Morphogen Gradients and Pattern Formation
https://reasonandscience.catsboard.com/t2354-morphogen-gradients-and-pattern-formation
Establishing an animal body plan depends on many mechanisms that go beyond genetic information.
The development of an organism from an undifferentiated single cell into a spatially complex structure requires spatial patterning of cell fates across tissues. Several different signaling pathways are essential for the formation of organized tissues, organismal form, and architecture. Spatial patterning of a developing animal requires that cells become different according to their positions in the embryo, which means that cells must respond to extracellular signals produced by other cells, especially their neighbors. In what is probably the most common mode of spatial patterning, a group of pluripotent cells start out with the same developmental potential, and a signal from cells outside the group then induces one or more members of the group to change their character. Some inductive signals depend on cell–cell contact; others act over a longer range and are mediated by molecules that diffuse through the extracellular medium or are transported in the bloodstream. Most of the known events in animal development are governed by a small number of signaling pathways.
A core set of key morphogens regulates development. Morphogens are signaling molecules forming long-range concentration gradients that pattern a field of cells. After morphogens are secreted from a group of cells, they are transported across tissues to establish a gradient. The transport of morphogens is mediated by kinesin and dynein motor proteins along the microtubule networks of axons. Morphogens near the local source get attached to the shuttle and the morphogen-shuttle complex is transported across tissues. Subsequently, the morphogen-shuttle complex is degraded, resulting in the morphogen being locally immobilized, and start forming a local gradient. Bioelectric signals and resting membrane potentials across tissues are also and additionally crucial for proper patterning in multiple organisms. Furthermore, there are also neurotransmitters ( chemical messengers that transmit a message from a nerve cell across the synapse to a target cell) establish left-right patterning in embryos through regulation of ion fluxes. Calcium (Ca2+) signaling also plays a key role in mediating cellular responses to morphogens. In addition to mediating cellular differentiation and proliferation, Ca2+ signals also influence cellular migration in response to morphogen gradients.
Robustness is a ubiquitous feature of biological systems that ensures specific functions of the system are maintained despite external and internal perturbations. Furthermore, directional transport of substances through the nervous system is necessary to achieve scale-free morphogen patterning and body axis polarity determination.
Cells sense and interpret their position as a function of the amount of signal they receive, thus obtaining ‘‘positional information’’. Within larger tissues, neural networks provide directed information, via physiological signaling, that supplements positional information through diffusion. This positional information specifies gene expression and subsequent fates of cells in tissues.
A few key-sentences: Cells operate upon biological design rules - Developing cells have a vocabulary - There is intercellular communication - Cells communicate with each other through cell-cell gap junctions, which exercise modulation, that is control, direct, induce, and regulate pattern formation - There is so-called physiological signaling, and there are positive diffusion regulators - There are as well signals that propagate modulation of ion fluxes through voltage-gated ion channels - There are signaling molecules that provide information for direction cues - There are as well bioelectrical signals - Cells know how to interpret their position by obtaining ‘‘positional information’ through a coordinate system, which is modulated and mediated through morphogens that permit the formation of specific patterns through gradients. These regulate development; those also specify cell fates.
There is a general importance of mechanical forces in regulating growth and morphogenesis. Mechanical forces, cellular mechanics, and tissue mechanics are key components of morphogenesis and can influence pattern formation.
As a general scheme, it can be observed, that there are not simply chemical reactions, but molecules and cells operate, behave, migrate, position themselves, organize, form tissues, specialize based on instructions through various signaling networks and mechanisms, which have as source various epigenetic signaling pathways. Integrated, network-based information systems permit little if no errors, which undoubtedly lead to disease. This is prevented by robustness which has to be set up from the beginning. This leads to my understanding to intelligent design as the best explanation.

Morphogen Gradients and Pattern Formation 1
Small Numbers of Conserved Cell–Cell Signaling Pathways Coordinate Spatial Patterning
Spatial patterning of a developing animal requires that cells become different according to their positions in the embryo, which means that cells must respond to extracellular signals produced by other cells, especially their neighbors. In what is probably the commonest mode of spatial patterning, a group of cells starts out with the same developmental potential, and a signal from cells outside the group then induces one or more members of the group to change their character. This process is called inductive signaling. Generally, the inductive signal is limited in time and space so that only a subset of the cells capable of responding—the cells close to the source of the signal—take on the induced character
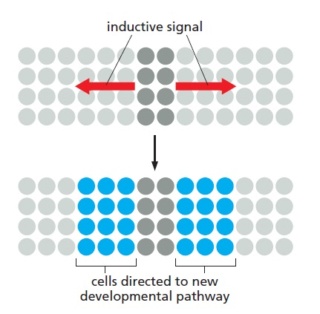
Inductive signaling.
Some inductive signals depend on cell–cell contact; others act over a longer range and are mediated by molecules that diffuse through the extracellular medium or are transported in the bloodstream. Most of the known inductive events in animal development are governed by a small number of highly conserved signaling pathways, including
transforming growth factor-β (TGFβ),
Wnt,
Hedgehog,
Notch,
receptor tyrosine kinase (RTK) pathways
The discovery of the limited vocabulary that developing cells use for intercellular communication has emerged over the past 25 years as one of the great simplifying features of developmental biology.
Compartment boundaries are the sources of morphogens. Morphogens are signaling molecules that are produced from a localized source forming long-range concentration gradients that pattern a field of cells (Figure 7a). Cells interpret their position as a function of the amount of signal they receive, thus obtaining ‘‘positional information’’ (Wolpert, 1989, 1996). Signaling molecules have to fulfill two stringent criteria to qualify as morphogens: (1) their effect must be exerted in a concentration-dependent manner and (2) they must act directly on target cells at a distance from the source (i.e., not through a secondary relay mechanism). When these criteria are met, the local concentration can be interpreted as a measure of distance from the source of the signal (Figure 7a). The three signaling proteins that qualify as morphogens in Drosophila wing development are Hedgehog (Hh), Decapentaplegic (Dpp), and Wingless (Wg) (e.g., Wg is shown in Figure 7).

Figure 7 Example of a morphogen gradient: Wingless (Wg).
(a) Drawing of a section of the wing pouch epithelium (upper panel) and of the wing pouch in an apical view (lower panel). The cells in the center express Wg which generates a gradient across the adjacent tissue. Cells at different distances receive different levels of Wg.
(b) Schematic drawing of the Wg gradient and target gene activation in the epithelium in response to Wg (upper panel). The Wg gradient activates the target genes hindsight (cyan) and distalless (blue) at different threshold levels. The expression domains of Hindsight and Distalless are depicted in the same colors in the lower panel. (c) Wg downregulates its own receptor Drosophila Frizzled 2 (DFz2; blue), shaping its gradient and rendering the
cells at a distance from the source of Wg more sensitive.
It is generally accepted that cells interpret the gradient by eliciting differential transcriptiona responses depending on the concentration of morphogen they are exposed to. This requires cells to make decisions depending on different threshold levels of signaling pathway activity. The concept implies that a single event – namely the production of a secreted molecule at a localized source – can lead to the formation of several different cell
types in a correct spatial relationship to each other (Figure 7). This represents a highly efficient way of generating complex patterns in previously uncommitted cells (review: Gurdon and Bourillot, 2001).
Different modes of morphogen movement/transport have been invoked to explain long-range gradient formation: extracellular diffusion of the secreted molecule, cycles of receptor- mediated endocytosis and resecretion (planar transcytosis) , membranous exosomes (argosomes), and cytoplasmic extensions (cytonemes). Although there is no compelling evidence to date that mechanisms other than diffusion contribute productively to gradient formation, it is certainly plausible that these mechanisms could do so. Cytonemes and argosomes exist, but have not yet been shown to be required to mediate ligand movement or to transduce signals. Evidence presented in favor of endocytosis and resecretion as a mechanism of ligand transport has been questioned. Further experimentalevidence is needed to support or refute these possibilities. Several factors have been implicated in shaping morphogen gradients: posttranslational modification of the ligand can influence diffusibility (e.g., acylation of Hh and Wg (Ingham, 2000, 2001)).
Regulation of receptor levels can influence ligand movement (Figure 7c). Cell surface heparan-sulfate proteoglycans, and secreted enzymes that modify them, can modulate ligand
movement . Secreted ligand binding proteins can influence movement or stability . In addition, cellular responses to these ligands can also be modulated, contributing to shaping the activity gradients rather than the ligand gradients per se.
Interplay between morphogen‐directed positional information systems and physiological signaling 03 December 2019 4
The development of an organism from an undifferentiated single cell into a spatially complex structure requires spatial patterning of cell fates across tissues. Positional information, proposed by Lewis Wolpert in 1969, has led to the characterization of many components involved in regulating morphogen signaling activity. Quantitative and systems-based approaches are increasingly needed to define general biological design rules that govern positional information systems in developing organisms. There are various roles of physiological signaling in modulating and mediating morphogen-based pattern formation. Similarities between neural transmission and morphogen-based pattern formation mechanisms suggest underlying shared principles of active cell-based communication. Within larger tissues, neural networks provide directed information, via physiological signaling, that supplements positional information through diffusion. Further, mounting evidence demonstrates that physiological signaling plays a role in ensuring robustness of morphogen-based signaling. We conclude by highlighting several outstanding questions regarding the role of physiological signaling in morphogen-based pattern formation. Elucidating how physiological signaling impacts positional information is critical for understanding the close coupling of developmental and cellular processes in the context of development, disease, and regeneration. 2
Lewis Wolpert proposed positional information as a mechanism whereby differential gene expression of cells results in spatial patterns of cellular differentiation. Within the concept of positional information, a coordinate system defines the magnitude and directionality of the positional information sensed by cells. This positional information specifies gene expression and subsequent fates of cells in tissues (Figure below)
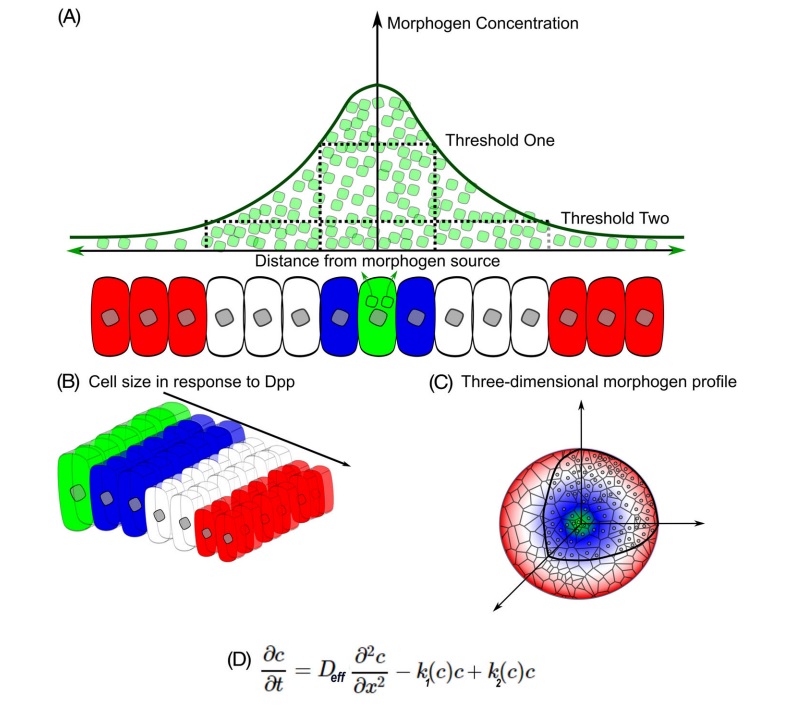
A, classical view of positional information and morphogenesis.
