Michael Rossmann, Purdue - T4 Bacteriophage Assembly
https://vimeo.com/10700469


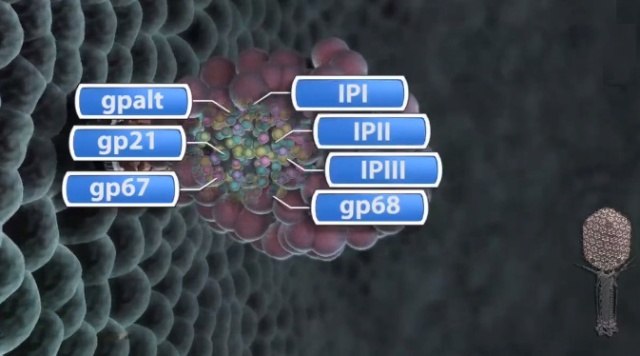





F.Arisaka (2005): Assembly of bacteriophage generally proceeds along a well-ordered pathway. About 45 genes or gene products GP are involved in the assembly of the virion. In order to assemble such a complex structure as T4, a number of intriguing “molecular devices” are employed. These “molecular devices” are considered to make the assembly efficient and free from errors.
Scaffold processing and expansion
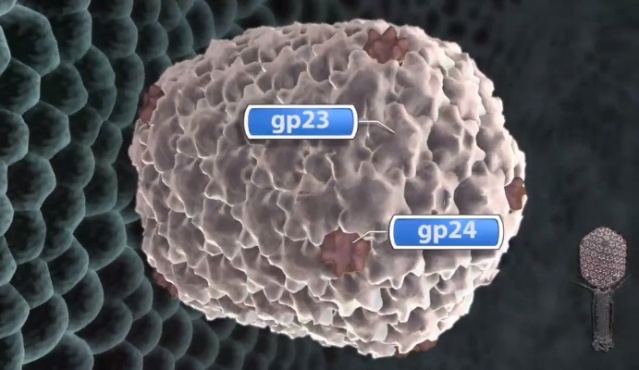
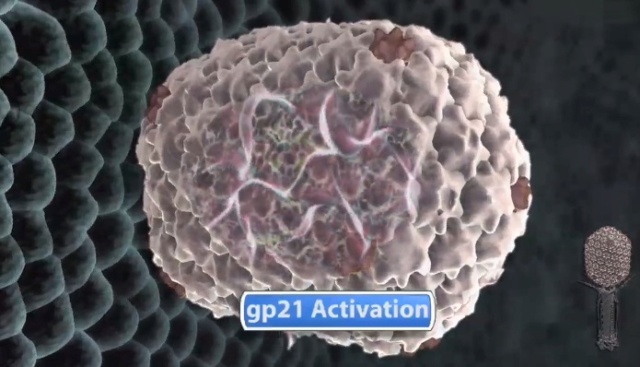
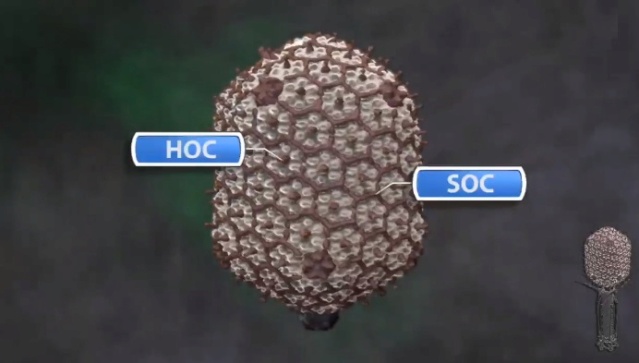
Head formation takes place on the inner membrane of the host E. coli. The initiation complex, made of twelve subunits of gp20, forms part of a neck or knob structure on which a scaffold of the head is formed. The main component of the scaffold is gp22, which is assumed to form a helix-rich ellipsoid of revolution together with minor components such as inner proteins I, II, III, and prohead protease gp21 or T4PPase. The major capsid protein gp23 forms the capsid, which is an icosahedron and elongated along the fivefold axis with T= 13 and Q= 20,6 where T is the triangulation number, which denotes the number of unit triangles in the p6 net, and Q is similar to the T number, but is related to the length of the elongated icosahedron. How the length of the head is determined is not yet fully understood, but the capsid formation starts as the scaffold formation is initiated and it is assumed to be determined by their interaction together with its intrinsic curvature. After the prohead is formed, most of the head proteins except for gp20 are processed or cleaved by T4PPase. T4PPase is specific for Glu residue, but appears to recognize higher order structure as well. The major capsid protein gp23 is cleaved between residue E65 and A66. The scaffold is cleaved into small peptides and is taken out of the prohead, making enough space for DNA packaging. As the processing of the prohead is completed, the prohead leaves the inner membrane and expansion takes place. It expands about 15%, accompanying the increase of the inner space by 50%. The expanded shell of the capsid is thinner but more rigid. The head is further strengthened by the accessory proteins, gp hoc highly immunogenic outer capsid and gp soc small outer capsid. Recently, the three-dimensional structure of gp24 was determined. Gp24 has a high homology to gp23 and forms pentamers at the vertices of the icosahedron, whereas gp23 forms hexamers on the