatten their apical surfaces, producing cells with a clear apical and basal domain. Concurrently, blastomeres polarize along their radial axis, accumulating members of the PAR–aPKC complex, including PAR3, PAR6 and PKCζ on their apical surface, and E-cadherin, PAR1 and JAM1 along their basal membranes(Fig. above). This association between compaction and polarization remains incompletely understood as it is difficult to perturb the two processes independently. For example, premature activation of PKC22,23 and blocking the intercellular engagement of E-cadherin molecules are two perturbations widely used to accelerate or interfere with embryo compaction, but these methods also influence apical–basal cell polarization. Isolated blastomeres lacking E-cadherin or cellular contacts remain capable of polarizing to form an apical domain-like structure on one end of the cell, suggesting that polarization could occur cell autonomously. This indicates that compaction is not an absolute prerequisite for polarization, but it remains open to what extent compaction facilitates the polarization process by mediating the formation of cell–cell interactions to establish the apical and basal domains of the embryo. The cytoskeleton is a primary regulator of cell shape due to its intrinsic mechanical properties allowing it to resist deformation. At the same time, cytoskeletal contractility can generate forces on the surface of the cell to influence cell morphology. During the early stages of embryogenesis, changes in cell and embryo morphology are accompanied by rearrangements in the cytoskeleton. Hence, these early compaction and polarization events have been tightly connected to the cytoskeleton and its dynamics.
Compaction and polarization are associated with cytoskeletal rearrangements.
At the time of compaction, a marked redistribution of microtubules occurs, from an even cortical localization in early blastomeres to a pronounced asymmetry following compaction, accumulating within the apical region of compacted blastomeres. At the same time, an opposite asymmetry in a stabler pool of acetylated microtubules is set up. Like microtubules, actin filaments are also enriched cortically during compaction, coupled with an apical reorganization of finger-like protrusions composed of linear arrays of actin filaments known as microvilli. This restriction of microvilli to the apical domain is likely controlled by spatial regulation of the phosphorylation of ezrin — a protein acting as a linker between the plasma membrane and the actin cytoskeleton — as shown in epithelial cells, although the precise roles of microvilli in the embryo remain unknown.
Following this initial apical accumulation of actin, keratin intermediate filaments also become enriched at the apical membrane (Fig. above). Disrupting the apically polarized pool of actin filaments in turn blocks the apical localization of keratin filaments, resulting in a more homogeneous keratin filament distribution throughout these early blastomeres. Therefore, all three major cytoskeletal systems undergo extensive reorganization early in development, setting up the first apical–basal cell asymmetries polarizing the early embryo. Cytoskeleton-generated mechanical forces driving compaction. The morphological changes occurring during embryo compaction are regulated in part by actomyosin driven tensile forces generated at the apical cell cortex. These forces can be probed by micropipette aspiration of 8-cell mouse embryos, revealing a role for increasing surface tension in driving embryo compaction. This is regulated by a complex interplay of actin regulators that is still not well understood: inhibiting actin polymerization with cytochalasin D blocks embryo compaction, but decreasing levels of cofilin 1, an actin-binding protein that also blocks actin polymerization, accelerates the compaction process. One driver of forces generated during compaction is filopodia that form specifically during the 8-cell to 16-cell stage of development (Fig. above). Some cells extend these filopodia containing actin, E-cadherin and myosin X over the apical surface of their neighbouring cells during embryo compaction. These filopodia were under tension, and disrupting filopodia by laser ablation or downregulation of filopodial components, including E-cadherin and myosin X, was sufficient to block compaction, resulting in more segregated, rounder cells. Conversely, forcing premature formation of filopodia by myosin X overexpression accelerated compaction. Together, these results suggest that the extension of filopodia over the surface of neighbouring cells facilitates the cell shape changes underlying compaction. Consistent with the role of filopodia in promoting compaction, cells retract their filopodia just before division, when they decompact and become spherical in preparation for mitosis. Retraction of filopodia could serve as a trigger for mitosis, although a role for filopodia in regulating cell division in the mouse embryo has not yet been reported. Alternatively, mitotic onset could regulate retraction of filopodia, given that mitotic cells undergo significant cytoskeletal reorganization. In other systems, filopodia have been shown to mediate the transport of signalling molecules such as Wnt in zebrafish embryos and SHH in chick embryos, both of which can act as morphogens to pattern the developing tissue. Future work investigating a potential role for filopodia in directional transport of signalling molecules between cells of the early mouse embryo could reveal parallel mechanisms between these systems. This would demonstrate how cytoskeletal structures can integrate cell shape changes with the regulation of the timing of cell division and lineage specification using biochemical cues. Beyond actin, it is plausible that more extensive cytoskeletal crosstalk between the three filament systems drives compaction in the 8-cell embryo. For example, the asymmetries in microtubule organization during compaction suggest that microtubules could interact with actin, via direct connections or by mediating intercellular trafficking to deliver proteins that differentially regulate actin organization to the apical membrane. This is in line with work demonstrating the importance of protein intracellular transport in driving compaction in the mouse embryo, along with a study showing transport of E-cadherin along microtubule bridges of the mouse embryo, although the latter has not been directly tested in the context of compaction. Moreover, inhibiting microtubule polymerization increased the rate of compaction in early 8-cell embryos. Another possibility for microtubule regulation of compaction is via cell shape changes, akin to the microtubule-driven flattening of differentiated mouse keratinocytes in the mature epidermis, for example. However, whether microtubules in the mouse embryo indeed have a mechanical role in regulating cell shape during compaction remains an important open question. Lastly, keratin filaments, too, anchor to the apical cell cortex following polarization, where they are known to stabilize actin filaments at later stages of development. It remains to be investigated whether the mechanical support conferred by keratin filaments is also involved in establishing a compacted embryo containing the first polarized cells in development.
