Chemical evolution of amino acids and proteins ? Impossible !!
https://reasonandscience.catsboard.com/t2887-chemical-evolution-of-amino-acids-and-proteins-impossible
Chemical evolution of amino acids and proteins ? Impossible !!
https://www.youtube.com/watch?v=1L1MfGrtk0A
In order to have a minimal functional proteome one would have to assume that:
1. Why are N-unsubstituted a-monoalkyl amino acids used and not b-, g- or d-amino acids, a-dialkylamino acids, or N-alkyl-a-amino acids?
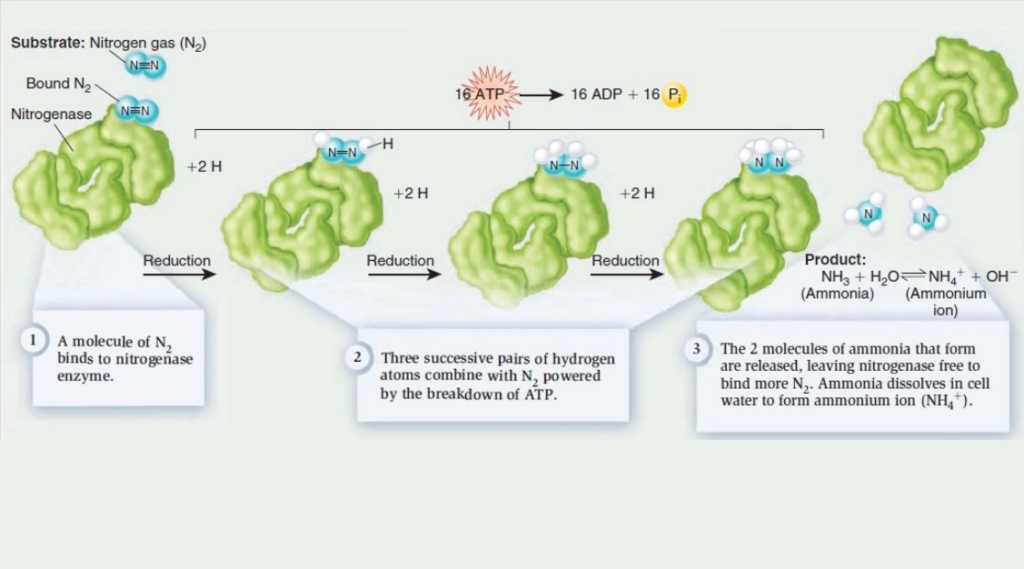
2. Nitrogen and carbon in fixed form, necessary elements of amino acids, was readily available, and that all of the twenty amino acids used in life did form naturally ( disregarding the lifetime of ammonia which would be short because of its photochemical dissociation)
3. Organosulfur compounds required in a few amino acids used in life would be readily available, even if in nature sulfur exists only in its most oxidized form (sulfate or SO4), and only some unique groups of prokaryotes mediate the reduction of SO4 to its most reduced state (sulfide or H2S)
4. Billions of each amino acid would be readily available. ( even if eight proteinogenic amino acids were never abiotically synthesized under prebiotic conditions)
5. The amino acids would be concentrated all together at one assembly site.
6. There would be selected twenty, and not more or less amino acids to make proteins.
7. Only the best suited would have been selected to enable the formation of soluble structures with close-packed cores, allowing the presence of ordered binding pockets inside proteins
8. Nature did somehow "know" that the set of amino acids selected appears to be near ideal and optimal.
9. The amino acids were only in homochiral, that is the left-handed configuration.
10. They would be pure, and without contaminating reactants, somehow avoiding the concomitant synthesis of undesired or irrelevant by-products.
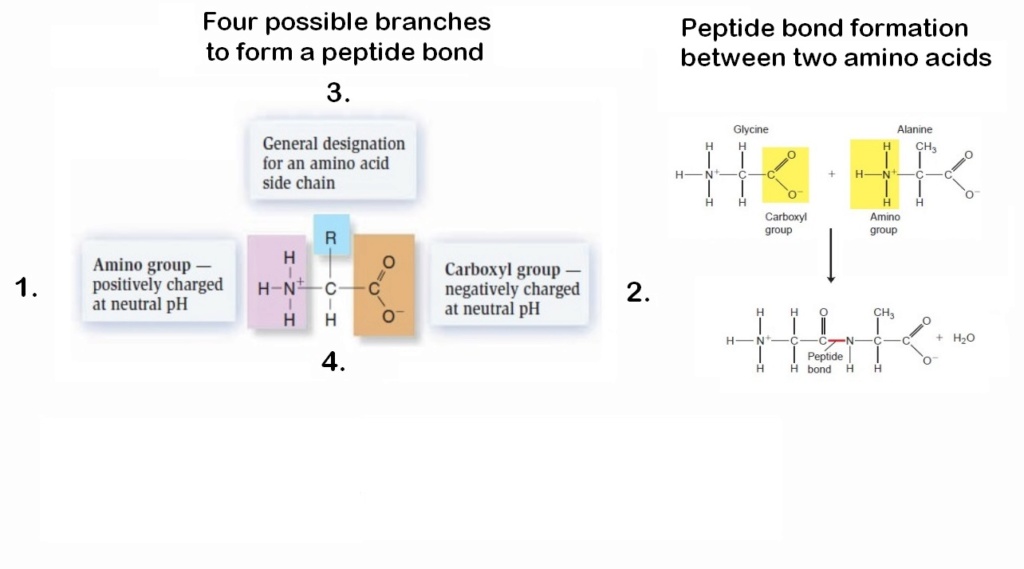
11. They would be all bifunctional monomers with amino groups and carboxyl groups. AA's with unifunctional monomers (with only one functional group) would have been sorted out somehow.
12. They would remain stable, and not DEVOLVE to give uselessly complex mixtures, “asphalts”
13. They would be able to bond and polymerize by non-enzymatic means, without the ribosome
14. There are four different ways to bond AA's together by the side chains. if bonded to the wrong side chain, no deal. They would, somehow, bond together at the right place.
15. The polypeptide chain would not hydrolyze to its constituent amino acids.
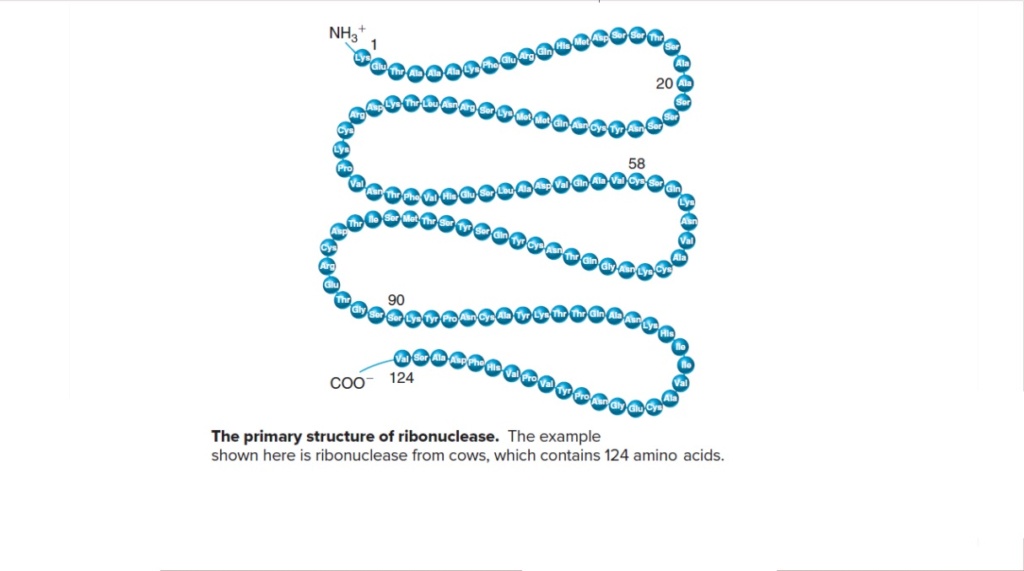
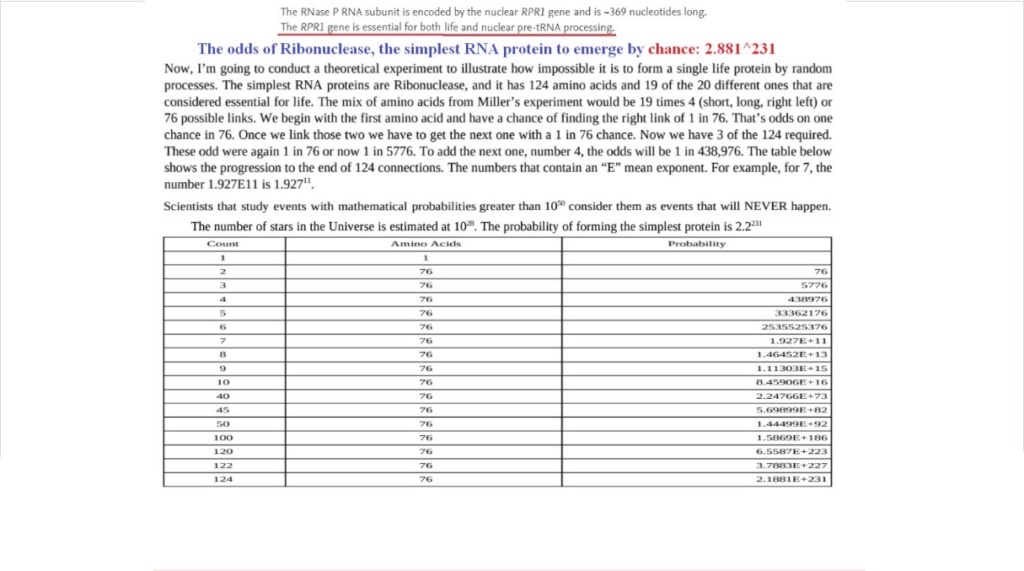
16. In each trial, the average protein would be 400 amino acid units in length
17. The rate to form and test each chain would be just one-third of a ten-million-billionth of a second! This is around 150 thousand trillion times the normal speed in living things.
