The Interdependency of Lipid Membranes and Membrane Proteins 1
https://reasonandscience.catsboard.com/t2397-the-interdependency-of-lipid-membranes-and-membrane-proteins
Cell membranes only come from cell membranes. A cell cannot produce the cell membrane de novo from scratch. It inherits it. Daughter cell membranes come only from mother cell membranes.
Even in the simplest cells, the membrane is a biological device of a staggering complexity that carries diverse protein complexes mediating energy-dependent – and tightly regulated - import and export of metabolites and polymers 2
Remarkably, even the author of the book: Agents Under Fire: Materialism and the Rationality of Science, pgs. 104-105 (Rowman & Littlefield, 2004). HT: ENV. asks the readers:
Hence a chicken and egg paradox: a lipid membrane would be useless without membrane proteins but how could membrane proteins have evolved in the absence of functional membranes?
Paul Davies, the fifht miracle, page 48:
All micro-organisms are separated from their surroundings by a membrane or cell wall. Indeed, it is hard to imagine life without a boundary of some sort. The question is, when did this cellular structure arise—before, during, or after the principal chemical steps ? Oparin was a cells-first advocate. He was impressed by the fact that oily substances and water don’t mix, and sometimes produce a suspension known as a coacervate, in which the oil retreats into tiny droplets. The oily blobs superficially resemble biological cells. Oparin’s theory assumed that the physical structure of the cell came first, providing a natural containment vessel in which some molecular marvels could proceed. This idea has some attraction, because there are many physical processes (not just oil in water) that produce vesicles of some sort. Also, fluid cells and droplets can become unstable and split in two, representing a crude form of reproduction. If a bag full of chemicals swells up and undergoes fission, each of the “daughter” bags will inherit the chemical mix of the parent.
The membrane needs to have some special properties. For instance, it must trap the life-sustaining molecules inside the cell, but let through the needed raw materials from the outside. Oparin’s idea of rooting the origin of life in the formation of cells partly reflects the state of knowledge of the day. Scientists at that time were still struggling to work out the processes of metabolism and the role of proteins within the cell, with only the vaguest idea of the nature of genes. Molecular biology and knowledge of DNA did not yet exist. It was perhaps only natural that Oparin deemphasized the genetic aspects of life and gave primacy to the physical aspects—cell formation and structure—which were better understood. That does not make the cells-first theory wrong, but it does warn us that the temptation to place the things we understand at the center of a theory risks putting the cart before the horse. Theorizing about the origin of life seemed altogether too speculative in the 1920s, and few people paid much attention to the ideas of Oparin and Haldane. One person who did take notice, however, was Harold Urey, an American chemist who would one day win the Nobel Prize for the discovery of deuterium. Urey realized that it might be possible to test the theory of the primordial soup in the laboratory. Many years later, in 1953, he set out to do just that.
A topologically closed membrane is a ubiquitous feature of all cellular life forms. This membrane is not a simple lipid bilayer enclosing the innards of the cell: far from that,
Membrane proteins, which contain hydrophobic stretches and are generally insoluble in water, could not have evolved in the absence of functional membranes, while purely lipid membranes would be impenetrable and hence useless without membrane proteins. The origins of biological membranes - as complex cellular devices that control the energetics of the cell and its interactions with the surrounding world - remain obscure 3
Even in the simplest cells, the membrane is a biological device of a staggering complexity that carries diverse protein complexes mediating energy-dependent – and tightly regulated - import and export of metabolites and polymers 2
Hence a chicken and egg paradox: a lipid membrane would be useless without membrane proteins but how could membrane proteins have evolved in the absence of functional membranes?
THE CELL Evolution of the First Organism, Joseph Panno, Ph.D., page 17:
The cell membrane, far from being featureless, contains a molecular forest that gives the cell its eyes, its ears, and the equipment it needs to capture food and to communicate with other cells. Phospholipids, the main component in cell membranes, are composed of a polar head group (usually an alcohol), a phosphate, glycerol, and two hydrophobic fatty acid tails. Fat that is stored in the body as an energy reserve has a structure similar to a phospholipid, being composed of three fatty acid chains attached to a molecule of glycerol. The third fatty acid takes the place of the phosphate and head group of a phospholipid. Sugars are polymerized to form chains of two or more monosaccharides. Disaccharides (two monosaccharides), and oligosaccharides (about 3–12 monosaccharides), are attached to proteins and lipids destined for the cell surface. Polysaccharides,
such as glycogen and starch, may contain several hundred monosaccharides and are stored in cells as an energy reserve.

All the molecules shown in the figure above are assumed to have formed in the prebiotic oceans, and this was followed by autoassembly of the macromolecules.Modern cells all have a membrane constructed from a phospholipid bilayer. From the very beginning, cells used the lipid bilayer to regulate their internal environment. The bilayer blocked, or impeded, the passive flow of most molecules into the cell, thus protecting the cell from the external environment. Cells exploited this property by embedding proteins in their membranes that would allow only certain molecules to gain entry. In this way, the cell could fine-tune the selection of what got in and what did not. Other proteins embedded in the membrane acted like sensory antennae, making it possible for cells to gain information about their immediate environment. Some of these proteins were used to detect the presence of food molecules, while others became specialized as transmitters and receivers, allowing the cells to communicate with each other. Cell-to-cell communication led to the next stage in the development of life on our planet. Single cells began to form colonies of increasing complexity, eventually transforming themselves into the multicellular creatures that now inhabit the Earth.
Additional proteins necessary for collecting glucose and other sugars are also located in the cell membrane. These proteins, called glucose transporters or carriers, are specially designed for bringing glucose into the cell. Glucose and other simple sugars can diffuse passively across the cell membrane, but it is a much slower process. Transporters provide a channel that allows the cell to take up glucose 100 times faster than by simple diffusion. The ability to make glucose carriers that are embedded in the cell membrane was probably the most important event leading to the transition from the first cells to the ancestral prokaryotes. Once cells learned this trick, they expanded on it very quickly. Proteins were embedded in the membrane that could detect and import other sugars, such as maltose or lactose. They even learned how to make sugar receptors, also embedded in the membrane, which could signal the cell when a high concentration of glucose or maltose was encountered so the activity of the transporters could be stepped up accordingly. The electron transport chain may have evolved from proteins that were originally embedded in the membrane to process or detect sugar molecules.