Actin regulation of apical polarity.
The establishment of apical polarity within the developing mouse embryo has recently been dissected in detail, and involves a complex interplay of actin filaments, polarity proteins and upstream molecular regulators. This polarization occurs in two main steps: first, an initiation phase whereby phospholipase C (PLC)-driven RHOA activation at the apical cell cortex promotes the apical enrichment of actin filaments and myosin II activity, followed by a second, maturation phase that recruits polarity proteins, including PAR3, PAR6 and aPKC, to establish a mature apical domain51,52. Interestingly, this two-step mechanism of apical polarization was also recently found to occur within human embryos, similarly regulated by PLC activation, suggesting that this process could be evolutionarily conserved among early mammalian embryos53,54. During the latter maturation phase, actin remodelling, downstream of the activity of the transcription factors TFAP2C and TEAD4, was shown to promote cooperative recruitment of apical polarity proteins such as ezrin55. Indeed, double knockdown of TFAP2C and TEAD4 reduced levels of actin regulators, including the ARP2/3 complex component ARPC1B, and completely blocked embryo polarization at the 8-cell stage. In addition to cooperative recruitment, which is driven by a positive-feedback mechanism promoting increasing rates of ezrin binding to the apical membrane, RHOA-dependent lateral mobility of ezrin is also required to establish the size of the rounded apical patch of accumulated polarity proteins that forms across the contact-free surface of these early blastomeres. The extent of apical polarization is robustly controlled by the balance between these two opposing mechanisms: actin-mediated cooperative recruitment of polarity factors alone without RHOA activation would generate only small abnormal apical protrusions, whereas lateral mobility by itself cannot establish proper apical–basal polarization, resulting in excessive distribution of proteins across the cell surface. Although a detailed mechanism for how actin dynamics can function to recruit and organize the apical domain remains unknown, these studies highlight the central role of the cytoskeleton in establishing the first polarized cells in development.
Cytoskeleton in the regulation of cell division patterns.
Eight-cell-stage blastomeres can divide with different angles relative to the surface of the embryo or apical–basal cell axis. Many studies have often referred to divisions occurring along the surface of the embryo and producing two outer daughter cells as ‘symmetric’, whereas divisions perpendicular to the embryo surface producing an outer daughter cell and an inner daughter cell have been referred to as ‘asymmetric’ (Fig. a).

Cytoskeletal heterogeneities between cells that promote inner cell versus outer cell segregation.
a | From the 8-cell stage, cells can divide with various angles, resulting in more spatially symmetric divisions that allocate two daughter cells to the outer surface of the embryo, or more asymmetric divisions that segregate daughters into outer and inner positions. The grey shapes represent the rest of the cells of the embryo.
b | Some cell divisions during the transition from the 8-cell stage to the 16-cell stage display tilted angles and can be followed by the internalization of one of the daughter cells to form the inner cell mass.
c | Cytoskeletal differences between sister cells that favour inner segregation events versus outer segregation events. Inheritance of keratin filaments stabilizes the F-actin-rich apical cell cortex and hinders cell internalization. By contrast, cells that do not inherit keratin filaments do not seem to repolarize their apical cell cortex and undergo apical constriction to form the inner cell mass, a process dependent on myosin II-mediated cortical tension. In parallel, the microtubule bridge connecting the two sister cells is retained after division, and it promotes increased transport of RAB11A-positive vesicles carrying E-cadherin to the cell membrane of the internalizing cell. This is essential to establish adhesion with basolateral neighbours as the cell internalizes and acquires a larger basolateral contact region compared with the sister cell remaining in the outer part of the embryo.
