Magnesium and magnesium transporters, another example of cell interdependence comes to light
https://reasonandscience.catsboard.com/t2440-magnesium-and-magnesium-transporters-another-example-of-cell-interdependence-comes-to-light
There are 24 metal and non metal elements , that are essential for life, amongst them magnesium, which plays a critical role in cellular metabolism, DNA repair, its also present in all deoxyribonucleic acid (DNA) and RNA activation processes, stabilizing macromolecular complexes and membranes. As activator of over 300 different enzymes, magnesium participates in many metabolic processes, such as glycolysis, Krebs cycle, β-oxidation or ion transport across cell membranes. Cells must have mechanisms to maintain physiological levels of Mg2+. It is indispensable for the nucleus ( in eukaryotes ) to function as a whole and for the maintenance of physical stability as well as aggregation of ribosomes into polysomes able to initiate protein synthesis. All these different essential roles elucidate that life could not have had a first go without magnesium.
But in order for the cell to be able to make use of it, Magnesium like other metal ions has to be transported inside cells across the cell membrane by specific membrane proteins. Three distinct classes of Mg2+ transporters have been identified in bacteria. MgtA transporter proteins can sense magnesium ions down to micromolar concentrations, which is the equivalent to a pinch (1 gram) of magnesium salt in 10,000 liters of water. Wow ! This detection system depends on a specific lipid molecule in the membrane called cardiolipin. MgtA and cardiolipin have to work together in an interdependent manner.
Organisms must maintain physiological levels of Mg2+ because this divalent cation is critical for the stabilization of membranes and ribosomes, the neutralization of nucleic acids, and as a cofactor in a variety of enzymatic reactions. Furthermore, specialized biosynthesis pathways and specialized proteins exist to make these import proteins and cardiolipin.
Question: How could cells have emerged gradually, if the cell membrane, magnesium uptake, regulation, use, and consequently transport/import proteins and magnesium regulation were not fully set up and existing right from the beginning? Had the cell membrane, transport proteins, cardiolipin that works in an interdependent manner with MgtA proteins, and the biosynthesis pathways to produce these proteins etc. not have to be fully setup from day one, ore life would not have had a beginning?
Is that not one more reason to believe that life had to start through cells fully setup and complex? Any gradual build-up would not be possible, giving one more good reason to believe life was designed, conceptualized, and made all at once by an intelligent creator.
Chemist Wilhelm Huck, professor at Radboud University Nijmegen :
"A working cell is more than the sum of its parts. "A functioning cell must be entirely correct at once, in all its complexity"
The significance of magnesium in prebiotic geochemistry
Magnesium
The significance of Mg in prebiotic geochemistry
Magnesium plays a special role in biochemistry because of its ability to coordinate six oxygen atoms efficiently in its first coordination shell. Such oxygen atoms may be part of one or two charged oxyanions, which means that Mg2+ can, for instance, tie together two different phosphate groups that are located at distance from each other in a macromolecule, and in this way be responsible for the folding of molecules like RNA. This property of Mg2+ also helps the stabilization of diphosphate and triphosphate groups of nucleotides, as well as promoting the condensation of orthophosphate to oligophosphates, like pyrophosphate and trimetaphosphate. Borates, on the other hand, are known to promote the formation of nucleobases and carbohydrates, ribose in particular, which is yet another constituent of nucleotides. The oldest borate minerals that we find on Earth today are magnesium borates. Dissolved borate stabilizes pentose sugars by forming complexes with cis-hydroxyl groups. In the furanose form of ribose, the preferential binding occurs to the 2 and 3 carbon, leaving the 5 carbon free for phosphorylation. The central role of Mg2+ in the function of ribozymes and its ‘archaic’ position in ribosomes, and the fact that magnesium generally has coordination properties different from other cations, suggests that the inorganic chemistry of magnesium had a key position in the first chemical processes leading to the origin and early evolution of life.
Magnesium (Mg) is a common element on Earth and the other terrestrial planets. It is one of the eight main elements of Earth’s crust and one of the four major elements making up the mass of the whole Earth. Furthermore, Mg is one of the principal constituents of silicate minerals that build up Earth, like olivine, pyroxenes, and Mg layer silicates (e.g., serpentines, talc, Mg smectites). The concentration of divalent magnesium (Mg2+) in contemporary ocean water is 52.8 mmol kg−1. The coordination geometry of magnesium is normally octahedral, that is, the Mg atom coordinates six atoms – almost always oxygen – around itself in its first coordination shell. Six-coordinated Mg2+ has a small ionic radius of 0.65 Å, at the same time as it has the largest hydrated radius of any common cation. The volume difference between hydrated and ionic Mg2+ is almost 400-fold. In the marine geochemical environment, magnesium is particularly important because the tri-octahedral layer of the common smectites in sediments consists primarily of brucite, the mineral name of magnesium hydroxide (Mg(OH)2). The ocean floor beneath the sediment layers consists of basalts and ultramafic rocks that have a high content of primary ferromagnesian silicate minerals (olivine and pyroxenes). Alteration of these minerals in contact with water leads to ‘serpentinization’, a process in which olivine and pyroxenes are transformed to serpentines. Serpentines like lizardite cannot accommodate all of the magnesium of the primary minerals, so brucite is formed as a separate mineral phase, often in veins of the serpentine, at temperatures below about 315 °C. Laboratory experiments of olivine alteration by our own research group show a spike of dissolved Mg2+ at about 25 ppm when the olivine surface is fresh and then a strong decrease in the concentration of the fluid phase with nucleation and precipitation of secondary Mg phases, such as brucite.