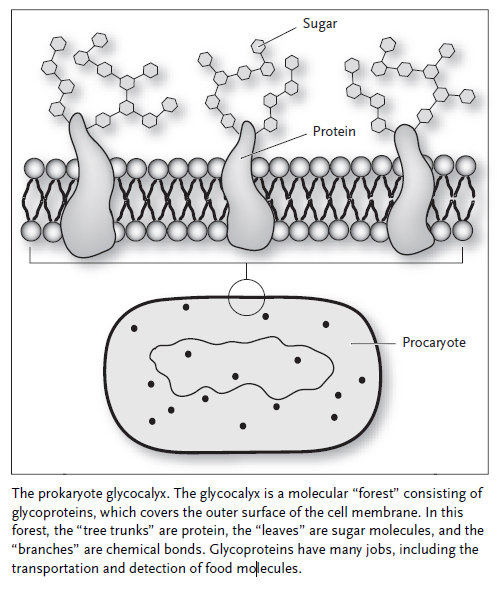
The sugar carriers, receptors, and components of the respiratory chain are all glycoproteins; that is, sugar molecules are attached to the proteins to enhance or modulate their behavior. Glycoproteins are like molecular trees, with the protein portion being the trunk and the sugar molecules forming the leaves and branches. It is almost as though the prokaryotes were building a forest with which to cover themselves, much in the way higher plants covered the surface of the Earth so many millions of years later. The molecular forest of a prokaryote is called the glycocalyx, and its importance to the cell cannot be overstated. This forest gives the cell its eyes, ears, and a sense of touch in addition to the energy-processing machinery. It is through the glycocalyx that cells learned how to communicate with one another, paving the way for the appearance of multicellular creatures like ourselves.
Cell's design, Fazale Rana, page 61:
The origin of cell membranes has to be one of the first steps in life's emergence. Researchers assume, for the most part, that once membrane components form or appear on early Earth, they readily self-assembled to form the first cell membranes. To explain this origin, and along with it the emergence of the first cell, most investigators simply attempt to identify compounds— likely present on early Earth—with the potential to spontaneously assemble into bilayer structures. These scientists also look to define mechanisms in which bilayer structures can encapsulate more complex self-replicating molecules and acquire proper- ties that resemble those of contemporary cell membranes such as transport and energy transduction.
biochemists have discovered that life also requires a series of steps. These steps include:
• assembly of boundary membranes
• formation of energy capturing capabilities by the boundary membrane
• encapsulation of macromolecules (like proteins, RNA, and DNA) within
the boundary membrane
• introduction of pores into the boundary membrane that can funnel raw
materials into the interior space
• production of systems that allow the macromolecules to grow
• generation of catalysts that speed up the growth of the encapsulated
macromolecules
• provision for the macromolecules to replicate
• introduction of information into one set of macromolecules that directs
the production of other macromolecules
• development of mechanisms to cause the boundary membrane to subdi-
vide into two smaller systems that can grow
• production of the means to pass information-containing macromole-
cules to the daughter products of the subdivision process
Biochemists now acknowledge that cell membranes consist of a careful arrangement of molecular pieces. This exquisite organization at the molecular level is integral to many functions performed by cell membranes. These supramolecular assemblies also require fine-tuning of their composition to exist as stable structures and carry out key operations. In addition, some biochemists think cell membranes harbor information ( glycan code). These three characteristics (organization, fine-tuning, and information) are part of the intelligent design inference. A few key advances in membrane biochemistry have transformed the way scientists view cell membranes. These new insights represent a case study of sorts and show how pattern recognition—using the intelligent design tem- plate—can be applied to specific features of the cell in order to reveal the work of the Creator.
An Intricate Design
Cell membranes are comprised of phospholipids that possess a wide range of chemical variability. Phospholipid head groups typically consist of a phosphate group bound to a glycerol (glycerin) backbone. That group binds one of several possible compounds that vary in their chemical and physical properties. Frequently, phospholipids are identified by their head group structure. Alternatively, phospholipid head groups bind ethanolamine, serine, glycerol, and inositol molecules. Phospholipids vary in tail length and structure. Their tails are typically long linear hydrocarbon chains linked to the glycerol backbone. The phospholipid hydrocarbon chains are commonly fourteen, sixteen, or eighteen carbon atoms long, but can be as short as twelve and as long as twenty- four. Sometimes one or both of the hydrocarbon chains possesses a permanent kink. These kinks can occur at different locations along the chain length. (Carbon-carbon double bonds inserted into the hydrocarbon chain cause the kinks.) In addition to phospholipids, the cell membranes of bacteria contain another type of lipid (lipopolysaccharides). The cell boundaries of eukaryotic organisms also consist of several different classes of lipids (such as cholesterol, plasmalogens, sphingolipids, and glycolipids) beyond phospholipids. Superficially, the complex chaotic lipid compositions of cell membranes appear to reflect a long history of undirected evolutionary events. According to this view, over vast periods of time, a variety of molecules were incorpo- rated into membranes as metabolic pathways that produced the various phospholipids randomly emerged and diversified under the auspices of natural selection. Recent advances suggest that the seemingly chaotic mix of phospholipids in cell membranes was deliberately planned. This molecular mix is necessary and points to a deep rationale that underlies the membrane's composition.
Using Just the Right Pieces
The variable length and geometry of phospholipids' hydrocarbon chains affect the physical properties of cell membranes. Bilayers composed of phospholipids with short hydrocarbon chains or hydrocarbon chains with kinks possess a fluid, liquidlike interior. On the other hand, cell membranes have solidlike interiors if formed from phospholipids with longer hydrocarbon chains and chains that are straight. The fluidity of the cell membrane's interior has important biological consequences. The bilayer's physical state regulates the function of integral proteins. Local variations in phospholipid composition also create regions of variable fluidity within the bilayer with some areas more solid and others more liquid. These differences in fluidity help segregate the cell membrane's components into functionally distinct domains within the bilayer. Without a large ensemble of phospholipids, precise regulation of membrane protein activity and creation of functionally distinct domains would be impossible. Phospholipids with a wide-range of hydrocarbon chain lengths and shapes (straight or kinked) make it possible for the cell to precisely adjust bilayer fluidity.
