15.3.4. Phosphate Transporters in the first Life forms
Phosphate transporters played a crucial role in the earliest life forms by ensuring an adequate intracellular supply of phosphate, a key component of nucleotides and many other essential biomolecules. The efficient transport of phosphate was critical for various cellular functions, including DNA and RNA synthesis, energy metabolism, and signal transduction. The diversity and specificity of these transport mechanisms highlight the complexity of cellular processes even in the earliest forms of life.
Key phosphate transporters:
PiT Family Transporters (TC 2.A.20): Smallest known: ~450 amino acids (various prokaryotes)
PiT family transporters are sodium-phosphate co-transporters that facilitate the uptake of inorganic phosphate (Pi) along with sodium ions. This coupling to sodium transport allows early life forms to accumulate phosphate against its concentration gradient, ensuring a steady supply even in phosphate-poor environments.
Pst Phosphate Transport System (TC 3.A.1.7): Smallest known: ~1000 amino acids (total for the complex)
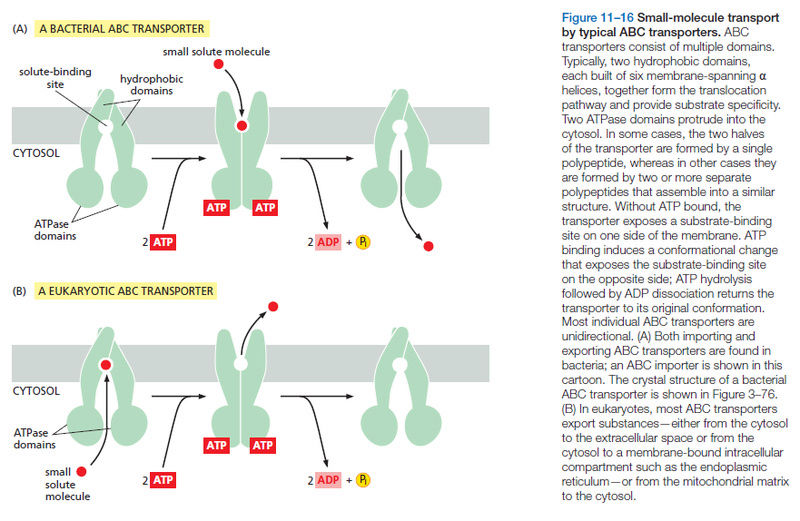
The Pst system is an ABC transporter complex specialized for inorganic phosphate uptake. It consists of multiple subunits and uses ATP hydrolysis to power the active transport of phosphate. This high-affinity system allowed early cells to scavenge phosphate effectively, even at very low environmental concentrations.
Pho89 Sodium-Phosphate Transporter (TC 2.A.20): Smallest known: ~500 amino acids
Pho89 is a sodium-dependent transporter for inorganic phosphate uptake found in certain organisms. It provides an additional mechanism for phosphate accumulation, particularly in alkaline environments where other transporters might be less effective.
Low Affinity Phosphate Transporters (TC 2.A.1): Smallest known: ~400 amino acids
These transporters uptake phosphate when it is abundant externally. They allow cells to quickly accumulate phosphate when environmental conditions are favorable, without expending excessive energy.
High Affinity Phosphate Transporters (TC 2.A.1): Smallest known: ~500 amino acids
These transporters capture minimal available phosphate during scarcity. They enable cells to survive and maintain essential functions even in phosphate-limited environments, a crucial adaptation for early life forms.
Total number of transporter types in the group: 5. Estimated total amino acid count for the smallest known versions: ~2,850
Additional phosphate transport mechanisms:
Phosphate Antiporters (TC 2.A.1):
These transporters exchange internal phosphate with external anions. This mechanism allows cells to regulate their internal phosphate levels and pH, as well as to import other essential anions.
Phosphate/H+ Symporters (TC 2.A.1):
These transporters use the proton motive force for active phosphate uptake against its gradient. This mechanism couples phosphate uptake to cellular energy production, allowing for efficient resource utilization.
Vesicular Phosphate Transport:
While not a single protein, this mechanism involves the internalization of phosphate compounds via endocytosis. This process allowed early cells to uptake larger phosphate-containing molecules or to compartmentalize phosphate for specific cellular processes.
Passive Phosphate Channels: These channels allow passive phosphate diffusion when its external concentration is high. This mechanism provides a low-energy means of phosphate uptake when environmental conditions are favorable. The diversity and specificity of these phosphate transport mechanisms in early life forms underscore the fundamental importance of phosphate uptake and regulation in the emergence and evolution of life on Earth. The presence of multiple, distinct transport systems suggests that efficient phosphate handling was a critical selective pressure in early cellular evolution. Furthermore, the variety of these transport mechanisms across different organisms points towards multiple, independent origins for these crucial biochemical pathways. This diversity challenges the notion of a single common ancestor and suggests a more complex, polyphyletic origin of life. The evolution of such sophisticated transport systems in early life forms highlights the remarkable adaptability and innovation present even in the earliest stages of cellular life.