Magnesium as a Catalyst
Magnesium ions play critical roles in cellular metabolism. They stabilize structures of proteins, nucleic acids, and cell membranes by binding to the macromolecule’s surface. They are also key to enzymatic reactions in various ways; for instance, they can generate magnesium-substrate scaffolds to which enzymes bind. However, the best-known example of divalent Mg in life processes is probably the central role that it has in photosynthesis as a component of chlorophylls. Anoxygenic photosynthesis occurred by photosynthesizing anaerobic bacteria already before the existence of cyanobacteria. Reported evidence suggests that the photosystem in the anoxygenic purple photosynthetic bacteria is the most ancient known. However, divalent magnesium has many more functions in biological systems; of the many metal cations that form complexes with nucleotides, nucleic acids and enzymes, perhaps none is more essential than Mg2+ . According to the ‘RNA World’ hypothesis, the first enzymes – the ribozymes – consisted of ribonucleic acid (RNA), which depended on Mg2+ for its self-cleavage. Divalent magnesium is also present in all deoxyribonucleic acid (DNA) and RNA activation processes. These circumstances point to a central role for Mg in the geochemistry that presumably led to the first life-like processes.
BIOCHEMISTRY OF MAGNESIUM
It is indispensable for the nucleus to function as a whole and for the maintenance of physical stability as well as aggregation of rybosomes into polysomes able to initiate protein synthesis. As an essential cofactor in NER, BER, MMR processes, Mg2+ is required for the removal of DNA damage. An activator of over 300 different enzymes, magnesium participates in many metabolic processes, such as glycolysis, Krebs cycle, β-oxidation or ion transport across cell membranes. magnesium is indispensable for the functioning of the cell nucleus as a whole as it is involved in the activation of enzymes important for DNA repair (endonuclease)
Magnesium and DNA
More than half the magnesium contained in the nucleus is closely associated with nucleic acids and free nucleotides. Since nucleic acids are polyanions, they require counterions in order to neutralize negatively charged phosphate groups.
In ribosomes, Mg2+ is associated with rRNA or proteins, which are essential for the maintenance of physical stability as well as aggregation of these structures into polysomes able to initiate protein synthesis. Cowan has shown that magnesium deficit leads to the cleaving of a ribosomal complex. Since the only function performed by ribosomes is protein
biosynthesis, it is the presence of Mg2+ in ribosomes that conditions the shape of RNA structures by stimulating the transformation of amino acids into active forms, polypeptide synthesis and stabilization of a protein structure.
In 1976 LOEB et al. noted that magnesium ions are indispensable for DNA replication fidelity. Although Co2+, Mn2+ and Ni2+ ions can be substituted for Mg2+, such an exchange causes a considerable decrease in the fidelity of the discussed process.
Magnesium in metabolic cycles
In higher organisms, metabolic processes such as glycolysis, Krebs cycle, β-oxidation, active transport of ions or electrochemical coupling are regulated by Mg-dependent enzymes. The main domain of magnesium action is the activation of enzymes responsible for formation, storing and using of high-energy compounds. All reactions involving ATP require the presence of magnesium ions. Perfect confirmation of the key role of cellular magnesium is glycolysis, especially in human erythrocytes, as many enzymes involved in this process are Mg-dependent.
Magnesium and calcium ion transport
Magnesium ions are important for maintaining cell homeostasis because they are essential to the stabilization of cell membranes, to the activation of sodium-potassium pump (Na-K-ATP-ase) or calcium pump (Ca-ATP-ase), and to the regulation of composition of intra- and extracellular liquid.
Magnesium, Nucleotides, and Folding
Magnesium plays a special role in the function and folding of nucleotides. As the coordination geometry of Mg2+ is octahedral, it may coordinate six oxygen atoms of different oxyanions. The divalent Mg ion has an enhanced ability compared to other cations to form bidentate clamps with RNA, because the oxyanions of RNA phosphates have significant affinity for Mg2+. Unlike other cations, Mg2+ may bring oxygens from two different (charged) phosphate groups in its first shell into direct contact with each other. The coordination capacity of Mg2+ is attributed to its size, which makes it possible to simultaneously coordinate negatively charged oxygen of two phosphate groups. Divalent magnesium therefore normally coordinates two adjacent oxygen atoms of pyro- and triphosphate. This is also the reason why, during RNA-folding, Mg2+ can offset the phosphate–phosphate repulsion by being in a central position between the two groups. For the same reason, Mg2+ has been shown to be an extremely effective catalyst for the synthesis of 2′,3′-cyclic AMP and ATP in aqueous solution. Divalent magnesium was shown to accelerate this reaction approximately one hundred times more compared with systems with other cations, like Ca2+. The difference in this respect between Mg2+ and Ca2+ is attributed to the difference in ionic radius. The smaller magnesium ion (0.65 Å, coordination number CN = 6) can form a complex with two or three oxygen atoms of different phosphate groups in a phosphate ester more strongly than a divalent calcium ion (0.99 Å, CN=6).
Importance of Mg for life
Adequate magnesium intake is also important in maintaining glucose and insulin homeostasis. Magnesium is an essential mineral needed for activation of over 300 enzymes, glucose transportation between membranes, glucose oxidation, all reactions involving phosphorylation, energy exchange, and for the proper activity of insulin. 1
Magnesium (Mg) is an essential mineral found abundantly in whole grains, leafy green vegetables, legumes, and nuts that plays a central role in hundreds of physiological processes in the human body.
Biological action of magnesium has three important roles in human biological systems :
1. It is the basic “biological competitor” of calcium, antagonizing it in binding in cellular membranes
and proteins.
2. It is a fundamental cofactor in more than 300 essential enzymatic reactions related to the transportation,
storage and use of energy.
3. It regulates the passage of electrolytes and other substances through cellular membranes.
It is an essential cofactor in reactions involving phosphorylation; thus, magnesium de fi ciency could impair the insulin signal transduction pathway
Magnesium is an essential element for bone and plays a major role in bone and mineral homeostasis by functioning on all phases of skeletal metabolism. It directly affects bone cell function, in fl uences hydroxyapatite crystal formation and growth, takes part in normal activity of calcitropic hormones, and plays an important role in calcium metabolism
Magnesium is an essential element in many biological functions; TRPM6, the ion channel protein, regulates magnesium balance in the body by intestinal and renal mechanisms, respectively.