A third category of ‘intermediate’ divisions has also been identified as divisions displaying tilted angles, whereby two outer daughter cells are initially generated, but one of them gradually internalizes via apical constriction (Fig. b above). It remains debated whether the orientation of these divisions occurs stochastically or whether it is prepatterned by heterogeneities in the molecular or cytoskeletal organization of the parental cells before division. One possible mechanism regulating division orientation is cell shape. It has long been postulated that cell shape can orient the mitotic spindle, a phenomenon described by Hertwig’s long-axis rule whereby cells divide along their long axis (or line of tension). This could explain the bias towards symmetric divisions occurring within the 16-cell embryo, where outer cells acquire a more elongated morphology extending across the surface of the embryo. This is also consistent with higher levels of myosin II phosphorylation and likely also of cortical tension at the apical surface of the 16-cell-stage embryo when compared with the preceding stage. However, cells in the 8-cell embryo display a diversity of division angles, despite having similar cellular morphologies. This suggests that the early mouse embryo could use distinct mechanisms during the divisions occurring from the 8-cell stage to the 16-cell stage. In various cellular and organismal contexts, the cytoskeleton plays a major role in the control of division orientation. For example, asymmetric division within the Caenorhabditis elegans zygote involves pulling forces exerted by the cell cortex on astral microtubules growing from centrosomes, mediated by cortical force regulators that position the mitotic spindle within the dividing cell. By contrast, the mechanisms controlling division orientation in the early mouse embryo are less well understood. The mouse embryo does not have functional centrosomes serving as the main microtubule organizing centres (MTOCs) until blastocyst stages, and is thought to lack clear astral microtubule arrays.
Therefore, division patterns could instead involve actin-driven mechanisms as seen in the mouse oocyte, which also lacks centrosomes. During meiosis I, the meiotic spindle migrates from the centre of the oocyte, where the spindle is formed, to the periphery of the cell to generate the polar body and produce a mature oocyte. This spindle positioning is controlled by a formin 2-dependent dense cytoplasmic F-actin meshwork that encases the mitotic spindle, exerting pulling forces via myosin II at the actin-rich cell cortex. It was recently shown that a cytoplasmic F-actin meshwork is also present in all cells of the early mouse embryo, yet it remains unknown whether spindle positioning in the embryo is similarly regulated by this F-actin meshwork, and how it may influence division patterns and inner cell–outer cell allocation. Some studies point to an important role of apical polarity proteins in positioning the mitotic spindle to regulate division orientation during development. This is the case in asymmetric cell divisions in a number of non-mammalian systems, including Drosophila melanogaster neuroblasts and the C. elegans zygote. Given that apical–basal polarization is already established at the 8-cell stage in the mouse embryo, it has been suggested that the apical domain could control spindle orientation also in these early mammalian blastomeres by accumulating MTOC components at the apical pole.
This could potentially be mediated by mechanical coupling between F-actin and microtubules at the mitotic spindle, as previously documented in Xenopus laevis embryos. In addition, the organization and regulation of the microtubule network during interphase could also influence division orientation. In support of this possibility, one study demonstrated that microtubule-dependent positioning of nuclei in 8-cell blastomeres is predictive of their subsequent division orientation. Mechanistically, it was shown that the expression of the apical domain protein aPKC regulates dynein-mediated versus kinesin-mediated pulling forces on the nucleus to position it apically or basally, thereby favouring symmetric or asymmetric divisions, respectively. Thus, the polarized apical domain of the 8-cell embryo could regulate spindle orientation via a number of mechanisms. Alternatively or in parallel, nuclear positioning and cell division orientation could be influenced by a recently identified non-centrosomal MTOC that regulates microtubule organization during interphase. In most dividing cells, the thin cytokinetic bridges formed at the end of cell division and connecting the daughter cells are abscised. However, the cells of the preimplantation mouse embryo retain their cytokinetic bridge and do not abscise it following cytokinesis. Instead, they maintain this structure throughout most of interphase and convert it into a non-centrosomal MTOC. Some of the microtubule bundles originating from this bridge may be in direct contact with the cell nucleus and help to position the nucleus within the cell. Interestingly, live-imaging experiments showed that the disassembly of this bridge occurs just before cell division and in a sequential manner: it starts with the depolymerization of microtubules of the bridge of one sister cell, which enters mitosis first, and is then followed by the depolymerization of the bridge of the neighbouring cell, which enters mitosis with a delay as compared with its sister. Thus, it is plausible that the temporal pattern of bridge disassembly could provide a mechanism to influence the division pattern or establish differences between sister cells. Overall, given that the mouse embryo is a unique acentrosomal system, further investigation into cytoskeletal regulation of division orientation will likely yield novel insights into how actin and microtubules, both individually and in combination, interact with the mitotic spindle apparatus to generate the diversity of spindle angles and division patterns during early development.