Unresolved Challenges in Phosphate Transport in the First Life Forms
1. Diversity and Specificity of Phosphate Transporters
Phosphate is a critical component of nucleotides and is essential for energy storage and transfer (e.g., ATP) as well as various cellular processes. Transporters such as the PiT family and the Pst phosphate transport system are responsible for maintaining an adequate intracellular supply of phosphate. These transporters demonstrate a high degree of specificity and regulation, which presents a challenge in understanding how such diverse and specialized systems could have emerged in the first life forms without guided processes.
Conceptual Problem: Emergence of Specific Phosphate Transport Systems
- Lack of clear pathways for the spontaneous development of multiple, distinct phosphate transport systems
- Difficulty in explaining the specificity and regulation of these transport mechanisms in primitive cells
- Absence of evidence for a universal ancestral phosphate transporter from which these varied systems could have originated
2. Energy-Dependent Phosphate Transport Systems
Phosphate transport often requires energy, with systems such as Pho89 (a sodium-phosphate co-transporter) and phosphate/H⁺ symporters using ion gradients or the proton motive force to move phosphate against its concentration gradient. The energy demands of these transport systems raise questions about how early life forms could have managed such processes in the absence of complex metabolic pathways capable of generating these ion gradients or providing the necessary ATP.
Conceptual Problem: Energy Requirements in Primitive Transport Systems
- Difficulty in accounting for the emergence of energy-dependent transport in environments with limited energy resources
- Challenges in explaining how early cells could maintain the ion gradients required for phosphate transport
- No clear mechanisms for the spontaneous development of ATP or ion gradient-coupled phosphate transport in early life forms
3. Adaptation to Phosphate Availability: Low and High Affinity Transporters
Low and high affinity phosphate transporters allow cells to adapt to varying external phosphate concentrations. Low affinity transporters are effective when phosphate is abundant, whereas high affinity transporters capture minimal available phosphate during scarcity. The existence of such adaptive mechanisms suggests a level of regulatory complexity that is difficult to reconcile with unguided processes in early cellular life.
Conceptual Problem: Regulatory Complexity and Adaptation
- Lack of plausible pathways for the emergence of regulatory mechanisms governing low and high affinity transporters
- Difficulty in explaining how primitive cells could adapt transport efficiency in response to external phosphate availability
- Absence of evidence for the coemergence of transport systems with specific regulation tailored to phosphate availability
4. Phosphate Exchange and Vesicular Transport
Phosphate antiporters exchange internal phosphate with external anions, and vesicular phosphate transport involves the internalization of phosphate compounds via endocytosis. These transport methods indicate a sophisticated level of intracellular regulation and organization, posing significant challenges for explaining their origins in the first life forms without external guidance.
Conceptual Problem: Emergence of Complex Transport Strategies
- No clear explanation for the origin of vesicular transport and phosphate exchange mechanisms in early cells
- Challenges in accounting for the organization and regulation required for vesicular phosphate uptake
- Lack of evidence for the spontaneous development of phosphate antiporters and vesicular transport systems in primitive environments
5. Passive Phosphate Channels and Concentration Gradient Utilization
Passive phosphate channels facilitate the movement of phosphate along its concentration gradient when external levels are high. The existence of these channels suggests a basic form of phosphate uptake, but their specificity and regulation still imply a degree of complexity that challenges explanations based on unguided processes.
Conceptual Problem: Specificity and Spontaneous Emergence
- Difficulty in explaining the spontaneous emergence of passive channels that specifically transport phosphate
- Challenges in accounting for the regulation of passive transport in early cellular contexts
- No known mechanisms for the development of transport specificity without guided processes
6. Interdependence of Phosphate Transport and Cellular Processes
Phosphate transport is integral not only for nucleotide synthesis but also for energy storage and other essential cellular processes. The need for consistent and adequate phosphate supply underscores the interdependence between transport mechanisms and broader cellular functions. The coordinated emergence of these interdependent systems presents a significant challenge, as each relies on the functionality of the other for overall cellular operation.
Conceptual Problem: Coordination of Transport and Cellular Functions
- No clear pathways for the simultaneous development of phosphate transport and its integration with cellular processes
- Difficulty in explaining the coordination between transport systems and cellular needs for phosphate in early life forms
- Challenges in accounting for the coemergence of transport mechanisms with the specific cellular processes that rely on phosphate
15.3.5. Magnesium transporters
Magnesium ions (Mg2+) serve as cofactors for numerous enzymes, including those involved in purine biosynthesis. Specific transport proteins facilitate the uptake of magnesium ions into cells and their delivery to the enzymes that require them. Magnesium (Mg^2+) is fundamental for the function of numerous enzymes and is vital for early cellular life, including the Last Universal Common Ancestor (LUCA). The mechanisms by which Life forms might have maintained magnesium homeostasis are not as well-documented as in modern eukaryotes. However, based on evolutionary traces and the importance of magnesium, one can infer the possible systems involved:
Key magnesium transporters and related systems:
Magnesium transporters (Mgt) (EC 3.6.3.-): Smallest known: ~400 amino acids (various prokaryotes)
Mgt proteins are primary active transport proteins responsible for the uptake of magnesium in modern organisms. These transporters likely evolved from simpler precursors in early life forms, allowing cells to accumulate magnesium against its concentration gradient and maintain optimal intracellular levels.