Mg is an essential element in many biological functions. It has been established that Mg is the fourth most abundant metal in living organisms. This element is distributed in three major compartments in the body: 65% in mineral phase of skeleton, 34% in the intracellular space, and 1% in the extracellular fluid.
Mg is an essential cofactor in many mechanisms of DNA repair such as the following: NER (nucleotide excision repair) and BER (base excision repair) by keeping many enzymes, for instance, nuclease, N-methylpurine-DNA glycosylase, metal-dependent endonuclease, activated when needed. Mg is a crucial factor for the action of ATPase, prevalent enzyme regulating cell energetics. In a physiological state, Mg together with potassium is bound in polynucleotides that stabilize the double helix of DNA. Moreover, both cations are critical factors for the maintenance of the chromatin structure in the compact state of so-called heterochromatin
Intake of Mg into cells
Uptake of magnesium is often blocked because the primary uptake mechanism, the calcium/magnesium ATPase enzyme is inhibited.2
Mg2+-importing ATPase
Magnesium transporter
Magnesium transporters are proteins that transport magnesium across the cell membrane. All forms of life require magnesium, yet the molecular mechanisms of Mg2+ uptake from the environment and the distribution of this vital element within the organism are only slowly being elucidated.
Three distinct classes of Mg2+ transporters have been identified in bacteria:
CorA,
MgtE,
MgtA
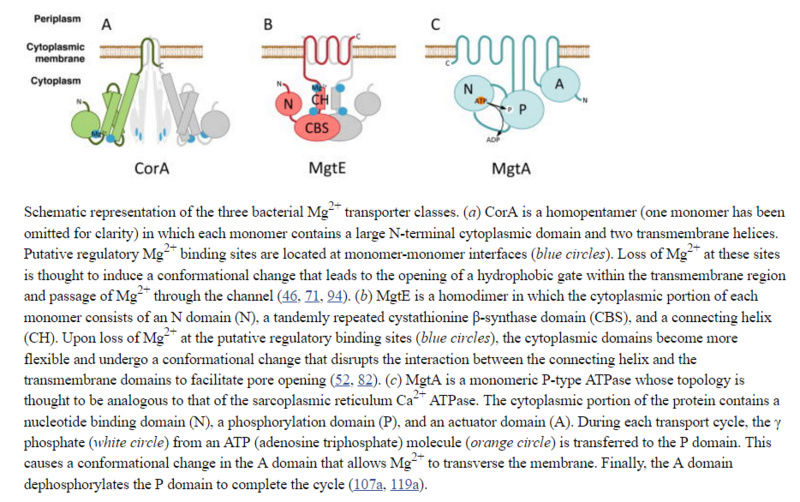
The magnesium transporter A is activated by cardiolipin and is highly sensitive to free magnesium in vitro
Magnesium is an essential element for living cells, meaning that organisms from bacteria to humans need magnesium to survive.
The magnesium transporter A (MgtA) is a specialized P-type ATPase, believed to import Mg2+ into the cytoplasm. In Salmonella typhimurium and Escherichia coli, the virulence determining two-component system PhoQ/PhoP regulates the transcription of mgtA gene by sensing Mg2+ concentrations in the periplasm. MgtA is highly dependent on anionic phospholipids and in particular, cardiolipin. Colocalization studies confirm that MgtA is found in the cardiolipin lipid domains in the membrane. The head group of cardiolipin plays major role in activation of MgtA suggesting that cardiolipin may act as a Mg2+ chaperone for MgtA. We further show that MgtA is highly sensitive to free Mg2+ (Mg2+free) levels in the solution. MgtA is activated when the Mg2+free concentration is reduced below 10 μM and is strongly inhibited above 1 mM, indicating that Mg2+free acts as product inhibitor. Combined, our findings conclude that MgtA may act as a sensor as well as a transporter of Mg2+.
All cells are surrounded by a membrane made of fatty molecules called lipids, which is also embedded with proteins. Magnesium, like other metal ions, is transported inside cells across the cell’s membrane by specific membrane proteins.
MgtA can sense magnesium ions down to micromolar concentrations, which is the equivalent to a pinch (1 gram) of magnesium salt in 10,000 liters of water. Wow !
The experiments also showed that this detection system depended on a specific lipid molecule in the membrane called cardiolipin. MgtA and cardiolipin were found together in the membrane of living E. coli suggesting that the two do indeed work together. The discovery that a membrane transporter that pumps ions needs cardiolipin to work suggests that cells could indirectly control the movement of ions by changing the levels of specific lipids in their membranes.
Cardiolipin
The magnesium transporter A is activated by cardiolipin and is highly sensitive to free magnesium in vitro
Anionic phospholipids, particularly cardiolipin (CL) are crucial for in vitro activation of MgtA.
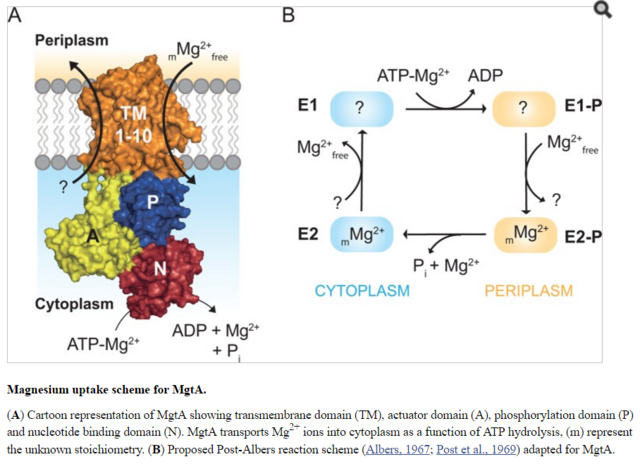
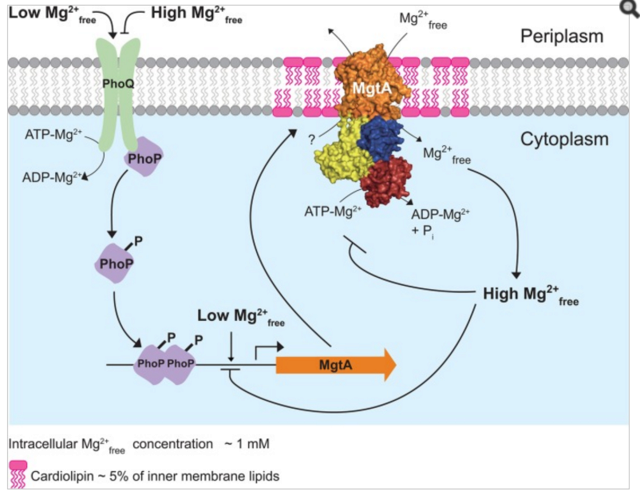
Model illustrating the regulation of Mg2+ uptake by MgtA.