18. If a usable sequence were obtained, the action would stop so that it would be preserved, and shuffling would restart to obtain all proteins required for life.
19. 1/3 of all proteins once folded require chaperones, other proteins, that help the protein to fold into its proper, functional shape. They were not required for the first protein folds.
20. The synthesized proteins would be able to merge and interlink into assembly lines and metabolic pathways, ready for working together in a living system.
21. Somehow, nature knew how to transition from prebiotic synthesis to cell synthesis of amino acids. A minimum of 112 enzymes is required to synthesize the 20 (+2) amino acids used in proteins.
22. Why are amino acids used, as opposed to say hydroxy acids, thio acids, or amino sulphonic or amino phosphinic acids?
Unsolved issues:
Unsolved issues about the origin of amino acids on early earth:
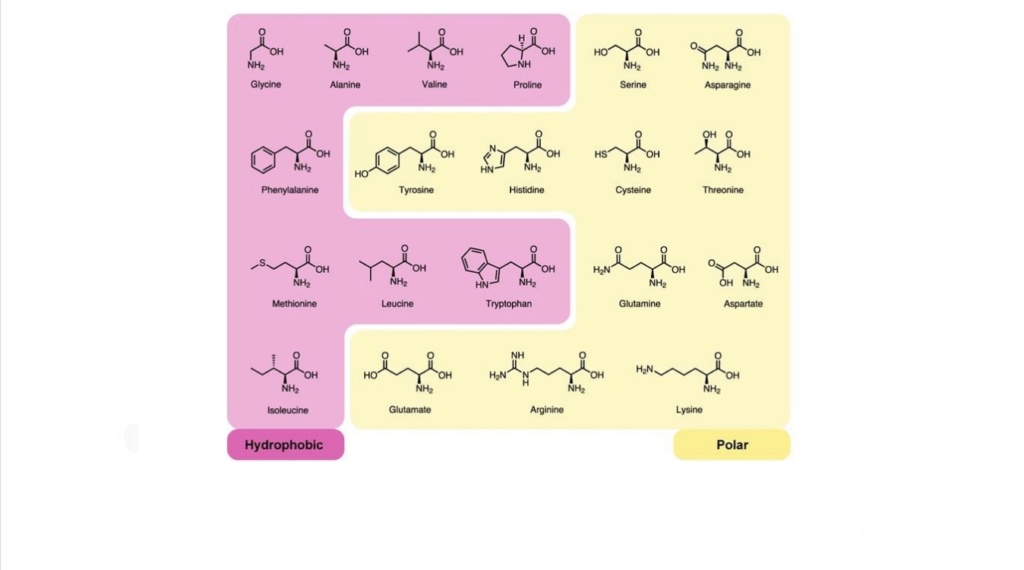
How did unguided nondesigned coincidence select the right amino acids amongst over 300 ( known, but the number is theoretically limitless ) that occur naturally on earth? All life on Earth uses the same 20 ( in some cases, 22 genetically encoded) amino acids to construct its proteins even though this represents a small subset of the amino acids available in nature?
How would twenty amino acids be selected (+2) and not more or less to make proteins?
How was the concomitant synthesis of undesired or irrelevant by-products avoided?
How were bifunctional monomers, that is, molecules with two functional groups so they combine with two others selected, and unfunctional monomers (with only one functional group) sorted out?
How were β, γ, δ… amino acids sorted out?
How did a prebiotic synthesis of biological amino acids avoid the concomitant synthesis of undesired or irrelevant by-products?
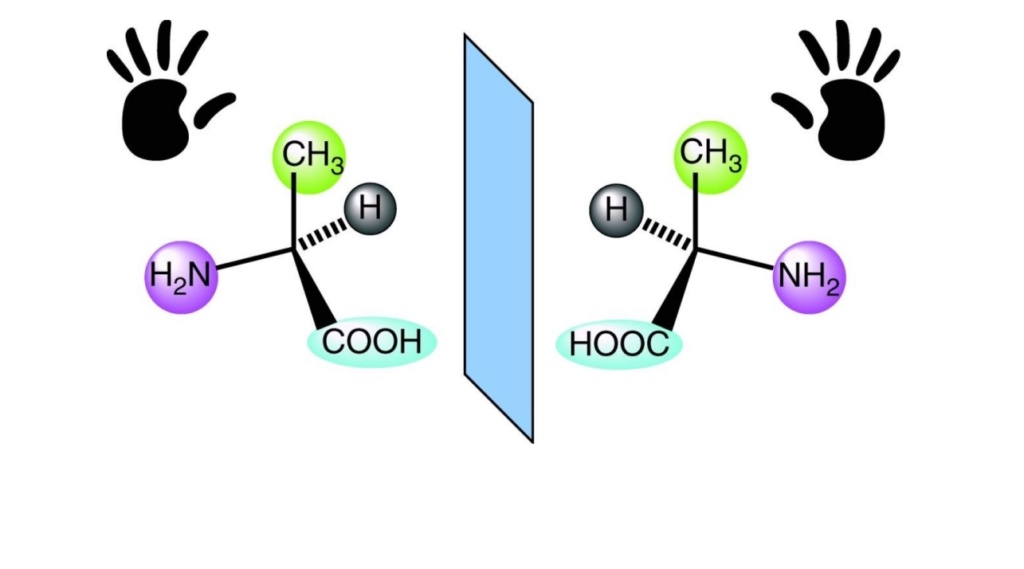
How could achiral precursors of amino acids have produced and concentrated only left-handed amino acids? ( The homochirality problem )?
How did the transition from prebiotic enantiomer selection to the enzymatic reaction of transamination occur that had to be extant when cellular self-replication and life began?
How did ammonia (NH3), the precursor for amino acid synthesis, accumulate on prebiotic earth, if the lifetime of ammonia would be short because of its photochemical dissociation?
How could prebiotic events have delivered organosulfur compounds required in a few amino acids used in life, if in nature sulfur exists only in its most oxidized form (sulfate or SO4), and only some unique groups of procaryotes mediate the reduction of SO4 to its most reduced state (sulfide or H2S)?
How did the transition from prebiotic enantiomer selection to the enzymatic reaction of transamination occur that had to be extant when cellular self-replication and life began?
How did natural events have foreknowledge that the selected amino acids are best suited to enable the formation of soluble structures with close-packed cores, allowing the presence of ordered binding pockets inside proteins?
How did nature select the set of amino acids which appears to be near-optimal in regard to size, charge, and hydrophobicity more broadly and more evenly than in 16 million alternative sets?
How did Amino acid synthesis regulation emerge? Biosynthetic pathways are often highly regulated such that building blocks are synthesized only when supplies are low.
