Phosphorus No Help for Chemical Evolution
https://reasonandscience.catsboard.com/t2297-phosphorus-no-help-for-chemical-evolution
Phosphorus (P) has the atomic number 15 and is a chemical element found in the earth's crust with a concentration of about 1 gram in each kilogram. It is required for energy production, DNA synthesis and protein synthesis. [url=https://drlwilson.com/articles/minerals for life.htm]32[/url]
Balkrishna Tiwari (2014): Phosphorus plays a very important role in the synthesis of nucleic acids, phospholipids, and many biochemical intermediates of the cell. Its role in cellular signaling and maintenance of biochemical energy pool makes it a very essential macronutrient for life. Inorganic phosphate (Pi) or orthophosphate is the only form of phosphorus that can be directly used by the living cell but it is limited in many ecosystems.
Prof. Dr. Ruth E. Blake (2020): From structural to functional, informational, and energetic roles, Phosphorus is absolutely essential to life. 21
Norio Kitadai (2017): It constitutes biomolecules that play central roles in replication and information (RNA and DNA), metabolism (ATP, NADPH, and other coenzymes), and structure (phospholipids) 58
Radosław W. Piast (2020): All life on Earth uses one universal biochemistry stemming from one universal common ancestor of all known living organisms. One of the most striking features of this universal biochemistry is its utter dependence on phosphate group transfer between biochemical molecules. Both nucleic acid and peptide biological synthesis relies heavily on phosphate group transfer. Such dependents strongly indicate very early incorporation of phosphate chemistry in the origin of life. Perhaps as early as prebiotic soup stage. 22
Mikhail Butusov wrote in the book: The Role of Phosphorus in the Origin of Life and in Evolution:
Phosphorus, in the form of phosphate, has played an important role in the origin and evolution of life on several different levels. It was, most likely, a key component in the early precursors of RNA. It plays an essential role in both the genetics and the energy systems of all living cells as well as in the cell membrane of all modern cells. Phosphorus has also had a decisive role in forming the climatic and atmospheric conditions that set the boundary conditions for evolution and led to us humans and the world we know now. 10
Stanley Miller gave a sobering verdict based on his investigation in regards of a prebiotic source of phosphate:
There are no known efficient prebiotic synthesis of high-energy phosphates or phosphate esters. There is no known robust synthesis of polyphosphates or even pyrophosphate, thereby raising the question of whether
polyphosphates were used in prebiotic reactions and indeed if the pre-RNA world had informational macromolecules that contained phosphate at all. These results suggest that it may not be possible to produce adequate concentrations of high-energy phosphates using electric discharges or volcanic sources. We recognize that we may have missed some high-energy compounds in these experiments so this statement needs to be taken with some reservation. It is perhaps significant that there have been few experiments in the last 20 years attempting to produce high-energy phosphates. This suggests that robust syntheses may not be possible. 59
De Duve (2005): “How did nature choose phosphates?” Unless one believes in intelligent design, fitness does not account for use, except through a process of selective optimization. But phosphate must have entered metabolism before replication and its correlates, mutation and selection, came on the scene, presumably with RNA. There must be a chemical explanation for nature’s choice of phosphates. As I have discussed elsewhere (de Duve, 1991, 2001), this explanation is far from obvious. 61
There was an attempt to solve the problem raised by Miller, which was pointed out by Sci-news: Chemical reactions that make the building blocks of living things need a lot of phosphorus, but phosphorus is scarce. 12
Jonathan D. Toner and colleagues supposedly found an answer to this problem in certain types of lakes. They write (2019): Phosphate is generally limited to micromolar levels in the environment because it precipitates with calcium as low-solubility apatite minerals. This disparity between laboratory conditions and environmental constraints is an enigma known as “the phosphate problem.” Here we show that carbonate-rich lakes are a marked exception to phosphate-poor natural waters. In principle, modern carbonate-rich lakes could accumulate up to ∼0.1 molal phosphate under steady-state conditions of evaporation and stream inflow because calcium is sequestered into carbonate minerals. This prevents the loss of dissolved phosphate to apatite precipitation. Even higher phosphate concentrations (>1 molal) can form during evaporation in the absence of inflows. On the prebiotic Earth, carbonate-rich lakes were likely abundant and phosphate-rich relative to the present day because of the lack of microbial phosphate sinks and enhanced chemical weathering of phosphate minerals under relatively CO2-rich atmospheres. 11
And co-author David Catling claimed: The extremely high phosphate levels in these lakes and ponds would have driven reactions that put phosphorus into the molecular building blocks of RNA, proteins, and fats, all of which were needed to get life going 60
How to put phosphorus into the molecular building blocks without the complex cellular machinery is entirely another feat, and unexplained. Miller was also pessimistic about that. He wrote: Phosphate is an unlikely reagent for the prebiotic world, and this may also apply to the preRNA world.