When PhoQ senses low Mg2+free (<50 μM) in periplasm, it phosphorylates PhoP. This activates PhoP and it promotes transcription of the mgtA gene. MgtA protein is targeted to CL-rich region in bacterial inner membrane. Association of MgtA with CL is essential for its activity. MgtA imports Mg2+free available in periplasm to the cytoplasm of bacteria, thereby increasing cytoplasmic Mg2+free concentration. When the cytoplasmic Mg2+free concentration reaches a threshold (~1 mM), MgtA is inhibited both at the transcriptional and the post-translational level.
The evolution of cardiolipin biosynthesis and maturation pathways and its implications for the evolution of eukaryotes
Cardiolipin (CL) is an important component in mitochondrial inner and bacterial membranes. Its appearance in these two biomembranes has been considered as evidence of the endosymbiotic origin of mitochondria. But CL was reported to be synthesized through two distinct enzymes--CLS_cap and CLS_pld in eukaryotes and bacteria. Therefore, how the CL biosynthesis pathway evolved is an interesting question.
Its not just an interesting question. Its evidence against the endosymbiotic theory.
Complex evolution of two types of cardiolipin synthase in the eukaryotic lineage stramenopiles
The chimeric origin of the cardiolipin biosynthetic pathway in the Eukarya domain
Anionic phospholipids such as phosphatidylglycerol (PG) and cardiolipin (CL) are key components of cellular membranes, where they have important roles in several cellular processes such as energy transduction, stress response, participation in the mechanism of translation coupled to transcription (transertion) in bacteria and the stabilization, maintenance and segregation of mitochondrial DNA
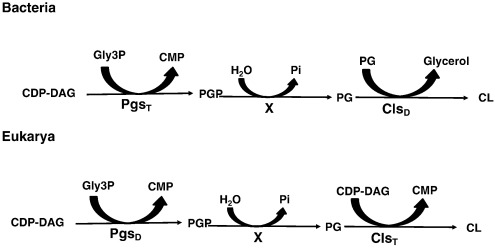
Fig. 1.
Cardiolipin biosynthesis in the Bacteria and Eukarya domain.
Although the first enzyme common for both domains uses the same substrates, the enzymatic mechanism varies between them. The bacterial enzyme is a CDP-DAG transferase whilst the eukaryote enzyme is a PLD. On the other hand, the Cls is a PLD in bacterial whilst a CDP-DAG transferase in Eukarya. The archeal pathway is believed to be identical to the bacterial. Pgs, phosphatidylglycerol phosphate synthase; Cls, cardiolipin synthase; CDP-DAG, cytidine diphosphate-diacylglycerol; PGP, phosphatidylglycerol phosphate; PG, phosphatidylglycerol; CMP, cytidine monophosphate. The X symbol represents a poorly conserved enzyme necessary for the hydrolysis of the ester bond between glycerol and phosphate from the head group.
Bacterial Mg2+ Homeostasis, Transport, and Virulence
Organisms must maintain physiological levels of Mg2+ because this divalent cation is critical for the stabilization of membranes and ribosomes, the neutralization of nucleic acids, and as a cofactor in a variety of enzymatic reactions. We describe the mechanisms that bacteria utilize to sense the levels of Mg2+ both outside and inside the cytoplasm. We examine how bacteria achieve Mg2+ homeostasis by adjusting the expression and activity of Mg2+ transporters, and by changing the composition of their cell envelope. We discuss the connections that exist between Mg2+ sensing, Mg2+ transport and bacterial virulence. Additionally, we explore the logic behind the fact that bacterial genomes encode multiple Mg2+ transporters and distinct sensing systems for cytoplasmic and extracytoplasmic Mg2+.
Mg2+ plays several essential roles, including stabilizing macromolecular complexes and membranes, neutralizing nucleic acids and nucleotides in the cytoplasm and phospholipid head groups and surface molecules outside the cytoplasm, and acting as a cofactor in a variety of enzymatic reactions. Certain cations can replace Mg2+ for some of these functions, but other activities strictly depend on Mg2+, indicating that cells must have mechanisms to maintain physiological levels of Mg2+.
Orthologous and nonorthologous Mg2+ transporters and Mg2+-responsive signal transduction systems have now been uncovered in a variety of bacterial species. These studies have established that bacteria possess the means to assess the levels of Mg2+, both in their surroundings and inside the cytoplasm, and to mount a response that helps maintain Mg2+ at the required levels. Such a response often entails modifying the amounts and/or activities of transporters that move Mg2+ from one compartment to another and of enzymes that chemically modify surface molecules harboring negative charges that are normally neutralized by Mg2+. The production of these proteins must be coordinated for a cell to survive and replicate in an environment that is limiting in Mg2+
HOW BACTERIA SENSE Mg2+
The Mg2+ sensor PhoQ from Salmonella harbors two transmembrane helices that delineate a periplasmic domain of 146 amino acids. This domain is required for PhoQ to respond to Mg2+ because a Salmonella strain harboring a chimeric PhoQ protein, containing the transmembrane and periplasmic domains from the sensor EnvZ fused to the cytoplasmic domain of PhoQ, expresses PhoP-activated genes in an Mg2+-independent fashion. Furthermore, as few as two amino acid substitutions in the periplasmic domain of PhoQ are sufficient to render this protein constitutively active (i.e., unresponsive to repression by Mg2+). A model for Mg2+ sensing based on the crystal structure of PhoQ from Salmonella has been proposed. According to this model, Mg2+ ions form metal bridges between a patch of negatively charged amino acid residues on PhoQ’s periplasmic domain and phospholipid head groups of the cytoplasmic membrane. This stabilizes PhoQ in a rigid conformation that promotes the unphosphorylated form of PhoP. Disruption of these metal bridges by removal of Mg2+ ions results in destabilization of this conformation, thereby furthering the phosphorylated form of PhoP (13). Mn2+ and Ca2+ can also silence the PhoP/PhoQ system during growth in defined media in vitro (13, 34). However, it is unclear whether the effect of these two cations is physiologically significant given that Salmonella is unlikely to experience high enough concentrations of Mn2+ and/or Ca2+ in its natural environment to silence the PhoP/PhoQ system.