The classical view of positional information stems from the French Flag Model in which source cells secrete morphogens that are transported through a tissue due to diffusion. A, Morphogen molecules (green) are secreted from source cells where morphogen concentration will be the highest. Morphogens travel to neighboring cells to establish a concentration gradient. Cells will have differential biological responses to the morphogen gradient dependent upon multiple threshold levels. Blue cells are located where morphogen concentrations are above the higher threshold. White cells sense morphogen concentrations that are above the lower threshold. Red cells detect low to no morphogens.
B, Cells respond to morphogens to determine cell fates and cell morphology in a dose-dependent response.
C, The ability of progenitor cells to create morphogen gradient-based patterns is dependent upon tissue geometry, size, temporal signaling of morphogens.
D, Governing diffusion equation of the morphogen concentration in accordance with Fick's second law of diffusion with a nonlinear degradation profile (k1), a source term dependent upon location (k2), and effective diffusion of the molecule (Deff). This results in a powerlaw relationship in which the gradient is position-dependent and reflects the local steepness of the gradient
Following Wolpert, Gierer and Meinhardt proposed models of morphogen distribution to demonstrate that relatively simple molecular mechanisms can explain the formation of a spatially patterned tissue. Subsequent experimental work has demonstrated that a core set of morphogens generate and relay positional information to cells to directly induce cellular responses based on the cells' location. Because morphogens play crucial roles during the specification of cell fates, they contribute to many aspects of development. A core set of key morphogens regulates development. Examples include members of the Hedgehog (Hh) family that are involved in Drosophila appendage formation and chick neural tube development. As a second example, Wingless (Wg)/Int-1 (Wnt) proteins contribute to Drosophila appendage development and human degenerative diseases. Bone morphogenetic proteins (BMPs), such as Drosophila Decapentaplegic (Dpp), are utilized in dorsal-ventral patterning of the Drosophila embryo and pattern formation and growth control of limb primordia. BMPs also regulate the formation of early germ layers of mammals. Morphogen signaling includes conveying positional information, and their ability to induce pattern formation through gradients. One morphogen can control the expression of another during morphogenetic processes, such as Hh-induced Dpp activity in developing Drosophila and Hh participating in crosstalk with Wnt in cancer. On the other hand, computational modeling has proven critical for explaining increasingly complex datasets and counter-intuitive results from genetic perturbations to morphogenetic patterning systems. Additional computational efforts have uncovered the role of the nervous system in facilitating regeneration in Planaria. This among many other studies support a close analogy between embryonic patterning and brain-like signal processing. However, in many cases the underlying kinetics and dynamics in gradient formation and maintenance remain poorly understood.
Morphogen-based signaling during development requires active cellular and physiological processes, as has been noted through analysis of the importance of lipid metabolism in morphogen transport. Here, we denote the term physiological signaling to represent cell regulatory mechanisms at the ionic and molecular level that control key cellular functions such as trafficking through exo- and endocytosis, metabolic processes of cellular growth and division, or the regulation of cell mechanics. Increasingly, evidence demonstrates the central role of physiological signaling events in mediating morphogen activity and emerging parallels between neurotransmission and morphogen transport across non-neural tissues. In particular, we highlight the functional roles of secondary messengers, such as calcium (Ca2+), in mediating morphogen secretion, transport, downstream information processing, and providing robustness of positional information.
CELLULAR MECHANICS DIRECTLY INFLUENCE PATTERN FORMATION AND MORPHOGEN GRADIENTS
Early studies of morphogenesis posited the notion that chemical-based morphogen gradients are the primary signals to pattern cell differentiation. However, there is emerging evidence demonstrating that mechanical events can also be a primary trigger in pattern formation. Mechanical forces drive cellular self-organization and structural rearrangements of a feather follicle's shape and gene expression in chicken embryos. In particular, mechanical activation of β-catenin initiated downstream follicle gene expression of bmp2, a key component of the TGF-β signaling pathway. This study thus serves as a striking example of the general importance of mechanical forces in regulating growth and morphogenesis. Mechanical forces, cellular mechanics, and tissue mechanics are key components of morphogenesis and can influence pattern formation. The underlying mechanisms in which mechanics provide positional information to cells require further elucidation.
MORPHOGEN SOURCES ARE DYNAMIC AND CONTROLLED BY PHYSIOLOGICAL SIGNALING
The conceptual French Flag model, an iconic description of positional information proposed by Wolpert, specifies that morphogens are secreted from a cluster of cells and form a graded distribution throughout the tissue to specify multiple cell types dependent on the concentration sensed by cells (Figure A above). Within this model, the secretion of morphogens from source cells is the initial step in the formation of positional information (Figure B above). This initial framework has since expanded from a static viewpoint to include dynamic changes to the size, geometry, location, mechanics, and temporal signaling of morphogen secreting cells (Figure C above). An example of a dynamic morphogen source occurs during the morphogenesis of dorsal appendages of the Drosophila melanogaster eggshell. The morphological boundaries of these structures depend on the spatial patterning transcription factor Broad r, which is regulated by the epidermal growth factor receptor (EGFR) signaling pathway through a feedback regulatory network. The patterning of Broad is established when Gurken, an EGFR ligand, is secreted from the underlying oocyte forming a posterior-to-anterior gradient. Later, a dorsoventral gradient forms after translocation of the oocyte nucleus to the dorsal anterior cortex.41-43 This suggests that morphogen sources are spatiotemporally dynamic and do not always adhere to the framework of passive diffusion-based transport from a stationary source (Figure D). This computational-based analysis of how subsequent rounds of EGFR activation refine spatial patterns was experimentally confirmed. Given the potential for spatiotemporally dynamic morphogen sources, secretion mechanisms of morphogens may provide insight into how this is possible.
Source cells secrete morphogens through exocytosis, an active transport mechanism of molecules to the cell membrane through vesicles. Fusion of vesicles to the cell membrane releases the morphogen. Therefore, morphogen secretion is influenced by physiological regulation of exocytosis. For example, inwardly rectifying potassium (Irk) channels regulate the release of Dpp and cytosolic Ca2+ in the developing Drosophila wing imaginal disc45 (Figure below).
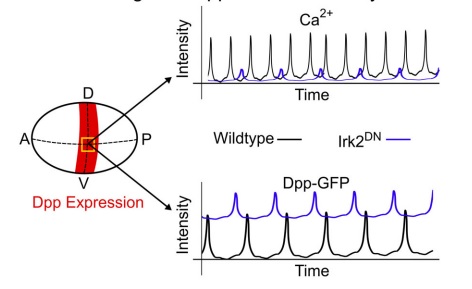
Irk channels regulate Dpp secretion and cytosolic Ca2+.
In the Drosophila wing disc, inwardly rectifying potassium channels (Irk) regulate both the secretion of bone morphogenic protein Dpp and cytosolic Ca2+ levels. Expression of Dpp along the dorsal-ventral (D-V) axis in the wing disc is indicated in red. Dpp expression is not present along the anterior-posterior (A-P) axis of the wing disc. Inhibition of Irk channels using dominant negative Irk2 mutants (Irk2DN) decreased the duration and amplitude of Ca2+ oscillations in Dpp-producing cells (yellow square indicates region of interest). Further, loss of Irk2 channel function increased the baseline concentration level of Dpp-GFP while simultaneously decreasing discrete Dpp release events. Thus, Irk2 inhibition in wing discs simultaneously alters intracellular Ca2+ activity and Dpp release
Irk channels facilitate flow of potassium (K+) into the cell and have a fundamental role in restoring the membrane potential to its resting potential. Inhibition of Irk channels in Dpp producing cells, reduced Dpp secretion independent of Dpp expression. Irk channels regulate vesicle release by changing membrane potential while also altering intracellular Ca2+ dynamics. A potential explanation for similar outcomes in Dpp and Ca2+ after Irk channel inhibition is that intracellular Ca2+ dynamics regulate Dpp release, although whether or not Ca2+ has a causative effect requires further study. Ca2+ is crucial to regulation of epithelial physiology during development. However, whether Ca2+ directly regulates Dpp secretion is currently unknown. Given that misregulation of Ca2+ is frequently associated with developmental genetic disorders resulting in neoplasms, it is necessary to further characterize the roles of Ca2+ and other second messengers in morphogen transport and morphogenesis.
PARALLELS BETWEEN NEUROTRANSMISSION AND MORPHOGEN-BASED TRANSPORT SYSTEMS
After morphogens are secreted from a group of cells, they are transported across tissues to establish a gradient. How morphogens disperse and form gradients is still under debate despite progress in understanding the molecular mechanisms of morphogen transport through imaging studies and biophysical measurements. Several morphogen transport models have been proposed in the literature. These range from passive mechanisms, such as free or hindered diffusion (first Figure D), to cell-based dispersal by transcytosis or cytonemes. Multiple transport mechanisms may be involved, and this likely varies across developmental contexts. The simplest mechanism of morphogen transport is passive diffusion where molecules disperse by random motion. However, this model does not fully capture the complexity of morphogen transport dynamics due to evidence demonstrating that a single source of morphogen is not always sufficient to establish a gradient. For example, during Drosophila wing disc development, DWnt6 is expressed in an identical pattern to Wg,57 while both BMP ligands Gbb and Dpp are necessary to establish proper morphogen gradients. In facilitated diffusion, morphogens are largely immobile until they bind to a positive diffusion regulator that enhances motility. Shuttling is a special case of facilitated diffusion in which molecular shuttles, not morphogens, are generated from a localized source. Morphogens near the local source get attached to the shuttle and the morphogen-shuttle complex is transported across tissues. Subsequently, the morphogen-shuttle complex is degraded, resulting in the morphogen being immobilized to stationary negative diffusion regulators and the formation of a gradient.
Beyond extracellular-diffusion-based morphogen transport, morphogens also can be transported through cell-based mechanisms via transcytosis or along cellular extensions known as cytonemes. In the case of transcytosis, signaling molecules are taken up by cells through endocytosis and subsequently released through exocytosis into the extracellular space. Endocytosis is the process in which cargo molecules, including morphogens, are absorbed and distributed into a series of endosomes with distinct physical and biochemical properties. An endosome's dynamics, such as fission, fusion, and maturation, influences its ability to sort and concentrate cargo molecules. For the case of morphogens, endosome dynamics are thus able to establish concentration gradients and signaling activities across tissues. Significant questions remain regarding which is the dominant mode of morphogen transport in a given developmental context. In the case of the Drosophila wing disc, Dpp can move through regions that are mutant for the type I transforming growth factor beta receptor thickveins (tkv) and downstream transcriptional repressor (Brinker). This is significant given that support for transcytosis stems from experiments utilizing the Dpp receptor Tkv. Due to the apparent conflicts between experimental studies, much work remains to clarify the roles of the physiological processes governing morphogen transport. An example of morphogen signaling being affected by exo- and endocytosis is seen in development of the Drosophila air sac primordia (ASP), which depends on Dpp signaling. Cytoneme-based signaling utilizes many of the same components found in neural synapses. In the Drosophila ASP (“receiving cells”), specialized filopodia-like cytonemes endocytose Dpp from the adjacent Drosophila wing disc cells (“sending cells”; Figure 3A).