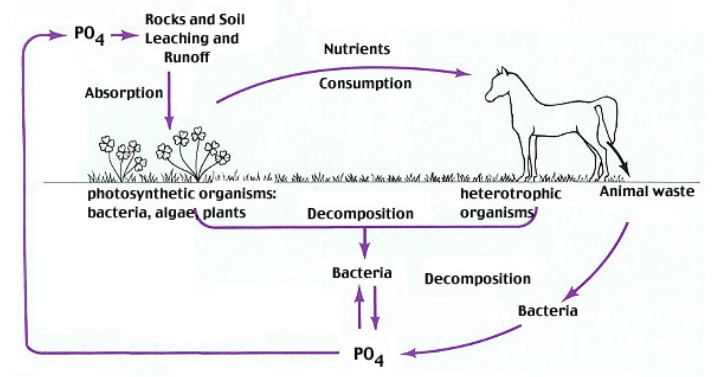
The phosphorus cycle
Living organisms utilize inorganic phosphate from the ecosystem and return it in the form of organic phosphorus. At this level of phosphorus, cycle microbes contribute significantly by adapting various mechanisms to mineralize dissolved organic phosphorus (DOP) which contribute a major part of the total dissolved phosphorus pool in oceanic fresh water and terrestrial ecosystem. Dissolved organic phosphorus contributes>80% of the total pool of dissolved phosphorus in the North Atlantic Ocean. 14
Libretexts explains: Rocks are a reservoir for phosphorus, and these rocks have their origins in the ocean. Phosphate-containing ocean sediments form primarily from the bodies of ocean organisms and from their excretions. However, volcanic ash, aerosols, and mineral dust may also be significant phosphate sources. This sediment then is moved to land over geologic time by the uplifting of Earth’s surface. Marine birds play a unique role in the phosphorous cycle. These birds take up phosphorous from ocean fish. Their droppings on land (guano) contain high levels of phosphorous. 14
Bacteria use sophisticated mechanisms to, sense, acquire and import phosphate and to maintain intracellular amounts at optimal levels
Juan Francisco Martín and colleagues explain in a scientific paper from 2021: Bacteria transport inorganic phosphate by the high-affinity phosphate transport system PstSCAB, and the low-affinity PitH transporters. The PstSCAB system consists of four components. PstS is the phosphate-binding protein and discriminates between arsenate and phosphate. 15
Vanessa R. Pegos and colleagues write in a scientific publication from 2017: Bacteria have developed specialized systems for phosphorus uptake such as the low-affinity transporter, PitA, and the Phosphate Specific Transporter (Pst), an ATP-Binding Cassette transporter (ABC transporter). Structurally, the Pst system consists of two transmembrane proteins, two associated cytoplasmic ATPases and a periplasmic protein responsible for the affinity and specificity of the system. 16
Armen Y. Mulkidjanian and colleagues (2019): A topologically closed membrane is a ubiquitous feature of all cellular life forms. This membrane is not a simple lipid bilayer enclosing the innards of the cell: far from that, even in the simplest cells, the membrane is a biological device of a staggering complexity that carries diverse protein complexes mediating energy-dependent – and tightly regulated - import and export of metabolites and polymers 17
Angus Menuge asks in his book: Agents Under Fire: Materialism and the Rationality of Science, pgs. 104-105: Hence a chicken and egg paradox: a lipid membrane would be useless without membrane proteins but how could membrane proteins have evolved in the absence of functional membranes? 18
Joseph Panno Ph.D. writes in: THE CELL Evolution of the First Organism, page 17: The cell membrane, far from being featureless, contains a molecular forest that gives the cell its eyes, its ears, and the equipment it needs to capture food and to communicate with other cells. Phospholipids, the main component in cell membranes, are composed of a polar head group (usually an alcohol), a phosphate, glycerol, and two hydrophobic fatty acid tails. Fat that is stored in the body as an energy reserve has a structure similar to a phospholipid, being composed of three fatty acid chains attached to a molecule of glycerol. The third fatty acid takes the place of the phosphate and head group of a phospholipid. Sugars are polymerized to form chains of two or more monosaccharides. Disaccharides (two monosaccharides), and oligosaccharides (about 3–12 monosaccharides), are attached to proteins and lipids destined for the cell surface. Polysaccharides, such as glycogen and starch, may contain several hundred monosaccharides and are stored in cells as an energy reserve. 19
So there is a further catch22 problem: Cell membranes require phosphorus. But the uptake of phosphorus into the cell to make daughter cells with membranes using phosphorus requires pre-existent cell membranes with phosphorus import channels. Cell membranes only come from cell membranes. A cell cannot produce the cell membrane de novo from scratch. It inherits it. Daughter cell membranes come only from mother cell membranes. The mother cell grows twice its starting size, expands its membrane, and once it reaches the right size, it splits. The process is called binary fission and is an enormously complex process, mediated by a multiprotein complex denominated the divisome. 20