Sensing Cytoplasmic Mg2+
In addition to the control of transcription initiation by extracytoplasmic Mg2+ levels, transcription elongation into the protein-coding regions of Mg2+ transporter genes can respond to the concentration of Mg2+ in the cytoplasm (19, 22). This enables expression of Mg2+ transporters to be shut down when Mg2+ needs in the cytoplasm have been met. Whereas bacteria depend on proteins to sense extracytoplasmic Mg2+, they use RNAs to detect Mg2+ in the cytoplasm. Specifically, the mRNAs corresponding to the Mg2+ transporter genes mgtA and mgtB in Salmonella and mgtE in Bacillus subtilis include long leader sequences that function as sensing devices for cytoplasmic Mg2+
1. (2013). Interrelations between Essential Metal Ions and Human Diseases. page 6
2. http://www.perque.com/wheybetterguard/wp-content/uploads/2013/05/PIH_MgCholinestudy-53012.pdf
https://reasonandscience.catsboard.com/t2440-magnesium-and-magnesium-transporters-another-example-of-cell-interdependence-comes-to-light
There are 24 metal and non metal elements , that are essential for life, amongst them magnesium, which plays a critical role in cellular metabolism, DNA repair, its also present in all deoxyribonucleic acid (DNA) and RNA activation processes, stabilizing macromolecular complexes and membranes. As activator of over 300 different enzymes, magnesium participates in many metabolic processes, such as glycolysis, Krebs cycle, β-oxidation or ion transport across cell membranes. Cells must have mechanisms to maintain physiological levels of Mg2+. It is indispensable for the nucleus ( in eukaryotes ) to function as a whole and for the maintenance of physical stability as well as aggregation of ribosomes into polysomes able to initiate protein synthesis. All these different essential roles elucidate that life could not have had a first go without magnesium.
But in order for the cell to be able to make use of it, Magnesium like other metal ions has to be transported inside cells across the cell membrane by specific membrane proteins. Three distinct classes of Mg2+ transporters have been identified in bacteria. MgtA transporter proteins can sense magnesium ions down to micromolar concentrations, which is the equivalent to a pinch (1 gram) of magnesium salt in 10,000 liters of water. Wow ! This detection system depends on a specific lipid molecule in the membrane called cardiolipin. MgtA and cardiolipin have to work together in an interdependent manner.
Organisms must maintain physiological levels of Mg2+ because this divalent cation is critical for the stabilization of membranes and ribosomes, the neutralization of nucleic acids, and as a cofactor in a variety of enzymatic reactions. Furthermore, specialized biosynthesis pathways and specialized proteins exist to make these import proteins and cardiolipin.
Question: How could cells have emerged gradually, if the cell membrane, magnesium uptake, regulation, use, and consequently transport/import proteins and magnesium regulation were not fully set up and existing right from the beginning? Had the cell membrane, transport proteins, cardiolipin that works in an interdependent manner with MgtA proteins, and the biosynthesis pathways to produce these proteins etc. not have to be fully setup from day one, ore life would not have had a beginning?
Is that not one more reason to believe that life had to start through cells fully setup and complex? Any gradual build-up would not be possible, giving one more good reason to believe life was designed, conceptualized, and made all at once by an intelligent creator.
Chemist Wilhelm Huck, professor at Radboud University Nijmegen :
"A working cell is more than the sum of its parts. "A functioning cell must be entirely correct at once, in all its complexity"
The significance of magnesium in prebiotic geochemistry
Magnesium
The significance of Mg in prebiotic geochemistry
Magnesium plays a special role in biochemistry because of its ability to coordinate six oxygen atoms efficiently in its first coordination shell. Such oxygen atoms may be part of one or two charged oxyanions, which means that Mg2+ can, for instance, tie together two different phosphate groups that are located at distance from each other in a macromolecule, and in this way be responsible for the folding of molecules like RNA. This property of Mg2+ also helps the stabilization of diphosphate and triphosphate groups of nucleotides, as well as promoting the condensation of orthophosphate to oligophosphates, like pyrophosphate and trimetaphosphate. Borates, on the other hand, are known to promote the formation of nucleobases and carbohydrates, ribose in particular, which is yet another constituent of nucleotides. The oldest borate minerals that we find on Earth today are magnesium borates. Dissolved borate stabilizes pentose sugars by forming complexes with cis-hydroxyl groups. In the furanose form of ribose, the preferential binding occurs to the 2 and 3 carbon, leaving the 5 carbon free for phosphorylation. The central role of Mg2+ in the function of ribozymes and its ‘archaic’ position in ribosomes, and the fact that magnesium generally has coordination properties different from other cations, suggests that the inorganic chemistry of magnesium had a key position in the first chemical processes leading to the origin and early evolution of life.