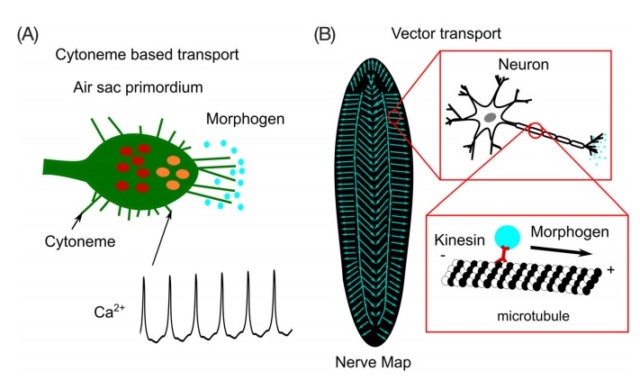
Morphogen transport mechanisms.
A, Extended filopodia called cytonemes are present in the Drosophila air sac primordium (ASP). Cytonemes projecting from the ASP take up Dpp from the adjacent wing imaginal disc. Correlated transients of Ca2+ concentrations are observed in cytonemes. Cytoneme mediated transport requires Synaptotagmin-4 (Syt4), which helps in vesicle fusion and receptor internalization, and glutamate receptor GluRII in the ASP. Wing disc cells secreting Dpp require Synaptobrevin, Synaptotagmin-1, the glutamate transporter, and voltage-gated calcium channels. Blue circles represent morphogens, red circles represent pMad molecules in cells, and orange circles represent dpERK molecules in cells. B, In Planaria, morphogens are transported on microtubule arrays along the axons from the nervous system to the wound edge during regeneration. Vector transport is identified as a fundamental requirement for scale-free self-assembly of morphogens during Planaria homeostasis and regeneration. Figure is adapted with permission from Reference
Dpp signaling was compromised when Synaptotagmin-1, Synaptobrevin, the glutamate receptor, or voltage-gated Ca2+ channels were inhibited in the secreting disc cells, resulting in a reduction in the size of the ASP. This result parallels neurotransmission as Synaptobrevin is an intrinsic membrane protein that regulates neurotransmitter release through Ca2 +-dependent exocytosis. The receiving cells of the ASP require Synaptotagmin-4 and the glutamate receptor GluRII. This is noteworthy given that Ca2+ is a crucial regulator of endocytosis 3 and neurotransmitter regulation. Huang et al further demonstrated that Ca2+ transients observed in cytonemes correlate with Dpp uptake. This suggests that signal uptake and transport within cytonemes depends on local Ca2+ concentrations. This study, thus, underscores the role of physiological signals, like Ca2+, in morphogen mediated transport and internalization that have not yet been fully explored. Further evidence for second messenger signaling and morphogen transport lies in the interaction between Ca2+ and endocytic and exocytic regulators. Knockdown of Ca2+ signaling-dependent exocytic components in the prothoracic gland in Drosophila brain, resulted in the accumulation of unreleased steroid hormone ecdysone. Whether the same exocytic machinery controls secretion of key morphogens is currently unknown. Reverse genetic RNAi screening could be employed to map out the components regulating morphogen secretion and transport through exocytosis. Additionally, synthesis of new alleles with mutations in Ca2+ binding domains of key morphogen regulatory proteins will further enable confirmation of the role of physiological signals in regulating morphogen secretion.
A key player in the endocytic process is the regulatory guanosine triphosphatase (GTP) protein Rab5. Rab5 proteins aid in the formation of transport vesicles and regulate molecular cargo degradation and recycling. Rab5 is required for endosome integrity in the presynaptic terminal in Drosophila neuromuscular synapses. Impaired Rab5 function affects both Exo- and endocytosis rates and decreases the ability of neurotransmitter release, while overexpression of Rab5 increases the release efficacy of neurotransmitter. This is particularly interesting because Ca2+ is an important regulator of neurotransmitter release. Recent work has shown that Ca2+ channels regulate bulk endocytosis, a form of endocytosis of synaptic vesicles at nerve terminals, in addition to coupling exo- and endocytosis. Further, Rab5-dependent endocytosis requires Ca2+ signaling to increase the rate of membrane capacitance, which determines how quickly the membrane potential can respond to a change in current and is linearly related to changes in membrane surface area. The converse was also shown in which low Ca2+ concentrations decreased membrane capacitance. Thus, an increase in surface area reflects increased exocytosis and decreased surface area reflects an increase in endocytosis. Additionally, discovery of a feedback loop showed BMP and Scrib, a scaffold protein associated with cellular proliferation, promotes Rab5 endosome-dependent BMP/Dpp signaling during morphogenesis in Drosophila. Given that Ca2+ affects Rab5-related endocytosis and Rab5 affects Dpp signaling, it can be inferred that Ca2+ concentrations may serve as a potential modulator of morphogen transport through coupled exo- and endocytosis. However, this inference remains to be tested directly. Another possible mechanism through which physiological signals such as Ca2+ affect transport of morphogens is through Ca2+ binding domains of transport proteins. Evidence for this lies in the presence of Ca2+ binding EF-domains in proteins involved in the formation of BMP and Dpp gradients in the Drosophila wing disc. For example, Drosophila Pentagone (Pent) directly interacts with Dally to provide long range distribution of the Dpp ligand. Structurally Pent has a similar domain composition to that of human SMOC protein, and both Pent and SMOC proteins contain Ca2+ binding EF domains. Xenopus SMOC-1 (XSMOC-1) protein acts as a BMP antagonist in Xenopus embryos even in the presence of constitutively active BMP receptor.84 Further analysis suggests that SMOC-1 antagonizes BMP signaling downstream of receptor binding through activation of MAPK signaling. Later studies demonstrated the ability of Drosophila-specific Pent to similarly inhibit BMP signaling in Xenopus downstream of the BMP receptor after injection of synthetic pent mRNA into Xenopus embryos.
Following this, Thomas et al utilized the SMOC deletion mutant constructs XSMOC-1ΔEC (lacking the extracellular Ca2+ binding domain) and XSMOC-1EC (containing the extracellular Ca2+ binding domain only) to demonstrate that normal XSMOC-1 and XSMOC1ΔEC, but not XSMOC-1EC convert the fate of naïve Xenopus ectoderm explants to anterior neural tissue. Thus, embryonic cell fate decisions to become neural tissue via SMOC, and BMP inhibition does not require the extracellular Ca2+ binding domain. However, the potentiation of BMP signaling by the extracellular Ca2+ binding domain was shown in further studies by observing the affinities of XSMOC-1EC and BMP2 for each other and other heparan sulfate proteoglycans (HSPGs). Experiments utilizing in vitro diffusion assays on agarose gels embedded with or without HSPGs demonstrated that the binding of BMPs to HSPGs restricts their range of effect and that BMP diffusion can be enhanced by the binding of SMOC to HSPGs to expand the range of effect. The SMOC extracellular Ca2+ binding domain expands the range of BMP signaling through competitive binding to HSPGs. These studies demonstrate the importance of the SMOC extracellular Ca2+ binding domain in the context of development. Further investigations are needed into the role of physiological signaling within the context of the extracellular environment.
The coupling between neuronal and non-neural tissues in defining positional information extends beyond similarities in signaling mechanisms. A recent study provided further insight into the role of the nervous system in morphogen-based pattern formation and regeneration in Planaria. A remarkable feature of regeneration in Planaria is the reformation of key morphogen gradients such as Hh and Notum Regulating Factor (NRF) after fragmentation or wounding of the organism. Pietak et al developed a quantitative model of regenerating Planaria to elucidate the mechanism of morphogen gradient activity that ensures robust body-plan regulation. The model predicts the fraction of heteromorphoses in regenerating Planaria fragments. Through an iterative combination of computational simulations and experiments, they found that vector transport of morphogens was required to explain regeneration of pattern formation. Morphogen vector-based transport is defined as the directional transport of morphogens by a vector field. The vector transport field coincided with the nerve polarity throughout regenerating planarian tissue. In their Markov chain model, the transport of morphogens, such as Hh and NRF, is mediated by kinesin and dynein motor proteins along the microtubule networks of axons (Figure B above)
They further demonstrated that the head-tail axis is controlled by the net polarity of neurons. In contrast, a purely diffusion-based model of patterning could not explain the scaling of steady-state concentrations of morphogens of fragments of various sizes. Further, diffusion would be too slow to regenerate the pattern (requiring more than a week on a 1 cm scale organism), while Planaria can reform within 72 hours or less. Thus, the nervous system plays an instructive role in regulating long-distance axial patterning repair during regeneration.
Bioelectric signals and resting membrane potentials across tissues are crucial for proper patterning in multiple organisms. Changes in membrane potential in regenerating Planaria, induced by ionophore treatment, permanently impacted gene expression, patterning, and polarity. After ionophore washout from the treated tissue, the induced changes in resting membrane potential persisted. This mechanism parallels synaptic plasticity in the brain where action potentials, modulated by voltage-gated ion channels, propagate signals. Work done in Xenopus and chick provides insight to this occurrence where serotonin (5-HT), an endogenous neurotransmitter, establishes left-right patterning in embryos through regulation of ion fluxes. A follow-up study showed that extracellular 5-HT availability drives innervation through tissues via gap junctional communication modulation. Combining their findings, Levin and colleagues propose that extracellular 5-HT, a positively charged molecule, navigates through gap junctions to accumulate in hyperpolarized cells, mimicking the reuptake function of 5-HT transporters (SERTs). These 5-HT sequestering, hyperpolarized cells can depolarize in response to loss of chloride ions via glycine-gated chloride channel activation. Without normal hyperpolarization, 5-HT is exported to the extracellular space by SERTs, which can induce growth and hyperinnervation of tissue. The importance of ion channels in facilitating communication in bacterial biofilm communities shows the generality of physiological signaling for mediating cell-to cell communication mechanisms. For example, bacterial biofilm communities utilize synchronized oscillations of short-range connectivity among a few cells and community-wide signaling, to minimize competition for resources. In sum, further quantitative investigations are needed to explore the role of neurotransmission, gap junction communication, and the nervous system in morphogen based transport systems. The establishment of morphogen gradients through neural signaling still needs to be integrated with known physiological regulators of patterning. This will be critical for considering how patterns form across large spatial domains where diffusion-based mechanisms become ineffective.
PHYSIOLOGICAL SIGNALS MODULATE MORPHOGEN-BASED POSITIONAL INFORMATION SYSTEMS
Ca2+ signaling also plays a key role in mediating cellular responses to morphogens. Several studies in various model systems have shown the interplay between Hh signaling and Ca2+ dynamics. For example, Shaw et al showed that intracellular Ca2+ mobilization regulates the level of Sonic hedgehog (Shh)-dependent expression domains of nkx2.2b, isl1, nkx6.1, and pax3 genes in the developing nervous system of zebrafish embryos during the 18-somite stage. Furthermore, they showed that reduced expression of ryanodine receptor (RyR), an intracellular Ca2+-release channel, shifted the allocation of Shh-dependent cell fates in the somitic muscle and neural tubes in a manner that resembled the effects of reduced Shh signaling. These findings were discovered by utilizing loss-of-function mutations, antisense morpholino knockdowns, and pharmacological treatments to perturb RyR activity.
In another study, Belgacem and colleagues demonstrated that Shh acutely increases Ca2+ through the activation of the transducer Smoothened (Smo), which then recruits heterotrimeric GTP-binding protein-dependent pathways. They demonstrated that Shh increases Ca2+ spike activity of developing spinal neurons and propose that Ca2+ spike frequency encodes Shh concentration and is required for proper neuronal differentiation (Figure A).
