The human immune system is irreducible, depending on several macromolecular complexes that work together in a joint venture
https://reasonandscience.catsboard.com/t3016-the-human-immune-system-is-irreducible-depending-on-several-macromolecular-complexes-that-work-in-a-joint-venture
1. Waste management (or waste disposal) includes the activities and actions required to manage waste from its inception to its final disposal. This includes the collection, transport, treatment, and disposal of waste, together with monitoring and regulation of the waste management process, and is always preceded by careful planning and foresight of the entire process by waste management engineers, and implemented virtually simultaneously.
2. Biological cells have cleverly engineered mechanisms that grind molecular protein garbage ( Proteasome Garbage Grinders ), coordinate loading and translocation of the waste products ( by superb Multisubunit peptide-loading complexes (PLC) ) to the waste disposal site, where the waste products are processed and sorted out (Histocompatibility complex class I (MHC-I) with 1,6 million atoms that to work with atomic precision ), and the final products are transported to the surface of the cell through the exquisite secretory pathway. There, T-Cells scan the MHC-I with receptors, and recognize when the cell was infected by foreign invaders, and induce their apoptosis ( cell suicide)
At least 9 macromolecular complexes need to work together in a joint venture, which communicate with each other to orchestrate this masterfully information-based process through signaling languages. If one of the complexes in the pathway is missing, no deal, the immune system cannot do its job, and the organism cannot survive and dies. Of course, all this incredible marvel of molecular engineering had to be born fully set up. No stepwise evolutionary process would lead to such a system.
3. Therefore, the intelligent design theorist is justified to posit an unfathomably clever intelligent designer with foresight, who knew how to implement such a masterfully crafted waste management system on a molecular scale.
The immune system is the body’s natural defense against infection and disease, including cancer, and protects the body from substances that can cause harm, such as bacteria and viruses (also called germs).
The cells of the immune system continuously flow through the body, looking for germs that may be invading the body. The immune system recognizes invaders by their antigens, which are proteins on the surface of the invading cells . Every cell or substance has its own specific antigens, and a person’s cells carry “self-antigens” that are unique to that individual.
People carry self-antigens on normal cells, such as liver, colon, and thyroid cells. Cells with self-antigens are typically not a threat. Invading germs, however, do not originate in the body and so do not carry self-antigens; instead, they carry what are called “nonself-antigens.”
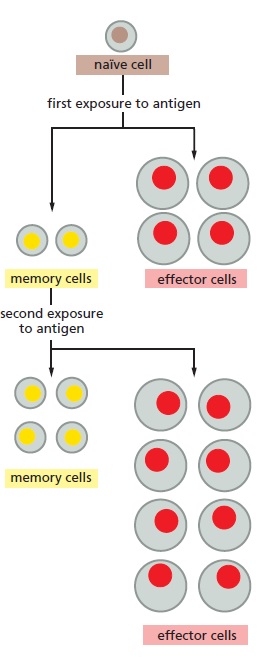
The immune system is designed to identify cells with nonself-antigens as harmful and respond appropriately. Most immune cells release cytokines (messengers) to help them communicate with other immune cells and control the response to any threats. When, for example, the immune system’s first barrier, the skin, is broken, harmful substances can easily enter the body. As soon as the injury occurs, immune cells in the injured tissue begin to respond and also call other immune cells that have been circulating in your body to gather at the site and release cytokines to call other immune cells to help defend the body against invasion. The immune cells can recognize any bacteria or foreign substances as invaders. Immune cells, known as natural killer cells, (Natural Killer (NK) Cells are lymphocytes in the same family as T and B cells) begin to destroy the invaders with a general attack. Now, we will give a closer look at how that happens. 4
Our body constantly encounters pathogens or malignant transformation. Cells that are infected by a virus or carry a carcinogenic mutation produce proteins foreign to the body. The adaptive immune system fights back unwanted invaders through highly sophisticated defense mechanisms. One of the central hinges of human adaptive immunity is the major histocompatibility complex (MHC) class I antigen presentation pathway.
Antigenic peptides result from the degradation of unwanted exogenous proteins ( proteasomal degradation products ) inside the cell. They are generated in the cytosol by proteasomal protein degradation. These antigens are, of the most part, short peptides (8 to 12 amino acids) resulting from the degradation of intracellular proteins.