Magnesium (Mg) is a common element on Earth and the other terrestrial planets. It is one of the eight main elements of Earth’s crust and one of the four major elements making up the mass of the whole Earth. Furthermore, Mg is one of the principal constituents of silicate minerals that build up Earth, like olivine, pyroxenes, and Mg layer silicates (e.g., serpentines, talc, Mg smectites). The concentration of divalent magnesium (Mg2+) in contemporary ocean water is 52.8 mmol kg−1. The coordination geometry of magnesium is normally octahedral, that is, the Mg atom coordinates six atoms – almost always oxygen – around itself in its first coordination shell. Six-coordinated Mg2+ has a small ionic radius of 0.65 Å, at the same time as it has the largest hydrated radius of any common cation. The volume difference between hydrated and ionic Mg2+ is almost 400-fold. In the marine geochemical environment, magnesium is particularly important because the tri-octahedral layer of the common smectites in sediments consists primarily of brucite, the mineral name of magnesium hydroxide (Mg(OH)2). The ocean floor beneath the sediment layers consists of basalts and ultramafic rocks that have a high content of primary ferromagnesian silicate minerals (olivine and pyroxenes). Alteration of these minerals in contact with water leads to ‘serpentinization’, a process in which olivine and pyroxenes are transformed to serpentines. Serpentines like lizardite cannot accommodate all of the magnesium of the primary minerals, so brucite is formed as a separate mineral phase, often in veins of the serpentine, at temperatures below about 315 °C. Laboratory experiments of olivine alteration by our own research group show a spike of dissolved Mg2+ at about 25 ppm when the olivine surface is fresh and then a strong decrease in the concentration of the fluid phase with nucleation and precipitation of secondary Mg phases, such as brucite.
Magnesium as a Catalyst
Magnesium ions play critical roles in cellular metabolism. They stabilize structures of proteins, nucleic acids, and cell membranes by binding to the macromolecule’s surface. They are also key to enzymatic reactions in various ways; for instance, they can generate magnesium-substrate scaffolds to which enzymes bind. However, the best-known example of divalent Mg in life processes is probably the central role that it has in photosynthesis as a component of chlorophylls. Anoxygenic photosynthesis occurred by photosynthesizing anaerobic bacteria already before the existence of cyanobacteria. Reported evidence suggests that the photosystem in the anoxygenic purple photosynthetic bacteria is the most ancient known. However, divalent magnesium has many more functions in biological systems; of the many metal cations that form complexes with nucleotides, nucleic acids and enzymes, perhaps none is more essential than Mg2+ . According to the ‘RNA World’ hypothesis, the first enzymes – the ribozymes – consisted of ribonucleic acid (RNA), which depended on Mg2+ for its self-cleavage. Divalent magnesium is also present in all deoxyribonucleic acid (DNA) and RNA activation processes. These circumstances point to a central role for Mg in the geochemistry that presumably led to the first life-like processes.
BIOCHEMISTRY OF MAGNESIUM
It is indispensable for the nucleus to function as a whole and for the maintenance of physical stability as well as aggregation of rybosomes into polysomes able to initiate protein synthesis. As an essential cofactor in NER, BER, MMR processes, Mg2+ is required for the removal of DNA damage. An activator of over 300 different enzymes, magnesium participates in many metabolic processes, such as glycolysis, Krebs cycle, β-oxidation or ion transport across cell membranes. magnesium is indispensable for the functioning of the cell nucleus as a whole as it is involved in the activation of enzymes important for DNA repair (endonuclease)
Magnesium and DNA
More than half the magnesium contained in the nucleus is closely associated with nucleic acids and free nucleotides. Since nucleic acids are polyanions, they require counterions in order to neutralize negatively charged phosphate groups.
In ribosomes, Mg2+ is associated with rRNA or proteins, which are essential for the maintenance of physical stability as well as aggregation of these structures into polysomes able to initiate protein synthesis. Cowan has shown that magnesium deficit leads to the cleaving of a ribosomal complex. Since the only function performed by ribosomes is protein
biosynthesis, it is the presence of Mg2+ in ribosomes that conditions the shape of RNA structures by stimulating the transformation of amino acids into active forms, polypeptide synthesis and stabilization of a protein structure.
In 1976 LOEB et al. noted that magnesium ions are indispensable for DNA replication fidelity. Although Co2+, Mn2+ and Ni2+ ions can be substituted for Mg2+, such an exchange causes a considerable decrease in the fidelity of the discussed process.
Magnesium in metabolic cycles
In higher organisms, metabolic processes such as glycolysis, Krebs cycle, β-oxidation, active transport of ions or electrochemical coupling are regulated by Mg-dependent enzymes. The main domain of magnesium action is the activation of enzymes responsible for formation, storing and using of high-energy compounds. All reactions involving ATP require the presence of magnesium ions. Perfect confirmation of the key role of cellular magnesium is glycolysis, especially in human erythrocytes, as many enzymes involved in this process are Mg-dependent.
Magnesium and calcium ion transport
Magnesium ions are important for maintaining cell homeostasis because they are essential to the stabilization of cell membranes, to the activation of sodium-potassium pump (Na-K-ATP-ase) or calcium pump (Ca-ATP-ase), and to the regulation of composition of intra- and extracellular liquid.
Magnesium, Nucleotides, and Folding
Magnesium plays a special role in the function and folding of nucleotides. As the coordination geometry of Mg2+ is octahedral, it may coordinate six oxygen atoms of different oxyanions. The divalent Mg ion has an enhanced ability compared to other cations to form bidentate clamps with RNA, because the oxyanions of RNA phosphates have significant affinity for Mg2+. Unlike other cations, Mg2+ may bring oxygens from two different (charged) phosphate groups in its first shell into direct contact with each other. The coordination capacity of Mg2+ is attributed to its size, which makes it possible to simultaneously coordinate negatively charged oxygen of two phosphate groups. Divalent magnesium therefore normally coordinates two adjacent oxygen atoms of pyro- and triphosphate. This is also the reason why, during RNA-folding, Mg2+ can offset the phosphate–phosphate repulsion by being in a central position between the two groups. For the same reason, Mg2+ has been shown to be an extremely effective catalyst for the synthesis of 2′,3′-cyclic AMP and ATP in aqueous solution. Divalent magnesium was shown to accelerate this reaction approximately one hundred times more compared with systems with other cations, like Ca2+. The difference in this respect between Mg2+ and Ca2+ is attributed to the difference in ionic radius. The smaller magnesium ion (0.65 Å, coordination number CN = 6) can form a complex with two or three oxygen atoms of different phosphate groups in a phosphate ester more strongly than a divalent calcium ion (0.99 Å, CN=6).