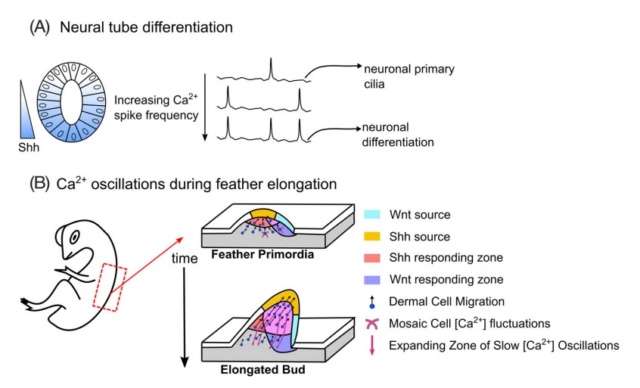
Physiological roles of Ca2+ in mediating morphogen response.
A, A gradient of the morphogen Sonic Hedgehog (Shh) directs the patterning of neuronal differentiation in the developing Xenopus spinal cord. Shh increases Ca2+ spike activity in developing spinal neurons. A current model suggests that Ca2+ spikes convert noisy Hh signaling into binary outputs to specify cell fates. This was demonstrated by a positive relationship between activation of Hedgehog signaling through activation of the Shh transducer Smoothened (Smo) and stimulating Ca2+ spike activity. Loss of the Ca2+ spikes resulted in decreased GABAergic neuronal cell fates. This suggests Ca2+ spike frequency encodes Shh concentration and is required for proper neuronal differentiation .
B, Tissue-wide, long-range Ca2+ oscillations have been observed in mesenchymal cells. Synergistic actions of Shh and Wnt signaling allowed synchronized Ca2+ oscillations to coordinate cell movements during chicken feather elongation
In addition to mediating cellular differentiation and proliferation, Ca2+ signals also influence cellular migration in response to morphogen gradients. Coordinated cell migration during chicken feather elongation is accompanied by dynamic changes of bioelectric currents and Ca2+ signaling. Specifically, Shh-responsive cells contained synchronized Ca2+ oscillations in which Shh plays a key role in mediating interactions between the epithelium and mesenchyme during feather morphogenesis. Voltage-gated Ca2+ channels and Connexin43-based gap junctions regulate Ca2+ dynamics during feather elongation. Shh signaling and β-Catenin signaling activates Connexin-43 expression transcriptionally to establish the gap junctional network (Figure B). The establishment of gap junctional networks in growing tissues thus alters the spatial range of Ca2+ signaling
Further, a review of the role of gap junctions in regulation of pattern formation details that left-right asymmetry in C. elegans neurons involves Ca2+ signaling and communication through gap junctions.94,95 These studies further demonstrate the role of second messenger systems in mediating morphogen-induced responses of cells and the role of physiological signaling in morphogen-based pattern formation. Quantitative experiments investigating the interplay between second messengers and gap junctions during pattern formation are greatly needed.
FEEDBACK FROM PHYSIOLOGICAL SIGNALING ENSURES ROBUSTNESS OF MORPHOGEN-BASED SIGNALING
Important aspects of morphogen gradients as a source of positional information are robustness in the presence of genetic or environmental noise and the proper scaling of morphological patterns with respect to size. Robustness is an ubiquitous feature of biological systems that ensures specific functions of the system are maintained despite external and internal perturbations. Robustness of positional information can influence development significantly. Several recent studies have begun to elucidate the mechanisms governing pattern robustness. For instance, in the context of Planarian regeneration, it was shown that pure reaction-diffusion mechanism of morphogens fails to provide scale-free morphogen gradients. The authors hypothesized that in addition to the classical mechanism involving diffusion, directional transport of substances through the nervous system is necessary to achieve scale-free morphogen patterning and body axis polarity determination.
How was that directing achieved? What if the robustness was not there right from the beginning?
Overall, this study supports the idea of scale invariance in developing systems in which morphogen gradients are scaled properly despite external and internal perturbations. However, despite extensive work into the scaling mechanisms that regulate scaling of patterns at the tissue, organ, and organism level, biochemical mechanisms underlying patterning robustness remain to be discovered Regulation of downstream responses by physiological signals also suggest that physiological signals alter the robustness of morphogenetic processes. Ca2+ gradients are generated along the dorsal-ventral axis of the Drosophila embryo. These concentration gradients are formed during embryonic stage 5 with higher Ca2+ levels on the dorsal side. They also showed that manipulation of the Ca2+ gradient affects the specification of amnioserosa, located dorsally in Drosophila. This study underscores the importance of physiological signaling pathways that utilize Ca2+ in contributing to the specification of the dorsal embryonic region. Ca2+ gradients affect robustness of morphogenetic processes. An outstanding question is whether this mechanism of specification of positional information is influenced by Ca2+ gradients in other model systems.
Increasingly, evidence is accumulating for significant crosstalk of Ca2+ and ROS between endoplasmic reticulum, an established site of Ca2+ storage, and mitochondria, a generation site of ROS.102 In particular, Ca2+ diminished ROS from ROS generation sites within the mitochondria under normal conditions and enhanced ROS generation when generation sites were inhibited by pharmacological agents.103 Further, quantitative in vivo microscopy in Drosophila and zebrafish embryos identified ROS as crucial signals that regulate cell polarity after wounding.104 Hunter et al utilized fluorescent imaging after wounding Drosophila embryos and demonstrated Ca2+-dependent mitochondrial ROS production correlates with the site of actomyosin cable assembly. This suggests that wound-induced ROS production promotes healing in Drosophila and zebrafish embryos. Taken together, these studies demonstrate that physiological signaling, in the form of ROS and Ca2+, act to regulate robustness of morphogenesis. Further experiments are needed to elucidate how ROS and Ca2+ affect robustness of positional information in the context of development, regeneration, and disease.
Analysis on gene modular network reveals morphogen-directed development robustness in Drosophila30 June 2020 4
Genetic robustness is an important characteristic to tolerate genetic or nongenetic perturbations and ensure phenotypic stability. Morphogens, a type of evolutionarily conserved ( no evolutionary change) diffusible molecules, govern tissue patterns in a direction-dependent or concentration-dependent manner by differentially regulating downstream gene expression. However, whether the morphogen-directed gene regulatory network possesses genetic robustness remains elusive. In the present study, we collected 4217 morphogen-responsive genes along A-P axis of Drosophila wing discs from the RNA-seq data, and clustered them into 12 modules. By applying mathematical model to the measured data, we constructed a gene modular network (GMN) to decipher the module regulatory interactions and robustness in morphogen-directed development. The computational analyses on asymptotical dynamics of this GMN demonstrated that this morphogen-directed gene modular network (GMN) is robust to tolerate a majority of genetic perturbations, which has been further validated by biological experiments. Furthermore, besides the genetic alterations, we further demonstrated that this morphogen-directed GMN can well tolerate nongenetic perturbations (Hh production changes) via computational analyses and experimental validation. Therefore, these findings clearly indicate that the morphogen-directed GMN is robust in response to perturbations and is important for Drosophila to ensure the proper tissue patterning in wing disc.
Multimodal transcriptional control of pattern formation in embryonic development December 27, 2019 5
Predicting how the gene expression patterns that specify animal body plans arise from interactions between transcription factor proteins and regulatory DNA remains a major challenge in physical biology. While the modulation of transcriptional bursting has been implicated as the primary lever for controlling gene expression, we find that this alone cannot quantitatively recapitulate pattern formation. Instead, we find that the pattern arises through the joint action of 2 regulatory strategies—control of bursting and control of the total duration of transcription—that originate from distinct underlying molecular mechanisms. During embryonic development, tightly choreographed patterns of gene expression—shallow gradients, sharp steps, narrow stripes—specify cell fates. The correct positioning, sharpness, and amplitude of these patterns of cytoplasmic mRNA and protein ensure the reliable determination of animal body plans. Predicting developmental outcomes demands a quantitative understanding of the flow of information along the central dogma: how input transcription factors dictate the output rate of mRNA production, how this rate of mRNA production dictates cytoplasmic patterns of mRNA, and how these mRNA patterns lead to protein patterns that feed back into the gene regulatory network. While the connection between transcription factor concentration and output mRNA production rate has been the subject of active research over the last 3 decades, the connection between this output rate and the resulting cytoplasmic patterns of mRNA has remained largely unexplored. For example, a graded stripe of cytoplasmic mRNA within an embryo could arise as a result of radically different transcriptional dynamics at the single-nucleus level
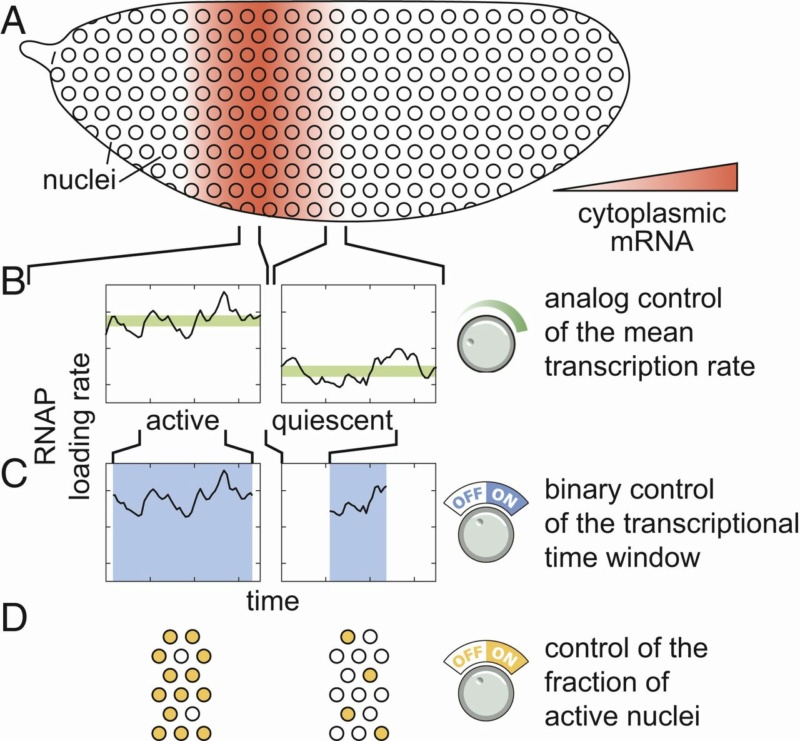
Multiple modes of pattern formation by single-cell transcriptional activity.
(A–D) Cytoplasmic mRNA patterns (A) could arise from transcription factors exerting control over the mean transcription rate (B), the transcriptional time window dictating when a nucleus is transcriptionally active or quiescent (C), or the fraction of active nuclei (D) or some combination thereof.
The detailed cytoplasmic distribution of mRNA that makes these stripes is key to the transmission of spatial information along the gene regulatory network that drives Drosophila development and reinforcing the need to develop models of gene regulation capable of connecting quantitative variations in input transcription factor patterns to graded output rates of transcription. We found that all 3 regulatory strategies outlined in Fig. above quantitatively contribute to the formation of eve stripe 2.