surface. As the fold of gp23 would be similar, we can now predict the atomic structure of the whole head. Tail proteins are not processed with one exception, i.e., gp5 or tail lysozyme, where the peptide bond between Ser351 and Ala352 is cleaved by E. coli protease. This cleavage makes it possible for the lysozyme domain to be released from the rest of protein molecule during penetration of the tail tube
HOST FACTORS ESSENTIAL FOR THE PHAGE ASSEMBLY
As a virus by definition, phage T4 utilizes all the necessary bacterial organelles or protein complexes for its growth. Smaller phages are more dependent on their host functions than are larger phages. T4 phage is one of the largest phages and many functions are self-supplied, but it still requires a number of host factors. Such factors include the inner membrane of E. coli, where the initiation complex of the head is formed, RNA polymerase, ribosome, GroEL, etc. RNA polymerase is not encoded in T4, but the E. coli RNA polymerase undergoes some modifications by T4 proteins. Aside from the ADP-ribosylation of the subunit of the holoenzyme, E. coli omega factor, 70, is replaced by T4-encoded omega factor gp55 to transcribe the late genes, which has a special consensus sequence of TATAAATA. In other words, the switch of transcription from early genes to late genes is accomplished by the replacement of the omega factor of RNA polymerase; from omega 70 to gp55. Most of the structural proteins for virion assembly are encoded in late genes. 19
Moh Lan Yap (2015): Bacteriophage T4 is classified as a member in the Myoviridae family of the Caudovirales order because it has a contractile tail. The head, the tail and the long tail fibers (LTFs) of T4 are assembled independently before they are joined together to produce a mature phage.The 168 kbp dsDNA genome of T4 is encapsidated in its head. A contractile tail is attached to a special portal vertex at one end of the head. A hexagonal baseplate is attached to the distal end of the contractile tail. Six long tail fibers (LTFs) are attached to the periphery of the hexagonal baseplate. The LTFs are the sensors that can recognize receptor molecules on the host. There are six short tail fibers (STFs) folded beneath the baseplate that unfold upon host recognition. After unfolding these STFs bind irreversibly to the host cell, thereby increasing the efficiency of infection. The contractile tail improves the efficiency of infection by making it possible for the tail tube to penetrate the outer host cell membrane prior to the delivery of phage DNA into the host cell.