A Living Kaleidoscope
Phospholipids play a critical role in controlling the activity of proteins associated with the cell membranes and, in some instances, those proteins located in the cytoplasm. This control extends beyond simply dictating bilayer fluidity. Phospholipids regulate protein function through direct and highly specific interactions. Through interactions mediated by the head group, phospholipids with glycerol (PGs) and serine (PSs) attachments bind to target proteins. PGs and PSs are both negatively charged. They also have distinct chemical structures. Both features factor into their association with proteins. PSs play a central role in activating proteins in the cytoplasm that are part of the cell signaling pathways. These pathways alter the cell's metabolism in response to changes taking place outside the cell. In bacteria, PGs activate some of the proteins involved in (1) replicating DNA, (2) assembling the outer membrane, and (3) moving proteins across cell membranes.
Phospholipids with choline (PCs) and ethanolamine (PEs) as part of their head groups are neutral in charge. Interestingly, these two types of phospholipids have regions both negatively and positively charged. (The charges cancel each other to yield overall electrical neutrality.) PCs and PEs are the major phospholipid components of cell membranes.
Even though PCs and PEs are neutrally charged, their chemical structures differ sufficiently so these two phospholipids play distinct roles in cell membranes. The primary role of PCs is bilayer formation. PEs also function in this capacity. Additionally, PE-rich regions in cell membranes can adopt nonbilayer structures. These nonbilayer phases regulate the activities of some proteins and play a central role in cell division and membrane fusion events. PEs also trigger the activity of membrane proteins that shuttle materials across cell membranes. Phospholipids are highly involved in a litany of biochemical processes. As biochemist William Dohan notes, "The wide range of processes in which specific involvement of phospholipids have been documented explains the need for diversity in phospholipid structure and why there are so many membrane lipids." Far from reflecting the aimlessness of evolutionary processes, the complexity of cell membranes appears intentional. Each phospholipid is an essential part of the biochemical mosaic that adorns the cell's surfaces.
Apart from cell membranes, phospholipids spontaneously assemble into multibilayer sheets. So how can cell membranes consist of a single bilayer phase? Single bilayer phases, similar to those that constitute cell membranes, can be permanently stable but only under unique conditions. Formation of single bilayer vesicles occurs only at a specific critical temperature. At this temperature, pure phospholipids spontaneously transform from either multiple bilayer sheets or unstable liposomes into stable single bilayers.Above or below this temperature, the unilamellar phase collapses into multilamellar structures. The critical temperature varies depending on the specific chemical makeup of the phospholipids. In the case of bilayers formed from a mixture of phospholipids, the critical temperatures depend on the bilayers' specific phospholipid composition. phospholipids extracted from rat and squid nervous system tissue assemble into single bilayer structures at critical temperatures that correspond to the physiological temperatures of these two organisms.
For the cold-blooded sea urchin L.pictus, the membrane composition of the earliest cells in the embryo varies in response to the environment's temperature. In these cases, changes in phospholipid composition allowed the bilayers to adopt a unilamellar phase at a critical temperature that corresponded to the environmental conditions. In addition, the bacterium E. coli also adjusts its cell membrane phospholipid composition to maintain a single bilayer phase as growth temperature varies.
These studies highlight the biological importance of the critical bilayer phenomena. So does other research that indicates how devastating it is for life when cell membranes deviate from critical conditions. Gershfeld identified a correlation between the rupture of human red blood cells (hemolysis) and incubation at temperatures exceeding 37°C (the normal human body temperature). Transformation of the cell membrane from a single bilayer to multiple bilayer stacks accompanies the red blood cells' hemolysis—a loss of the cell membrane's critical state. Gershfeld and his collaborators even provided some evidence that cell membrane defects at the sites of neurodegeneration may play a role in Al- zheimer's disease. Presumably, collapse of the cell membrane's single bilayer state into a multiple bilayer condition stems from altered membrane phospholipid composition.
Cell membranes are carefully crafted structures that appear to be the product of a Creator's painstaking handiwork. It indicates that cell membranes are highly fine-tuned molecular structures dependent on an exacting set of physical and chemical conditions.
Each Piece Placed by Hand
The fine-tuning of cell membrane lipid compositions does more than stabilize the single bilayer phase. One recent study demonstrates that it also appears to critically influence the interactions between cell membranes and proteins. A team of Russian scientists, exploring the role that phospholipids play when the cell's machinery inserts proteins into membranes, discovered that successful insertion of the protein colicin E (a bacterial toxin) requires a fairly exacting phospholipid composition. Introduction of colicin E into bilayers requires PG levels between 25 and 30 percent. PGs bear a negative charge that sets up an electrical potential at the membrane's surface. This potential plays a key role in the insertion process. If the surface potential varies outside a narrow range of values, protein insertion doesn't properly take place. This restrictive range of values occurs only for specific concentrations of PGs. Surface potential depends on the salt concentration in the environment. The researchers noted that as they changed the salinity, the PG concentra- tions in the membranes had to vary accordingly to maintain the just-right surface potential for protein insertion to take place. Interestingly, the content of negatively charged phospholipids (PGs and PSs) in most membranes is between 25 and 30 percent. In fact, most bacteria adjust the level of PGs in their membranes in response to changes in the salinity of the growth medium. These observations suggest that the compositional fine-tuning of phospholipids needed for the import of colicin E into bilayers may be a general requirement for the insertion of most membrane proteins.
Membrane proteins, which contain hydrophobic stretches and are generally insoluble in water, could not have evolved in the absence of functional membranes, while purely lipid membranes would be impenetrable and hence useless without membrane proteins. The origins of biological membranes - as complex cellular devices that control the energetics of the cell and its interactions with the surrounding world - remain obscure.
