Origin and development of bones ( Osteogenesis)
https://reasonandscience.catsboard.com/t2296-origin-and-development-of-bones-osteogenesis
It seems perfectly apt to say that bones are well-designed: indeed, to describe them in any other way seems pedantic. 3
Osteoblasts, 1 cells with single nuclei that synthesize bone., arise from mesenchymal stem cells. Mesenchymal stem cells are found in large numbers in the periosteum, the fibrous-like layer on the outside surface of bones, and in the bone marrow. During cellular differentiation of osteoblasts, the developing progenitor cells express the regulatory transcription factor Cbfa1/Runx2, which is also active in chondrocytes. A second important transcription factor required for osteoblastic differentiation is osterix.[5] Osteoprogenitors differentiate under the influence of growth factors, although isolated mesenchymal stem cells in tissue culture form osteoblasts under permissive conditions that include vitamin C and substrates for alkaline phosphatase, a key enzyme that provides high concentrations of phosphate at the site of mineral deposition.[6]
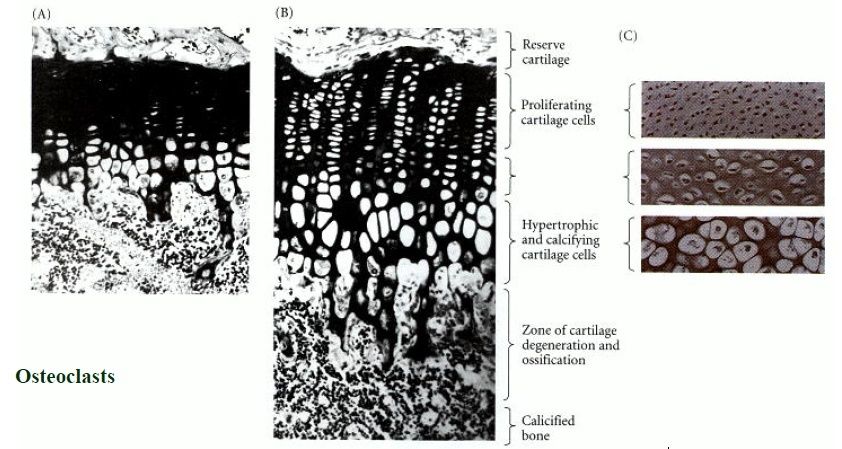
In the living organism, bone development is very complex; in most cases it follows the formation of a first skeleton of cartilage made by chondrocytes, which is then removed and replaced by bone, made by osteoblasts. Key growth factors in skeletal differentiation include bone morphogenetic proteins (BMPs), which determine to a major extent where bone differentiation occurs and where joint spaces are left between bones. The system of cartilage replacement by bone in the living organism has a complex regulatory system. It includes the bone morphogenetic proteins, in particular BMP2, that also regulate early patterning of the skeleton. Other growth factors that are important include transforming growth factor beta (TGF-β), which is part of a superfamily of proteins that include BMPs, which possess common signaling elements in the TGF beta signaling pathway. TGF-β is particularly important in cartilage differentiation, which in most cases precedes osteoblast-mediated bone formation. An additional family of essential bone regulatory factors is the fibroblast growth factors (FGFs), which determine where skeletal elements occur in relation to the skin.
bones in adults that contain red marrow serve an essential function in hemopoiesis (blood cell formation). Bones also serve as crucial reservoirs for calcium, phosphate, and other essential minerals. Almost all (99%) of the calcium in the body is stored in bones, from which the body draws its daily calcium needs.
Bone building: perfect protein 2
Bones are an amazing example of design, present in all vertebrates. They have a huge advantage over man-made girders, in that they are constantly rebuilding and redesigning themselves to cope with changing stress directions.1
This involves a fine balance of the activity of bone-depositing cells (osteoblasts) and bone resorbing cells (osteoclasts). It’s been recently shown that thyroid-stimulating hormone (TSH), best known for what its name says—stimulating the production of hormones in the thyroid gland—has an important role. It oversees both types of cells—without it, bones have osteoporosis in some parts (too little bone, so very weak), and are too dense in other patches.2 So both are essential.