Importance of Mg for life
Adequate magnesium intake is also important in maintaining glucose and insulin homeostasis. Magnesium is an essential mineral needed for activation of over 300 enzymes, glucose transportation between membranes, glucose oxidation, all reactions involving phosphorylation, energy exchange, and for the proper activity of insulin. 1
Magnesium (Mg) is an essential mineral found abundantly in whole grains, leafy green vegetables, legumes, and nuts that plays a central role in hundreds of physiological processes in the human body.
Biological action of magnesium has three important roles in human biological systems :
1. It is the basic “biological competitor” of calcium, antagonizing it in binding in cellular membranes
and proteins.
2. It is a fundamental cofactor in more than 300 essential enzymatic reactions related to the transportation,
storage and use of energy.
3. It regulates the passage of electrolytes and other substances through cellular membranes.
It is an essential cofactor in reactions involving phosphorylation; thus, magnesium de fi ciency could impair the insulin signal transduction pathway
Magnesium is an essential element for bone and plays a major role in bone and mineral homeostasis by functioning on all phases of skeletal metabolism. It directly affects bone cell function, in fl uences hydroxyapatite crystal formation and growth, takes part in normal activity of calcitropic hormones, and plays an important role in calcium metabolism
Magnesium is an essential element in many biological functions; TRPM6, the ion channel protein, regulates magnesium balance in the body by intestinal and renal mechanisms, respectively.
Mg is an essential element in many biological functions. It has been established that Mg is the fourth most abundant metal in living organisms. This element is distributed in three major compartments in the body: 65% in mineral phase of skeleton, 34% in the intracellular space, and 1% in the extracellular fluid.
Mg is an essential cofactor in many mechanisms of DNA repair such as the following: NER (nucleotide excision repair) and BER (base excision repair) by keeping many enzymes, for instance, nuclease, N-methylpurine-DNA glycosylase, metal-dependent endonuclease, activated when needed. Mg is a crucial factor for the action of ATPase, prevalent enzyme regulating cell energetics. In a physiological state, Mg together with potassium is bound in polynucleotides that stabilize the double helix of DNA. Moreover, both cations are critical factors for the maintenance of the chromatin structure in the compact state of so-called heterochromatin
Intake of Mg into cells
Uptake of magnesium is often blocked because the primary uptake mechanism, the calcium/magnesium ATPase enzyme is inhibited.2
Mg2+-importing ATPase
Magnesium transporter
Magnesium transporters are proteins that transport magnesium across the cell membrane. All forms of life require magnesium, yet the molecular mechanisms of Mg2+ uptake from the environment and the distribution of this vital element within the organism are only slowly being elucidated.
Three distinct classes of Mg2+ transporters have been identified in bacteria:
CorA,
MgtE,
MgtA

The magnesium transporter A is activated by cardiolipin and is highly sensitive to free magnesium in vitro
Magnesium is an essential element for living cells, meaning that organisms from bacteria to humans need magnesium to survive.
The magnesium transporter A (MgtA) is a specialized P-type ATPase, believed to import Mg2+ into the cytoplasm. In Salmonella typhimurium and Escherichia coli, the virulence determining two-component system PhoQ/PhoP regulates the transcription of mgtA gene by sensing Mg2+ concentrations in the periplasm. MgtA is highly dependent on anionic phospholipids and in particular, cardiolipin. Colocalization studies confirm that MgtA is found in the cardiolipin lipid domains in the membrane. The head group of cardiolipin plays major role in activation of MgtA suggesting that cardiolipin may act as a Mg2+ chaperone for MgtA. We further show that MgtA is highly sensitive to free Mg2+ (Mg2+free) levels in the solution. MgtA is activated when the Mg2+free concentration is reduced below 10 μM and is strongly inhibited above 1 mM, indicating that Mg2+free acts as product inhibitor. Combined, our findings conclude that MgtA may act as a sensor as well as a transporter of Mg2+.
All cells are surrounded by a membrane made of fatty molecules called lipids, which is also embedded with proteins. Magnesium, like other metal ions, is transported inside cells across the cell’s membrane by specific membrane proteins.
MgtA can sense magnesium ions down to micromolar concentrations, which is the equivalent to a pinch (1 gram) of magnesium salt in 10,000 liters of water. Wow !
The experiments also showed that this detection system depended on a specific lipid molecule in the membrane called cardiolipin. MgtA and cardiolipin were found together in the membrane of living E. coli suggesting that the two do indeed work together. The discovery that a membrane transporter that pumps ions needs cardiolipin to work suggests that cells could indirectly control the movement of ions by changing the levels of specific lipids in their membranes.
Cardiolipin
The magnesium transporter A is activated by cardiolipin and is highly sensitive to free magnesium in vitro
Anionic phospholipids, particularly cardiolipin (CL) are crucial for in vitro activation of MgtA.


Model illustrating the regulation of Mg2+ uptake by MgtA.
When PhoQ senses low Mg2+free (<50 μM) in periplasm, it phosphorylates PhoP. This activates PhoP and it promotes transcription of the mgtA gene. MgtA protein is targeted to CL-rich region in bacterial inner membrane. Association of MgtA with CL is essential for its activity. MgtA imports Mg2+free available in periplasm to the cytoplasm of bacteria, thereby increasing cytoplasmic Mg2+free concentration. When the cytoplasmic Mg2+free concentration reaches a threshold (~1 mM), MgtA is inhibited both at the transcriptional and the post-translational level.
The evolution of cardiolipin biosynthesis and maturation pathways and its implications for the evolution of eukaryotes
Cardiolipin (CL) is an important component in mitochondrial inner and bacterial membranes. Its appearance in these two biomembranes has been considered as evidence of the endosymbiotic origin of mitochondria. But CL was reported to be synthesized through two distinct enzymes--CLS_cap and CLS_pld in eukaryotes and bacteria. Therefore, how the CL biosynthesis pathway evolved is an interesting question.