The Interdependency of Lipid Membranes and Membrane Proteins
The cell membrane contains various types of proteins, including ion channel proteins, proton pumps, G proteins, and enzymes. These membrane proteins function cooperatively to allow ions to penetrate the lipid bilayer. The interdependency of lipid membranes and membrane proteins suggests that lipid bilayers and membrane proteins co-evolved together with membrane bioenergetics.
Its remarkable that a scientific mainstream source resorts to evolution to explain the origin of cell membranes, despite the fact that evolution was not a driving force prior to DNA replication. Secondly, interdependence is openly admitted. The question arises, how could and would a) the membrane and b) the indispensable membrane proteins emerge together ? The synthesis of proteins requires the hardware and software to produce proteins fully in place, setup, and working, and a protecting cell membrane.... Thats a classicle chicken/egg problem, which is simply neglected , making up a just so scneario without detailled explanations.
Water-soluble proteins evolved gradually into highly hydrophobic membrane proteins. Primordial membranes initially contained pores, which enabled ions, small molecules, and polymers to be exchanged passively between protocells and their environment.
If these pores were not specific enough, without a elaborate mechanism to distinguish ions, small molecules, and polymers that were useful and permittet to enter the cell, the "proto-cell" would certainly die. In fact, these ion channel proteins, proton pumps, G proteins, and enzymes had to be fully developed and working from day 1, or nothing would go. They are life-essential for a working cell. A gradual evolutionary development is simply not feasable.
In contrast, modern membrane proteins must be inserted into the membrane by membrane protein complexes. Membrane protein complexes could not have logically existed prior to the existence of membrane proteins.
That makes the whole picture even more difficult. Beside the membrane, the membrane proteins, also protein complexes that insert the membrane proteins into the right place had to emerge, with the program of how, which proteins, and when to insert them at the right place in the membrane. That adds a significant hurdle of difficulty, which origin must be explained.
Which indicates that there was no common ancestry. They had to emerge separately.
It is seemingly impossible to have the formation of impermeable membranes without membrane proteins and translocases to shuttle essential materials in and out of the cell. Consequently, it is also unlikely that very specialized membrane proteins were able to form without a membrane initially present. It is hypothesized that the development of a permeable, porous membrane eventually integrated evolving proteins, to later evolve into a highly specialized and efficient impermeable membrane.
The author makes implicitly a strong case for intelligent design. How would and could a random process originating from a prebiotic soup have coordinated the production of membranes and membrane proteins, membrane protein complexes required for the assembly of the membrane proteins , all at the same time, and the information for the correct assembly process ?
Co-evolution of primordial membranes and membrane proteins
The chemical compositions and biogenesis pathways of archaeal and bacterial membranes are fundamentally different [4,5,12].The glycerol moieties of the membrane phospholipids in all archaea and bacteria are of the opposite chiralities. With a few exceptions, the hydrophobic chains differ as well, being based on fatty acids in bacteria and on isoprenoids in archaea; furthermore, in bacterial lipids, the hydrophobic tails are usually linked to the glycerol moiety by ester bonds whereas archaeal lipids contain ether bonds [4,5,12]. The difference extends beyond the chemical structures of the phospholipids, to the evolutionary provenance of the enzymes involved in membrane biogenesis that are either non-homologous or distantly related but not orthologous in bacteria and archaea [4,5,12,13].
It has been argued that fatty acids are the simplest amphiphilic molecules that could form abiogenically to be subsequently recruited by first organisms
Question: If fatty acids would form abiogenetically, why would the cell "invent" extraordinarly compex biosynthesis pathways to produce it, and nanofactories of extraordinary complexity ? :
The first step of fatty acid biosynthesis in modern cells requires the participation of malonyl-CoA, a three-carbon intermediate. The formation of malonyl-CoA from acetyl-CoA is an irreversible process, catalyzed by acetyl-CoA carboxylase enzymes. a multifunctional protein with 3 subunits, which is carefully regulated.
In the second step, fatty acid synthase ( FAS) proteins come into action. FAS most striking feature is the “high degree of architectural complexity” – some 48 active sites, complete with moving parts.
http://reasonandscience.heavenforum.org/t2168-the-amazing-fatty-acid-synthase-nano-factories-and-origin-of-life-scenarios#3950
Then they go on and argue that they " could be subsequently recruited by first organisms".
That seems to me another simplistic pseudo scientific just so assertion without any detailled explanation of how that would have happened without guiding intelligence. In order for this to be possible in the way they assert the five following conditions would all have to be met::
C1: Availability. Among the parts available for recruitment to form the system, there would need to be ones capable of performing the highly specialized tasks of individual parts, even though all of these items serve some other function or no function.
C2: Synchronization. The availability of these parts would have to be synchronized so that at some point, either individually or in combination, they are all available at the same time.
C3: Localization. The selected parts must all be made available at the same ‘construction site,’ perhaps not simultaneously but certainly at the time they are needed.
C4: Coordination. The parts must be coordinated in just the right way: even if all of the parts of a system are available at the right time, it is clear that the majority of ways of assembling them will be non-functional or irrelevant.
C5: Interface compatibility. The parts must be mutually compatible, that is, ‘well-matched’ and capable of properly ‘interacting’: even if sub systems or parts are put together in the right order, they also need to interface correctly.
( Agents Under Fire: Materialism and the Rationality of Science, pgs. 104-105 (Rowman & Littlefield, 2004). HT: ENV.)
A pure lipid bilayer, however, is not a practical solution for the membrane of a primordial cellular life form because it would effectively prevent exchange between the inside of a vesicle and the environment. Therefore another unsolved question is how electrically charged compounds could be transferred across the primordial membranes. Because of the hydrophobic barrier, ions penetrate the lipid bilayer with the help of specialized membrane proteins, such as channels or translocases. The membrane-embedded portions of these proteins consist, largely, of hydrophobic amino acids, and the proteins themselves are water-insoluble. Hence a chicken and egg paradox: a lipid membrane would be useless without membrane proteins but how could membrane proteins have evolved in the absence of functional membranes?
Its particularly interesting when mainstream scientific papers admit chicken-egg difficulties ....
Much uncertainty remains regarding the chemical nature of membranes in the LUCA and earlier.