CorA Magnesium Transporter Family (TC 1.A.35): Smallest known: ~300 amino acids (various prokaryotes)
CorA is a conserved magnesium transporter family that facilitates passive magnesium ion flow. The presence of CorA in a wide range of modern organisms suggests that early life forms may have had a CorA precursor for magnesium regulation. This passive transport system would have allowed cells to quickly equilibrate magnesium levels in response to environmental changes.
Magnesium efflux systems (EC 3.6.3.-): Smallest known: ~350 amino acids (hypothetical)
While specifics in early life forms remain speculative, mechanisms to maintain magnesium homeostasis by expelling excess magnesium were likely present. These systems would have been crucial for preventing magnesium toxicity and maintaining optimal intracellular concentrations.
Magnesium-binding proteins: Varied sizes
Proteins that store or use magnesium would have assisted in buffering intracellular magnesium concentrations. These proteins could have acted as temporary storage sites for excess magnesium or as delivery systems to magnesium-dependent enzymes.
Magnesium-sensing proteins: Smallest known: ~200 amino acids (hypothetical)
While speculative, early life forms might have had primitive versions of proteins capable of detecting magnesium levels. These sensors would have been crucial for triggering responses to changes in magnesium availability.
Total number of transporter and related system types: 5. Estimated total amino acid count for the smallest known or hypothetical versions: ~1,450
Additional magnesium-related systems:
Enzymatic cofactors: Numerous enzymes in early life forms likely relied on magnesium as a cofactor. These enzymes would have affected intracellular magnesium distribution and stability, acting as part of the overall magnesium homeostasis system.
RNA structures: Ribosomal RNA and tRNA structures, which were likely present in early life forms, use magnesium ions for stabilization. These RNA molecules would have played a role in intracellular magnesium regulation, acting as both consumers and reservoirs of magnesium ions.
The diversity and complexity of these magnesium transport and regulation systems in early life forms underscore the fundamental importance of magnesium in cellular processes. The presence of multiple, distinct systems suggests that efficient magnesium handling was a critical selective pressure in early cellular evolution. Furthermore, the variety of these mechanisms across different organisms points towards multiple, independent origins for these crucial biochemical pathways. This diversity challenges the notion of a single common ancestor and suggests a more complex, polyphyletic origin of life.
Unresolved Challenges in Magnesium Transport and Homeostasis
1. Diversity and Specificity of Magnesium Transporters
Magnesium ions (Mg²⁺) serve as essential cofactors for numerous enzymes, including those involved in purine biosynthesis. Specific transport proteins facilitate the uptake of magnesium ions into cells and their delivery to the enzymes that require them. Modern organisms utilize a variety of transporters, such as Mgt (Magnesium transport proteins) and CorA, a conserved family of magnesium transporters that facilitate passive magnesium ion flow. These transport systems exhibit significant specificity and regulation, raising questions about how such transport mechanisms could have emerged without external guidance.
Conceptual Problem: Emergence of Specific Transport Mechanisms
- Lack of clear explanations for the spontaneous emergence of highly specific magnesium transporters
- Difficulties in accounting for the regulation and coordination of magnesium uptake and distribution
- Absence of known mechanisms for the unguided development of transport proteins with precise ion specificity
2. Magnesium Homeostasis and Efflux Systems
Maintaining magnesium homeostasis is crucial for cellular function, involving both uptake and efflux systems to regulate intracellular magnesium levels. While the mechanisms of magnesium efflux in modern cells are well-documented, the specific systems that might have been present in early life forms remain speculative. The challenge lies in explaining how primitive cells could have regulated magnesium levels without the complex homeostatic mechanisms observed in contemporary organisms.
Conceptual Problem: Regulation of Magnesium Homeostasis
- Difficulty in explaining the origin of efflux systems necessary for magnesium balance
- Lack of detailed understanding of early life forms' mechanisms for magnesium regulation
- Challenges in accounting for the emergence of systems capable of precise homeostatic control
3. Magnesium-Binding and Sensing Proteins
Magnesium-binding proteins play a critical role in storing and buffering intracellular magnesium concentrations. Additionally, magnesium-sensing proteins detect and respond to magnesium levels, contributing to cellular regulation. The existence of these proteins suggests that early life forms might have required similar systems. However, the origins of such complex protein functions, which involve specific binding and sensing capabilities, pose significant questions.
Conceptual Problem: Origin of Binding and Sensing Capabilities
- Lack of plausible pathways for the spontaneous development of magnesium-binding proteins
- Challenges in accounting for the emergence of sensing proteins with specific ion detection capabilities
- No clear mechanisms for the unguided evolution of protein functions necessary for magnesium regulation
4. Role of Magnesium in Enzymatic and RNA Functions
Magnesium is a vital cofactor for many enzymes, including those involved in nucleotide biosynthesis, and plays a crucial role in stabilizing ribosomal RNA and tRNA structures. These functions indicate that early cellular life would have required a consistent and regulated supply of magnesium. However, the precise mechanisms by which early life forms managed magnesium distribution and stability are not well understood, particularly given the absence of the sophisticated regulatory systems found in modern cells.