Its not just an interesting question. Its evidence against the endosymbiotic theory.
Complex evolution of two types of cardiolipin synthase in the eukaryotic lineage stramenopiles
The chimeric origin of the cardiolipin biosynthetic pathway in the Eukarya domain
Anionic phospholipids such as phosphatidylglycerol (PG) and cardiolipin (CL) are key components of cellular membranes, where they have important roles in several cellular processes such as energy transduction, stress response, participation in the mechanism of translation coupled to transcription (transertion) in bacteria and the stabilization, maintenance and segregation of mitochondrial DNA

Fig. 1.
Cardiolipin biosynthesis in the Bacteria and Eukarya domain.
Although the first enzyme common for both domains uses the same substrates, the enzymatic mechanism varies between them. The bacterial enzyme is a CDP-DAG transferase whilst the eukaryote enzyme is a PLD. On the other hand, the Cls is a PLD in bacterial whilst a CDP-DAG transferase in Eukarya. The archeal pathway is believed to be identical to the bacterial. Pgs, phosphatidylglycerol phosphate synthase; Cls, cardiolipin synthase; CDP-DAG, cytidine diphosphate-diacylglycerol; PGP, phosphatidylglycerol phosphate; PG, phosphatidylglycerol; CMP, cytidine monophosphate. The X symbol represents a poorly conserved enzyme necessary for the hydrolysis of the ester bond between glycerol and phosphate from the head group.
Bacterial Mg2+ Homeostasis, Transport, and Virulence
Organisms must maintain physiological levels of Mg2+ because this divalent cation is critical for the stabilization of membranes and ribosomes, the neutralization of nucleic acids, and as a cofactor in a variety of enzymatic reactions. We describe the mechanisms that bacteria utilize to sense the levels of Mg2+ both outside and inside the cytoplasm. We examine how bacteria achieve Mg2+ homeostasis by adjusting the expression and activity of Mg2+ transporters, and by changing the composition of their cell envelope. We discuss the connections that exist between Mg2+ sensing, Mg2+ transport and bacterial virulence. Additionally, we explore the logic behind the fact that bacterial genomes encode multiple Mg2+ transporters and distinct sensing systems for cytoplasmic and extracytoplasmic Mg2+.
Mg2+ plays several essential roles, including stabilizing macromolecular complexes and membranes, neutralizing nucleic acids and nucleotides in the cytoplasm and phospholipid head groups and surface molecules outside the cytoplasm, and acting as a cofactor in a variety of enzymatic reactions. Certain cations can replace Mg2+ for some of these functions, but other activities strictly depend on Mg2+, indicating that cells must have mechanisms to maintain physiological levels of Mg2+.
Orthologous and nonorthologous Mg2+ transporters and Mg2+-responsive signal transduction systems have now been uncovered in a variety of bacterial species. These studies have established that bacteria possess the means to assess the levels of Mg2+, both in their surroundings and inside the cytoplasm, and to mount a response that helps maintain Mg2+ at the required levels. Such a response often entails modifying the amounts and/or activities of transporters that move Mg2+ from one compartment to another and of enzymes that chemically modify surface molecules harboring negative charges that are normally neutralized by Mg2+. The production of these proteins must be coordinated for a cell to survive and replicate in an environment that is limiting in Mg2+
HOW BACTERIA SENSE Mg2+
The Mg2+ sensor PhoQ from Salmonella harbors two transmembrane helices that delineate a periplasmic domain of 146 amino acids. This domain is required for PhoQ to respond to Mg2+ because a Salmonella strain harboring a chimeric PhoQ protein, containing the transmembrane and periplasmic domains from the sensor EnvZ fused to the cytoplasmic domain of PhoQ, expresses PhoP-activated genes in an Mg2+-independent fashion. Furthermore, as few as two amino acid substitutions in the periplasmic domain of PhoQ are sufficient to render this protein constitutively active (i.e., unresponsive to repression by Mg2+). A model for Mg2+ sensing based on the crystal structure of PhoQ from Salmonella has been proposed. According to this model, Mg2+ ions form metal bridges between a patch of negatively charged amino acid residues on PhoQ’s periplasmic domain and phospholipid head groups of the cytoplasmic membrane. This stabilizes PhoQ in a rigid conformation that promotes the unphosphorylated form of PhoP. Disruption of these metal bridges by removal of Mg2+ ions results in destabilization of this conformation, thereby furthering the phosphorylated form of PhoP (13). Mn2+ and Ca2+ can also silence the PhoP/PhoQ system during growth in defined media in vitro (13, 34). However, it is unclear whether the effect of these two cations is physiologically significant given that Salmonella is unlikely to experience high enough concentrations of Mn2+ and/or Ca2+ in its natural environment to silence the PhoP/PhoQ system.
Sensing Cytoplasmic Mg2+
In addition to the control of transcription initiation by extracytoplasmic Mg2+ levels, transcription elongation into the protein-coding regions of Mg2+ transporter genes can respond to the concentration of Mg2+ in the cytoplasm (19, 22). This enables expression of Mg2+ transporters to be shut down when Mg2+ needs in the cytoplasm have been met. Whereas bacteria depend on proteins to sense extracytoplasmic Mg2+, they use RNAs to detect Mg2+ in the cytoplasm. Specifically, the mRNAs corresponding to the Mg2+ transporter genes mgtA and mgtB in Salmonella and mgtE in Bacillus subtilis include long leader sequences that function as sensing devices for cytoplasmic Mg2+
1. (2013). Interrelations between Essential Metal Ions and Human Diseases. page 6
2. http://www.perque.com/wheybetterguard/wp-content/uploads/2013/05/PIH_MgCholinestudy-53012.pdf
Last edited by Admin on Mon Feb 20, 2017 11:06 am; edited 5 times in total