How did the transition from prebiotic synthesis to the synthesis through metabolic pathways of amino acids occur? A minimum of 112 enzymes is required to synthesize the 20 (+2) amino acids used in proteins.
In order to have a functional protein, you need to have amino acids.
In order to have the amino acids used in life, you have to select the right ones amongst over 500 that occur naturally on earth.
To get functional ones, you need to sort them out between left-handed and right-handed ones ( the homochirality problem). Only left-handed amino acids are used in cells.
There is no selection process known besides the one used in cells by sophisticated enzymes, which produce only left-handed amino acids.
Amino acids used for life have amino groups and carboxyl groups. To form a chain, it is necessary to have the reaction of bifunctional monomers, that is, molecules with two functional groups so they combine with two others. If a unifunctional monomer (with only one functional group) reacts with the end of the chain, the chain can grow no further at this end. If only a small fraction of unifunctional molecules were present, long polymers could not form. But all ‘prebiotic simulation’ experiments produce at least three times more unifunctional molecules than bifunctional molecules.
The useful amino acids would have to be joined and brought together at the same assembly site in enough quantity.
There are four different ways to bond them together by the side chains. if bonded to the wrong side chain, no deal.
The formation of amide bonds without the assistance of enzymes poses a major challenge for theories of the origin of life.
Instructional/specified complex information is required to get the right amino acid sequence which is essential to get the functionality in a vast sequence space ( amongst trillions os possible sequences, rare are the ones that provide function )
Before amino acids would join into a sequence providing functional folding, it would disintegrate if hit by UV radiation.
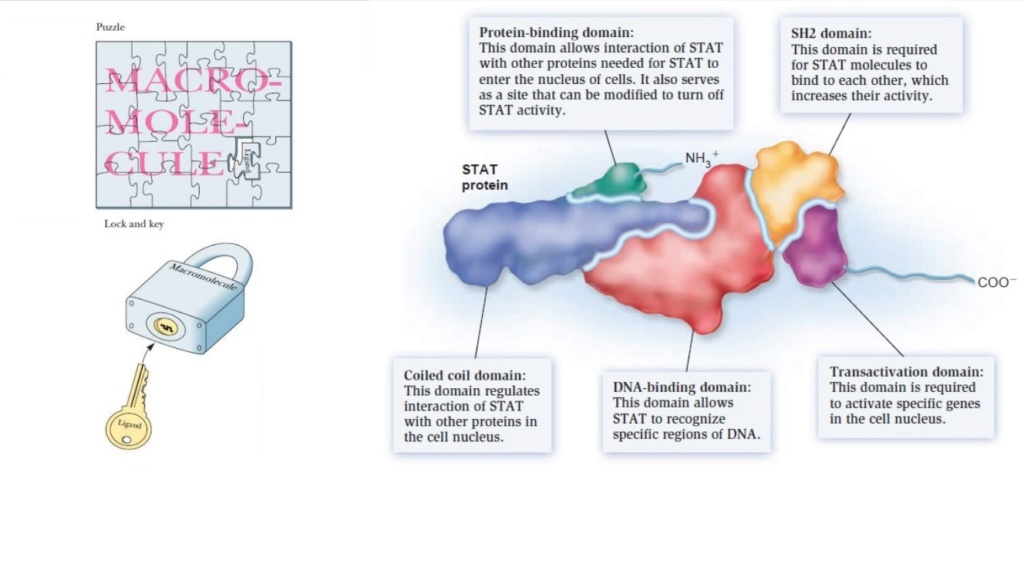
But even IF that would not be the case, most proteins become only functional, if they are joined into holo-enzymes, where various amino acid chains come together like lock and key.
If that would occur, the tertiary or quaternary structure in most cases would bear no function without the insertion of a co-factor inside the pocket, like retinal in the opsin pocket, forming rhodopsin.
But even IF there would emerge a functional protein on the early earth, by itself, it would be like a piston outside the engine block of an automobile. Many proteins bear only function once they are integrated in an assembly line, producing sophisticated molecular products used in life.
But even IF we had an assembly line of enzymes producing a functional product, what good would there be for that product, if the cell would not know where that product is required in the Cell?
For example, chlorophyll requires the complex biosynthesis process of 17 enzymes, lined up in the right order, each producing the substrate used by the next enzyme. But chlorophyll has no function unless inserted in the light-harvesting antenna complex used in photosynthesis to capture light and funnel it to the reaction center.
But even if that complex, chlorophyll and the LHC would be fully set up, they have no function without all over 30 protein complexes forming photosynthesis, used to make hydrocarbons, essential for all advanced life forms.
Now, let's suppose all this would assemble by a freaky random accident on early earth, there would still be no mechanisms of transition from a prebiotic assembly, to Cell factory synthesis.
Tan; Stadler: The Stairway To Life:
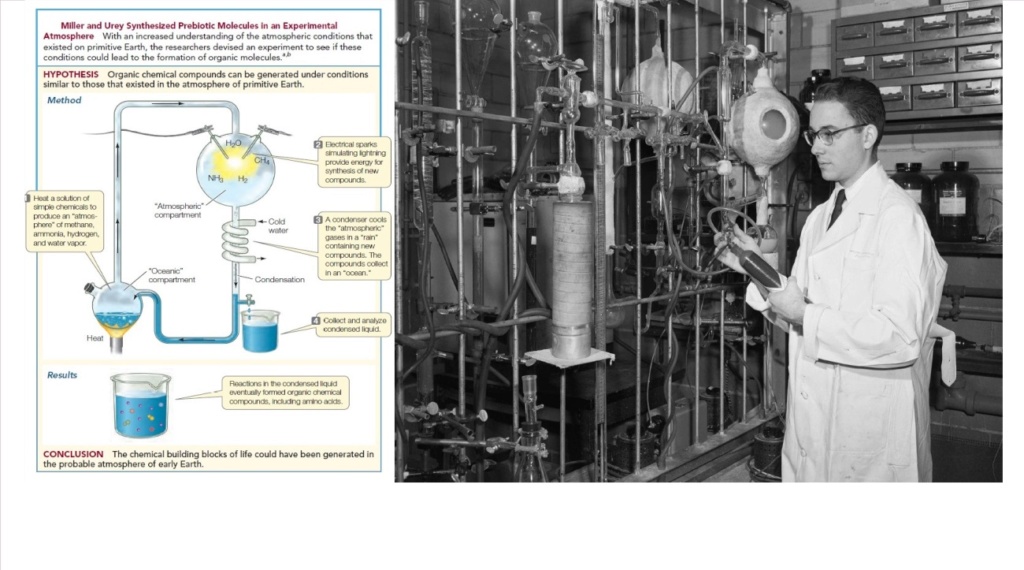
Countless biology textbooks describe the Miller-Urey experiment and others like it as strong evidence that life began spontaneously. This argument seemingly equates the simplicity of a handful of amino acids (formed by a natural process) with the unimaginable complexity of living organisms. This is like finding sand and concluding that microprocessors (computer chips based upon silicon) must be able to assemble spontaneously. Indeed, the chasm between simple organic molecules and living organisms is frequently dismissed in a single sentence. Take, for example, this quote from a popular biology textbook: “The first spark of life ignited when simple chemical reactions began to convert small molecules into larger, more complex molecules with novel 3-D structures and activities”. In his 2014 book, Undeniable, Bill Nye similarly applies “the spark of life” to dismiss complexity: “The origin of life just requires some raw material that could allow the spark of life to emerge”. Walt Disney made a movie about a wooden puppet that turned into a boy through a spark of life. Perhaps such fantasy inspired our current biology textbooks and Bill Nye because these statements are certainly not based on scientific evidence or rational thought. In The Vital Question, a 2015 book that Bill Gates praised as “an amazing inquiry into the origins of life,” biochemist Nick Lane expects his readers to join him in dismissing the great complexity separating simple building blocks and living cells: The formation of organic matter from H2 and CO2 is thermodynamically favoured under alkaline hydrothermal conditions, so long as oxygen is excluded…This means that organic matter should form spontaneously from H2 and CO2 under these conditions. The formation of cells releases energy and increases overall entropy! The average reader of The Vital Question may not have noticed the colossal leap that occurred in the last sentence, where Lane equates spontaneous formation of organic building blocks with the spontaneous formation of cells. This could indeed be possible if cells were “nothing more than a shapeless, mobile, little lump of mucus or slime, consisting of an albuminous combination of carbon,” as thought by Haeckel in the late nineteenth century, but each new year of research exposes previously unimagined layers of cellular complexity, even among the simplest known forms of life. Bestselling author Dan Brown, in his 2017 book Origin, includes a respected scholarly character Robert Langdon who concludes, “Life arose spontaneously from the laws of physics”. Addy Pross, a professor of chemistry at Ben Gurion University, concludes the following in his 2012 book, What is Life?: “Life then is just the chemical consequences that derive from the power of exponential growth operating on certain replicating systems”. Jeremy England, a professor of physics at MIT who also happens to appear in Dan Brown’s Origin, similarly sweeps all the complexity of life under the rug with one sentence: “You start with a random clump of atoms, and if you shine light on it for long enough, it should not be so surprising that you get a plant” . Carl Sagan offered similar words, which are strikingly discordant with all observable evidence: “The origin of life must be a highly probable affair; as soon as conditions permit, up it pops!” . Daily news articles on astronomy and astrobiology barrage us with suggestions that life probably exists on other planets. These articles lead us to believe that the simple discovery of water on a planet virtually guarantees the spontaneous formation of life. A reality check is long overdue. The fantastic complexity of all known life-forms stands in stark contrast to what our schools are teaching, what some scientists believe, and what popular media suggests.