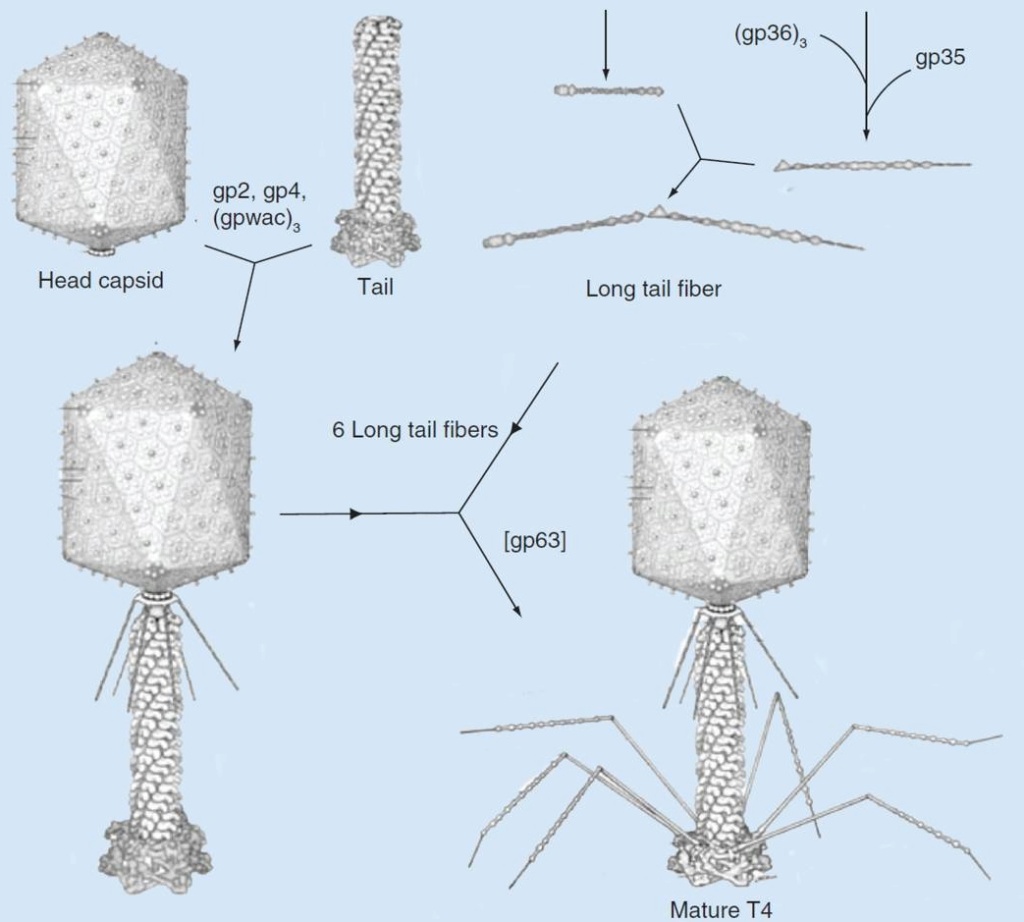
Assembly pathway of bacteriophage T4
The assembly of T4 can be divided into three independent subassemblies: the head, the tail and the long tail fibers. The tail binds to the head followed by attachment of the fibritin protein at the neck region. Six long tail fibers then attach to form a viable T4 virion. 18

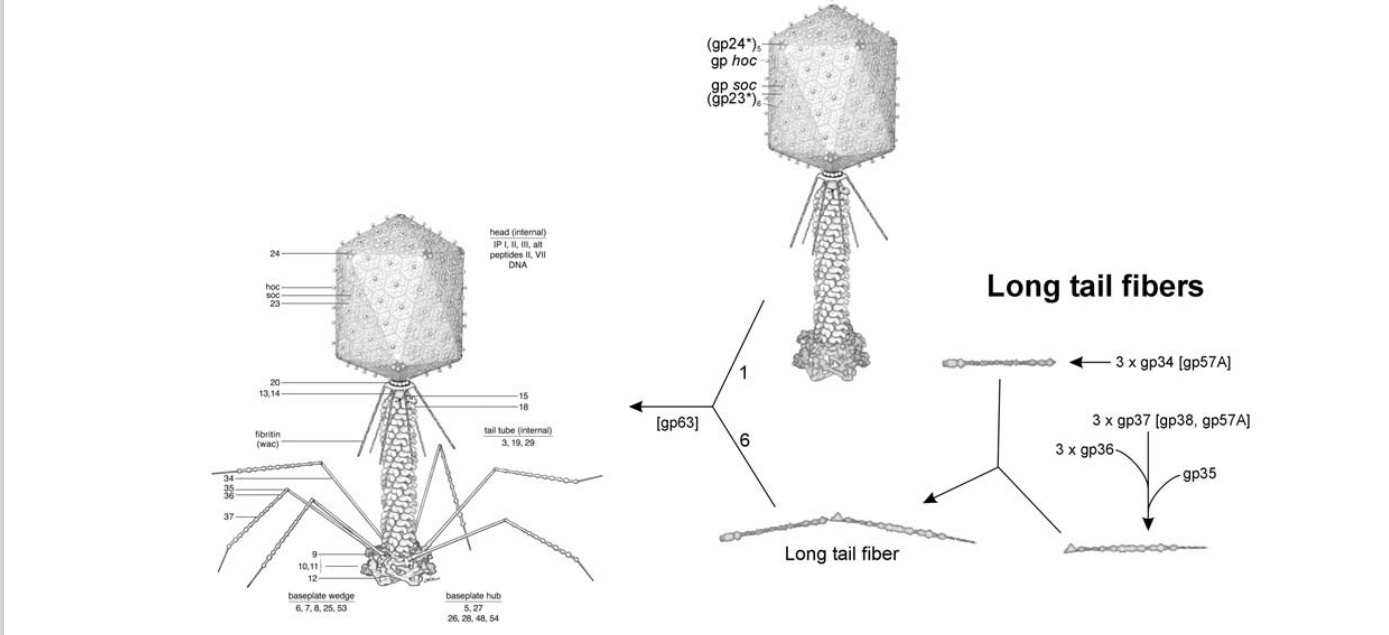
Morphogenesis of the bacteriophage T4 virion.