First, a smaller fraction of nuclei become active and engage in transcription in the periphery of the stripe than in the center, although this regulation of the fraction of active nuclei makes only a minor contribution to stripe formation. Second, the rate of mRNA production is significantly elevated in the center of the stripe. This analog control of the transcription rate is insufficient to quantitatively recapitulate the cytoplasmic mRNA stripe pattern. In addition to the control of the rate of mRNA production among nuclei, there is a pronounced regulation of the window of time during which eve loci were engaged in transcription across the stripe, with those in the stripe center expressing for approximately 3 times longer than those in the flanks. While it is widely appreciated that genes are transcriptionally competent for limited windows of time during development, we found that—in the case of eve stripe 2—this binary transcriptionally engaged/disengaged logic is not merely a necessary precondition for pattern formation—it is the primary driver thereof. Thus, we conclude that the regulation of eve stripe 2 is multimodal in nature, with contributions from 3 distinct regulatory strategies (Fig. B–D above). Nonetheless, stripe formation can be quantitatively explained almost entirely through the interplay between 2 distinct control strategies: binary control of the duration of transcriptional engagement. (Fig. C) and control of the mean rate of transcription (Fig. B).
To describe eve stripe 2 transcriptional dynamics, we need to account for both the short, transient ON periods dictated by transcriptional bursts and a longer transcriptional time window that describes the period over which loci engage in this transcriptional bursting. One or more of these bursting parameters are subject to spatially controlled regulation.
In Drosophila development, information encoded in a handful of maternally deposited protein gradients propagates through increasingly complex layers of interacting genes, culminating in the specification of the adult body plan. A priori, there are several distinct regulatory strategies at the single-cell level capable of generating spatially differentiated patterns of cytoplasmic mRNA each with distinct implications for the nature of the underlying molecular processes at play. The average rate of transcription is mainly modulated across the embryo by tuning the frequency of transcriptional bursting. It has remained unclear whether this modulation of the rate of transcription (and thereby mRNA production) is the dominant modality by which input concentrations of transcription factors drive the formation of patterns of gene expression or whether, instead, it is simply the most readily apparent mechanism among multiple distinct control strategies.
While it is widely appreciated that genes are expressed for discrete windows of time over the course of development, in the case of this eve stripe 2 reporter—this binary transcriptionally engaged/quiescent logic is actively regulated by transcription factors to drive pattern formation. Important is the temporal component of transcriptional regulation in specifying developmental outcomes. The limited readout time imposed by short nuclear cycles in early Drosophila development places strict constraints on the kinds of regulatory architecture that could be responsible for driving observed patterns of hunchback gene expression. The pioneer factor Zelda plays a key role in regulating both the timing and probability of transcriptional activation following mitosis. Our work complements these previous observations by exploring yet another aspect of the interplay between timing and transcriptional regulation.
1. INSECT DEVELOPMENT MORPHOGENESIS, MOLTING AND METAMORPHOSIS, page 70
2. https://anatomypubs.onlinelibrary.wiley.com/doi/pdf/10.1002/dvdy.140
3. https://en.wikipedia.org/wiki/Endocytosis
4. https://www.nature.com/articles/s41421-020-0173-z
5. https://www.pnas.org/content/117/2/836
6. https://sci-hub.tw/https://www.sciencedirect.com/science/article/pii/S0960982219303173
https://reasonandscience.catsboard.com/t2354-morphogen-gradients-and-pattern-formation
Establishing an animal body plan depends on many mechanisms that go beyond genetic information.
The development of an organism from an undifferentiated single cell into a spatially complex structure requires spatial patterning of cell fates across tissues. Several different signaling pathways are essential for the formation of organized tissues, organismal form, and architecture. Spatial patterning of a developing animal requires that cells become different according to their positions in the embryo, which means that cells must respond to extracellular signals produced by other cells, especially their neighbors. In what is probably the most common mode of spatial patterning, a group of pluripotent cells start out with the same developmental potential, and a signal from cells outside the group then induces one or more members of the group to change their character. Some inductive signals depend on cell–cell contact; others act over a longer range and are mediated by molecules that diffuse through the extracellular medium or are transported in the bloodstream. Most of the known events in animal development are governed by a small number of signaling pathways.
A core set of key morphogens regulates development. Morphogens are signaling molecules forming long-range concentration gradients that pattern a field of cells. After morphogens are secreted from a group of cells, they are transported across tissues to establish a gradient. The transport of morphogens is mediated by kinesin and dynein motor proteins along the microtubule networks of axons. Morphogens near the local source get attached to the shuttle and the morphogen-shuttle complex is transported across tissues. Subsequently, the morphogen-shuttle complex is degraded, resulting in the morphogen being locally immobilized, and start forming a local gradient. Bioelectric signals and resting membrane potentials across tissues are also and additionally crucial for proper patterning in multiple organisms. Furthermore, there are also neurotransmitters ( chemical messengers that transmit a message from a nerve cell across the synapse to a target cell) establish left-right patterning in embryos through regulation of ion fluxes. Calcium (Ca2+) signaling also plays a key role in mediating cellular responses to morphogens. In addition to mediating cellular differentiation and proliferation, Ca2+ signals also influence cellular migration in response to morphogen gradients.
Robustness is a ubiquitous feature of biological systems that ensures specific functions of the system are maintained despite external and internal perturbations. Furthermore, directional transport of substances through the nervous system is necessary to achieve scale-free morphogen patterning and body axis polarity determination.
Cells sense and interpret their position as a function of the amount of signal they receive, thus obtaining ‘‘positional information’’. Within larger tissues, neural networks provide directed information, via physiological signaling, that supplements positional information through diffusion. This positional information specifies gene expression and subsequent fates of cells in tissues.
A few key-sentences: Cells operate upon biological design rules - Developing cells have a vocabulary - There is intercellular communication - Cells communicate with each other through cell-cell gap junctions, which exercise modulation, that is control, direct, induce, and regulate pattern formation - There is so-called physiological signaling, and there are positive diffusion regulators - There are as well signals that propagate modulation of ion fluxes through voltage-gated ion channels - There are signaling molecules that provide information for direction cues - There are as well bioelectrical signals - Cells know how to interpret their position by obtaining ‘‘positional information’ through a coordinate system, which is modulated and mediated through morphogens that permit the formation of specific patterns through gradients. These regulate development; those also specify cell fates.
There is a general importance of mechanical forces in regulating growth and morphogenesis. Mechanical forces, cellular mechanics, and tissue mechanics are key components of morphogenesis and can influence pattern formation.
As a general scheme, it can be observed, that there are not simply chemical reactions, but molecules and cells operate, behave, migrate, position themselves, organize, form tissues, specialize based on instructions through various signaling networks and mechanisms, which have as source various epigenetic signaling pathways. Integrated, network-based information systems permit little if no errors, which undoubtedly lead to disease. This is prevented by robustness which has to be set up from the beginning. This leads to my understanding to intelligent design as the best explanation.

Morphogen Gradients and Pattern Formation 1
Small Numbers of Conserved Cell–Cell Signaling Pathways Coordinate Spatial Patterning
Spatial patterning of a developing animal requires that cells become different according to their positions in the embryo, which means that cells must respond to extracellular signals produced by other cells, especially their neighbors. In what is probably the commonest mode of spatial patterning, a group of cells starts out with the same developmental potential, and a signal from cells outside the group then induces one or more members of the group to change their character. This process is called inductive signaling. Generally, the inductive signal is limited in time and space so that only a subset of the cells capable of responding—the cells close to the source of the signal—take on the induced character

Inductive signaling.
Some inductive signals depend on cell–cell contact; others act over a longer range and are mediated by molecules that diffuse through the extracellular medium or are transported in the bloodstream. Most of the known inductive events in animal development are governed by a small number of highly conserved signaling pathways, including
transforming growth factor-β (TGFβ),
Wnt,
Hedgehog,
Notch,
receptor tyrosine kinase (RTK) pathways
The discovery of the limited vocabulary that developing cells use for intercellular communication has emerged over the past 25 years as one of the great simplifying features of developmental biology.
Compartment boundaries are the sources of morphogens. Morphogens are signaling molecules that are produced from a localized source forming long-range concentration gradients that pattern a field of cells (Figure 7a). Cells interpret their position as a function of the amount of signal they receive, thus obtaining ‘‘positional information’’ (Wolpert, 1989, 1996). Signaling molecules have to fulfill two stringent criteria to qualify as morphogens: (1) their effect must be exerted in a concentration-dependent manner and (2) they must act directly on target cells at a distance from the source (i.e., not through a secondary relay mechanism). When these criteria are met, the local concentration can be interpreted as a measure of distance from the source of the signal (Figure 7a). The three signaling proteins that qualify as morphogens in Drosophila wing development are Hedgehog (Hh), Decapentaplegic (Dpp), and Wingless (Wg) (e.g., Wg is shown in Figure 7).

Figure 7 Example of a morphogen gradient: Wingless (Wg).
(a) Drawing of a section of the wing pouch epithelium (upper panel) and of the wing pouch in an apical view (lower panel). The cells in the center express Wg which generates a gradient across the adjacent tissue. Cells at different distances receive different levels of Wg.
(b) Schematic drawing of the Wg gradient and target gene activation in the epithelium in response to Wg (upper panel). The Wg gradient activates the target genes hindsight (cyan) and distalless (blue) at different threshold levels. The expression domains of Hindsight and Distalless are depicted in the same colors in the lower panel. (c) Wg downregulates its own receptor Drosophila Frizzled 2 (DFz2; blue), shaping its gradient and rendering the
cells at a distance from the source of Wg more sensitive.
It is generally accepted that cells interpret the gradient by eliciting differential transcriptiona responses depending on the concentration of morphogen they are exposed to. This requires cells to make decisions depending on different threshold levels of signaling pathway activity. The concept implies that a single event – namely the production of a secreted molecule at a localized source – can lead to the formation of several different cell
types in a correct spatial relationship to each other (Figure 7). This represents a highly efficient way of generating complex patterns in previously uncommitted cells (review: Gurdon and Bourillot, 2001).
Different modes of morphogen movement/transport have been invoked to explain long-range gradient formation: extracellular diffusion of the secreted molecule, cycles of receptor- mediated endocytosis and resecretion (planar transcytosis) , membranous exosomes (argosomes), and cytoplasmic extensions (cytonemes). Although there is no compelling evidence to date that mechanisms other than diffusion contribute productively to gradient formation, it is certainly plausible that these mechanisms could do so. Cytonemes and argosomes exist, but have not yet been shown to be required to mediate ligand movement or to transduce signals. Evidence presented in favor of endocytosis and resecretion as a mechanism of ligand transport has been questioned. Further experimentalevidence is needed to support or refute these possibilities. Several factors have been implicated in shaping morphogen gradients: posttranslational modification of the ligand can influence diffusibility (e.g., acylation of Hh and Wg (Ingham, 2000, 2001)).
Regulation of receptor levels can influence ligand movement (Figure 7c). Cell surface heparan-sulfate proteoglycans, and secreted enzymes that modify them, can modulate ligand
movement . Secreted ligand binding proteins can influence movement or stability . In addition, cellular responses to these ligands can also be modulated, contributing to shaping the activity gradients rather than the ligand gradients per se.