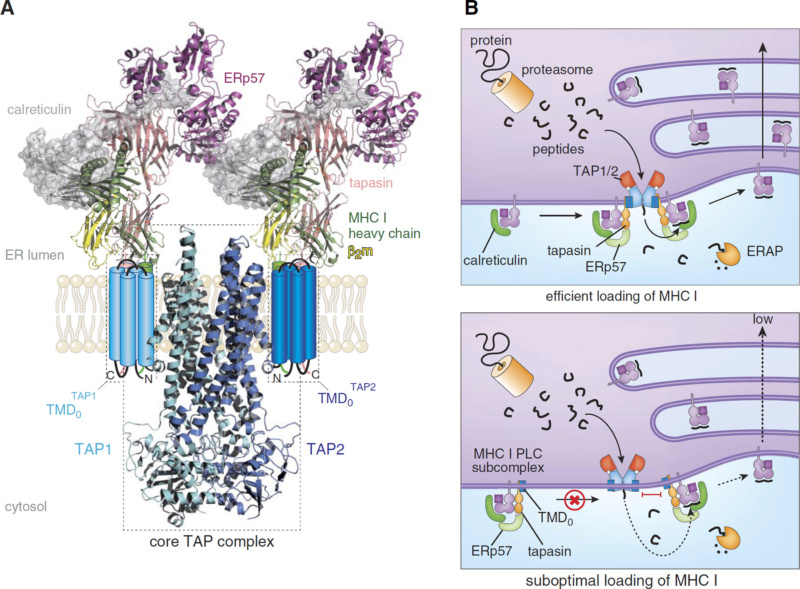
Triaging the vast pool of cytosolic degradation products to find the few peptides indicating infection or antigenic invasion requires sophisticated machinery, the highly dynamic multisubunit peptide-loading complex (PLC). The products for degradation are edited by PLC.
PLC contains as key constituents a transporter associated with antigen processing (TAP) and the MHC I-specific chaperone tapasin (Tsn).
The antigenic invaders are loaded, and TAP transports the proteasomal degradation (antigen) products from the cytosol intracellularly to the endoplasmic reticulum ER, where they are loaded and proofread by major histocompatibility complex class I proteins (MHC-I), which play a pivotal role, they are a cornerstone of the human adaptive immune system.5, being responsible for processing antigens that allow killer T cells to distinguish between healthy and compromised cells. Proofreading by MHC-I editing complexes guarantees that only very stable peptide/MHC-I complexes are released to the cell surface.
The peptide-MHC-I complexes then move via a secretory pathway to the cell surface, presenting their antigenic load to cytotoxic T-cells.
Peptides exposed at the cell surface, therefore, mirror cellular contents: In healthy cells, only peptides from the “self” are exposed; in cells compromised by a virus or cancer-causing mutation, both self and “nonself” peptides, either viral or mutated, are exposed and displayed. A sampling of myriads of different peptide/MHC-I allomorphs ( different protein forms) requires a precisely coordinated quality control network in a single macromolecular assembly, including the transporter associated with antigen processing TAP1/2, the MHC-I heterodimer, the oxidoreductase ERp57, and the ER chaperones tapasin and calreticulin. 3 The stability of the human MHC-I PLC requires two editing modules. A single-module PLC cannot assemble the circular belt formed. There is also a crucial role of Tsn in bridging PLC components together.
Patrolling T lymphocytes ( T cells ) detect and identify tainted cells by scanning the peptide MHC complexes via T cell receptors and induce their apoptosis ( cell suicide). This is how our immune system defends us against pathogens.
Consider what is required for this pathway to be able to operate:
1. Proteasome Garbage Grinders 6 are analogous to shredders or garbage disposals, indispensable to destroy cellular components, breaking them down to their constituent parts, which can then be recycled
2. Multisubunit peptide-loading complex (PLC) is essential for establishing a hierarchical immune response. 7
3. A transporter associated with antigen processing (TAP1/2) is essential for peptide presentation to the major histocompatibility complex (MHC) class I molecules on the cell surface and necessary for T-cell recognition 8
4. MHC I-specific chaperone tapasin (Tsn) is essential for the assembly of the PLC and for efficient MHC I antigen presentation. 9
5. Oxidoreductase ERp57 is essential through the participation in the assembly of the major histocompatibility complex class 1. 10
6. ER chaperones tapasin: Tapasin is an essential adapter protein recruiting MHC I molecules to TAP, catalyzes peptide loading of MHC I. 11
7. Histocompatibility complex class I (MHC-I) is extremely important and essential for the adaptive immune system. 12, 15
8. The secretory pathway is ubiquitous to all cells and essential for the export of proteins.13
9. Cytotoxic T-cells are essential in host defense against pathogens that live in the cytosol, the commonest of which are viruses. These cytotoxic T cells can kill any cell harboring such pathogens by recognizing foreign peptides. 14
1. https://phys.org/news/2020-08-giant-nanomachine-aids-immune.html?fbclid=IwAR2JepKjofCYjTYA_x1uBoz8yqGW81rP6Vh6s7iPL1yg5ishSq3S47Ew3bo
2. https://sci-hub.tw/https://www.pnas.org/content/117/34/20597
3. https://cordis.europa.eu/project/id/789121
4. https://www.sitcancer.org/connectedold/p/patient/resources/melanoma-guide/immune-system
5. https://www.frontiersin.org/articles/10.3389/fimmu.2017.00292/full
6. https://reasonandscience.catsboard.com/t1851-proteasome-garbage-grinders
7. https://pubmed.ncbi.nlm.nih.gov/29107940/
8. https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/transporter-associated-with-antigen-processing
9. https://www.nature.com/articles/srep17341
10. https://link.springer.com/article/10.2478/s11658-011-0022-z
11. https://faseb.onlinelibrary.wiley.com/doi/full/10.1096/fj.12-217489
12. https://en.wikipedia.org/wiki/Major_histocompatibility_complex
13. https://jlb.onlinelibrary.wiley.com/doi/pdf/10.1189/jlb.1208774
14. https://www.ncbi.nlm.nih.gov/books/NBK27101/#:~:text=Armed%20effector%20cytotoxic%20CD8%20T,to%20MHC%20class%20I%20molecules.