The overall assembly pathway can be divided into three independent stages: head, tail, and long tail fiber assembly. The chaperonines and catalytic proteins are indicated in brackets near the protein, or assembly step, that
requires the chaperonine. Known protein stoichiometries are given as subscripts. Crystal structures of structural proteins are shown as ribbon drawings. 16
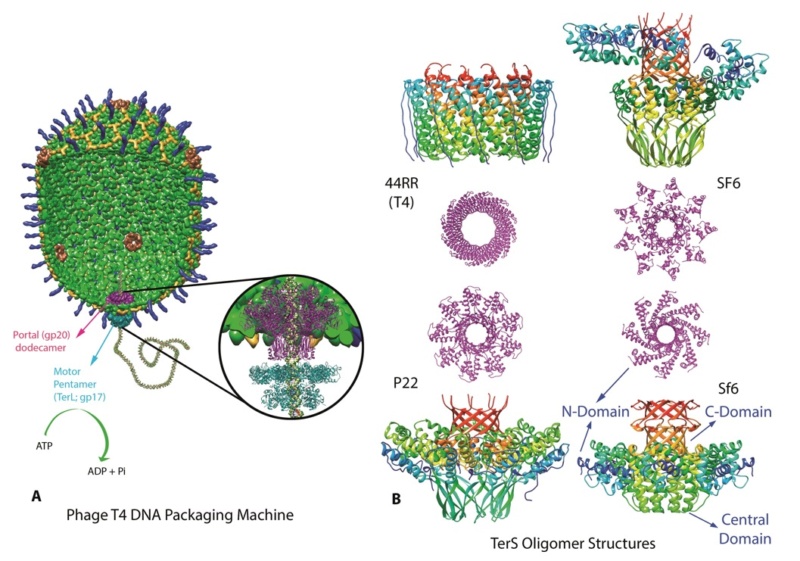
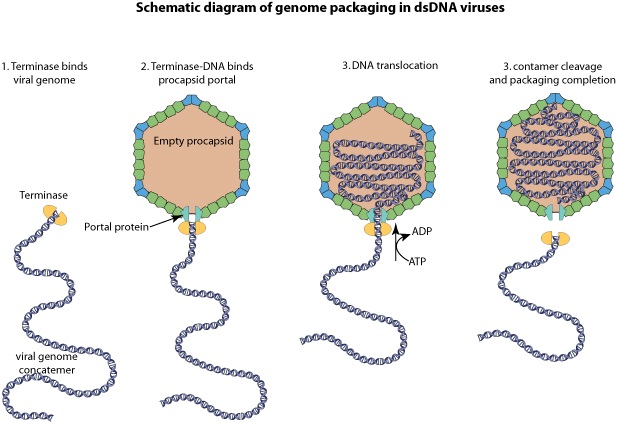
Zhihong Zhang (2011): Complex viruses are assembled from simple protein subunits by sequential and irreversible assembly. During genome packaging in bacteriophages, a powerful molecular motor assembles at the special portal vertex of an empty prohead to initiate packaging. The capsid expands after about 10%–25% of the genome is packaged. When the head is full, the motor cuts the concatemeric DNA and dissociates from the head. These viruses encode powerful machines to package their genomes tightly inside an icosahedral-shaped capsid “head.” Packaging requires precise orchestration of a series of steps: assembly of an empty prohead, concatemer cutting and attachment of the motor-DNA complex to the portal vertex, ATP-fueled DNA translocation until the head is full, DNA cutting to terminate packaging, detachment of the motor, and sealing of the packaged head by “neck” assembly. The virion consists of a head into which the genome is packaged, and a tail that delivers the genome into the bacterial cell. A capsid of precise dimensions is first assembled, often with a single type of protein subunit polymerizing around a protein scaffold
A capsid of precise dimensions is first assembled, often with a single type of protein subunit polymerizing around a protein scaffold. A cone-shaped dodecameric portal initiates assembly and remains at the special five-fold vertex of the isometric capsid (prehead), facilitating all subsequent transactions: DNA entry, tail attachment, and DNA ejection. The scaffold is removed, creating an empty space inside the capsid (prohead or procapsid) for encapsidating the viral genome.