Like all proteins, the instructions for OC are in the DNA,5 but there is more to its manufacture than simply decoding / translating the code and synthesizing the OC on a ribosome. Firstly, the transcription (DNA→mRNA) is regulated by 1,25-dihydroxy-Vitamin D3, one reason that Vitamin D is so important for healthy bones. It is then first decoded (translated) as a preproosteocalcin, which is 98 amino acids long. This comprises three parts: a 23-residue signal protein that is cleaved during translation,5 a 26-residue target propeptide, and the 49-residue mature protein.6
Even this does not complete the process; this requires another vitamin—K. Vitamin K1 or phylloquinone, best known for its vital role in the blood clotting cascade, is an essential co-factor in -carboxylation. That is, the specific glutamyl residues (Glu, from the amino acid glutamic acid) at positions 17, 21 and 24 have a second carboxyl group (–COOH) added to form -carboxyglutamyl residues (Gla). This changes the structure and stabilizes the -helical portion of the protein.6
Even now, the OC protein is fairly shapeless. But when OC meets calcium ions, it folds to a special structure.8 The two carboxyl groups on the -carboxyglutamyl residues chelate1 to the Ca2+ ions, as shown by Fourier-Transform Infrared spectroscopy (FTIR).9 There was no spectral change when Ca2+ was added to decarboxylated OC (i.e. as it would be before converted by Vitamin K), showing that there is no binding without the carboxylation.9 Amazingly (for uniformitarians), enough osteocalcin to produce an immune reaction was found in bones of an Iguanodon ‘dated’ to 120 Ma,10 yet proteins could not last for millions of years. And the fact that it’s a bone protein shows it can’t be contamination from outside.
To do this, OC’s building blocks, the amino acids, must be in a very precise sequence. For example, there is a tightly packed core involving the hydrophobic residues Leu 16, Leu 32, Phe 38, Ala 41, Tyr 42, Phe 45 and Tyr 46. There is also hydrogen bonding to stabilize the connection between different α-helices, Asn 26 in the helix α1–α2 linker and Tyr 46 in α3. The helices α1 and α2 form a V-shaped arrangement stabilized by a disulphide bridge between Cys 23 and Cys 29.
Thus the very precise sequence of OC, as well as the metabolism to form the essential -carboxyglutamyl residues, seems to be yet another example of irreducible complexity, a hallmark of design.11
This means this is yet another component that must be exactly right for the alleged transition from invertebrate to vertebrate. So it is not surprising that proponents of evolution have no fossil evidence for how the transition occurred—this protein alone shows it could never have happened. 2
Thus bone construction is irreducibly complex.
Which explains why there is not only no fossil intermediate between invertebrate and vertebrate, but why it could not exist (and mainly because God didn’t create one!).
What is required for bone formation (osteogenesis) ?
1) https://en.wikipedia.org/wiki/Osteoblast
2) http://creation.com/bone-building-perfect-protein-osteocalcin
3. http://www.esf.edu/efb/turner/publication%20pdfs/turner%20stellenbosch%20complexity.pdf
https://reasonandscience.catsboard.com/t2296-origin-and-development-of-bones-osteogenesis
It seems perfectly apt to say that bones are well-designed: indeed, to describe them in any other way seems pedantic. 3
Osteoblasts, 1 cells with single nuclei that synthesize bone., arise from mesenchymal stem cells. Mesenchymal stem cells are found in large numbers in the periosteum, the fibrous-like layer on the outside surface of bones, and in the bone marrow. During cellular differentiation of osteoblasts, the developing progenitor cells express the regulatory transcription factor Cbfa1/Runx2, which is also active in chondrocytes. A second important transcription factor required for osteoblastic differentiation is osterix.[5] Osteoprogenitors differentiate under the influence of growth factors, although isolated mesenchymal stem cells in tissue culture form osteoblasts under permissive conditions that include vitamin C and substrates for alkaline phosphatase, a key enzyme that provides high concentrations of phosphate at the site of mineral deposition.[6]
In the living organism, bone development is very complex; in most cases it follows the formation of a first skeleton of cartilage made by chondrocytes, which is then removed and replaced by bone, made by osteoblasts. Key growth factors in skeletal differentiation include bone morphogenetic proteins (BMPs), which determine to a major extent where bone differentiation occurs and where joint spaces are left between bones. The system of cartilage replacement by bone in the living organism has a complex regulatory system. It includes the bone morphogenetic proteins, in particular BMP2, that also regulate early patterning of the skeleton. Other growth factors that are important include transforming growth factor beta (TGF-β), which is part of a superfamily of proteins that include BMPs, which possess common signaling elements in the TGF beta signaling pathway. TGF-β is particularly important in cartilage differentiation, which in most cases precedes osteoblast-mediated bone formation. An additional family of essential bone regulatory factors is the fibroblast growth factors (FGFs), which determine where skeletal elements occur in relation to the skin.