Chemical evolution of amino acids and proteins ? Impossible !!
https://www.youtube.com/watch?v=1L1MfGrtk0A
The problem of getting nitrogen to make amino acids and DNA on early earth 2:41
The problem of getting all amino acids used in llife by origin of life experiments 4:20
The problem of selecting 20 amino acids prebiotically out of hundreds supposedly existing on early earth. 6:08
The problem of concentrating the amino acids used in life at one assembly site. 7:15
The problem of understanding why life uses 20 amino acids, and not more or less. 9:00
The problem of homochirality 12:23
The problem of amino acid synthesis regulation 13:43
The problem of peptide bonding of amino acids to form proteins 14:12
The problem of linking the right amino acid side sequence together 17:15
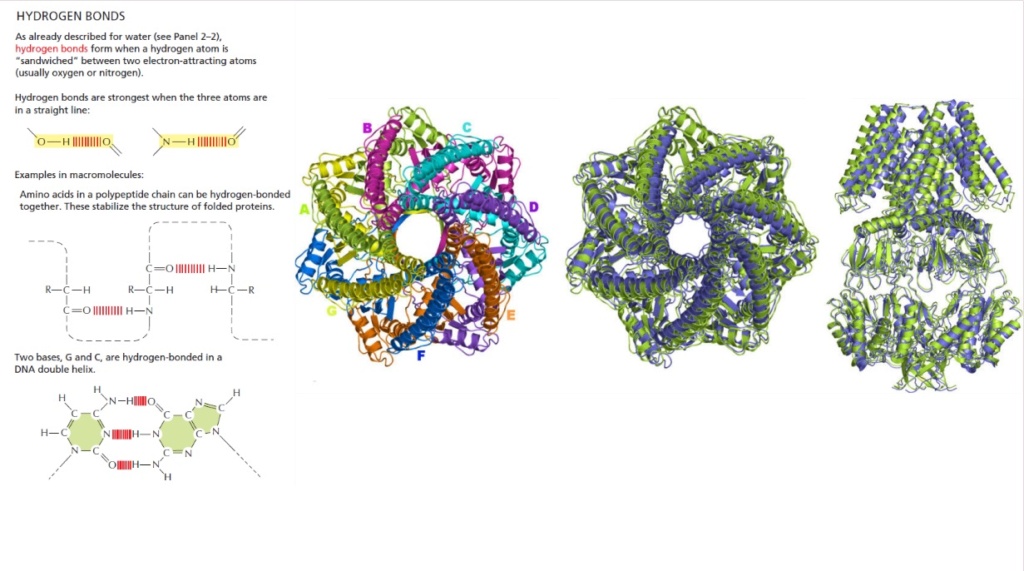
The problem of getting the right forces to stabilize proteins - essential for their correct folding 19:32
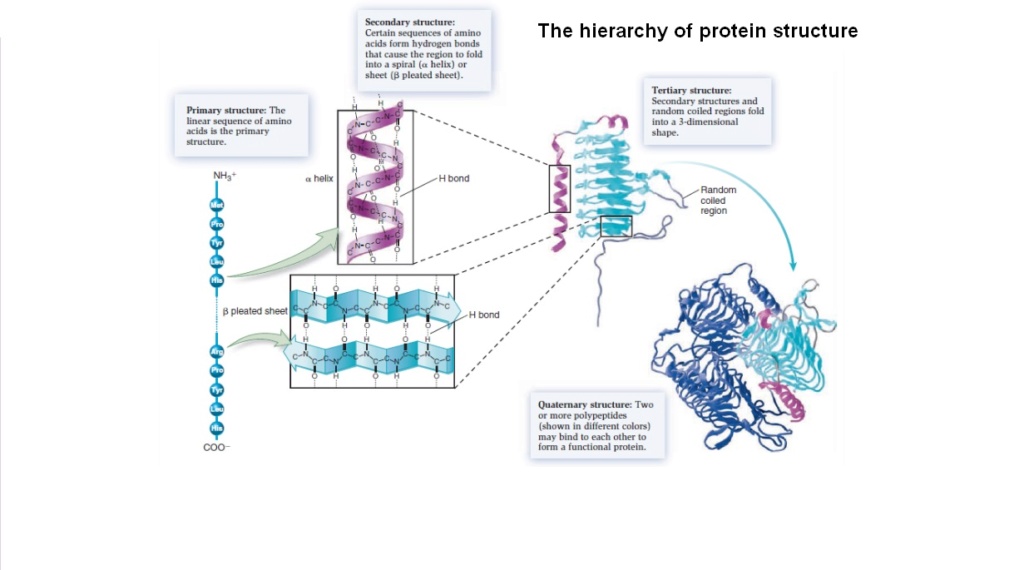
The problem of hierarchical structures of proteins 19:50
Scripps Research Institute: [A chemical clue to how life started on Earth AUGUST 1, 2019
https://www.sciencedaily.com/releases/2019/08/190801093310.htm
Claim: "In the prebiotic Earth, there would have been a much larger set of amino acids," says Leman, who also is scientific collaborator at the Center for Chemical Evolution. "Is there something special about these 20 amino acids, or did these just get frozen at a moment in time by evolution?"
The new study suggests that life's dependence on these 20 amino acids is no accident. The researchers show that the kinds of amino acids used in proteins are more likely to link up together because they react together more efficiently and have few inefficient side reactions.
https://www.universetoday.com/143056/all-life-on-earth-is-made-up-of-the-same-20-amino-acids-scientist-now-think-they-know-why/
Response:
This is a classic example to how methodological naturalism drives to the attempt to provide explanations, which reveal to be nothing more than pseudo-scientific nonsensical explanations.