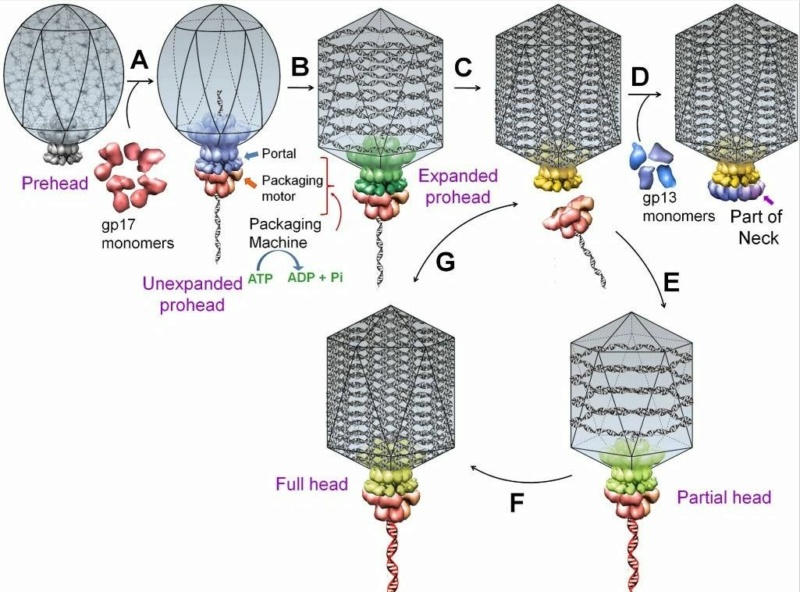
A schematic of DNA packaging by sequential assembly and promiscuous assembly.
The major capsid protein assembles around a scaffolding core into a prehead. The core is removed by proteolysis to produce an empty unexpanded prohead
(A). The unexpanded prohead normally has a round shape, but in phage T4 it has angular geometry. The packaging motor–DNA complex docks on portal and initiates packaging. The prohead expands after about 10%–25% of the DNA is packaged
(B). After headful packaging, the motor cuts the concatemeric DNA and dissociates from the DNA-full head
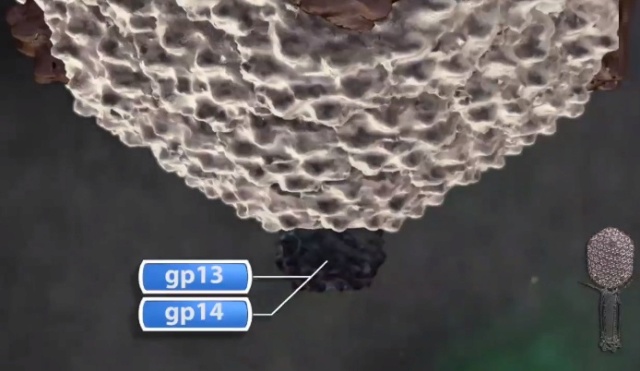
(C). The neck proteins (gp13, gp14, and gp15) assemble on portal to seal off the DNA-full head and provide a platform for tail assembly
(D). The various colors of portal represent different conformational states. In promiscuous assembly, the packaging motor assembles on a partial head produced by ejection of packaged DNA
(E) or a full head
(G), and refills the head with new fragments of DNA
([F] and [G]; new DNA fragments shown in red).