1. https://en.wikibooks.org/wiki/Structural_Biochemistry/The_Evolution_of_Membranes
2. Co-evolution of primordial membranes and membrane proteins
3. http://www.macromol.uni-osnabrueck.de/paperMulk/Mulkidjanian_Galperin_Evolution_of_membr_proteins_Figures.pdf
https://reasonandscience.catsboard.com/t2397-the-interdependency-of-lipid-membranes-and-membrane-proteins
Cell membranes only come from cell membranes. A cell cannot produce the cell membrane de novo from scratch. It inherits it. Daughter cell membranes come only from mother cell membranes.
Even in the simplest cells, the membrane is a biological device of a staggering complexity that carries diverse protein complexes mediating energy-dependent – and tightly regulated - import and export of metabolites and polymers 2
Remarkably, even the author of the book: Agents Under Fire: Materialism and the Rationality of Science, pgs. 104-105 (Rowman & Littlefield, 2004). HT: ENV. asks the readers:
Hence a chicken and egg paradox: a lipid membrane would be useless without membrane proteins but how could membrane proteins have evolved in the absence of functional membranes?
Paul Davies, the fifht miracle, page 48:
All micro-organisms are separated from their surroundings by a membrane or cell wall. Indeed, it is hard to imagine life without a boundary of some sort. The question is, when did this cellular structure arise—before, during, or after the principal chemical steps ? Oparin was a cells-first advocate. He was impressed by the fact that oily substances and water don’t mix, and sometimes produce a suspension known as a coacervate, in which the oil retreats into tiny droplets. The oily blobs superficially resemble biological cells. Oparin’s theory assumed that the physical structure of the cell came first, providing a natural containment vessel in which some molecular marvels could proceed. This idea has some attraction, because there are many physical processes (not just oil in water) that produce vesicles of some sort. Also, fluid cells and droplets can become unstable and split in two, representing a crude form of reproduction. If a bag full of chemicals swells up and undergoes fission, each of the “daughter” bags will inherit the chemical mix of the parent.
The membrane needs to have some special properties. For instance, it must trap the life-sustaining molecules inside the cell, but let through the needed raw materials from the outside. Oparin’s idea of rooting the origin of life in the formation of cells partly reflects the state of knowledge of the day. Scientists at that time were still struggling to work out the processes of metabolism and the role of proteins within the cell, with only the vaguest idea of the nature of genes. Molecular biology and knowledge of DNA did not yet exist. It was perhaps only natural that Oparin deemphasized the genetic aspects of life and gave primacy to the physical aspects—cell formation and structure—which were better understood. That does not make the cells-first theory wrong, but it does warn us that the temptation to place the things we understand at the center of a theory risks putting the cart before the horse. Theorizing about the origin of life seemed altogether too speculative in the 1920s, and few people paid much attention to the ideas of Oparin and Haldane. One person who did take notice, however, was Harold Urey, an American chemist who would one day win the Nobel Prize for the discovery of deuterium. Urey realized that it might be possible to test the theory of the primordial soup in the laboratory. Many years later, in 1953, he set out to do just that.
A topologically closed membrane is a ubiquitous feature of all cellular life forms. This membrane is not a simple lipid bilayer enclosing the innards of the cell: far from that,
Membrane proteins, which contain hydrophobic stretches and are generally insoluble in water, could not have evolved in the absence of functional membranes, while purely lipid membranes would be impenetrable and hence useless without membrane proteins. The origins of biological membranes - as complex cellular devices that control the energetics of the cell and its interactions with the surrounding world - remain obscure 3
Even in the simplest cells, the membrane is a biological device of a staggering complexity that carries diverse protein complexes mediating energy-dependent – and tightly regulated - import and export of metabolites and polymers 2
Hence a chicken and egg paradox: a lipid membrane would be useless without membrane proteins but how could membrane proteins have evolved in the absence of functional membranes?
THE CELL Evolution of the First Organism, Joseph Panno, Ph.D., page 17:
The cell membrane, far from being featureless, contains a molecular forest that gives the cell its eyes, its ears, and the equipment it needs to capture food and to communicate with other cells. Phospholipids, the main component in cell membranes, are composed of a polar head group (usually an alcohol), a phosphate, glycerol, and two hydrophobic fatty acid tails. Fat that is stored in the body as an energy reserve has a structure similar to a phospholipid, being composed of three fatty acid chains attached to a molecule of glycerol. The third fatty acid takes the place of the phosphate and head group of a phospholipid. Sugars are polymerized to form chains of two or more monosaccharides. Disaccharides (two monosaccharides), and oligosaccharides (about 3–12 monosaccharides), are attached to proteins and lipids destined for the cell surface. Polysaccharides,
such as glycogen and starch, may contain several hundred monosaccharides and are stored in cells as an energy reserve.

All the molecules shown in the figure above are assumed to have formed in the prebiotic oceans, and this was followed by autoassembly of the macromolecules.Modern cells all have a membrane constructed from a phospholipid bilayer. From the very beginning, cells used the lipid bilayer to regulate their internal environment. The bilayer blocked, or impeded, the passive flow of most molecules into the cell, thus protecting the cell from the external environment. Cells exploited this property by embedding proteins in their membranes that would allow only certain molecules to gain entry. In this way, the cell could fine-tune the selection of what got in and what did not. Other proteins embedded in the membrane acted like sensory antennae, making it possible for cells to gain information about their immediate environment. Some of these proteins were used to detect the presence of food molecules, while others became specialized as transmitters and receivers, allowing the cells to communicate with each other. Cell-to-cell communication led to the next stage in the development of life on our planet. Single cells began to form colonies of increasing complexity, eventually transforming themselves into the multicellular creatures that now inhabit the Earth.
Additional proteins necessary for collecting glucose and other sugars are also located in the cell membrane. These proteins, called glucose transporters or carriers, are specially designed for bringing glucose into the cell. Glucose and other simple sugars can diffuse passively across the cell membrane, but it is a much slower process. Transporters provide a channel that allows the cell to take up glucose 100 times faster than by simple diffusion. The ability to make glucose carriers that are embedded in the cell membrane was probably the most important event leading to the transition from the first cells to the ancestral prokaryotes. Once cells learned this trick, they expanded on it very quickly. Proteins were embedded in the membrane that could detect and import other sugars, such as maltose or lactose. They even learned how to make sugar receptors, also embedded in the membrane, which could signal the cell when a high concentration of glucose or maltose was encountered so the activity of the transporters could be stepped up accordingly. The electron transport chain may have evolved from proteins that were originally embedded in the membrane to process or detect sugar molecules.