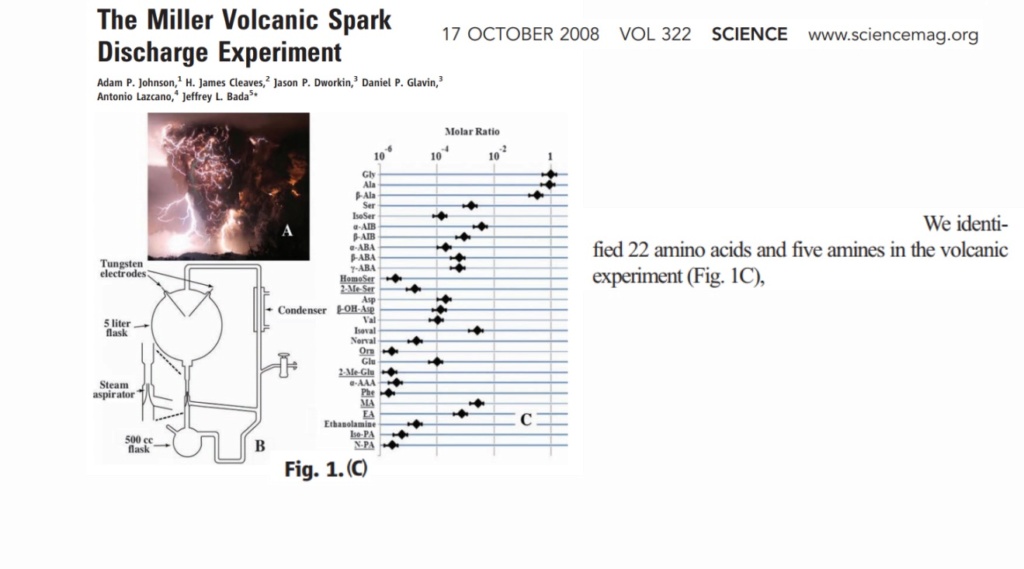
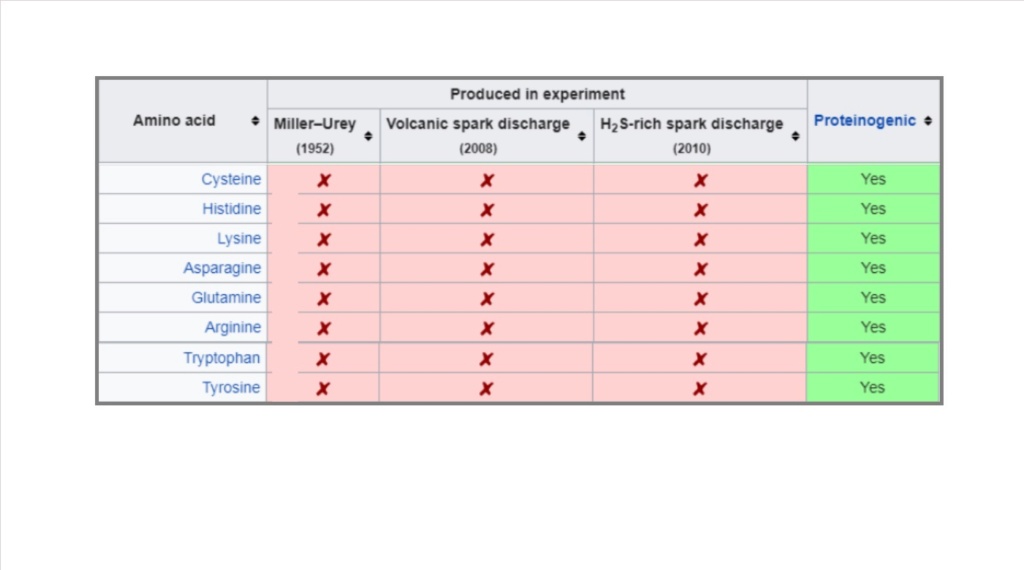
First of all: The authors neglect and obfuscate the fact that twelve of the twenty life essential amino acids, which are synthesized in hypercomplex metabolic pathways in the Cell, which are central to all life, namely: Cysteine Histidine Lysine Asparagine Pyrrolysine Proline Glutamine Arginine Threonine Selenocysteine Tryptophan Tyrosine have never been synthesized in the lab.
Claim: Scientists believe that there were over 500 naturally-occurring acids present on Earth during the Hadean Eon. As Loren Williams, a professor of biochemistry at Georgia Tech, explained:
“Our idea is that life started with the many building blocks that were there and selected a subset of them, but we don’t know how much was selected on the basis of pure chemistry or how much biological processes did the selecting.
Response: There is no reasonable natural unguided mechanism selecting twenty amino acids out of a pool of 500 supposedly existing. Why would it select anyway? See, the word " selecting" is misused so much today, and it is ignored that selecting is something that conscious minds do with a specific purpose. Mindless processes do not select anything in the literal meaning of the word.
Even IF they were likely to link up together because they react together more efficiently and have few inefficient side reactions, that says nothing about the fact that functional sequences in sequence space are EXTREMELY rare.
Functionally Acceptable Substitutions in Two a-Helical Regions of X Repressor
"The estimated number of sequences capable of adopting the h repressor fold is still 10^63 an exceedingly small fraction, about one in of the total number of possible 92-residue sequences."
http://sci-hub.tw/https://onlinelibrary.wiley.com/doi/pdf/10.1002/prot.340070403
Truth said: The information barrier is a problem that CANNOT be solved, and puts all abiogenesis explanations into the realm of SCIENCE FICTION. The ONLY reasonable, logical, and sound inference is that an immensly intelligent, powerful, eternal creator, created a universe, suited to host life, and created life in accordance to his eternal purposes. The fact that we do not know how the interface mind/matter works, says nothing about the possibility/impossibility.
Eliminative inductions argue for the truth of a proposition by arguing that competitors to that proposition are false. Provided the proposition, together with its competitors, form a mutually exclusive and exhaustive class, eliminating all the competitors entails that the proposition is true. Since either there is a God, or not, either one or the other is true. As Sherlock Holmes famous dictum says: when you have eliminated the impossible, whatever remains, however not fully comprehensible, but logically possible, must be the truth. Eliminative inductions, in fact, become deductions.
Amino acids used for life have amino groups and carboxyl groups. To form a chain, it is necessary to have the reaction of bifunctional monomers, that is, molecules with two functional groups so they combine with two others. If a unifunctional monomer (with only one functional group) reacts with the end of the chain, the chain can grow no further at this end. If only a small fraction of unifunctional molecules were present, long polymers could not form. But all ‘prebiotic simulation’ experiments produce at least three times more unifunctional molecules than bifunctional molecules.
The chart from Richard E. Dickerson's article, "Chemical Evolution and the Origin of Life," published in Scientific American in 1978, provides insights into the yield of one of Stanley Miller's experiments, which aimed to simulate conditions that could have led to the formation of organic compounds necessary for life. In Miller's experiment, 59,000 millimoles (mmol) of carbon in the form of methane were used as a starting material.
The main unifunctional products obtained from this experiment were as follows:
Formic acid: 2,330 mmol
Lactic acid: 310 mmol
Acetic acid: 150 mmol
Propionic acid: 130 mmol
In addition to the organic acids, four amino acids that are found in modern proteins were also produced in the experiment. The quantities of these amino acids obtained were:
Glycine: 630 mmol
Alanine: 340 mmol
Glutamic acid: 6 mmol
Aspartic acid: 4 mmol
Stanley Miller's pioneering experiments, including the one mentioned in the article, have contributed significantly to our understanding of how organic compounds, such as amino acids and organic acids, could have formed on early Earth and potentially led to the emergence of life.
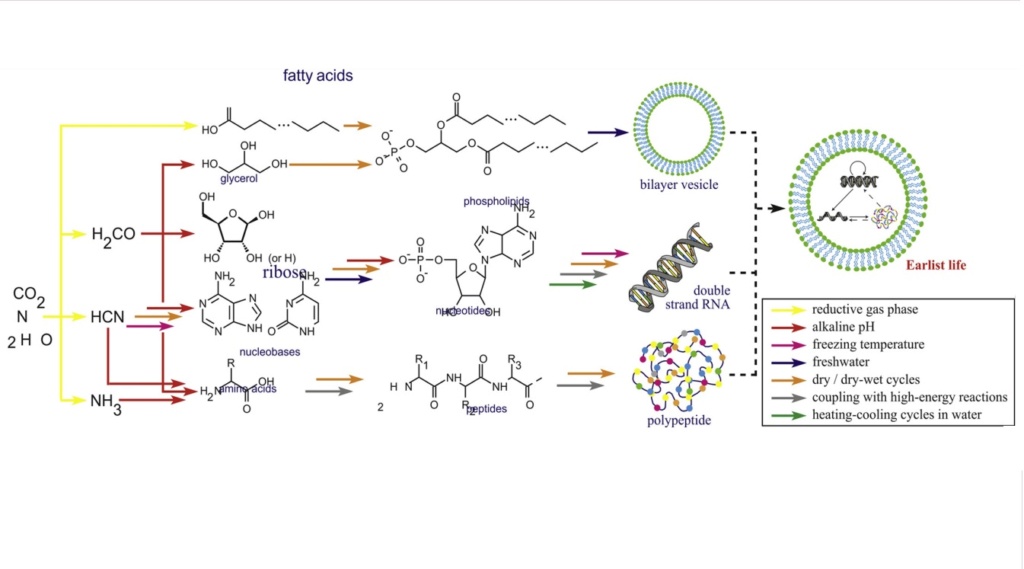
The presence of both amino groups and carboxyl groups in amino acids is indeed essential for the formation of peptide chains. The formation of peptide bonds between amino acids requires the reaction of bifunctional monomers, meaning molecules that possess two functional groups capable of participating in the bonding process. In this case, amino acids act as the bifunctional monomers. However, the problem arises when considering the abundance of unifunctional molecules, those with only one functional group, in comparison to bifunctional molecules in prebiotic simulations. All 'prebiotic simulation' experiments produce at least three times more unifunctional molecules than bifunctional molecules. This poses a challenge for the formation of long polymers, such as proteins. If unifunctional monomers react with the growing chain at the end, they will essentially terminate the chain's growth at that point because they cannot form further bonds. If a significant fraction of the available monomers are unifunctional, it becomes difficult for long polymers to form because the unifunctional monomers will tend to hinder further growth. This scenario contradicts the requirements for the formation of complex polymers like proteins, which necessitate a higher abundance of bifunctional monomers. The imbalance in favor of unifunctional molecules in prebiotic simulations makes it challenging to explain the emergence of long, functional chains in a plausible and efficient manner. This discrepancy between the abundance of bifunctional and unifunctional molecules raises questions about the plausibility of the proposed mechanisms for the origin of life.