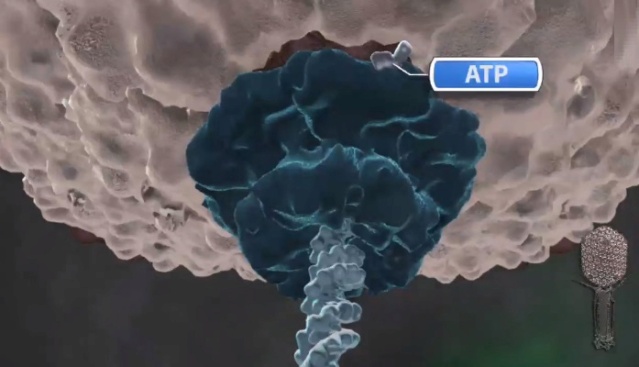
A packaging ATPase motor, also known as the “terminase,” recognizes and cuts the concatemeric viral DNA and docks at the narrow protruding end of the prohead portal, inserting the DNA end into the portal channel. The packaging machine thus assembled drives DNA translocation utilizing the free energy of ATP hydrolysis (Figure B above).
After filling the head (“headful” packaging), the motor cuts the DNA and dissociates from the DNA-full head (Figure C above). The neck and tail proteins assemble on the portal, completing the infectious virus assembly (Figure D above).
A fundamental feature of virus assembly is “sequential assembly” in which “simple” components assemble in a strict sequence to generate a complex nanomachine with unique biological properties. Each assembly step generates a new site or conformational state to which the next component binds with exquisite specificity, essentially irreversibly. A series of such steps, as documented by elegant studies in phage T4 and numerous other viruses, leads to rapid and high-fidelity assembly of a complex infectious virion. In phage T4, this process assembles virions approaching a theoretical infection efficiency of 1.
The sequence of steps in the head morphogenesis of phage T4, as well as in other phages and dsDNA viruses (e.g., herpes viruses), is as follows:
(i) assembly of the packaging motor on a nascent (unexpanded) empty prohead (Figure A)
(ii) expansion of the capsid after about 10%–25% of the genome is packaged (Figure B)
(iii) packaging until the head is full
(iv) cutting of DNA and dissociation of the motor (Figure C)
(v) assembly of neck proteins to seal the packaged heads (Figure D)
Conformational changes in the portal are reported to drive these sequential irreversible transitions (Figure above; different colors of portal represent different conformational states) 12
Overview of Major Steps in T4 Head Assembly
Andreas Kuhn (2022): T4 head assembly initiates at the cytoplasmic surface of the inner membrane of E. coli by the interaction of the gp20 portal protein with the membrane insertase YidC (Figure 1).

Figure 1. Overview of the major steps in T4 phage head assembly.
(A,B) Assembly initiates via the formation of a protein core anchored to the E. coli inner membrane via the portal, and around which the major capsid protein concomitantly assembles. Only in the absence of gp23 can naked cores be observed.
(A) Electron microscopy of proheads produced by 21- mutants in vivo (upper, thin section) and in vitro (lower). White arrow indicates a central hole in proheads assembled in the absence of the prohead protease (adapted from van Driel, Traub and Showe [6]). (C,D) Proteolytic maturation involves cleavage and removal of scaffold/core proteins as well as the propeptides of the internal and shell proteins, release of the prohead from the inner membrane and semi-expansion of the shell.
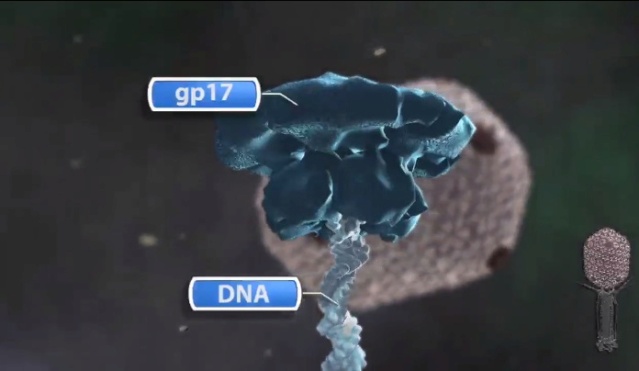
(E,F) Packaging of the genome into the prohead occurs via the action of the main packaging proteins, TerS (gp16) and TerL (gp17).
(C,E). Electron microscopy of thin sections of wild-type T4-infected E. coli (reproduced from Black and Thomas [7]).
(F) Scheme of how DNA packaging in vivo is integrated with late transcription, and DNA replication (reproduced from Black and Peng [8]).