One of my favorite blues tunes is "Born Under a Bad Sign," a song about someone who just can't catch a break. If there is a scientific discipline that is characterized by "havin' bad luck all of [its] days," it's origin-of-life research. This trend of bad luck continues for a collaborative team from the University of South Florida (USF) and the Georgia Institute of Technology (GT) who are seeking to identify a chemical process that could produce organic phosphates on early Earth, a necessary step in any origin-of-life scenario. Ironically, in their attempts to support a naturalistic origin-of-life scenario, these researchers have demonstrated the critical role an intelligent agency must play in life's genesis. The emergence of organic phosphates stands as one of the most significant challenges facing any naturalistic origin-of-life scenario. Organic phosphates include DNA; RNA; the biomolecules that form cell membranes; and ATP, the compound that serves as the cell's energy currency.
The Problem of Organic Phosphates
Phosphorylation reactions are the chemical processes that generate organic phosphates in the cell. Phosphorylation occurs when enzymes catalyze the addition of phosphate groups to a target molecule. One conceivable way that phosphorylation could have happened during abiogenesis is through the direct addition of a phosphate group to prebiotic molecules. However, this reaction doesn't take place readily in water—it requires dehydrating conditions and relatively high temperatures. Origin-of-life researchers aren't sure where these types of conditions would exist on early Earth. Additionally, they are concerned that high temperatures would have caused fragile prebiotic materials to break down. Another problem origin-of-life researchers have identified with this chemical route relates to phosphates' solubility. These compounds tend to be highly insoluble in the presence of calcium and magnesium ions, both of which would have been abundant in early Earth's oceans. The insolubility of calcium and magnesium phosphates would have rendered these compounds unavailable for any prebiotic reactions. (For a more complete discussion of the problems associated with generating organic phosphates on early Earth see my book Creating Life in the Lab.)
Have Researchers Found a Solution?
A few years ago, the team from USF proposed a way around these problems. They suggested that organic phosphates could be produced from the iron phosphide and iron-nickel phosphide composing schreibersite (a mineral found in meteorites).1 The USF scientists speculated that abundant schreibersite would have been delivered to early Earth when the planet was pummeled with asteroids during its early history. To confirm their suspicion, these researchers analyzed carbonate minerals from a geological formation in Australia that dates to around 3.5 billion years ago. The team identified phosphites in the carbonate minerals at levels that indicated these minerals would have been a prominent species in early Earth's oceans. Phosphites do not have a biological origin and the phosphites in the carbonate minerals were most likely generated from the phosphides in schreibersite.
Phosphites are much more chemically reactive than phosphates and can phosphorylate organic materials in water. This makes them—and schreibersite—a potential source of phosphorus for phosphorylation reactions on early Earth.
To confirm that schreibersite could, indeed, phosphorylate organic compounds, the researchers heated an aqueous solution of glycerol and schreibersite to 150°F for two days. Afterwards, they found phosphite in the solution along with low levels of glycerol phosphate.
In a follow-up study, the USF team, in collaboration with researchers from GT, assessed whether or not schreibersite could phosphorylate adenosine anduridine nucleosides. The phosphorylated forms of these molecules comprise two of the four building blocks of RNA.2 These building block materials factor significantly into the RNA world hypothesis, one of the most important origin-of-life scenarios. The scientists showed that these two nucleosides could be phosphorylated when heated with schreibersite for several days at 175°F, when the solution was slightly alkaline. They even showed that this reaction would proceed in the presence of magnesium ions.