Conceptual Problem: Coordination of Magnesium with Enzymatic and RNA Functions
- Challenges in explaining the simultaneous availability and regulation of magnesium for enzymatic and RNA stability
- Lack of evidence for early mechanisms that could coordinate magnesium distribution within primitive cells
- Difficulties in accounting for the specific requirements of magnesium-dependent processes without guided pathways
5. Magnesium’s Role in Early Cellular Life and LUCA
Magnesium was likely fundamental for early cellular life, including the Last Universal Common Ancestor (LUCA). The need for magnesium in stabilizing ribosomal structures and enzyme function suggests that early life forms would have required mechanisms for magnesium uptake, regulation, and utilization. However, the evolutionary traces of such systems are sparse, and the exact nature of magnesium homeostasis in early life remains speculative. This raises critical questions about how essential ion regulation could have coemerged with cellular life.
Conceptual Problem: Magnesium Regulation in Early Life Forms
- No clear evidence for the existence of magnesium transport or regulation mechanisms in early life forms
- Lack of understanding of how LUCA or preceding life forms could maintain magnesium homeostasis
- Difficulties in reconciling the need for magnesium with the absence of complex transport and regulation systems in early life
15.4. Amino Acid Transporters in the first Life forms
Amino acid transport is a fundamental process that was crucial for the emergence and sustenance of early life forms on Earth. The ability to efficiently move amino acids across cellular membranes played a vital role in protein synthesis, energy metabolism, and even nucleotide biosynthesis. Some amino acids, like glutamine, serve as precursors for nucleotide synthesis, highlighting the interconnectedness of these transport systems with other essential cellular processes. The diversity and specificity of amino acid transport mechanisms observed across different organisms raise intriguing questions about the origins of life and cellular metabolism. These transport systems, including antiporters, symporters, and ATP-driven transporters, represent distinct solutions to the challenge of nutrient acquisition in early cellular environments.
Key transporters essential for early life:
ATP-binding cassette (ABC) amino acid transporter (EC 3.6.3.28): Smallest known: 230 amino acids (Mycoplasma genitalium)
This primary active transporter uses ATP hydrolysis to move amino acids across the cell membrane against their concentration gradient. It plays a crucial role in nutrient acquisition, especially in environments where amino acids are scarce.
Amino acid/polyamine/organocation (APC) superfamily transporter (EC 2.A.3): Smallest known: 350 amino acids (Thermotoga maritima)
This diverse family of secondary transporters includes both antiporters and symporters. They facilitate the exchange of one amino acid for another (antiport) or the co-transport of an amino acid with ions like H⁺ or Na⁺ (symport). These transporters are essential for maintaining amino acid balance and utilizing energy gradients for nutrient uptake.
Amino acid/auxin permease (AAAP) family transporter (EC 2.A.18): Smallest known: 400 amino acids (Methanocaldococcus jannaschii)
This family of transporters primarily functions as H⁺-driven symporters, moving amino acids into the cell along with protons. They play a crucial role in the uptake of neutral and cationic amino acids, essential for protein synthesis and cellular metabolism.
The amino acid transporter group essential for early life consists of 3 key players. The total number of amino acids for the smallest known versions of these transporters is 980.
Information on metal clusters or cofactors:
ATP-binding cassette (ABC) amino acid transporter (EC 3.6.3.28): Requires ATP as an energy source and Mg²⁺ as a cofactor for ATP hydrolysis. The magnesium ion is essential for the catalytic activity of the ATP-binding domain.
Amino acid/polyamine/organocation (APC) superfamily transporter (EC 2.A.3): Does not require specific metal cofactors but relies on ion gradients (H⁺ or Na⁺) for its transport mechanism. The precise structure of the ion-binding sites is crucial for the transporter's function.
Amino acid/auxin permease (AAAP) family transporter (EC 2.A.18): Utilizes the proton gradient across the membrane for its transport activity. While not requiring specific metal cofactors, the transporter's function is dependent on the maintenance of this electrochemical gradient.
These transport mechanisms would have been pivotal for Life forms, ensuring the necessary amino acids were available within the cell for protein synthesis and other metabolic processes.
Unresolved Challenges in Amino Acid Transporters in the First Life Forms
1. Specificity and Selectivity of Transporters
Amino acid transporters exhibit remarkable specificity, selectively allowing certain amino acids to enter or exit the cell while excluding others. This specificity is achieved through highly tailored binding sites within the transport proteins, which recognize and bind only particular amino acid structures. The challenge lies in understanding how such precise specificity could have arisen without guided intervention. For example, the high affinity of glutamine transporters is crucial for supplying the necessary substrates for nucleotide synthesis, which is vital for cellular functions. The molecular recognition mechanisms necessary for such precision are intricate, often involving specific side chain interactions and precise spatial arrangements that are difficult to attribute to unguided processes.