Interplay between morphogen‐directed positional information systems and physiological signaling 03 December 2019 4
The development of an organism from an undifferentiated single cell into a spatially complex structure requires spatial patterning of cell fates across tissues. Positional information, proposed by Lewis Wolpert in 1969, has led to the characterization of many components involved in regulating morphogen signaling activity. Quantitative and systems-based approaches are increasingly needed to define general biological design rules that govern positional information systems in developing organisms. There are various roles of physiological signaling in modulating and mediating morphogen-based pattern formation. Similarities between neural transmission and morphogen-based pattern formation mechanisms suggest underlying shared principles of active cell-based communication. Within larger tissues, neural networks provide directed information, via physiological signaling, that supplements positional information through diffusion. Further, mounting evidence demonstrates that physiological signaling plays a role in ensuring robustness of morphogen-based signaling. We conclude by highlighting several outstanding questions regarding the role of physiological signaling in morphogen-based pattern formation. Elucidating how physiological signaling impacts positional information is critical for understanding the close coupling of developmental and cellular processes in the context of development, disease, and regeneration. 2
Lewis Wolpert proposed positional information as a mechanism whereby differential gene expression of cells results in spatial patterns of cellular differentiation. Within the concept of positional information, a coordinate system defines the magnitude and directionality of the positional information sensed by cells. This positional information specifies gene expression and subsequent fates of cells in tissues (Figure below)

A, classical view of positional information and morphogenesis.
The classical view of positional information stems from the French Flag Model in which source cells secrete morphogens that are transported through a tissue due to diffusion. A, Morphogen molecules (green) are secreted from source cells where morphogen concentration will be the highest. Morphogens travel to neighboring cells to establish a concentration gradient. Cells will have differential biological responses to the morphogen gradient dependent upon multiple threshold levels. Blue cells are located where morphogen concentrations are above the higher threshold. White cells sense morphogen concentrations that are above the lower threshold. Red cells detect low to no morphogens.
B, Cells respond to morphogens to determine cell fates and cell morphology in a dose-dependent response.
C, The ability of progenitor cells to create morphogen gradient-based patterns is dependent upon tissue geometry, size, temporal signaling of morphogens.
D, Governing diffusion equation of the morphogen concentration in accordance with Fick's second law of diffusion with a nonlinear degradation profile (k1), a source term dependent upon location (k2), and effective diffusion of the molecule (Deff). This results in a powerlaw relationship in which the gradient is position-dependent and reflects the local steepness of the gradient
Following Wolpert, Gierer and Meinhardt proposed models of morphogen distribution to demonstrate that relatively simple molecular mechanisms can explain the formation of a spatially patterned tissue. Subsequent experimental work has demonstrated that a core set of morphogens generate and relay positional information to cells to directly induce cellular responses based on the cells' location. Because morphogens play crucial roles during the specification of cell fates, they contribute to many aspects of development. A core set of key morphogens regulates development. Examples include members of the Hedgehog (Hh) family that are involved in Drosophila appendage formation and chick neural tube development. As a second example, Wingless (Wg)/Int-1 (Wnt) proteins contribute to Drosophila appendage development and human degenerative diseases. Bone morphogenetic proteins (BMPs), such as Drosophila Decapentaplegic (Dpp), are utilized in dorsal-ventral patterning of the Drosophila embryo and pattern formation and growth control of limb primordia. BMPs also regulate the formation of early germ layers of mammals. Morphogen signaling includes conveying positional information, and their ability to induce pattern formation through gradients. One morphogen can control the expression of another during morphogenetic processes, such as Hh-induced Dpp activity in developing Drosophila and Hh participating in crosstalk with Wnt in cancer. On the other hand, computational modeling has proven critical for explaining increasingly complex datasets and counter-intuitive results from genetic perturbations to morphogenetic patterning systems. Additional computational efforts have uncovered the role of the nervous system in facilitating regeneration in Planaria. This among many other studies support a close analogy between embryonic patterning and brain-like signal processing. However, in many cases the underlying kinetics and dynamics in gradient formation and maintenance remain poorly understood.
Morphogen-based signaling during development requires active cellular and physiological processes, as has been noted through analysis of the importance of lipid metabolism in morphogen transport. Here, we denote the term physiological signaling to represent cell regulatory mechanisms at the ionic and molecular level that control key cellular functions such as trafficking through exo- and endocytosis, metabolic processes of cellular growth and division, or the regulation of cell mechanics. Increasingly, evidence demonstrates the central role of physiological signaling events in mediating morphogen activity and emerging parallels between neurotransmission and morphogen transport across non-neural tissues. In particular, we highlight the functional roles of secondary messengers, such as calcium (Ca2+), in mediating morphogen secretion, transport, downstream information processing, and providing robustness of positional information.
CELLULAR MECHANICS DIRECTLY INFLUENCE PATTERN FORMATION AND MORPHOGEN GRADIENTS
Early studies of morphogenesis posited the notion that chemical-based morphogen gradients are the primary signals to pattern cell differentiation. However, there is emerging evidence demonstrating that mechanical events can also be a primary trigger in pattern formation. Mechanical forces drive cellular self-organization and structural rearrangements of a feather follicle's shape and gene expression in chicken embryos. In particular, mechanical activation of β-catenin initiated downstream follicle gene expression of bmp2, a key component of the TGF-β signaling pathway. This study thus serves as a striking example of the general importance of mechanical forces in regulating growth and morphogenesis. Mechanical forces, cellular mechanics, and tissue mechanics are key components of morphogenesis and can influence pattern formation. The underlying mechanisms in which mechanics provide positional information to cells require further elucidation.
MORPHOGEN SOURCES ARE DYNAMIC AND CONTROLLED BY PHYSIOLOGICAL SIGNALING
The conceptual French Flag model, an iconic description of positional information proposed by Wolpert, specifies that morphogens are secreted from a cluster of cells and form a graded distribution throughout the tissue to specify multiple cell types dependent on the concentration sensed by cells (Figure A above). Within this model, the secretion of morphogens from source cells is the initial step in the formation of positional information (Figure B above). This initial framework has since expanded from a static viewpoint to include dynamic changes to the size, geometry, location, mechanics, and temporal signaling of morphogen secreting cells (Figure C above). An example of a dynamic morphogen source occurs during the morphogenesis of dorsal appendages of the Drosophila melanogaster eggshell. The morphological boundaries of these structures depend on the spatial patterning transcription factor Broad r, which is regulated by the epidermal growth factor receptor (EGFR) signaling pathway through a feedback regulatory network. The patterning of Broad is established when Gurken, an EGFR ligand, is secreted from the underlying oocyte forming a posterior-to-anterior gradient. Later, a dorsoventral gradient forms after translocation of the oocyte nucleus to the dorsal anterior cortex.41-43 This suggests that morphogen sources are spatiotemporally dynamic and do not always adhere to the framework of passive diffusion-based transport from a stationary source (Figure D). This computational-based analysis of how subsequent rounds of EGFR activation refine spatial patterns was experimentally confirmed. Given the potential for spatiotemporally dynamic morphogen sources, secretion mechanisms of morphogens may provide insight into how this is possible.
Source cells secrete morphogens through exocytosis, an active transport mechanism of molecules to the cell membrane through vesicles. Fusion of vesicles to the cell membrane releases the morphogen. Therefore, morphogen secretion is influenced by physiological regulation of exocytosis. For example, inwardly rectifying potassium (Irk) channels regulate the release of Dpp and cytosolic Ca2+ in the developing Drosophila wing imaginal disc45 (Figure below).

Irk channels regulate Dpp secretion and cytosolic Ca2+.
In the Drosophila wing disc, inwardly rectifying potassium channels (Irk) regulate both the secretion of bone morphogenic protein Dpp and cytosolic Ca2+ levels. Expression of Dpp along the dorsal-ventral (D-V) axis in the wing disc is indicated in red. Dpp expression is not present along the anterior-posterior (A-P) axis of the wing disc. Inhibition of Irk channels using dominant negative Irk2 mutants (Irk2DN) decreased the duration and amplitude of Ca2+ oscillations in Dpp-producing cells (yellow square indicates region of interest). Further, loss of Irk2 channel function increased the baseline concentration level of Dpp-GFP while simultaneously decreasing discrete Dpp release events. Thus, Irk2 inhibition in wing discs simultaneously alters intracellular Ca2+ activity and Dpp release
Irk channels facilitate flow of potassium (K+) into the cell and have a fundamental role in restoring the membrane potential to its resting potential. Inhibition of Irk channels in Dpp producing cells, reduced Dpp secretion independent of Dpp expression. Irk channels regulate vesicle release by changing membrane potential while also altering intracellular Ca2+ dynamics. A potential explanation for similar outcomes in Dpp and Ca2+ after Irk channel inhibition is that intracellular Ca2+ dynamics regulate Dpp release, although whether or not Ca2+ has a causative effect requires further study. Ca2+ is crucial to regulation of epithelial physiology during development. However, whether Ca2+ directly regulates Dpp secretion is currently unknown. Given that misregulation of Ca2+ is frequently associated with developmental genetic disorders resulting in neoplasms, it is necessary to further characterize the roles of Ca2+ and other second messengers in morphogen transport and morphogenesis.
PARALLELS BETWEEN NEUROTRANSMISSION AND MORPHOGEN-BASED TRANSPORT SYSTEMS
After morphogens are secreted from a group of cells, they are transported across tissues to establish a gradient. How morphogens disperse and form gradients is still under debate despite progress in understanding the molecular mechanisms of morphogen transport through imaging studies and biophysical measurements. Several morphogen transport models have been proposed in the literature. These range from passive mechanisms, such as free or hindered diffusion (first Figure D), to cell-based dispersal by transcytosis or cytonemes. Multiple transport mechanisms may be involved, and this likely varies across developmental contexts. The simplest mechanism of morphogen transport is passive diffusion where molecules disperse by random motion. However, this model does not fully capture the complexity of morphogen transport dynamics due to evidence demonstrating that a single source of morphogen is not always sufficient to establish a gradient. For example, during Drosophila wing disc development, DWnt6 is expressed in an identical pattern to Wg,57 while both BMP ligands Gbb and Dpp are necessary to establish proper morphogen gradients. In facilitated diffusion, morphogens are largely immobile until they bind to a positive diffusion regulator that enhances motility. Shuttling is a special case of facilitated diffusion in which molecular shuttles, not morphogens, are generated from a localized source. Morphogens near the local source get attached to the shuttle and the morphogen-shuttle complex is transported across tissues. Subsequently, the morphogen-shuttle complex is degraded, resulting in the morphogen being immobilized to stationary negative diffusion regulators and the formation of a gradient.
Beyond extracellular-diffusion-based morphogen transport, morphogens also can be transported through cell-based mechanisms via transcytosis or along cellular extensions known as cytonemes. In the case of transcytosis, signaling molecules are taken up by cells through endocytosis and subsequently released through exocytosis into the extracellular space. Endocytosis is the process in which cargo molecules, including morphogens, are absorbed and distributed into a series of endosomes with distinct physical and biochemical properties. An endosome's dynamics, such as fission, fusion, and maturation, influences its ability to sort and concentrate cargo molecules. For the case of morphogens, endosome dynamics are thus able to establish concentration gradients and signaling activities across tissues. Significant questions remain regarding which is the dominant mode of morphogen transport in a given developmental context. In the case of the Drosophila wing disc, Dpp can move through regions that are mutant for the type I transforming growth factor beta receptor thickveins (tkv) and downstream transcriptional repressor (Brinker). This is significant given that support for transcytosis stems from experiments utilizing the Dpp receptor Tkv. Due to the apparent conflicts between experimental studies, much work remains to clarify the roles of the physiological processes governing morphogen transport. An example of morphogen signaling being affected by exo- and endocytosis is seen in development of the Drosophila air sac primordia (ASP), which depends on Dpp signaling. Cytoneme-based signaling utilizes many of the same components found in neural synapses. In the Drosophila ASP (“receiving cells”), specialized filopodia-like cytonemes endocytose Dpp from the adjacent Drosophila wing disc cells (“sending cells”; Figure 3A).