Based on these two studies, the researchers posit that they have made significant strides towards understanding how organic phosphates formed on early Earth and provided support for chemical evolution and abiogenesis:
The reactions we observed in our experiments have shown that the necessary prebiotic molecules were likely present on the early Earth and that the Earth was predisposed to phosphorylated biomolecules. Our results suggest a potential role for meteoric phosphorus in the development and origin of early life.
Why the Proposed Solution Doesn't Hold Up
Careful analysis of these two studies identifies significant problems with their conclusion. First, the yields of these reactions are low, raising questions about the significance of schreibersite-mediated phosphorylation reactions. When schreibersite was incubated with glycerol, the yield was only 2.5 percent; and when incubated with adenosine and uridine nucleosides, the yields were only 1 to 6 percent. Second, both studies were conducted under chemically pristine conditions, in which the researchers carefully excluded materials that would compete with the desired phosphorylation reactions. Other compounds would have likely been present on early Earth—many at relatively high levels—that could take part in phosphorylation reactions. These competing side reactions would dramatically reduce the already low yields of the desired products.
Selectivity of these reactions also raises concern. In biological systems, nucleosides are phosphorylated at a specific site (the 5' position) in the molecule, but in the laboratory studies, the 2' and 3' positions were also phosphorylated. This lack of selectivity is problematic and further reduces the yield of desired phosphorylation products.
Finally, phosphorylation of nucleosides by schreibersite is pH dependent. The researchers discovered that unless the reaction mixture was alkaline, phosphorylation would not occur. Unfortunately, early Earth's oceans were acidic. This fact alone makes it unlikely that schreibersite-mediated phosphorylation could have ever occurred to any appreciable extent on early Earth.
The USF and GT researchers have identified a chemical process that could, in principle, yield key organic phosphates. However, they failed to show that this process would operate efficiently enough on early Earth to contribute to a naturalistic origin of life. If it were not for the researchers' careful design and the controlled lab conditions, the schreibersite-mediated phosphorylation reactions wouldn't have been successful. In other words, the researchers acted as intelligent agents. As Simon Conway Morris pointed out in his book Life's Solution, "Many of the experiments designed to explain one or other step in the origin of life are either of tenuous relevance to any believable prebiotic setting or involve an experimental rig in which the hand of the researcher becomes for all intents and purposes the hand of God."4
http://www.reasons.org/articles/phosphorus-no-help-for-chemical-evolution
https://reasonandscience.catsboard.com/t2297-phosphorus-no-help-for-chemical-evolution
Phosphorus (P) has the atomic number 15 and is a chemical element found in the earth's crust with a concentration of about 1 gram in each kilogram. It is required for energy production, DNA synthesis and protein synthesis. [url=https://drlwilson.com/articles/minerals for life.htm]32[/url]
Balkrishna Tiwari (2014): Phosphorus plays a very important role in the synthesis of nucleic acids, phospholipids, and many biochemical intermediates of the cell. Its role in cellular signaling and maintenance of biochemical energy pool makes it a very essential macronutrient for life. Inorganic phosphate (Pi) or orthophosphate is the only form of phosphorus that can be directly used by the living cell but it is limited in many ecosystems.