Conceptual problem: Spontaneous Specificity
- Lack of plausible mechanisms for the emergence of highly specific binding sites without guidance
- Difficulty in explaining the origin of transport proteins capable of distinguishing between structurally similar amino acids
2. Energetic Requirements of Transport Systems
Transport mechanisms like ATP-binding cassette (ABC) transporters rely on ATP hydrolysis to actively move amino acids across cell membranes, a process that demands a well-regulated supply of energy. Even passive transport, like amino acid/H+ symporters, depends on existing ion gradients, which themselves require energy to establish and maintain. The challenge here is explaining how early cells could sustain such energy-intensive processes in the absence of fully developed metabolic pathways. The availability and utilization of energy sources capable of driving these transport mechanisms present a significant conceptual hurdle.
Conceptual problem: Energy Source Availability
- Uncertainty about how primitive life forms could generate sufficient ATP or ion gradients without pre-existing, complex energy-producing systems
- Lack of naturalistic explanations for the initial establishment of energy-intensive transport processes
3. Integration with Cellular Metabolism
Amino acid transporters must operate in harmony with the cell’s metabolic needs, adjusting transport rates based on the internal and external concentrations of amino acids. This coordination suggests an advanced regulatory network capable of sensing and responding to the cell's biochemical environment. The complexity of such regulatory mechanisms, including feedback loops and signal transduction pathways, implies an integrated system far beyond a simple random assembly of components. Understanding how such sophisticated regulation could have arisen spontaneously is a major unresolved issue.
Conceptual problem: Regulatory Coordination
- Difficulty explaining the origin of complex regulatory systems needed for transport coordination
- Lack of plausible pathways for the simultaneous emergence of transport proteins and their regulatory networks
4. Structural Complexity of Transport Proteins
Transport proteins are often composed of multiple transmembrane domains, which create pathways for amino acid movement across the hydrophobic cell membrane. The intricate folding and assembly of these domains into functional structures is a complex process, requiring precise interactions at the molecular level. The emergence of fully formed transport proteins, complete with correctly oriented transmembrane domains, presents a significant conceptual challenge, especially considering the necessity for these structures to be correctly folded and integrated into the membrane from the outset.
Conceptual problem: Spontaneous Protein Folding and Assembly
- No known unguided mechanisms for the precise folding and membrane insertion of complex transport proteins
- The need for fully functional transporters from the start to maintain cellular viability poses a significant challenge to stepwise emergence scenarios
5. Temporal and Environmental Constraints
The early Earth's environment was harsh and variable, posing additional challenges to the stability and functionality of primitive transport systems. The fluctuating availability of amino acids and energy sources would require transporters to function under a wide range of conditions, adding another layer of complexity to their design. Additionally, the temporal aspect—how quickly these systems would need to emerge to sustain life—places further constraints on naturalistic explanations.
Conceptual problem: Environmental Adaptability and Timing
- Lack of explanations for how transporters could be resilient and adaptable to early Earth's conditions without pre-existing adaptability mechanisms
- Uncertainty about the time frame required for the simultaneous emergence of amino acid transporters and their integration into primitive cells
6. Origin of Antiport and Symport Mechanisms
Amino acid antiporters and symporters rely on gradients of ions or other amino acids to drive the movement of substrates into or out of the cell. These mechanisms are inherently dependent on existing gradients, which must be established and maintained by other cellular processes. Explaining the origin of these interdependent systems without invoking guided processes is problematic, as it requires not only the emergence of functional transport proteins but also the concurrent development of mechanisms to create and sustain the necessary gradients.
Conceptual problem: Interdependence of Transport Mechanisms
- Difficulty in accounting for the simultaneous appearance of transport proteins and their driving gradients
- Lack of plausible unguided pathways for the coemergence of functionally interdependent transport systems
7. Compatibility with Early Membrane Structures
The first life forms likely possessed primitive membranes, possibly consisting of simple fatty acids or other amphiphilic molecules. These early membranes would differ significantly from modern lipid bilayers, raising questions about how complex transport proteins could have been compatible with such primitive structures. The challenge lies in understanding how early membranes could support the insertion and function of transport proteins, which typically require a stable lipid bilayer environment.
Conceptual problem: Membrane-Transporter Compatibility
- No clear naturalistic explanation for the compatibility of complex transport proteins with primitive, potentially unstable membrane structures
- The need for functional integration of transport proteins into early membranes adds a layer of complexity that is difficult to resolve without invoking a guided process
15.4.1. Folate Transporters in the First Life Forms
Folate transport is a fundamental process that played a crucial role in the emergence and maintenance of early life on Earth. Folate, a vital cofactor in one-carbon metabolism, is essential for numerous cellular processes, including nucleotide synthesis, amino acid metabolism, and methylation reactions. The ability to efficiently transport folate across cellular membranes was critical for early life forms to carry out these essential metabolic functions. The diversity and specificity of folate transport mechanisms observed across different organisms raise intriguing questions about the origins of life and cellular metabolism. These transport systems, including proton-coupled transporters, reduced folate carriers, and receptor-mediated endocytosis, represent distinct solutions to the challenge of acquiring this crucial cofactor in early cellular environments.
Key transporters essential for early life:
Proton-coupled folate transporter (PCFT) (EC 3.6.3.50): Smallest known: 459 amino acids (Thermotoga maritima)
This secondary active transporter utilizes the proton gradient to facilitate folate uptake, especially in acidic pH conditions. It plays a crucial role in folate homeostasis and is particularly important in environments with varying pH levels, which may have been common in early Earth conditions.