The sugar carriers, receptors, and components of the respiratory chain are all glycoproteins; that is, sugar molecules are attached to the proteins to enhance or modulate their behavior. Glycoproteins are like molecular trees, with the protein portion being the trunk and the sugar molecules forming the leaves and branches. It is almost as though the prokaryotes were building a forest with which to cover themselves, much in the way higher plants covered the surface of the Earth so many millions of years later. The molecular forest of a prokaryote is called the glycocalyx, and its importance to the cell cannot be overstated. This forest gives the cell its eyes, ears, and a sense of touch in addition to the energy-processing machinery. It is through the glycocalyx that cells learned how to communicate with one another, paving the way for the appearance of multicellular creatures like ourselves.

Cell's design, Fazale Rana, page 61:
The origin of cell membranes has to be one of the first steps in life's emergence. Researchers assume, for the most part, that once membrane components form or appear on early Earth, they readily self-assembled to form the first cell membranes. To explain this origin, and along with it the emergence of the first cell, most investigators simply attempt to identify compounds— likely present on early Earth—with the potential to spontaneously assemble into bilayer structures. These scientists also look to define mechanisms in which bilayer structures can encapsulate more complex self-replicating molecules and acquire proper- ties that resemble those of contemporary cell membranes such as transport and energy transduction.
biochemists have discovered that life also requires a series of steps. These steps include:
• assembly of boundary membranes
• formation of energy capturing capabilities by the boundary membrane
• encapsulation of macromolecules (like proteins, RNA, and DNA) within
the boundary membrane
• introduction of pores into the boundary membrane that can funnel raw
materials into the interior space
• production of systems that allow the macromolecules to grow
• generation of catalysts that speed up the growth of the encapsulated
macromolecules
• provision for the macromolecules to replicate
• introduction of information into one set of macromolecules that directs
the production of other macromolecules
• development of mechanisms to cause the boundary membrane to subdi-
vide into two smaller systems that can grow
• production of the means to pass information-containing macromole-
cules to the daughter products of the subdivision process
Biochemists now acknowledge that cell membranes consist of a careful arrangement of molecular pieces. This exquisite organization at the molecular level is integral to many functions performed by cell membranes. These supramolecular assemblies also require fine-tuning of their composition to exist as stable structures and carry out key operations. In addition, some biochemists think cell membranes harbor information ( glycan code). These three characteristics (organization, fine-tuning, and information) are part of the intelligent design inference. A few key advances in membrane biochemistry have transformed the way scientists view cell membranes. These new insights represent a case study of sorts and show how pattern recognition—using the intelligent design tem- plate—can be applied to specific features of the cell in order to reveal the work of the Creator.
An Intricate Design
Cell membranes are comprised of phospholipids that possess a wide range of chemical variability. Phospholipid head groups typically consist of a phosphate group bound to a glycerol (glycerin) backbone. That group binds one of several possible compounds that vary in their chemical and physical properties. Frequently, phospholipids are identified by their head group structure. Alternatively, phospholipid head groups bind ethanolamine, serine, glycerol, and inositol molecules. Phospholipids vary in tail length and structure. Their tails are typically long linear hydrocarbon chains linked to the glycerol backbone. The phospholipid hydrocarbon chains are commonly fourteen, sixteen, or eighteen carbon atoms long, but can be as short as twelve and as long as twenty- four. Sometimes one or both of the hydrocarbon chains possesses a permanent kink. These kinks can occur at different locations along the chain length. (Carbon-carbon double bonds inserted into the hydrocarbon chain cause the kinks.) In addition to phospholipids, the cell membranes of bacteria contain another type of lipid (lipopolysaccharides). The cell boundaries of eukaryotic organisms also consist of several different classes of lipids (such as cholesterol, plasmalogens, sphingolipids, and glycolipids) beyond phospholipids. Superficially, the complex chaotic lipid compositions of cell membranes appear to reflect a long history of undirected evolutionary events. According to this view, over vast periods of time, a variety of molecules were incorpo- rated into membranes as metabolic pathways that produced the various phospholipids randomly emerged and diversified under the auspices of natural selection. Recent advances suggest that the seemingly chaotic mix of phospholipids in cell membranes was deliberately planned. This molecular mix is necessary and points to a deep rationale that underlies the membrane's composition.
Using Just the Right Pieces
The variable length and geometry of phospholipids' hydrocarbon chains affect the physical properties of cell membranes. Bilayers composed of phospholipids with short hydrocarbon chains or hydrocarbon chains with kinks possess a fluid, liquidlike interior. On the other hand, cell membranes have solidlike interiors if formed from phospholipids with longer hydrocarbon chains and chains that are straight. The fluidity of the cell membrane's interior has important biological consequences. The bilayer's physical state regulates the function of integral proteins. Local variations in phospholipid composition also create regions of variable fluidity within the bilayer with some areas more solid and others more liquid. These differences in fluidity help segregate the cell membrane's components into functionally distinct domains within the bilayer. Without a large ensemble of phospholipids, precise regulation of membrane protein activity and creation of functionally distinct domains would be impossible. Phospholipids with a wide-range of hydrocarbon chain lengths and shapes (straight or kinked) make it possible for the cell to precisely adjust bilayer fluidity.
A Living Kaleidoscope
Phospholipids play a critical role in controlling the activity of proteins associated with the cell membranes and, in some instances, those proteins located in the cytoplasm. This control extends beyond simply dictating bilayer fluidity. Phospholipids regulate protein function through direct and highly specific interactions. Through interactions mediated by the head group, phospholipids with glycerol (PGs) and serine (PSs) attachments bind to target proteins. PGs and PSs are both negatively charged. They also have distinct chemical structures. Both features factor into their association with proteins. PSs play a central role in activating proteins in the cytoplasm that are part of the cell signaling pathways. These pathways alter the cell's metabolism in response to changes taking place outside the cell. In bacteria, PGs activate some of the proteins involved in (1) replicating DNA, (2) assembling the outer membrane, and (3) moving proteins across cell membranes.