Prof. Dr. Ruth E. Blake (2020): From structural to functional, informational, and energetic roles, Phosphorus is absolutely essential to life. 21
Norio Kitadai (2017): It constitutes biomolecules that play central roles in replication and information (RNA and DNA), metabolism (ATP, NADPH, and other coenzymes), and structure (phospholipids) 58
Radosław W. Piast (2020): All life on Earth uses one universal biochemistry stemming from one universal common ancestor of all known living organisms. One of the most striking features of this universal biochemistry is its utter dependence on phosphate group transfer between biochemical molecules. Both nucleic acid and peptide biological synthesis relies heavily on phosphate group transfer. Such dependents strongly indicate very early incorporation of phosphate chemistry in the origin of life. Perhaps as early as prebiotic soup stage. 22
Mikhail Butusov wrote in the book: The Role of Phosphorus in the Origin of Life and in Evolution:
Phosphorus, in the form of phosphate, has played an important role in the origin and evolution of life on several different levels. It was, most likely, a key component in the early precursors of RNA. It plays an essential role in both the genetics and the energy systems of all living cells as well as in the cell membrane of all modern cells. Phosphorus has also had a decisive role in forming the climatic and atmospheric conditions that set the boundary conditions for evolution and led to us humans and the world we know now. 10
Stanley Miller gave a sobering verdict based on his investigation in regards of a prebiotic source of phosphate:
There are no known efficient prebiotic synthesis of high-energy phosphates or phosphate esters. There is no known robust synthesis of polyphosphates or even pyrophosphate, thereby raising the question of whether
polyphosphates were used in prebiotic reactions and indeed if the pre-RNA world had informational macromolecules that contained phosphate at all. These results suggest that it may not be possible to produce adequate concentrations of high-energy phosphates using electric discharges or volcanic sources. We recognize that we may have missed some high-energy compounds in these experiments so this statement needs to be taken with some reservation. It is perhaps significant that there have been few experiments in the last 20 years attempting to produce high-energy phosphates. This suggests that robust syntheses may not be possible. 59
De Duve (2005): “How did nature choose phosphates?” Unless one believes in intelligent design, fitness does not account for use, except through a process of selective optimization. But phosphate must have entered metabolism before replication and its correlates, mutation and selection, came on the scene, presumably with RNA. There must be a chemical explanation for nature’s choice of phosphates. As I have discussed elsewhere (de Duve, 1991, 2001), this explanation is far from obvious. 61
There was an attempt to solve the problem raised by Miller, which was pointed out by Sci-news: Chemical reactions that make the building blocks of living things need a lot of phosphorus, but phosphorus is scarce. 12
Jonathan D. Toner and colleagues supposedly found an answer to this problem in certain types of lakes. They write (2019): Phosphate is generally limited to micromolar levels in the environment because it precipitates with calcium as low-solubility apatite minerals. This disparity between laboratory conditions and environmental constraints is an enigma known as “the phosphate problem.” Here we show that carbonate-rich lakes are a marked exception to phosphate-poor natural waters. In principle, modern carbonate-rich lakes could accumulate up to ∼0.1 molal phosphate under steady-state conditions of evaporation and stream inflow because calcium is sequestered into carbonate minerals. This prevents the loss of dissolved phosphate to apatite precipitation. Even higher phosphate concentrations (>1 molal) can form during evaporation in the absence of inflows. On the prebiotic Earth, carbonate-rich lakes were likely abundant and phosphate-rich relative to the present day because of the lack of microbial phosphate sinks and enhanced chemical weathering of phosphate minerals under relatively CO2-rich atmospheres. 11
And co-author David Catling claimed: The extremely high phosphate levels in these lakes and ponds would have driven reactions that put phosphorus into the molecular building blocks of RNA, proteins, and fats, all of which were needed to get life going 60
How to put phosphorus into the molecular building blocks without the complex cellular machinery is entirely another feat, and unexplained. Miller was also pessimistic about that. He wrote: Phosphate is an unlikely reagent for the prebiotic world, and this may also apply to the preRNA world.