Morphogen transport mechanisms.
A, Extended filopodia called cytonemes are present in the Drosophila air sac primordium (ASP). Cytonemes projecting from the ASP take up Dpp from the adjacent wing imaginal disc. Correlated transients of Ca2+ concentrations are observed in cytonemes. Cytoneme mediated transport requires Synaptotagmin-4 (Syt4), which helps in vesicle fusion and receptor internalization, and glutamate receptor GluRII in the ASP. Wing disc cells secreting Dpp require Synaptobrevin, Synaptotagmin-1, the glutamate transporter, and voltage-gated calcium channels. Blue circles represent morphogens, red circles represent pMad molecules in cells, and orange circles represent dpERK molecules in cells. B, In Planaria, morphogens are transported on microtubule arrays along the axons from the nervous system to the wound edge during regeneration. Vector transport is identified as a fundamental requirement for scale-free self-assembly of morphogens during Planaria homeostasis and regeneration. Figure is adapted with permission from Reference
Dpp signaling was compromised when Synaptotagmin-1, Synaptobrevin, the glutamate receptor, or voltage-gated Ca2+ channels were inhibited in the secreting disc cells, resulting in a reduction in the size of the ASP. This result parallels neurotransmission as Synaptobrevin is an intrinsic membrane protein that regulates neurotransmitter release through Ca2 +-dependent exocytosis. The receiving cells of the ASP require Synaptotagmin-4 and the glutamate receptor GluRII. This is noteworthy given that Ca2+ is a crucial regulator of endocytosis 3 and neurotransmitter regulation. Huang et al further demonstrated that Ca2+ transients observed in cytonemes correlate with Dpp uptake. This suggests that signal uptake and transport within cytonemes depends on local Ca2+ concentrations. This study, thus, underscores the role of physiological signals, like Ca2+, in morphogen mediated transport and internalization that have not yet been fully explored. Further evidence for second messenger signaling and morphogen transport lies in the interaction between Ca2+ and endocytic and exocytic regulators. Knockdown of Ca2+ signaling-dependent exocytic components in the prothoracic gland in Drosophila brain, resulted in the accumulation of unreleased steroid hormone ecdysone. Whether the same exocytic machinery controls secretion of key morphogens is currently unknown. Reverse genetic RNAi screening could be employed to map out the components regulating morphogen secretion and transport through exocytosis. Additionally, synthesis of new alleles with mutations in Ca2+ binding domains of key morphogen regulatory proteins will further enable confirmation of the role of physiological signals in regulating morphogen secretion.
A key player in the endocytic process is the regulatory guanosine triphosphatase (GTP) protein Rab5. Rab5 proteins aid in the formation of transport vesicles and regulate molecular cargo degradation and recycling. Rab5 is required for endosome integrity in the presynaptic terminal in Drosophila neuromuscular synapses. Impaired Rab5 function affects both Exo- and endocytosis rates and decreases the ability of neurotransmitter release, while overexpression of Rab5 increases the release efficacy of neurotransmitter. This is particularly interesting because Ca2+ is an important regulator of neurotransmitter release. Recent work has shown that Ca2+ channels regulate bulk endocytosis, a form of endocytosis of synaptic vesicles at nerve terminals, in addition to coupling exo- and endocytosis. Further, Rab5-dependent endocytosis requires Ca2+ signaling to increase the rate of membrane capacitance, which determines how quickly the membrane potential can respond to a change in current and is linearly related to changes in membrane surface area. The converse was also shown in which low Ca2+ concentrations decreased membrane capacitance. Thus, an increase in surface area reflects increased exocytosis and decreased surface area reflects an increase in endocytosis. Additionally, discovery of a feedback loop showed BMP and Scrib, a scaffold protein associated with cellular proliferation, promotes Rab5 endosome-dependent BMP/Dpp signaling during morphogenesis in Drosophila. Given that Ca2+ affects Rab5-related endocytosis and Rab5 affects Dpp signaling, it can be inferred that Ca2+ concentrations may serve as a potential modulator of morphogen transport through coupled exo- and endocytosis. However, this inference remains to be tested directly. Another possible mechanism through which physiological signals such as Ca2+ affect transport of morphogens is through Ca2+ binding domains of transport proteins. Evidence for this lies in the presence of Ca2+ binding EF-domains in proteins involved in the formation of BMP and Dpp gradients in the Drosophila wing disc. For example, Drosophila Pentagone (Pent) directly interacts with Dally to provide long range distribution of the Dpp ligand. Structurally Pent has a similar domain composition to that of human SMOC protein, and both Pent and SMOC proteins contain Ca2+ binding EF domains. Xenopus SMOC-1 (XSMOC-1) protein acts as a BMP antagonist in Xenopus embryos even in the presence of constitutively active BMP receptor.84 Further analysis suggests that SMOC-1 antagonizes BMP signaling downstream of receptor binding through activation of MAPK signaling. Later studies demonstrated the ability of Drosophila-specific Pent to similarly inhibit BMP signaling in Xenopus downstream of the BMP receptor after injection of synthetic pent mRNA into Xenopus embryos.
Following this, Thomas et al utilized the SMOC deletion mutant constructs XSMOC-1ΔEC (lacking the extracellular Ca2+ binding domain) and XSMOC-1EC (containing the extracellular Ca2+ binding domain only) to demonstrate that normal XSMOC-1 and XSMOC1ΔEC, but not XSMOC-1EC convert the fate of naïve Xenopus ectoderm explants to anterior neural tissue. Thus, embryonic cell fate decisions to become neural tissue via SMOC, and BMP inhibition does not require the extracellular Ca2+ binding domain. However, the potentiation of BMP signaling by the extracellular Ca2+ binding domain was shown in further studies by observing the affinities of XSMOC-1EC and BMP2 for each other and other heparan sulfate proteoglycans (HSPGs). Experiments utilizing in vitro diffusion assays on agarose gels embedded with or without HSPGs demonstrated that the binding of BMPs to HSPGs restricts their range of effect and that BMP diffusion can be enhanced by the binding of SMOC to HSPGs to expand the range of effect. The SMOC extracellular Ca2+ binding domain expands the range of BMP signaling through competitive binding to HSPGs. These studies demonstrate the importance of the SMOC extracellular Ca2+ binding domain in the context of development. Further investigations are needed into the role of physiological signaling within the context of the extracellular environment.
The coupling between neuronal and non-neural tissues in defining positional information extends beyond similarities in signaling mechanisms. A recent study provided further insight into the role of the nervous system in morphogen-based pattern formation and regeneration in Planaria. A remarkable feature of regeneration in Planaria is the reformation of key morphogen gradients such as Hh and Notum Regulating Factor (NRF) after fragmentation or wounding of the organism. Pietak et al developed a quantitative model of regenerating Planaria to elucidate the mechanism of morphogen gradient activity that ensures robust body-plan regulation. The model predicts the fraction of heteromorphoses in regenerating Planaria fragments. Through an iterative combination of computational simulations and experiments, they found that vector transport of morphogens was required to explain regeneration of pattern formation. Morphogen vector-based transport is defined as the directional transport of morphogens by a vector field. The vector transport field coincided with the nerve polarity throughout regenerating planarian tissue. In their Markov chain model, the transport of morphogens, such as Hh and NRF, is mediated by kinesin and dynein motor proteins along the microtubule networks of axons (Figure B above)
They further demonstrated that the head-tail axis is controlled by the net polarity of neurons. In contrast, a purely diffusion-based model of patterning could not explain the scaling of steady-state concentrations of morphogens of fragments of various sizes. Further, diffusion would be too slow to regenerate the pattern (requiring more than a week on a 1 cm scale organism), while Planaria can reform within 72 hours or less. Thus, the nervous system plays an instructive role in regulating long-distance axial patterning repair during regeneration.
Bioelectric signals and resting membrane potentials across tissues are crucial for proper patterning in multiple organisms. Changes in membrane potential in regenerating Planaria, induced by ionophore treatment, permanently impacted gene expression, patterning, and polarity. After ionophore washout from the treated tissue, the induced changes in resting membrane potential persisted. This mechanism parallels synaptic plasticity in the brain where action potentials, modulated by voltage-gated ion channels, propagate signals. Work done in Xenopus and chick provides insight to this occurrence where serotonin (5-HT), an endogenous neurotransmitter, establishes left-right patterning in embryos through regulation of ion fluxes. A follow-up study showed that extracellular 5-HT availability drives innervation through tissues via gap junctional communication modulation. Combining their findings, Levin and colleagues propose that extracellular 5-HT, a positively charged molecule, navigates through gap junctions to accumulate in hyperpolarized cells, mimicking the reuptake function of 5-HT transporters (SERTs). These 5-HT sequestering, hyperpolarized cells can depolarize in response to loss of chloride ions via glycine-gated chloride channel activation. Without normal hyperpolarization, 5-HT is exported to the extracellular space by SERTs, which can induce growth and hyperinnervation of tissue. The importance of ion channels in facilitating communication in bacterial biofilm communities shows the generality of physiological signaling for mediating cell-to cell communication mechanisms. For example, bacterial biofilm communities utilize synchronized oscillations of short-range connectivity among a few cells and community-wide signaling, to minimize competition for resources. In sum, further quantitative investigations are needed to explore the role of neurotransmission, gap junction communication, and the nervous system in morphogen based transport systems. The establishment of morphogen gradients through neural signaling still needs to be integrated with known physiological regulators of patterning. This will be critical for considering how patterns form across large spatial domains where diffusion-based mechanisms become ineffective.
PHYSIOLOGICAL SIGNALS MODULATE MORPHOGEN-BASED POSITIONAL INFORMATION SYSTEMS
Ca2+ signaling also plays a key role in mediating cellular responses to morphogens. Several studies in various model systems have shown the interplay between Hh signaling and Ca2+ dynamics. For example, Shaw et al showed that intracellular Ca2+ mobilization regulates the level of Sonic hedgehog (Shh)-dependent expression domains of nkx2.2b, isl1, nkx6.1, and pax3 genes in the developing nervous system of zebrafish embryos during the 18-somite stage. Furthermore, they showed that reduced expression of ryanodine receptor (RyR), an intracellular Ca2+-release channel, shifted the allocation of Shh-dependent cell fates in the somitic muscle and neural tubes in a manner that resembled the effects of reduced Shh signaling. These findings were discovered by utilizing loss-of-function mutations, antisense morpholino knockdowns, and pharmacological treatments to perturb RyR activity.
In another study, Belgacem and colleagues demonstrated that Shh acutely increases Ca2+ through the activation of the transducer Smoothened (Smo), which then recruits heterotrimeric GTP-binding protein-dependent pathways. They demonstrated that Shh increases Ca2+ spike activity of developing spinal neurons and propose that Ca2+ spike frequency encodes Shh concentration and is required for proper neuronal differentiation (Figure A).

Physiological roles of Ca2+ in mediating morphogen response.