Phospholipids with choline (PCs) and ethanolamine (PEs) as part of their head groups are neutral in charge. Interestingly, these two types of phospholipids have regions both negatively and positively charged. (The charges cancel each other to yield overall electrical neutrality.) PCs and PEs are the major phospholipid components of cell membranes.
Even though PCs and PEs are neutrally charged, their chemical structures differ sufficiently so these two phospholipids play distinct roles in cell membranes. The primary role of PCs is bilayer formation. PEs also function in this capacity. Additionally, PE-rich regions in cell membranes can adopt nonbilayer structures. These nonbilayer phases regulate the activities of some proteins and play a central role in cell division and membrane fusion events. PEs also trigger the activity of membrane proteins that shuttle materials across cell membranes. Phospholipids are highly involved in a litany of biochemical processes. As biochemist William Dohan notes, "The wide range of processes in which specific involvement of phospholipids have been documented explains the need for diversity in phospholipid structure and why there are so many membrane lipids." Far from reflecting the aimlessness of evolutionary processes, the complexity of cell membranes appears intentional. Each phospholipid is an essential part of the biochemical mosaic that adorns the cell's surfaces.
Apart from cell membranes, phospholipids spontaneously assemble into multibilayer sheets. So how can cell membranes consist of a single bilayer phase? Single bilayer phases, similar to those that constitute cell membranes, can be permanently stable but only under unique conditions. Formation of single bilayer vesicles occurs only at a specific critical temperature. At this temperature, pure phospholipids spontaneously transform from either multiple bilayer sheets or unstable liposomes into stable single bilayers.Above or below this temperature, the unilamellar phase collapses into multilamellar structures. The critical temperature varies depending on the specific chemical makeup of the phospholipids. In the case of bilayers formed from a mixture of phospholipids, the critical temperatures depend on the bilayers' specific phospholipid composition. phospholipids extracted from rat and squid nervous system tissue assemble into single bilayer structures at critical temperatures that correspond to the physiological temperatures of these two organisms.
For the cold-blooded sea urchin L.pictus, the membrane composition of the earliest cells in the embryo varies in response to the environment's temperature. In these cases, changes in phospholipid composition allowed the bilayers to adopt a unilamellar phase at a critical temperature that corresponded to the environmental conditions. In addition, the bacterium E. coli also adjusts its cell membrane phospholipid composition to maintain a single bilayer phase as growth temperature varies.
These studies highlight the biological importance of the critical bilayer phenomena. So does other research that indicates how devastating it is for life when cell membranes deviate from critical conditions. Gershfeld identified a correlation between the rupture of human red blood cells (hemolysis) and incubation at temperatures exceeding 37°C (the normal human body temperature). Transformation of the cell membrane from a single bilayer to multiple bilayer stacks accompanies the red blood cells' hemolysis—a loss of the cell membrane's critical state. Gershfeld and his collaborators even provided some evidence that cell membrane defects at the sites of neurodegeneration may play a role in Al- zheimer's disease. Presumably, collapse of the cell membrane's single bilayer state into a multiple bilayer condition stems from altered membrane phospholipid composition.
Cell membranes are carefully crafted structures that appear to be the product of a Creator's painstaking handiwork. It indicates that cell membranes are highly fine-tuned molecular structures dependent on an exacting set of physical and chemical conditions.
Each Piece Placed by Hand
The fine-tuning of cell membrane lipid compositions does more than stabilize the single bilayer phase. One recent study demonstrates that it also appears to critically influence the interactions between cell membranes and proteins. A team of Russian scientists, exploring the role that phospholipids play when the cell's machinery inserts proteins into membranes, discovered that successful insertion of the protein colicin E (a bacterial toxin) requires a fairly exacting phospholipid composition. Introduction of colicin E into bilayers requires PG levels between 25 and 30 percent. PGs bear a negative charge that sets up an electrical potential at the membrane's surface. This potential plays a key role in the insertion process. If the surface potential varies outside a narrow range of values, protein insertion doesn't properly take place. This restrictive range of values occurs only for specific concentrations of PGs. Surface potential depends on the salt concentration in the environment. The researchers noted that as they changed the salinity, the PG concentra- tions in the membranes had to vary accordingly to maintain the just-right surface potential for protein insertion to take place. Interestingly, the content of negatively charged phospholipids (PGs and PSs) in most membranes is between 25 and 30 percent. In fact, most bacteria adjust the level of PGs in their membranes in response to changes in the salinity of the growth medium. These observations suggest that the compositional fine-tuning of phospholipids needed for the import of colicin E into bilayers may be a general requirement for the insertion of most membrane proteins.
Membrane proteins, which contain hydrophobic stretches and are generally insoluble in water, could not have evolved in the absence of functional membranes, while purely lipid membranes would be impenetrable and hence useless without membrane proteins. The origins of biological membranes - as complex cellular devices that control the energetics of the cell and its interactions with the surrounding world - remain obscure.
The Interdependency of Lipid Membranes and Membrane Proteins
The cell membrane contains various types of proteins, including ion channel proteins, proton pumps, G proteins, and enzymes. These membrane proteins function cooperatively to allow ions to penetrate the lipid bilayer. The interdependency of lipid membranes and membrane proteins suggests that lipid bilayers and membrane proteins co-evolved together with membrane bioenergetics.
Its remarkable that a scientific mainstream source resorts to evolution to explain the origin of cell membranes, despite the fact that evolution was not a driving force prior to DNA replication. Secondly, interdependence is openly admitted. The question arises, how could and would a) the membrane and b) the indispensable membrane proteins emerge together ? The synthesis of proteins requires the hardware and software to produce proteins fully in place, setup, and working, and a protecting cell membrane.... Thats a classicle chicken/egg problem, which is simply neglected , making up a just so scneario without detailled explanations.