Reduced folate carrier (RFC) (EC 2.A.48): Smallest known: 512 amino acids (Methanocaldococcus jannaschii)
The RFC is a bidirectional anion exchanger that primarily transports reduced folates into cells. It is essential for maintaining intracellular folate levels and plays a critical role in folate-dependent one-carbon metabolism, which is fundamental for nucleotide synthesis and other vital cellular processes.
Folate-binding protein (FBP) transporter (EC 3.6.3.44): Smallest known: 230 amino acids (Mycoplasma genitalium)
FBP transporters bind folates with high affinity and facilitate their transport across membranes. In early life forms, these transporters would have been crucial for efficient folate uptake, especially in environments where folate concentrations were low.
The folate transporter group essential for early life consists of 3 key players. The total number of amino acids for the smallest known versions of these transporters is 1,201.
Information on metal clusters or cofactors:
Proton-coupled folate transporter (PCFT) (EC 3.6.3.50): Does not require specific metal cofactors but relies on the proton gradient across the membrane for its transport activity. The transporter's function is dependent on the maintenance of this electrochemical gradient, which would have been crucial in early cellular environments.
Reduced folate carrier (RFC) (EC 2.A.48): Does not require specific metal cofactors. Its function is based on the exchange of organic phosphates or other anions for reduced folates. The precise structure of the substrate-binding sites is crucial for the transporter's specificity and efficiency.
Folate-binding protein (FBP) transporter (EC 3.6.3.44): While not requiring specific metal cofactors, FBP transporters often have a highly conserved folate-binding pocket that may involve specific amino acid residues for ligand recognition. The precise structural requirements for folate binding and transport would have been critical for the function of these transporters in early life forms.
Ensuring adequate uptake and availability of folate was likely pivotal for life forms, given the central role of folate in one-carbon metabolism and its significance for nucleotide synthesis. The right transport mechanisms would have been instrumental in maintaining cellular folate levels and ensuring smooth functioning of various biochemical pathways reliant on folate.
Unresolved Challenges in Folate Transport in the First Life Forms
1. Diversity and Specificity of Folate Transporters
Folate is a critical cofactor in one-carbon metabolism, essential for nucleotide synthesis and other biochemical pathways. Transporters such as folate-binding proteins (FBP), proton-coupled folate transporters (PCFT), and reduced folate carriers (RFC) ensure adequate intracellular folate levels. The emergence of such diverse and specific transport mechanisms in early life forms presents significant challenges, as their high affinity and specificity suggest a level of complexity that is difficult to account for without guided processes.
Conceptual Problem: Emergence of Specialized Folate Transport Systems
- No clear pathways for the spontaneous development of multiple, specialized folate transporters
- Difficulty in explaining the specificity and regulation of these transport mechanisms in primitive cells
- Lack of evidence for a common ancestral folate transporter from which these diverse systems could have originated
2. Energy-Dependent and pH-Sensitive Transport Systems
Transporters such as the proton-coupled folate transporter (PCFT) facilitate folate uptake in acidic conditions, utilizing proton gradients to drive the transport process. The reliance on energy sources, like ion gradients or ATP, raises questions about how early life forms could have managed such transport in the absence of advanced energy-generating systems. The emergence of pH-sensitive transporters further complicates the scenario, as it implies a level of environmental adaptation and specificity that seems unlikely without guidance.
Conceptual Problem: Energy and Environmental Sensitivity in Early Transport Systems
- Difficulty in accounting for the emergence of energy-dependent transport systems in early cells with limited energy resources
- Challenges in explaining how early life forms could adapt transport processes to specific environmental conditions such as pH
- No known mechanisms for the spontaneous development of proton-coupled transport in primitive environments
3. Adaptation and Regulation of Folate Transport Mechanisms
Transporters like the reduced folate carrier (RFC) play a key role in maintaining folate homeostasis by regulating the uptake of reduced folates. The existence of such regulatory mechanisms suggests a level of cellular control and adaptability that is difficult to reconcile with unguided processes. The ability to adjust folate uptake based on cellular needs implies a sophisticated network of signals and responses that are not easily explained by random processes.
Conceptual Problem: Regulation and Adaptation Without Guidance
- Lack of clear pathways for the emergence of regulatory systems governing folate transport
- Difficulty in explaining how primitive cells could regulate folate levels without advanced control mechanisms
- Absence of evidence for the coemergence of transport systems with specific regulatory adaptations tailored to folate needs
4. Endocytic and Multidrug Transport Mechanisms
Folate receptors (FRs) facilitate folate uptake via endocytosis, while some multidrug resistance protein (MRP) transporters also handle folate compounds. The involvement of such complex transport processes raises significant questions about how early cells could have managed the coordination and regulation required for these mechanisms. The endocytic pathway, in particular, suggests a high level of cellular organization and directionality that seems implausible without guided development.