A, A gradient of the morphogen Sonic Hedgehog (Shh) directs the patterning of neuronal differentiation in the developing Xenopus spinal cord. Shh increases Ca2+ spike activity in developing spinal neurons. A current model suggests that Ca2+ spikes convert noisy Hh signaling into binary outputs to specify cell fates. This was demonstrated by a positive relationship between activation of Hedgehog signaling through activation of the Shh transducer Smoothened (Smo) and stimulating Ca2+ spike activity. Loss of the Ca2+ spikes resulted in decreased GABAergic neuronal cell fates. This suggests Ca2+ spike frequency encodes Shh concentration and is required for proper neuronal differentiation .
B, Tissue-wide, long-range Ca2+ oscillations have been observed in mesenchymal cells. Synergistic actions of Shh and Wnt signaling allowed synchronized Ca2+ oscillations to coordinate cell movements during chicken feather elongation
In addition to mediating cellular differentiation and proliferation, Ca2+ signals also influence cellular migration in response to morphogen gradients. Coordinated cell migration during chicken feather elongation is accompanied by dynamic changes of bioelectric currents and Ca2+ signaling. Specifically, Shh-responsive cells contained synchronized Ca2+ oscillations in which Shh plays a key role in mediating interactions between the epithelium and mesenchyme during feather morphogenesis. Voltage-gated Ca2+ channels and Connexin43-based gap junctions regulate Ca2+ dynamics during feather elongation. Shh signaling and β-Catenin signaling activates Connexin-43 expression transcriptionally to establish the gap junctional network (Figure B). The establishment of gap junctional networks in growing tissues thus alters the spatial range of Ca2+ signaling
Further, a review of the role of gap junctions in regulation of pattern formation details that left-right asymmetry in C. elegans neurons involves Ca2+ signaling and communication through gap junctions.94,95 These studies further demonstrate the role of second messenger systems in mediating morphogen-induced responses of cells and the role of physiological signaling in morphogen-based pattern formation. Quantitative experiments investigating the interplay between second messengers and gap junctions during pattern formation are greatly needed.
FEEDBACK FROM PHYSIOLOGICAL SIGNALING ENSURES ROBUSTNESS OF MORPHOGEN-BASED SIGNALING
Important aspects of morphogen gradients as a source of positional information are robustness in the presence of genetic or environmental noise and the proper scaling of morphological patterns with respect to size. Robustness is an ubiquitous feature of biological systems that ensures specific functions of the system are maintained despite external and internal perturbations. Robustness of positional information can influence development significantly. Several recent studies have begun to elucidate the mechanisms governing pattern robustness. For instance, in the context of Planarian regeneration, it was shown that pure reaction-diffusion mechanism of morphogens fails to provide scale-free morphogen gradients. The authors hypothesized that in addition to the classical mechanism involving diffusion, directional transport of substances through the nervous system is necessary to achieve scale-free morphogen patterning and body axis polarity determination.
How was that directing achieved? What if the robustness was not there right from the beginning?
Overall, this study supports the idea of scale invariance in developing systems in which morphogen gradients are scaled properly despite external and internal perturbations. However, despite extensive work into the scaling mechanisms that regulate scaling of patterns at the tissue, organ, and organism level, biochemical mechanisms underlying patterning robustness remain to be discovered Regulation of downstream responses by physiological signals also suggest that physiological signals alter the robustness of morphogenetic processes. Ca2+ gradients are generated along the dorsal-ventral axis of the Drosophila embryo. These concentration gradients are formed during embryonic stage 5 with higher Ca2+ levels on the dorsal side. They also showed that manipulation of the Ca2+ gradient affects the specification of amnioserosa, located dorsally in Drosophila. This study underscores the importance of physiological signaling pathways that utilize Ca2+ in contributing to the specification of the dorsal embryonic region. Ca2+ gradients affect robustness of morphogenetic processes. An outstanding question is whether this mechanism of specification of positional information is influenced by Ca2+ gradients in other model systems.
Increasingly, evidence is accumulating for significant crosstalk of Ca2+ and ROS between endoplasmic reticulum, an established site of Ca2+ storage, and mitochondria, a generation site of ROS.102 In particular, Ca2+ diminished ROS from ROS generation sites within the mitochondria under normal conditions and enhanced ROS generation when generation sites were inhibited by pharmacological agents.103 Further, quantitative in vivo microscopy in Drosophila and zebrafish embryos identified ROS as crucial signals that regulate cell polarity after wounding.104 Hunter et al utilized fluorescent imaging after wounding Drosophila embryos and demonstrated Ca2+-dependent mitochondrial ROS production correlates with the site of actomyosin cable assembly. This suggests that wound-induced ROS production promotes healing in Drosophila and zebrafish embryos. Taken together, these studies demonstrate that physiological signaling, in the form of ROS and Ca2+, act to regulate robustness of morphogenesis. Further experiments are needed to elucidate how ROS and Ca2+ affect robustness of positional information in the context of development, regeneration, and disease.
Analysis on gene modular network reveals morphogen-directed development robustness in Drosophila30 June 2020 4
Genetic robustness is an important characteristic to tolerate genetic or nongenetic perturbations and ensure phenotypic stability. Morphogens, a type of evolutionarily conserved ( no evolutionary change) diffusible molecules, govern tissue patterns in a direction-dependent or concentration-dependent manner by differentially regulating downstream gene expression. However, whether the morphogen-directed gene regulatory network possesses genetic robustness remains elusive. In the present study, we collected 4217 morphogen-responsive genes along A-P axis of Drosophila wing discs from the RNA-seq data, and clustered them into 12 modules. By applying mathematical model to the measured data, we constructed a gene modular network (GMN) to decipher the module regulatory interactions and robustness in morphogen-directed development. The computational analyses on asymptotical dynamics of this GMN demonstrated that this morphogen-directed gene modular network (GMN) is robust to tolerate a majority of genetic perturbations, which has been further validated by biological experiments. Furthermore, besides the genetic alterations, we further demonstrated that this morphogen-directed GMN can well tolerate nongenetic perturbations (Hh production changes) via computational analyses and experimental validation. Therefore, these findings clearly indicate that the morphogen-directed GMN is robust in response to perturbations and is important for Drosophila to ensure the proper tissue patterning in wing disc.
Multimodal transcriptional control of pattern formation in embryonic development December 27, 2019 5
Predicting how the gene expression patterns that specify animal body plans arise from interactions between transcription factor proteins and regulatory DNA remains a major challenge in physical biology. While the modulation of transcriptional bursting has been implicated as the primary lever for controlling gene expression, we find that this alone cannot quantitatively recapitulate pattern formation. Instead, we find that the pattern arises through the joint action of 2 regulatory strategies—control of bursting and control of the total duration of transcription—that originate from distinct underlying molecular mechanisms. During embryonic development, tightly choreographed patterns of gene expression—shallow gradients, sharp steps, narrow stripes—specify cell fates. The correct positioning, sharpness, and amplitude of these patterns of cytoplasmic mRNA and protein ensure the reliable determination of animal body plans. Predicting developmental outcomes demands a quantitative understanding of the flow of information along the central dogma: how input transcription factors dictate the output rate of mRNA production, how this rate of mRNA production dictates cytoplasmic patterns of mRNA, and how these mRNA patterns lead to protein patterns that feed back into the gene regulatory network. While the connection between transcription factor concentration and output mRNA production rate has been the subject of active research over the last 3 decades, the connection between this output rate and the resulting cytoplasmic patterns of mRNA has remained largely unexplored. For example, a graded stripe of cytoplasmic mRNA within an embryo could arise as a result of radically different transcriptional dynamics at the single-nucleus level

Multiple modes of pattern formation by single-cell transcriptional activity.
(A–D) Cytoplasmic mRNA patterns (A) could arise from transcription factors exerting control over the mean transcription rate (B), the transcriptional time window dictating when a nucleus is transcriptionally active or quiescent (C), or the fraction of active nuclei (D) or some combination thereof.
The detailed cytoplasmic distribution of mRNA that makes these stripes is key to the transmission of spatial information along the gene regulatory network that drives Drosophila development and reinforcing the need to develop models of gene regulation capable of connecting quantitative variations in input transcription factor patterns to graded output rates of transcription. We found that all 3 regulatory strategies outlined in Fig. above quantitatively contribute to the formation of eve stripe 2.
First, a smaller fraction of nuclei become active and engage in transcription in the periphery of the stripe than in the center, although this regulation of the fraction of active nuclei makes only a minor contribution to stripe formation. Second, the rate of mRNA production is significantly elevated in the center of the stripe. This analog control of the transcription rate is insufficient to quantitatively recapitulate the cytoplasmic mRNA stripe pattern. In addition to the control of the rate of mRNA production among nuclei, there is a pronounced regulation of the window of time during which eve loci were engaged in transcription across the stripe, with those in the stripe center expressing for approximately 3 times longer than those in the flanks. While it is widely appreciated that genes are transcriptionally competent for limited windows of time during development, we found that—in the case of eve stripe 2—this binary transcriptionally engaged/disengaged logic is not merely a necessary precondition for pattern formation—it is the primary driver thereof. Thus, we conclude that the regulation of eve stripe 2 is multimodal in nature, with contributions from 3 distinct regulatory strategies (Fig. B–D above). Nonetheless, stripe formation can be quantitatively explained almost entirely through the interplay between 2 distinct control strategies: binary control of the duration of transcriptional engagement. (Fig. C) and control of the mean rate of transcription (Fig. B).
To describe eve stripe 2 transcriptional dynamics, we need to account for both the short, transient ON periods dictated by transcriptional bursts and a longer transcriptional time window that describes the period over which loci engage in this transcriptional bursting. One or more of these bursting parameters are subject to spatially controlled regulation.
In Drosophila development, information encoded in a handful of maternally deposited protein gradients propagates through increasingly complex layers of interacting genes, culminating in the specification of the adult body plan. A priori, there are several distinct regulatory strategies at the single-cell level capable of generating spatially differentiated patterns of cytoplasmic mRNA each with distinct implications for the nature of the underlying molecular processes at play. The average rate of transcription is mainly modulated across the embryo by tuning the frequency of transcriptional bursting. It has remained unclear whether this modulation of the rate of transcription (and thereby mRNA production) is the dominant modality by which input concentrations of transcription factors drive the formation of patterns of gene expression or whether, instead, it is simply the most readily apparent mechanism among multiple distinct control strategies.
While it is widely appreciated that genes are expressed for discrete windows of time over the course of development, in the case of this eve stripe 2 reporter—this binary transcriptionally engaged/quiescent logic is actively regulated by transcription factors to drive pattern formation. Important is the temporal component of transcriptional regulation in specifying developmental outcomes. The limited readout time imposed by short nuclear cycles in early Drosophila development places strict constraints on the kinds of regulatory architecture that could be responsible for driving observed patterns of hunchback gene expression. The pioneer factor Zelda plays a key role in regulating both the timing and probability of transcriptional activation following mitosis. Our work complements these previous observations by exploring yet another aspect of the interplay between timing and transcriptional regulation.
1. INSECT DEVELOPMENT MORPHOGENESIS, MOLTING AND METAMORPHOSIS, page 70
2. https://anatomypubs.onlinelibrary.wiley.com/doi/pdf/10.1002/dvdy.140
3. https://en.wikipedia.org/wiki/Endocytosis
4. https://www.nature.com/articles/s41421-020-0173-z
5. https://www.pnas.org/content/117/2/836
6. https://sci-hub.tw/https://www.sciencedirect.com/science/article/pii/S0960982219303173
Last edited by Otangelo on Sat Mar 06, 2021 2:24 pm; edited 7 times in total