The phosphorus cycle
Living organisms utilize inorganic phosphate from the ecosystem and return it in the form of organic phosphorus. At this level of phosphorus, cycle microbes contribute significantly by adapting various mechanisms to mineralize dissolved organic phosphorus (DOP) which contribute a major part of the total dissolved phosphorus pool in oceanic fresh water and terrestrial ecosystem. Dissolved organic phosphorus contributes>80% of the total pool of dissolved phosphorus in the North Atlantic Ocean. 14
Libretexts explains: Rocks are a reservoir for phosphorus, and these rocks have their origins in the ocean. Phosphate-containing ocean sediments form primarily from the bodies of ocean organisms and from their excretions. However, volcanic ash, aerosols, and mineral dust may also be significant phosphate sources. This sediment then is moved to land over geologic time by the uplifting of Earth’s surface. Marine birds play a unique role in the phosphorous cycle. These birds take up phosphorous from ocean fish. Their droppings on land (guano) contain high levels of phosphorous. 14
Bacteria use sophisticated mechanisms to, sense, acquire and import phosphate and to maintain intracellular amounts at optimal levels
Juan Francisco Martín and colleagues explain in a scientific paper from 2021: Bacteria transport inorganic phosphate by the high-affinity phosphate transport system PstSCAB, and the low-affinity PitH transporters. The PstSCAB system consists of four components. PstS is the phosphate-binding protein and discriminates between arsenate and phosphate. 15
Vanessa R. Pegos and colleagues write in a scientific publication from 2017: Bacteria have developed specialized systems for phosphorus uptake such as the low-affinity transporter, PitA, and the Phosphate Specific Transporter (Pst), an ATP-Binding Cassette transporter (ABC transporter). Structurally, the Pst system consists of two transmembrane proteins, two associated cytoplasmic ATPases and a periplasmic protein responsible for the affinity and specificity of the system. 16
Armen Y. Mulkidjanian and colleagues (2019): A topologically closed membrane is a ubiquitous feature of all cellular life forms. This membrane is not a simple lipid bilayer enclosing the innards of the cell: far from that, even in the simplest cells, the membrane is a biological device of a staggering complexity that carries diverse protein complexes mediating energy-dependent – and tightly regulated - import and export of metabolites and polymers 17
Angus Menuge asks in his book: Agents Under Fire: Materialism and the Rationality of Science, pgs. 104-105: Hence a chicken and egg paradox: a lipid membrane would be useless without membrane proteins but how could membrane proteins have evolved in the absence of functional membranes? 18
Joseph Panno Ph.D. writes in: THE CELL Evolution of the First Organism, page 17: The cell membrane, far from being featureless, contains a molecular forest that gives the cell its eyes, its ears, and the equipment it needs to capture food and to communicate with other cells. Phospholipids, the main component in cell membranes, are composed of a polar head group (usually an alcohol), a phosphate, glycerol, and two hydrophobic fatty acid tails. Fat that is stored in the body as an energy reserve has a structure similar to a phospholipid, being composed of three fatty acid chains attached to a molecule of glycerol. The third fatty acid takes the place of the phosphate and head group of a phospholipid. Sugars are polymerized to form chains of two or more monosaccharides. Disaccharides (two monosaccharides), and oligosaccharides (about 3–12 monosaccharides), are attached to proteins and lipids destined for the cell surface. Polysaccharides, such as glycogen and starch, may contain several hundred monosaccharides and are stored in cells as an energy reserve. 19
So there is a further catch22 problem: Cell membranes require phosphorus. But the uptake of phosphorus into the cell to make daughter cells with membranes using phosphorus requires pre-existent cell membranes with phosphorus import channels. Cell membranes only come from cell membranes. A cell cannot produce the cell membrane de novo from scratch. It inherits it. Daughter cell membranes come only from mother cell membranes. The mother cell grows twice its starting size, expands its membrane, and once it reaches the right size, it splits. The process is called binary fission and is an enormously complex process, mediated by a multiprotein complex denominated the divisome. 20
One of my favorite blues tunes is "Born Under a Bad Sign," a song about someone who just can't catch a break. If there is a scientific discipline that is characterized by "havin' bad luck all of [its] days," it's origin-of-life research. This trend of bad luck continues for a collaborative team from the University of South Florida (USF) and the Georgia Institute of Technology (GT) who are seeking to identify a chemical process that could produce organic phosphates on early Earth, a necessary step in any origin-of-life scenario. Ironically, in their attempts to support a naturalistic origin-of-life scenario, these researchers have demonstrated the critical role an intelligent agency must play in life's genesis. The emergence of organic phosphates stands as one of the most significant challenges facing any naturalistic origin-of-life scenario. Organic phosphates include DNA; RNA; the biomolecules that form cell membranes; and ATP, the compound that serves as the cell's energy currency.
The Problem of Organic Phosphates
Phosphorylation reactions are the chemical processes that generate organic phosphates in the cell. Phosphorylation occurs when enzymes catalyze the addition of phosphate groups to a target molecule. One conceivable way that phosphorylation could have happened during abiogenesis is through the direct addition of a phosphate group to prebiotic molecules. However, this reaction doesn't take place readily in water—it requires dehydrating conditions and relatively high temperatures. Origin-of-life researchers aren't sure where these types of conditions would exist on early Earth. Additionally, they are concerned that high temperatures would have caused fragile prebiotic materials to break down. Another problem origin-of-life researchers have identified with this chemical route relates to phosphates' solubility. These compounds tend to be highly insoluble in the presence of calcium and magnesium ions, both of which would have been abundant in early Earth's oceans. The insolubility of calcium and magnesium phosphates would have rendered these compounds unavailable for any prebiotic reactions. (For a more complete discussion of the problems associated with generating organic phosphates on early Earth see my book Creating Life in the Lab.)