The problem of interacting side chains
The prebiotic polymerization of amino acids, which is a key process in the origin of life, presents some challenges due to the interactions between the side chains of amino acids. These interactions can hinder the formation of long, linear chains and affect the overall efficiency of the polymerization reaction. One of the primary challenges arises from the fact that the side chains of amino acids can react with each other, forming bonds between them instead of allowing the formation of peptide bonds that link amino acids together in a linear chain. This side-chain reactivity can lead to the formation of cyclic structures or branching in the resulting polymers, rather than the desired linear sequences. These side-chain interactions can limit the extent of polymerization and the production of long, biologically relevant chains. The presence of branching or cyclic structures can disrupt the formation of stable secondary structures like alpha-helices and beta-sheets, which are important for the proper folding and functioning of proteins. Furthermore, the side-chain interactions can also affect the stability and solubility of the resulting polymers. Depending on the nature of the side chains involved, they can introduce structural constraints or hydrophobic interactions that make the polymers less stable or less soluble in water. The consequence of side-chain interactions during prebiotic polymerization is that it can limit the formation of long, linear sequences of amino acids necessary for the development of functional proteins. The complexity and diversity of proteins as we observe them in living organisms today is a result of the precise arrangement and sequence of amino acids, which allows for specific interactions and functions.
Prebiotic nature requires that polymerization reactions occur selectively between specific amino acids. However, in a prebiotic environment where different amino acids are present in varying concentrations, achieving selectivity in the formation of peptide bonds between the desired amino acids is challenging. During the polymerization reaction, reactive intermediates are formed. These intermediates are unstable under prebiotic conditions and can be easily hydrolyzed before they can join with other amino acids. The prebiotic synthesis of amino acids may be limited in primitive environments. While the synthesis of simple amino acids has been demonstrated in laboratory conditions simulating early Earth conditions, obtaining all the necessary amino acids for protein formation remains a challenge. Prebiotic polymerization reactions of amino acids are generally slow in the absence of suitable catalysts. Kinetic barriers: Polymerization reactions face kinetic barriers, meaning the reactions may be energetically unfavorable or have high activation energies. Overcoming these barriers in a prebiotic setting, where energy sources may be limited, is a significant challenge. Prebiotic environments are complex and may contain various impurities and reactive species that can interfere with the polymerization process. Competing reactions, such as hydrolysis or non-specific bonding, can reduce the yield and efficiency of peptide bond formation. In modern biological systems, enzymes act as templates to guide and accelerate the polymerization of amino acids. However, in a prebiotic context, the emergence of such template molecules is a fundamental question. The spontaneous formation of well-defined templates that can facilitate polymerization selectively is a significant challenge. Living organisms on Earth primarily use left-handed (L-amino acids) in protein synthesis. However, prebiotic reactions often produce both left- and right-handed (D-amino acids) forms. Achieving the selective production of L-amino acids and maintaining homochirality is crucial for the formation of functional proteins. Prebiotic polymerization reactions are highly sensitive to environmental conditions such as pH, temperature, presence of metal ions, and redox potential. Recreating the specific environmental conditions of early Earth and understanding how they influenced the polymerization process is a complex task. Demonstrating prebiotic polymerization reactions on a laboratory scale is one thing, but scaling up these reactions to a level where they can account for the formation of polymers and proteins in a primordial soup scenario is a considerable challenge.
https://reasonandscience.catsboard.com/t2887-chemical-evolution-of-amino-acids-and-proteins-impossible
Chemical evolution of amino acids and proteins ? Impossible !!
https://www.youtube.com/watch?v=1L1MfGrtk0A
In order to have a minimal functional proteome one would have to assume that:
1. Why are N-unsubstituted a-monoalkyl amino acids used and not b-, g- or d-amino acids, a-dialkylamino acids, or N-alkyl-a-amino acids?
2. Nitrogen and carbon in fixed form, necessary elements of amino acids, was readily available, and that all of the twenty amino acids used in life did form naturally ( disregarding the lifetime of ammonia which would be short because of its photochemical dissociation)
3. Organosulfur compounds required in a few amino acids used in life would be readily available, even if in nature sulfur exists only in its most oxidized form (sulfate or SO4), and only some unique groups of prokaryotes mediate the reduction of SO4 to its most reduced state (sulfide or H2S)
4. Billions of each amino acid would be readily available. ( even if eight proteinogenic amino acids were never abiotically synthesized under prebiotic conditions)
5. The amino acids would be concentrated all together at one assembly site.
6. There would be selected twenty, and not more or less amino acids to make proteins.
7. Only the best suited would have been selected to enable the formation of soluble structures with close-packed cores, allowing the presence of ordered binding pockets inside proteins
8. Nature did somehow "know" that the set of amino acids selected appears to be near ideal and optimal.
9. The amino acids were only in homochiral, that is the left-handed configuration.
10. They would be pure, and without contaminating reactants, somehow avoiding the concomitant synthesis of undesired or irrelevant by-products.
11. They would be all bifunctional monomers with amino groups and carboxyl groups. AA's with unifunctional monomers (with only one functional group) would have been sorted out somehow.
12. They would remain stable, and not DEVOLVE to give uselessly complex mixtures, “asphalts”
13. They would be able to bond and polymerize by non-enzymatic means, without the ribosome
14. There are four different ways to bond AA's together by the side chains. if bonded to the wrong side chain, no deal. They would, somehow, bond together at the right place.
15. The polypeptide chain would not hydrolyze to its constituent amino acids.
16. In each trial, the average protein would be 400 amino acid units in length
17. The rate to form and test each chain would be just one-third of a ten-million-billionth of a second! This is around 150 thousand trillion times the normal speed in living things.
18. If a usable sequence were obtained, the action would stop so that it would be preserved, and shuffling would restart to obtain all proteins required for life.
19. 1/3 of all proteins once folded require chaperones, other proteins, that help the protein to fold into its proper, functional shape. They were not required for the first protein folds.
20. The synthesized proteins would be able to merge and interlink into assembly lines and metabolic pathways, ready for working together in a living system.
21. Somehow, nature knew how to transition from prebiotic synthesis to cell synthesis of amino acids. A minimum of 112 enzymes is required to synthesize the 20 (+2) amino acids used in proteins.
22. Why are amino acids used, as opposed to say hydroxy acids, thio acids, or amino sulphonic or amino phosphinic acids?
Unsolved issues:
Unsolved issues about the origin of amino acids on early earth:
How did unguided nondesigned coincidence select the right amino acids amongst over 300 ( known, but the number is theoretically limitless ) that occur naturally on earth? All life on Earth uses the same 20 ( in some cases, 22 genetically encoded) amino acids to construct its proteins even though this represents a small subset of the amino acids available in nature?
How would twenty amino acids be selected (+2) and not more or less to make proteins?
How was the concomitant synthesis of undesired or irrelevant by-products avoided?
How were bifunctional monomers, that is, molecules with two functional groups so they combine with two others selected, and unfunctional monomers (with only one functional group) sorted out?
How were β, γ, δ… amino acids sorted out?
How did a prebiotic synthesis of biological amino acids avoid the concomitant synthesis of undesired or irrelevant by-products?
How could achiral precursors of amino acids have produced and concentrated only left-handed amino acids? ( The homochirality problem )?
How did the transition from prebiotic enantiomer selection to the enzymatic reaction of transamination occur that had to be extant when cellular self-replication and life began?
How did ammonia (NH3), the precursor for amino acid synthesis, accumulate on prebiotic earth, if the lifetime of ammonia would be short because of its photochemical dissociation?