15. https://onlinelibrary.wiley.com/doi/full/10.1002/ece3.5373
https://reasonandscience.catsboard.com/t3016-the-human-immune-system-is-irreducible-depending-on-several-macromolecular-complexes-that-work-in-a-joint-venture
1. Waste management (or waste disposal) includes the activities and actions required to manage waste from its inception to its final disposal. This includes the collection, transport, treatment, and disposal of waste, together with monitoring and regulation of the waste management process, and is always preceded by careful planning and foresight of the entire process by waste management engineers, and implemented virtually simultaneously.
2. Biological cells have cleverly engineered mechanisms that grind molecular protein garbage ( Proteasome Garbage Grinders ), coordinate loading and translocation of the waste products ( by superb Multisubunit peptide-loading complexes (PLC) ) to the waste disposal site, where the waste products are processed and sorted out (Histocompatibility complex class I (MHC-I) with 1,6 million atoms that to work with atomic precision ), and the final products are transported to the surface of the cell through the exquisite secretory pathway. There, T-Cells scan the MHC-I with receptors, and recognize when the cell was infected by foreign invaders, and induce their apoptosis ( cell suicide)
At least 9 macromolecular complexes need to work together in a joint venture, which communicate with each other to orchestrate this masterfully information-based process through signaling languages. If one of the complexes in the pathway is missing, no deal, the immune system cannot do its job, and the organism cannot survive and dies. Of course, all this incredible marvel of molecular engineering had to be born fully set up. No stepwise evolutionary process would lead to such a system.
3. Therefore, the intelligent design theorist is justified to posit an unfathomably clever intelligent designer with foresight, who knew how to implement such a masterfully crafted waste management system on a molecular scale.
The immune system is the body’s natural defense against infection and disease, including cancer, and protects the body from substances that can cause harm, such as bacteria and viruses (also called germs).
The cells of the immune system continuously flow through the body, looking for germs that may be invading the body. The immune system recognizes invaders by their antigens, which are proteins on the surface of the invading cells . Every cell or substance has its own specific antigens, and a person’s cells carry “self-antigens” that are unique to that individual.
People carry self-antigens on normal cells, such as liver, colon, and thyroid cells. Cells with self-antigens are typically not a threat. Invading germs, however, do not originate in the body and so do not carry self-antigens; instead, they carry what are called “nonself-antigens.”
The immune system is designed to identify cells with nonself-antigens as harmful and respond appropriately. Most immune cells release cytokines (messengers) to help them communicate with other immune cells and control the response to any threats. When, for example, the immune system’s first barrier, the skin, is broken, harmful substances can easily enter the body. As soon as the injury occurs, immune cells in the injured tissue begin to respond and also call other immune cells that have been circulating in your body to gather at the site and release cytokines to call other immune cells to help defend the body against invasion. The immune cells can recognize any bacteria or foreign substances as invaders. Immune cells, known as natural killer cells, (Natural Killer (NK) Cells are lymphocytes in the same family as T and B cells) begin to destroy the invaders with a general attack. Now, we will give a closer look at how that happens. 4
Our body constantly encounters pathogens or malignant transformation. Cells that are infected by a virus or carry a carcinogenic mutation produce proteins foreign to the body. The adaptive immune system fights back unwanted invaders through highly sophisticated defense mechanisms. One of the central hinges of human adaptive immunity is the major histocompatibility complex (MHC) class I antigen presentation pathway.
Antigenic peptides result from the degradation of unwanted exogenous proteins ( proteasomal degradation products ) inside the cell. They are generated in the cytosol by proteasomal protein degradation. These antigens are, of the most part, short peptides (8 to 12 amino acids) resulting from the degradation of intracellular proteins.