The interplay of cytoskeletal components in driving apical constriction.
Inner cells can also be allocated by cell internalization, following intermediate or tilted divisions (Fig. b above). This process is driven by apical constriction, characterized by a reduction in apical cell area of constricting cells that results in an altered wedge-shaped cell geometry. On a larger scale, the coordinated regulation of apical constriction within a continuous tissue ultimately results in the acquisition of higher-order tissue structures, such as three-dimensional tubes or furrows. Therefore, apical constriction is a key mechanism utilized in many developmental contexts to guide tissue morphogenesis, ranging from tissue invagination in the D. melanogaster mesoderm to extrusion of apoptotic cells. Apical constriction is primarily driven by a conserved set of molecular components, including actomyosin and the upstream regulators RhoGEF, RHOA and ROCK. Nevertheless, apical constriction mechanisms display key differences in actomyosin localization, organization and contractile behaviour across the divergent model systems. During D. melanogaster ventral furrow formation, myosin II accumulates as a medio-apical pool together with the actin cytoskeleton, linked to adherens junction complexes at the cell surface. This medio-apical actomyosin network draws the opposing adherens junctions closer together during apical constriction via periodic cycles of contraction and stabilization in a ratchet-like manner. By contrast, neural tube closure in chick embryos is driven by a different pool of myosin II localized to cell junctions. Here, contractile actomyosin cables around the apical domain constrict the apical cell surface to drive apical constriction and tissue folding. In the early mouse embryo, apical constriction events occur heterogeneously in only a few cells that internalize to form the ICM, resulting in changes in cell position without grossly affecting embryo architecture (Fig. c above). Myosin II accumulates both along cell junctions and at the apical cortex of internalizing cells, increasing tensile forces relative to neighbouring cells. At the same time, cortical tension in cells adjacent to internalizing cells is also important for apical constriction, as wild-type blastomeres with myosin II-knockdown neighbours fail to constrict. This indicates that all cells need to have a minimal level of cortical tension to exert forces against each other to support inner cell–outer cell segregation. However, how these tension anisotropies are first established within the embryo to drive cell internalization is an open question. It has been suggested that the apical domain of outer cells could play a role in blocking actomyosin contractility, thus preventing their internalization. However, not all 16-cell-stage outer cells re-establish an apical domain immediately after cell division, and there remain temporal differences in the timing of reassembly of the apical domain that cannot fully explain the internalization patterns visualized in the embryo. Differences in keratin filament assembly between blastomeres have been shown to correlate with cell internalization. Cells assembling a keratin filament network before their neighbours do not undergo apical constriction, thereby remaining in the outer layer of the embryo. In other systems, keratins are known to confer cellular stiffness and promote overall tissue integrity. It is thus likely that keratin filaments in the early mammalian embryo may also contribute to cell stiffness to prevent cell internalization. Additionally, keratins may regulate the dynamics of the F-actin cortex, as these two cytoskeletal components have been found to interact in other systems, and also in the mammalian embryo. Apart from apical cytoskeletal changes, apical constriction also depends on cell–cell adhesion along basolateral junctions, as cell internalization is associated with cells acquiring a more extensive basolateral membrane area. Cadherin–cadherin interactions are necessary to couple cells for internalization and ICM establishment, in line with E-cadherin inhibition by the DECMA-1 blocking antibody resulting in apical constriction failures and fewer cells being allocated to the ICM56. This phenomenon is also observed in embryos with disrupted microtubule bridges, the microtubule-based connections between sister cells that are retained after cytokinesis and persist throughout interphase without abscission. Downregulation of the minus-end microtubule-binding protein CAMSAP3, which stabilizes microtubule ends, results in impaired cell internalization and ICM formation. This is consistent with the role of the bridges in supporting cell adhesion, as their microtubule bundles have been shown to facilitate the transport of vesicular structures labelled by RAB11A that carry E-cadherin to the plasma membrane (Fig. c above). Together, actomyosin-driven apical constriction, keratin regulation of cell stiffness and microtubule-regulated E-cadherin transport exemplify how the three major cytoskeletal components could act in a coordinate manner to bias inner cell versus outer cell segregation during early mammalian development.
1. https://pubmed.ncbi.nlm.nih.gov/33927361/