bones in adults that contain red marrow serve an essential function in hemopoiesis (blood cell formation). Bones also serve as crucial reservoirs for calcium, phosphate, and other essential minerals. Almost all (99%) of the calcium in the body is stored in bones, from which the body draws its daily calcium needs.
Bone building: perfect protein 2
Bones are an amazing example of design, present in all vertebrates. They have a huge advantage over man-made girders, in that they are constantly rebuilding and redesigning themselves to cope with changing stress directions.1
This involves a fine balance of the activity of bone-depositing cells (osteoblasts) and bone resorbing cells (osteoclasts). It’s been recently shown that thyroid-stimulating hormone (TSH), best known for what its name says—stimulating the production of hormones in the thyroid gland—has an important role. It oversees both types of cells—without it, bones have osteoporosis in some parts (too little bone, so very weak), and are too dense in other patches.2 So both are essential.
Osteocalcin and hydroxyapatite
The strength of bones mainly comes from the hexagonal mineral hydroxyapatite (HA, formula Ca5(PO4)3OH).3 But this must be built up in the right patterns. In vertebrate bones, this is built up with a special protein called osteocalcin (OC). It is a small protein, 49 amino acids long (5.8 kDa), and is ‘highly conserved’, meaning that its sequence is almost identical among vertebrates. Human OC has the sequence Tyr Leu Tyr Gln Trp Leu Gly Ala Pro Val Pro Tyr Pro Asp Pro Leu Gla Pro Arg Arg Gla Val Cys Gla Leu Asn Pro Asp Cys Asp Glu Leu Ala Asp His Ile Gly Phe Gln Glu Ala Tyr Arg Arg Phe Tyr Gly Pro Val.4Like all proteins, the instructions for OC are in the DNA,5 but there is more to its manufacture than simply decoding / translating the code and synthesizing the OC on a ribosome. Firstly, the transcription (DNA→mRNA) is regulated by 1,25-dihydroxy-Vitamin D3, one reason that Vitamin D is so important for healthy bones. It is then first decoded (translated) as a preproosteocalcin, which is 98 amino acids long. This comprises three parts: a 23-residue signal protein that is cleaved during translation,5 a 26-residue target propeptide, and the 49-residue mature protein.6
Even this does not complete the process; this requires another vitamin—K. Vitamin K1 or phylloquinone, best known for its vital role in the blood clotting cascade, is an essential co-factor in -carboxylation. That is, the specific glutamyl residues (Glu, from the amino acid glutamic acid) at positions 17, 21 and 24 have a second carboxyl group (–COOH) added to form -carboxyglutamyl residues (Gla). This changes the structure and stabilizes the -helical portion of the protein.6
Even now, the OC protein is fairly shapeless. But when OC meets calcium ions, it folds to a special structure.8 The two carboxyl groups on the -carboxyglutamyl residues chelate1 to the Ca2+ ions, as shown by Fourier-Transform Infrared spectroscopy (FTIR).9 There was no spectral change when Ca2+ was added to decarboxylated OC (i.e. as it would be before converted by Vitamin K), showing that there is no binding without the carboxylation.9 Amazingly (for uniformitarians), enough osteocalcin to produce an immune reaction was found in bones of an Iguanodon ‘dated’ to 120 Ma,10 yet proteins could not last for millions of years. And the fact that it’s a bone protein shows it can’t be contamination from outside.