Triaging the vast pool of cytosolic degradation products to find the few peptides indicating infection or antigenic invasion requires sophisticated machinery, the highly dynamic multisubunit peptide-loading complex (PLC). The products for degradation are edited by PLC.
PLC contains as key constituents a transporter associated with antigen processing (TAP) and the MHC I-specific chaperone tapasin (Tsn).
The antigenic invaders are loaded, and TAP transports the proteasomal degradation (antigen) products from the cytosol intracellularly to the endoplasmic reticulum ER, where they are loaded and proofread by major histocompatibility complex class I proteins (MHC-I), which play a pivotal role, they are a cornerstone of the human adaptive immune system.5, being responsible for processing antigens that allow killer T cells to distinguish between healthy and compromised cells. Proofreading by MHC-I editing complexes guarantees that only very stable peptide/MHC-I complexes are released to the cell surface.
The peptide-MHC-I complexes then move via a secretory pathway to the cell surface, presenting their antigenic load to cytotoxic T-cells.
Peptides exposed at the cell surface, therefore, mirror cellular contents: In healthy cells, only peptides from the “self” are exposed; in cells compromised by a virus or cancer-causing mutation, both self and “nonself” peptides, either viral or mutated, are exposed and displayed. A sampling of myriads of different peptide/MHC-I allomorphs ( different protein forms) requires a precisely coordinated quality control network in a single macromolecular assembly, including the transporter associated with antigen processing TAP1/2, the MHC-I heterodimer, the oxidoreductase ERp57, and the ER chaperones tapasin and calreticulin. 3 The stability of the human MHC-I PLC requires two editing modules. A single-module PLC cannot assemble the circular belt formed. There is also a crucial role of Tsn in bridging PLC components together.
Patrolling T lymphocytes ( T cells ) detect and identify tainted cells by scanning the peptide MHC complexes via T cell receptors and induce their apoptosis ( cell suicide). This is how our immune system defends us against pathogens.
Consider what is required for this pathway to be able to operate:
1. Proteasome Garbage Grinders 6 are analogous to shredders or garbage disposals, indispensable to destroy cellular components, breaking them down to their constituent parts, which can then be recycled
2. Multisubunit peptide-loading complex (PLC) is essential for establishing a hierarchical immune response. 7
3. A transporter associated with antigen processing (TAP1/2) is essential for peptide presentation to the major histocompatibility complex (MHC) class I molecules on the cell surface and necessary for T-cell recognition 8
4. MHC I-specific chaperone tapasin (Tsn) is essential for the assembly of the PLC and for efficient MHC I antigen presentation. 9
5. Oxidoreductase ERp57 is essential through the participation in the assembly of the major histocompatibility complex class 1. 10
6. ER chaperones tapasin: Tapasin is an essential adapter protein recruiting MHC I molecules to TAP, catalyzes peptide loading of MHC I. 11
7. Histocompatibility complex class I (MHC-I) is extremely important and essential for the adaptive immune system. 12, 15
8. The secretory pathway is ubiquitous to all cells and essential for the export of proteins.13
9. Cytotoxic T-cells are essential in host defense against pathogens that live in the cytosol, the commonest of which are viruses. These cytotoxic T cells can kill any cell harboring such pathogens by recognizing foreign peptides. 14
1. https://phys.org/news/2020-08-giant-nanomachine-aids-immune.html?fbclid=IwAR2JepKjofCYjTYA_x1uBoz8yqGW81rP6Vh6s7iPL1yg5ishSq3S47Ew3bo
2. https://sci-hub.tw/https://www.pnas.org/content/117/34/20597
3. https://cordis.europa.eu/project/id/789121
4. https://www.sitcancer.org/connectedold/p/patient/resources/melanoma-guide/immune-system
5. https://www.frontiersin.org/articles/10.3389/fimmu.2017.00292/full
6. https://reasonandscience.catsboard.com/t1851-proteasome-garbage-grinders
7. https://pubmed.ncbi.nlm.nih.gov/29107940/
8. https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/transporter-associated-with-antigen-processing
9. https://www.nature.com/articles/srep17341
10. https://link.springer.com/article/10.2478/s11658-011-0022-z
11. https://faseb.onlinelibrary.wiley.com/doi/full/10.1096/fj.12-217489
12. https://en.wikipedia.org/wiki/Major_histocompatibility_complex
13. https://jlb.onlinelibrary.wiley.com/doi/pdf/10.1189/jlb.1208774
14. https://www.ncbi.nlm.nih.gov/books/NBK27101/#:~:text=Armed%20effector%20cytotoxic%20CD8%20T,to%20MHC%20class%20I%20molecules.
15. https://onlinelibrary.wiley.com/doi/full/10.1002/ece3.5373