How could prebiotic events have delivered organosulfur compounds required in a few amino acids used in life, if in nature sulfur exists only in its most oxidized form (sulfate or SO4), and only some unique groups of procaryotes mediate the reduction of SO4 to its most reduced state (sulfide or H2S)?
How did the transition from prebiotic enantiomer selection to the enzymatic reaction of transamination occur that had to be extant when cellular self-replication and life began?
How did natural events have foreknowledge that the selected amino acids are best suited to enable the formation of soluble structures with close-packed cores, allowing the presence of ordered binding pockets inside proteins?
How did nature select the set of amino acids which appears to be near-optimal in regard to size, charge, and hydrophobicity more broadly and more evenly than in 16 million alternative sets?
How did Amino acid synthesis regulation emerge? Biosynthetic pathways are often highly regulated such that building blocks are synthesized only when supplies are low.
How did the transition from prebiotic synthesis to the synthesis through metabolic pathways of amino acids occur? A minimum of 112 enzymes is required to synthesize the 20 (+2) amino acids used in proteins.
In order to have a functional protein, you need to have amino acids.
In order to have the amino acids used in life, you have to select the right ones amongst over 500 that occur naturally on earth.
To get functional ones, you need to sort them out between left-handed and right-handed ones ( the homochirality problem). Only left-handed amino acids are used in cells.
There is no selection process known besides the one used in cells by sophisticated enzymes, which produce only left-handed amino acids.
Amino acids used for life have amino groups and carboxyl groups. To form a chain, it is necessary to have the reaction of bifunctional monomers, that is, molecules with two functional groups so they combine with two others. If a unifunctional monomer (with only one functional group) reacts with the end of the chain, the chain can grow no further at this end. If only a small fraction of unifunctional molecules were present, long polymers could not form. But all ‘prebiotic simulation’ experiments produce at least three times more unifunctional molecules than bifunctional molecules.
The useful amino acids would have to be joined and brought together at the same assembly site in enough quantity.
There are four different ways to bond them together by the side chains. if bonded to the wrong side chain, no deal.
The formation of amide bonds without the assistance of enzymes poses a major challenge for theories of the origin of life.
Instructional/specified complex information is required to get the right amino acid sequence which is essential to get the functionality in a vast sequence space ( amongst trillions os possible sequences, rare are the ones that provide function )
Before amino acids would join into a sequence providing functional folding, it would disintegrate if hit by UV radiation.
But even IF that would not be the case, most proteins become only functional, if they are joined into holo-enzymes, where various amino acid chains come together like lock and key.
If that would occur, the tertiary or quaternary structure in most cases would bear no function without the insertion of a co-factor inside the pocket, like retinal in the opsin pocket, forming rhodopsin.
But even IF there would emerge a functional protein on the early earth, by itself, it would be like a piston outside the engine block of an automobile. Many proteins bear only function once they are integrated in an assembly line, producing sophisticated molecular products used in life.
But even IF we had an assembly line of enzymes producing a functional product, what good would there be for that product, if the cell would not know where that product is required in the Cell?
For example, chlorophyll requires the complex biosynthesis process of 17 enzymes, lined up in the right order, each producing the substrate used by the next enzyme. But chlorophyll has no function unless inserted in the light-harvesting antenna complex used in photosynthesis to capture light and funnel it to the reaction center.
But even if that complex, chlorophyll and the LHC would be fully set up, they have no function without all over 30 protein complexes forming photosynthesis, used to make hydrocarbons, essential for all advanced life forms.
Now, let's suppose all this would assemble by a freaky random accident on early earth, there would still be no mechanisms of transition from a prebiotic assembly, to Cell factory synthesis.
Tan; Stadler: The Stairway To Life:
Countless biology textbooks describe the Miller-Urey experiment and others like it as strong evidence that life began spontaneously. This argument seemingly equates the simplicity of a handful of amino acids (formed by a natural process) with the unimaginable complexity of living organisms. This is like finding sand and concluding that microprocessors (computer chips based upon silicon) must be able to assemble spontaneously. Indeed, the chasm between simple organic molecules and living organisms is frequently dismissed in a single sentence. Take, for example, this quote from a popular biology textbook: “The first spark of life ignited when simple chemical reactions began to convert small molecules into larger, more complex molecules with novel 3-D structures and activities”. In his 2014 book, Undeniable, Bill Nye similarly applies “the spark of life” to dismiss complexity: “The origin of life just requires some raw material that could allow the spark of life to emerge”. Walt Disney made a movie about a wooden puppet that turned into a boy through a spark of life. Perhaps such fantasy inspired our current biology textbooks and Bill Nye because these statements are certainly not based on scientific evidence or rational thought. In The Vital Question, a 2015 book that Bill Gates praised as “an amazing inquiry into the origins of life,” biochemist Nick Lane expects his readers to join him in dismissing the great complexity separating simple building blocks and living cells: The formation of organic matter from H2 and CO2 is thermodynamically favoured under alkaline hydrothermal conditions, so long as oxygen is excluded…This means that organic matter should form spontaneously from H2 and CO2 under these conditions. The formation of cells releases energy and increases overall entropy! The average reader of The Vital Question may not have noticed the colossal leap that occurred in the last sentence, where Lane equates spontaneous formation of organic building blocks with the spontaneous formation of cells. This could indeed be possible if cells were “nothing more than a shapeless, mobile, little lump of mucus or slime, consisting of an albuminous combination of carbon,” as thought by Haeckel in the late nineteenth century, but each new year of research exposes previously unimagined layers of cellular complexity, even among the simplest known forms of life. Bestselling author Dan Brown, in his 2017 book Origin, includes a respected scholarly character Robert Langdon who concludes, “Life arose spontaneously from the laws of physics”. Addy Pross, a professor of chemistry at Ben Gurion University, concludes the following in his 2012 book, What is Life?: “Life then is just the chemical consequences that derive from the power of exponential growth operating on certain replicating systems”. Jeremy England, a professor of physics at MIT who also happens to appear in Dan Brown’s Origin, similarly sweeps all the complexity of life under the rug with one sentence: “You start with a random clump of atoms, and if you shine light on it for long enough, it should not be so surprising that you get a plant” . Carl Sagan offered similar words, which are strikingly discordant with all observable evidence: “The origin of life must be a highly probable affair; as soon as conditions permit, up it pops!” . Daily news articles on astronomy and astrobiology barrage us with suggestions that life probably exists on other planets. These articles lead us to believe that the simple discovery of water on a planet virtually guarantees the spontaneous formation of life. A reality check is long overdue. The fantastic complexity of all known life-forms stands in stark contrast to what our schools are teaching, what some scientists believe, and what popular media suggests.
Chemical evolution of amino acids and proteins ? Impossible !!
https://www.youtube.com/watch?v=1L1MfGrtk0A
The problem of getting nitrogen to make amino acids and DNA on early earth 2:41
The problem of getting all amino acids used in llife by origin of life experiments 4:20
The problem of selecting 20 amino acids prebiotically out of hundreds supposedly existing on early earth. 6:08
The problem of concentrating the amino acids used in life at one assembly site. 7:15
The problem of understanding why life uses 20 amino acids, and not more or less. 9:00
The problem of homochirality 12:23
The problem of amino acid synthesis regulation 13:43
The problem of peptide bonding of amino acids to form proteins 14:12
The problem of linking the right amino acid side sequence together 17:15
The problem of getting the right forces to stabilize proteins - essential for their correct folding 19:32
The problem of hierarchical structures of proteins 19:50
Scripps Research Institute: [A chemical clue to how life started on Earth AUGUST 1, 2019
https://www.sciencedaily.com/releases/2019/08/190801093310.htm
Claim: "In the prebiotic Earth, there would have been a much larger set of amino acids," says Leman, who also is scientific collaborator at the Center for Chemical Evolution. "Is there something special about these 20 amino acids, or did these just get frozen at a moment in time by evolution?"
The new study suggests that life's dependence on these 20 amino acids is no accident. The researchers show that the kinds of amino acids used in proteins are more likely to link up together because they react together more efficiently and have few inefficient side reactions.
https://www.universetoday.com/143056/all-life-on-earth-is-made-up-of-the-same-20-amino-acids-scientist-now-think-they-know-why/
Response:
This is a classic example to how methodological naturalism drives to the attempt to provide explanations, which reveal to be nothing more than pseudo-scientific nonsensical explanations.