Conceptual Problem: Complexity of Endocytic and Multidrug Transport
- No clear explanation for the origin of endocytic transport systems for folate in early cells
- Challenges in accounting for the regulation and specificity required for multidrug transporters that also handle folate
- Lack of mechanisms for the spontaneous development of complex, coordinated transport processes in primitive life forms
5. Role of ABC Transporters in Folate Transport
Some members of the ABC transporter family are involved in folate transport, utilizing ATP hydrolysis to drive the process. The emergence of such energy-dependent systems in early life forms is problematic, as it necessitates the presence of ATP and the ability to efficiently couple its hydrolysis to folate transport. This implies a level of biochemical sophistication that is difficult to account for without invoking guided processes.
Conceptual Problem: ATP-Dependent Transport and Energy Constraints
- Difficulty in explaining the spontaneous emergence of ATP-coupled folate transport systems in early life forms
- Challenges in accounting for the energy requirements of ATP hydrolysis in environments with limited ATP availability
- No plausible mechanisms for the coemergence of ATP-generating pathways and their integration with folate transport
6. Interdependence of Folate Transport and Cellular Metabolism
Folate transport is tightly interlinked with one-carbon metabolism and nucleotide synthesis. The need for consistent and adequate folate uptake underscores the interdependence between transport mechanisms and cellular metabolic processes. The coordinated emergence of these interdependent systems presents a significant challenge, as each relies on the functionality of the other for overall cellular operation.
Conceptual Problem: Coordinated Emergence of Interdependent Systems
- No clear pathways for the simultaneous development of folate transport and its integration with cellular metabolism
- Difficulty in explaining the coordination between transport systems and the metabolic needs for folate in early life forms
- Challenges in accounting for the coemergence of transport mechanisms with the specific metabolic processes that depend on folate
15.4.2. SAM Transporters in the first Life Forms
S-Adenosyl methionine (SAM) is a crucial biological molecule, serving as the primary methyl donor in numerous cellular processes. The transport of SAM across cellular compartments is essential for maintaining methylation reactions, which are fundamental to life. This overview focuses on the key transporters involved in SAM movement in the earliest life forms, highlighting their significance in the emergence and maintenance of life.
Key transporters involved in SAM transport in early life forms:
SAM Transporter (SAMT) (EC 3.6.3.-): Smallest known: Approximately 250-300 amino acids (based on modern bacterial homologs)
SAMTs are specialized membrane proteins that facilitate the transport of SAM across cellular membranes. These transporters are crucial for maintaining SAM concentrations in different cellular compartments, ensuring its availability for various methylation reactions. In early life forms, SAMTs likely played a vital role in regulating SAM-dependent processes, which are essential for DNA methylation, protein modification, and metabolite synthesis.
ATP-Binding Cassette (ABC) Transporters (EC 3.6.3.-): Smallest known: Approximately 400-600 amino acids (based on minimal ABC transporter structures)
Some ABC transporters are capable of transporting SAM along with other molecules. These versatile transporters use the energy from ATP hydrolysis to move substrates across membranes. In early life forms, ABC transporters may have contributed to SAM transport, especially in organisms lacking specialized SAMTs. Their role in SAM transport would have been crucial for maintaining cellular methylation processes and overall metabolic balance.
Solute Carrier Family Transporters (SLC) (EC 2.A.1.-): Smallest known: Approximately 300-400 amino acids (based on minimal SLC transporter structures)
Some members of the SLC family are capable of transporting SAM. While their presence in the earliest life forms is speculative, these transporters could have played a role in SAM movement across membranes. If present, they would have contributed to the regulation of SAM-dependent processes, potentially influencing early cellular metabolism and gene regulation.
Multidrug Resistance Proteins (MRPs) (EC 3.6.3.44): Smallest known: Approximately 600-800 amino acids (based on minimal MRP structures)
Some MRPs are capable of transporting SAM and related compounds. While these transporters are more complex and may not have been present in the earliest life forms, they represent a potential evolutionary development in SAM transport. If present in early life, they would have contributed to the regulation of intracellular SAM levels and potentially played a role in cellular detoxification processes.
Total number of transporter types in the group: 4. Total amino acid count for the smallest known versions (approximate): 1550-2100
Information on metal clusters or cofactors:
SAM Transporter (SAMT) (EC 3.6.3.-): SAMTs typically do not require metal clusters or cofactors for their function. However, they may be sensitive to the membrane potential and ion gradients across cellular membranes.
ATP-Binding Cassette (ABC) Transporters (EC 3.6.3.-): ABC transporters require ATP as a cofactor for their function. They also typically contain metal-binding domains, often involving Mg²⁺ ions, which are essential for ATP hydrolysis and the subsequent conformational changes that drive substrate transport.
Solute Carrier Family Transporters (SLC) (EC 2.A.1.-): Most SLC transporters do not require metal clusters or cofactors. However, some may be sensitive to ion gradients or membrane potential, which can influence their transport activity.
Multidrug Resistance Proteins (MRPs) (EC 3.6.3.44): Like ABC transporters, MRPs require ATP as a cofactor and typically contain metal-binding domains, often involving Mg²⁺ ions, which are essential for their transport function.
The presence and diversity of SAM transporters in early life forms underscore the critical importance of methylation reactions in the emergence and maintenance of life. These transporters would have played a crucial role in regulating SAM availability across cellular compartments, thereby influencing fundamental processes such as DNA methylation, protein modification, and metabolite synthesis. The evolution of these transport systems likely contributed significantly to the increasing complexity and efficiency of early cellular metabolism, paving the way for the diverse life forms we observe today.