Have Researchers Found a Solution?
A few years ago, the team from USF proposed a way around these problems. They suggested that organic phosphates could be produced from the iron phosphide and iron-nickel phosphide composing schreibersite (a mineral found in meteorites).1 The USF scientists speculated that abundant schreibersite would have been delivered to early Earth when the planet was pummeled with asteroids during its early history. To confirm their suspicion, these researchers analyzed carbonate minerals from a geological formation in Australia that dates to around 3.5 billion years ago. The team identified phosphites in the carbonate minerals at levels that indicated these minerals would have been a prominent species in early Earth's oceans. Phosphites do not have a biological origin and the phosphites in the carbonate minerals were most likely generated from the phosphides in schreibersite.
Phosphites are much more chemically reactive than phosphates and can phosphorylate organic materials in water. This makes them—and schreibersite—a potential source of phosphorus for phosphorylation reactions on early Earth.
To confirm that schreibersite could, indeed, phosphorylate organic compounds, the researchers heated an aqueous solution of glycerol and schreibersite to 150°F for two days. Afterwards, they found phosphite in the solution along with low levels of glycerol phosphate.
In a follow-up study, the USF team, in collaboration with researchers from GT, assessed whether or not schreibersite could phosphorylate adenosine anduridine nucleosides. The phosphorylated forms of these molecules comprise two of the four building blocks of RNA.2 These building block materials factor significantly into the RNA world hypothesis, one of the most important origin-of-life scenarios. The scientists showed that these two nucleosides could be phosphorylated when heated with schreibersite for several days at 175°F, when the solution was slightly alkaline. They even showed that this reaction would proceed in the presence of magnesium ions.
Based on these two studies, the researchers posit that they have made significant strides towards understanding how organic phosphates formed on early Earth and provided support for chemical evolution and abiogenesis:
The reactions we observed in our experiments have shown that the necessary prebiotic molecules were likely present on the early Earth and that the Earth was predisposed to phosphorylated biomolecules. Our results suggest a potential role for meteoric phosphorus in the development and origin of early life.
Why the Proposed Solution Doesn't Hold Up
Careful analysis of these two studies identifies significant problems with their conclusion. First, the yields of these reactions are low, raising questions about the significance of schreibersite-mediated phosphorylation reactions. When schreibersite was incubated with glycerol, the yield was only 2.5 percent; and when incubated with adenosine and uridine nucleosides, the yields were only 1 to 6 percent. Second, both studies were conducted under chemically pristine conditions, in which the researchers carefully excluded materials that would compete with the desired phosphorylation reactions. Other compounds would have likely been present on early Earth—many at relatively high levels—that could take part in phosphorylation reactions. These competing side reactions would dramatically reduce the already low yields of the desired products.
Selectivity of these reactions also raises concern. In biological systems, nucleosides are phosphorylated at a specific site (the 5' position) in the molecule, but in the laboratory studies, the 2' and 3' positions were also phosphorylated. This lack of selectivity is problematic and further reduces the yield of desired phosphorylation products.
Finally, phosphorylation of nucleosides by schreibersite is pH dependent. The researchers discovered that unless the reaction mixture was alkaline, phosphorylation would not occur. Unfortunately, early Earth's oceans were acidic. This fact alone makes it unlikely that schreibersite-mediated phosphorylation could have ever occurred to any appreciable extent on early Earth.
The USF and GT researchers have identified a chemical process that could, in principle, yield key organic phosphates. However, they failed to show that this process would operate efficiently enough on early Earth to contribute to a naturalistic origin of life. If it were not for the researchers' careful design and the controlled lab conditions, the schreibersite-mediated phosphorylation reactions wouldn't have been successful. In other words, the researchers acted as intelligent agents. As Simon Conway Morris pointed out in his book Life's Solution, "Many of the experiments designed to explain one or other step in the origin of life are either of tenuous relevance to any believable prebiotic setting or involve an experimental rig in which the hand of the researcher becomes for all intents and purposes the hand of God."4
http://www.reasons.org/articles/phosphorus-no-help-for-chemical-evolution
Last edited by Otangelo on Wed Sep 28, 2022 8:28 am; edited 3 times in total