Water-soluble proteins evolved gradually into highly hydrophobic membrane proteins. Primordial membranes initially contained pores, which enabled ions, small molecules, and polymers to be exchanged passively between protocells and their environment.
If these pores were not specific enough, without a elaborate mechanism to distinguish ions, small molecules, and polymers that were useful and permittet to enter the cell, the "proto-cell" would certainly die. In fact, these ion channel proteins, proton pumps, G proteins, and enzymes had to be fully developed and working from day 1, or nothing would go. They are life-essential for a working cell. A gradual evolutionary development is simply not feasable.
In contrast, modern membrane proteins must be inserted into the membrane by membrane protein complexes. Membrane protein complexes could not have logically existed prior to the existence of membrane proteins.
That makes the whole picture even more difficult. Beside the membrane, the membrane proteins, also protein complexes that insert the membrane proteins into the right place had to emerge, with the program of how, which proteins, and when to insert them at the right place in the membrane. That adds a significant hurdle of difficulty, which origin must be explained.
The Co-Evolution of Lipid Bilayers, Membrane Bioenergetics, and Membrane Proteins
In order to maintain proper bioenergetics, cells meticulously regulate the flow of matter and therefore energy through the cellular membrane. There is an inverse relationship between membrane permeability and the number of enzymatic pathways present in a cell.LUCA and Early Membranes
Archaea and bacteria differ greatly in their biogenesis pathways as well as in the hydrophobic chains contained in the membrane structure.Which indicates that there was no common ancestry. They had to emerge separately.
It is seemingly impossible to have the formation of impermeable membranes without membrane proteins and translocases to shuttle essential materials in and out of the cell. Consequently, it is also unlikely that very specialized membrane proteins were able to form without a membrane initially present. It is hypothesized that the development of a permeable, porous membrane eventually integrated evolving proteins, to later evolve into a highly specialized and efficient impermeable membrane.
The author makes implicitly a strong case for intelligent design. How would and could a random process originating from a prebiotic soup have coordinated the production of membranes and membrane proteins, membrane protein complexes required for the assembly of the membrane proteins , all at the same time, and the information for the correct assembly process ?
Co-evolution of primordial membranes and membrane proteins
The chemical compositions and biogenesis pathways of archaeal and bacterial membranes are fundamentally different [4,5,12].The glycerol moieties of the membrane phospholipids in all archaea and bacteria are of the opposite chiralities. With a few exceptions, the hydrophobic chains differ as well, being based on fatty acids in bacteria and on isoprenoids in archaea; furthermore, in bacterial lipids, the hydrophobic tails are usually linked to the glycerol moiety by ester bonds whereas archaeal lipids contain ether bonds [4,5,12]. The difference extends beyond the chemical structures of the phospholipids, to the evolutionary provenance of the enzymes involved in membrane biogenesis that are either non-homologous or distantly related but not orthologous in bacteria and archaea [4,5,12,13].
It has been argued that fatty acids are the simplest amphiphilic molecules that could form abiogenically to be subsequently recruited by first organisms
Question: If fatty acids would form abiogenetically, why would the cell "invent" extraordinarly compex biosynthesis pathways to produce it, and nanofactories of extraordinary complexity ? :
The first step of fatty acid biosynthesis in modern cells requires the participation of malonyl-CoA, a three-carbon intermediate. The formation of malonyl-CoA from acetyl-CoA is an irreversible process, catalyzed by acetyl-CoA carboxylase enzymes. a multifunctional protein with 3 subunits, which is carefully regulated.
In the second step, fatty acid synthase ( FAS) proteins come into action. FAS most striking feature is the “high degree of architectural complexity” – some 48 active sites, complete with moving parts.
http://reasonandscience.heavenforum.org/t2168-the-amazing-fatty-acid-synthase-nano-factories-and-origin-of-life-scenarios#3950
Then they go on and argue that they " could be subsequently recruited by first organisms".
That seems to me another simplistic pseudo scientific just so assertion without any detailled explanation of how that would have happened without guiding intelligence. In order for this to be possible in the way they assert the five following conditions would all have to be met::
C1: Availability. Among the parts available for recruitment to form the system, there would need to be ones capable of performing the highly specialized tasks of individual parts, even though all of these items serve some other function or no function.
C2: Synchronization. The availability of these parts would have to be synchronized so that at some point, either individually or in combination, they are all available at the same time.
C3: Localization. The selected parts must all be made available at the same ‘construction site,’ perhaps not simultaneously but certainly at the time they are needed.
C4: Coordination. The parts must be coordinated in just the right way: even if all of the parts of a system are available at the right time, it is clear that the majority of ways of assembling them will be non-functional or irrelevant.
C5: Interface compatibility. The parts must be mutually compatible, that is, ‘well-matched’ and capable of properly ‘interacting’: even if sub systems or parts are put together in the right order, they also need to interface correctly.
( Agents Under Fire: Materialism and the Rationality of Science, pgs. 104-105 (Rowman & Littlefield, 2004). HT: ENV.)
A pure lipid bilayer, however, is not a practical solution for the membrane of a primordial cellular life form because it would effectively prevent exchange between the inside of a vesicle and the environment. Therefore another unsolved question is how electrically charged compounds could be transferred across the primordial membranes. Because of the hydrophobic barrier, ions penetrate the lipid bilayer with the help of specialized membrane proteins, such as channels or translocases. The membrane-embedded portions of these proteins consist, largely, of hydrophobic amino acids, and the proteins themselves are water-insoluble. Hence a chicken and egg paradox: a lipid membrane would be useless without membrane proteins but how could membrane proteins have evolved in the absence of functional membranes?
Its particularly interesting when mainstream scientific papers admit chicken-egg difficulties ....
Much uncertainty remains regarding the chemical nature of membranes in the LUCA and earlier.
1. https://en.wikibooks.org/wiki/Structural_Biochemistry/The_Evolution_of_Membranes
2. Co-evolution of primordial membranes and membrane proteins
3. http://www.macromol.uni-osnabrueck.de/paperMulk/Mulkidjanian_Galperin_Evolution_of_membr_proteins_Figures.pdf
Last edited by Admin on Sat Oct 03, 2020 5:09 pm; edited 1 time in total