Osteocalcin’s crystal structure
Now, pig OC’s crystal structure has been discovered, using a type of X-ray diffraction called the iterative single anomalous scattering method. This provides new insights into how finely designed it must be to work.7 The active site has a negatively charged region that binds the positively charged Ca2+ ions. Five Ca2+ ions are coordinated by three special Gla residues and an Asp at position 30. But not in just any old way—five calcium ions are bound in the same arrangement as in the exposed face of a HA crystal, parallel to the c axis. So the OC can dock on the HA and add the calcium, and thus grow the crystal, making the bone grow in the area needed.To do this, OC’s building blocks, the amino acids, must be in a very precise sequence. For example, there is a tightly packed core involving the hydrophobic residues Leu 16, Leu 32, Phe 38, Ala 41, Tyr 42, Phe 45 and Tyr 46. There is also hydrogen bonding to stabilize the connection between different α-helices, Asn 26 in the helix α1–α2 linker and Tyr 46 in α3. The helices α1 and α2 form a V-shaped arrangement stabilized by a disulphide bridge between Cys 23 and Cys 29.
Thus the very precise sequence of OC, as well as the metabolism to form the essential -carboxyglutamyl residues, seems to be yet another example of irreducible complexity, a hallmark of design.11
This means this is yet another component that must be exactly right for the alleged transition from invertebrate to vertebrate. So it is not surprising that proponents of evolution have no fossil evidence for how the transition occurred—this protein alone shows it could never have happened. 2
Summary
Bones are dynamic supports, constantly rebuilding to cope with changing stresses. Bone shape is a delicate balance of bone deposition and resorption.Bone strength is largely from the mineral hydroxyapatite (HA).One vital component for bone growth is the small, highly conserved protein osteocalcin (OC).Large fragments have been found in dinosaur bones ‘dated’ over 100 million years old, although measured rates of breakdown mean that nothing should have survived that long.Vitamin K is essential to modify three amino acid residues of OC, otherwise it can’t bind calcium at all.Recent discovery of OC’s crystal structure shows that it binds calcium in exactly the right geometry to add to a certain crystal face of HA.Thus bone construction is irreducibly complex.
Which explains why there is not only no fossil intermediate between invertebrate and vertebrate, but why it could not exist (and mainly because God didn’t create one!).
What is required for bone formation (osteogenesis) ?
| Osteoblasts | cells with single nuclei that synthesize bone |
| Mesenchymal stem cells | Multipotent stromal cells that can differentiate into a variety of cell types |
| Regulatory transcription factor Cbfa1/Runx2 | |
| osterix | |
| bone morphogenetic proteins (BMPs) | determine to a major extent where bone differentiation occurs and where joint spaces are left between bones |
| alkaline phosphatase | provides high concentrations of phosphate at the site of mineral deposition |
| chondrocytes | formation of a first skeleton of cartilage |
| transforming growth factor beta (TGF-β) | important in cartilage differentiation, |
| fibroblast growth factors (FGFs) | |
| osteocalcin (OC) | determine where skeletal elements occur in relation to the skin |
| 3-residue signal protein | |
| 1,25-dihydroxy-Vitamin D32 | regulates transcription (DNA→mRNA) |
| 26-residue target propeptide | |
| 49-residue mature protein | |
| Vitamin K1 or phylloquinone | essential co-factor in -carboxylation |
| calcium ions | |
| osteopontin | |
| paracrine factors | induce the nearby mesodermal cells to express two transcription factors, Pax1 and Scleraxis |
| transcription factors, Pax1 and Scleraxis | |
| N-cadherin and N-Cam | important in the initiation of the condensation of the committed mesenchyme cells into compact nodules and differentiate into chondrocytes, the cartilage cells. |
| collagen X | |
| fibronectin | |
| enzymes | active in the generation of calcium and phosphate ions and initiate the mineralization process within the cartilaginous matrix |
| Estrogen Receptors |
1) https://en.wikipedia.org/wiki/Osteoblast
2) http://creation.com/bone-building-perfect-protein-osteocalcin
3. http://www.esf.edu/efb/turner/publication%20pdfs/turner%20stellenbosch%20complexity.pdf
Last edited by Admin on Tue Sep 11, 2018 3:04 am; edited 13 times in total