Unresolved Challenges in SAM Transporters in the First Life Forms
1. Specificity and Functionality of SAM Transporters
S-adenosylmethionine (SAM) transporters are crucial for moving SAM across cellular compartments to ensure its availability for methylation reactions, which are vital for a wide range of biochemical processes. SAM transporters exhibit specificity in recognizing and transporting SAM, requiring precise binding sites and transport mechanisms. The emergence of such specific transport systems in early life forms presents a significant challenge, as it requires highly selective interactions with SAM molecules, a complexity that is difficult to attribute to unguided processes.
Conceptual problem: Spontaneous Specificity
- No naturalistic mechanisms adequately explain the emergence of transport proteins with highly specific binding sites for SAM
- The difficulty in accounting for the precise recognition and transport of SAM among other similar metabolites
2. Energetic Demands of SAM Transport Systems
Transporting SAM across membranes often requires energy input, especially when moving against concentration gradients. ABC transporters and other active transport systems utilize ATP hydrolysis, whereas other potential transport mechanisms might rely on ion gradients. The challenge lies in understanding how primitive cells could generate the necessary energy to support such active transport mechanisms, especially in the absence of fully developed metabolic networks capable of ATP synthesis or maintaining ion gradients.
Conceptual problem: Energy Source Availability
- Uncertainty about the source and regulation of energy needed for the active transport of SAM in early cells
- No clear naturalistic explanations for how energy-intensive transport processes were established and maintained in the earliest life forms
3. Coordination with Cellular Methylation Reactions
SAM plays a central role as a methyl donor in numerous biochemical reactions, including DNA, RNA, and protein methylation. Efficient SAM transport requires integration with cellular metabolic pathways to ensure the timely and adequate supply of SAM to enzymes that perform these reactions. The coordination and regulation of SAM transport in conjunction with cellular demand for methylation pose significant unresolved questions, as it implies an advanced level of regulatory oversight and metabolic integration.
Conceptual problem: Regulatory Coordination
- Lack of plausible mechanisms for the emergence of sophisticated regulatory systems needed to coordinate SAM transport with cellular methylation needs
- Difficulty explaining how transporters and methylation pathways could coemerge in a functionally integrated manner
4. Structural Complexity of SAM Transport Proteins
SAM transporters, like many transport proteins, consist of multiple transmembrane domains that create specific pathways for SAM movement across membranes. The intricate structure, folding, and proper integration of these proteins into cell membranes represent a substantial challenge. Explaining the spontaneous formation of fully functional SAM transport proteins, complete with correctly oriented domains and binding sites, remains a significant unresolved issue, particularly given the critical role these transporters play in early cellular function.
Conceptual problem: Spontaneous Protein Folding and Assembly
- No known unguided processes account for the precise folding and membrane integration of complex SAM transport proteins
- The necessity for operational transporters from the start to maintain SAM availability complicates the concept of gradual emergence
5. Environmental and Temporal Constraints
Early Earth environments were variable and often harsh, posing additional challenges for the stability and function of primitive SAM transport systems. The fluctuating availability of SAM and the energy sources required for its transport necessitate robust and adaptable transport mechanisms. Additionally, the rapid emergence of SAM transport systems capable of supporting essential methylation reactions imposes stringent temporal constraints, complicating naturalistic scenarios that lack coordination or pre-existing adaptability.
Conceptual problem: Environmental Adaptability and Timing
- Uncertainty about how early SAM transporters could have functioned effectively in the diverse and challenging conditions of early Earth
- Difficulty explaining the quick emergence of integrated SAM transport and methylation systems without invoking pre-existing coordination mechanisms
6. Origin of Multicomponent Transport Mechanisms
SAM transport can involve complex multicomponent systems, including ABC transporters and vesicular transport mechanisms. These systems are inherently dependent on the coordinated function of multiple proteins and cellular structures, which raises significant questions about their simultaneous emergence. The coemergence of transport proteins, vesicular components, and regulatory elements without guided processes remains a major conceptual challenge, as it necessitates highly specific interactions and cooperation among distinct cellular components.
Conceptual problem: Interdependence of Transport Mechanisms
- Lack of naturalistic explanations for the concurrent emergence of SAM transport proteins and their associated cellular components
- Difficulty accounting for the coordinated function of complex, multicomponent transport systems without invoking guidance
7. Compatibility with Primitive Cellular Membranes
The early life forms likely had basic membrane structures that may not have been fully developed lipid bilayers. SAM transporters, however, require stable and specific membrane environments to function correctly. The challenge lies in understanding how these transport proteins could have been compatible with primitive membranes that might not have provided the stability or specific lipid environment needed for their proper function, posing significant questions about the compatibility and functionality of SAM transport systems in early cells.
Conceptual problem: Membrane-Transporter Compatibility
- No clear explanations for how complex SAM transport proteins could have integrated into and functioned within primitive, potentially unstable membrane structures
- The need for functional integration of SAM transporters into early membranes adds complexity that is difficult to resolve without invoking a guided process