First of all: The authors neglect and obfuscate the fact that twelve of the twenty life essential amino acids, which are synthesized in hypercomplex metabolic pathways in the Cell, which are central to all life, namely: Cysteine Histidine Lysine Asparagine Pyrrolysine Proline Glutamine Arginine Threonine Selenocysteine Tryptophan Tyrosine have never been synthesized in the lab.
Claim: Scientists believe that there were over 500 naturally-occurring acids present on Earth during the Hadean Eon. As Loren Williams, a professor of biochemistry at Georgia Tech, explained:
“Our idea is that life started with the many building blocks that were there and selected a subset of them, but we don’t know how much was selected on the basis of pure chemistry or how much biological processes did the selecting.
Response: There is no reasonable natural unguided mechanism selecting twenty amino acids out of a pool of 500 supposedly existing. Why would it select anyway? See, the word " selecting" is misused so much today, and it is ignored that selecting is something that conscious minds do with a specific purpose. Mindless processes do not select anything in the literal meaning of the word.
Even IF they were likely to link up together because they react together more efficiently and have few inefficient side reactions, that says nothing about the fact that functional sequences in sequence space are EXTREMELY rare.
Functionally Acceptable Substitutions in Two a-Helical Regions of X Repressor
"The estimated number of sequences capable of adopting the h repressor fold is still 10^63 an exceedingly small fraction, about one in of the total number of possible 92-residue sequences."
http://sci-hub.tw/https://onlinelibrary.wiley.com/doi/pdf/10.1002/prot.340070403
Truth said: The information barrier is a problem that CANNOT be solved, and puts all abiogenesis explanations into the realm of SCIENCE FICTION. The ONLY reasonable, logical, and sound inference is that an immensly intelligent, powerful, eternal creator, created a universe, suited to host life, and created life in accordance to his eternal purposes. The fact that we do not know how the interface mind/matter works, says nothing about the possibility/impossibility.
Eliminative inductions argue for the truth of a proposition by arguing that competitors to that proposition are false. Provided the proposition, together with its competitors, form a mutually exclusive and exhaustive class, eliminating all the competitors entails that the proposition is true. Since either there is a God, or not, either one or the other is true. As Sherlock Holmes famous dictum says: when you have eliminated the impossible, whatever remains, however not fully comprehensible, but logically possible, must be the truth. Eliminative inductions, in fact, become deductions.
Amino acids used for life have amino groups and carboxyl groups. To form a chain, it is necessary to have the reaction of bifunctional monomers, that is, molecules with two functional groups so they combine with two others. If a unifunctional monomer (with only one functional group) reacts with the end of the chain, the chain can grow no further at this end. If only a small fraction of unifunctional molecules were present, long polymers could not form. But all ‘prebiotic simulation’ experiments produce at least three times more unifunctional molecules than bifunctional molecules.
The chart from Richard E. Dickerson's article, "Chemical Evolution and the Origin of Life," published in Scientific American in 1978, provides insights into the yield of one of Stanley Miller's experiments, which aimed to simulate conditions that could have led to the formation of organic compounds necessary for life. In Miller's experiment, 59,000 millimoles (mmol) of carbon in the form of methane were used as a starting material.
The main unifunctional products obtained from this experiment were as follows:
Formic acid: 2,330 mmol
Lactic acid: 310 mmol
Acetic acid: 150 mmol
Propionic acid: 130 mmol
In addition to the organic acids, four amino acids that are found in modern proteins were also produced in the experiment. The quantities of these amino acids obtained were:
Glycine: 630 mmol
Alanine: 340 mmol
Glutamic acid: 6 mmol
Aspartic acid: 4 mmol
Stanley Miller's pioneering experiments, including the one mentioned in the article, have contributed significantly to our understanding of how organic compounds, such as amino acids and organic acids, could have formed on early Earth and potentially led to the emergence of life.
The presence of both amino groups and carboxyl groups in amino acids is indeed essential for the formation of peptide chains. The formation of peptide bonds between amino acids requires the reaction of bifunctional monomers, meaning molecules that possess two functional groups capable of participating in the bonding process. In this case, amino acids act as the bifunctional monomers. However, the problem arises when considering the abundance of unifunctional molecules, those with only one functional group, in comparison to bifunctional molecules in prebiotic simulations. All 'prebiotic simulation' experiments produce at least three times more unifunctional molecules than bifunctional molecules. This poses a challenge for the formation of long polymers, such as proteins. If unifunctional monomers react with the growing chain at the end, they will essentially terminate the chain's growth at that point because they cannot form further bonds. If a significant fraction of the available monomers are unifunctional, it becomes difficult for long polymers to form because the unifunctional monomers will tend to hinder further growth. This scenario contradicts the requirements for the formation of complex polymers like proteins, which necessitate a higher abundance of bifunctional monomers. The imbalance in favor of unifunctional molecules in prebiotic simulations makes it challenging to explain the emergence of long, functional chains in a plausible and efficient manner. This discrepancy between the abundance of bifunctional and unifunctional molecules raises questions about the plausibility of the proposed mechanisms for the origin of life.
The problem of interacting side chains
The prebiotic polymerization of amino acids, which is a key process in the origin of life, presents some challenges due to the interactions between the side chains of amino acids. These interactions can hinder the formation of long, linear chains and affect the overall efficiency of the polymerization reaction. One of the primary challenges arises from the fact that the side chains of amino acids can react with each other, forming bonds between them instead of allowing the formation of peptide bonds that link amino acids together in a linear chain. This side-chain reactivity can lead to the formation of cyclic structures or branching in the resulting polymers, rather than the desired linear sequences. These side-chain interactions can limit the extent of polymerization and the production of long, biologically relevant chains. The presence of branching or cyclic structures can disrupt the formation of stable secondary structures like alpha-helices and beta-sheets, which are important for the proper folding and functioning of proteins. Furthermore, the side-chain interactions can also affect the stability and solubility of the resulting polymers. Depending on the nature of the side chains involved, they can introduce structural constraints or hydrophobic interactions that make the polymers less stable or less soluble in water. The consequence of side-chain interactions during prebiotic polymerization is that it can limit the formation of long, linear sequences of amino acids necessary for the development of functional proteins. The complexity and diversity of proteins as we observe them in living organisms today is a result of the precise arrangement and sequence of amino acids, which allows for specific interactions and functions.
Prebiotic nature requires that polymerization reactions occur selectively between specific amino acids. However, in a prebiotic environment where different amino acids are present in varying concentrations, achieving selectivity in the formation of peptide bonds between the desired amino acids is challenging. During the polymerization reaction, reactive intermediates are formed. These intermediates are unstable under prebiotic conditions and can be easily hydrolyzed before they can join with other amino acids. The prebiotic synthesis of amino acids may be limited in primitive environments. While the synthesis of simple amino acids has been demonstrated in laboratory conditions simulating early Earth conditions, obtaining all the necessary amino acids for protein formation remains a challenge. Prebiotic polymerization reactions of amino acids are generally slow in the absence of suitable catalysts. Kinetic barriers: Polymerization reactions face kinetic barriers, meaning the reactions may be energetically unfavorable or have high activation energies. Overcoming these barriers in a prebiotic setting, where energy sources may be limited, is a significant challenge. Prebiotic environments are complex and may contain various impurities and reactive species that can interfere with the polymerization process. Competing reactions, such as hydrolysis or non-specific bonding, can reduce the yield and efficiency of peptide bond formation. In modern biological systems, enzymes act as templates to guide and accelerate the polymerization of amino acids. However, in a prebiotic context, the emergence of such template molecules is a fundamental question. The spontaneous formation of well-defined templates that can facilitate polymerization selectively is a significant challenge. Living organisms on Earth primarily use left-handed (L-amino acids) in protein synthesis. However, prebiotic reactions often produce both left- and right-handed (D-amino acids) forms. Achieving the selective production of L-amino acids and maintaining homochirality is crucial for the formation of functional proteins. Prebiotic polymerization reactions are highly sensitive to environmental conditions such as pH, temperature, presence of metal ions, and redox potential. Recreating the specific environmental conditions of early Earth and understanding how they influenced the polymerization process is a complex task. Demonstrating prebiotic polymerization reactions on a laboratory scale is one thing, but scaling up these reactions to a level where they can account for the formation of polymers and proteins in a primordial soup scenario is a considerable challenge.
Last edited by Otangelo on Fri Jul 05, 2024 6:58 am; edited 24 times in total