(G) Recombinant Δhoc phage particles after immuno-gold labelling with an anti-Hoc antibody (inset WT phage particle). The visualization of the “gold necklace” provided evidence that the portal structure contained fusion proteins (gp20-Hoc). Confirming the recombinant phenotype was important, as these particles had a central role in refuting the rotary portal packaging model (reproduced from Baumann, Mullaney and Black [9]).
(H) Cryo-electron micrograph of a T4 Alt mutant imaged after the eighth exposure of a dose series of 16.5 el/Å2 per exposure). The bubbles are generated from the internal proteins, which are inferred to be randomly positioned within the DNA, but excluded from a zone of about 100–110 Å directly under the outer shell (reproduced from Wu et al. [10]).
The resulting dodecameric ring structure forms the foundation onto which a protein-only structure, the “core”, forms and acts as a scaffold to direct the correct assembly of the prolate exterior shell. Concomitantly with core formation, or immediately after, hexagons of the major shell protein gp23 and pentagons of gp24 assemble around the core, leading to the formation of a “prohead”. The core contains the proteins gp21, gp22, gp67 and gp68, together with the internal proteins Alt, IP1, IPII and IPIII. Proheads undergo proteolytic maturation by gp21, detach from the membrane, and the capsid shell expands to its final symmetry. DNA is then packaged into the capsid via interactions of the terminase with the portal protein. Once packaging is completed, a neck structure composed of gp13 and gp14 assembles onto the portal, allowing the addition of the tail and tail fibers. These steps in T4 assembly are typically referred to individually (e.g., assembly, maturation, packaging), but it is important to recognize that each is interlinked. Hence, each step is somewhat dependent on the former and/or concomitant steps, but also part of the overall process. 20
Assembly of the capsid shell
Moh Lan Yap (2015): There are several stages in the assembly of the head (Figure below): prohead formation, prohead proteolysis, DNA packaging, expansion of the prolate head and binding of the Hoc and Soc accessory proteins. The T4 prohead starts to assemble with the formation of the membrane-bound initiation complex, which comprises the portal protein gp20 mediated by the chaperone protein gp40. The initiation complex first attaches to the inner side of the host cytoplasmic membrane followed by binding of the prohead core (scaffold) proteins gp21, gp22, gp67, gp68, the initiation proteins IPI, IPII, IPIII and gpalt. Subsequently, the capsid proteins gp23 and gp24 start to form a shell around the core proteins to assemble into a prohead. Both the core and shell assemble concurrently. The major capsid protein gp23 requires two chaperone proteins, the host-encoded GroEL and the phage-encoded gp31, for proper folding. A total of 930 copies of the gp23 and 55 copies of gp24 are needed for the assembly of the prohead. The volume of the prohead is approximately 15–20% smaller than that of the mature head. The gp21 is then self-cleaved and activated to become a protease (T4PPase) in the prohead. The amino terminal peptides of gp23, gp24, IPI, IPII, IPIII and gpalt are cleaved, whereas gp21, gp22, gp67 and gp68 are extensively digested into small peptide fragments. All digested peptide fragments except for peptide II and IV of gp67 and gp22, respectively, are then expelled from the prohead. The cleaved head is released from the membrane into the cytosol and the gp23* and probably gp24* proteins undergo a large conformational rearrangement resulting in the expansion of the prohead. During this process, the capsid’s facets are flattened and the wall of the capsid becomes thinner. The processed prohead is then released from the membrane for DNA packaging. 18
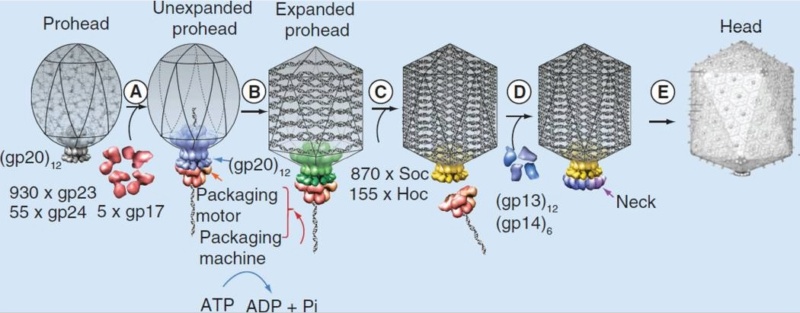
T4 head assembly
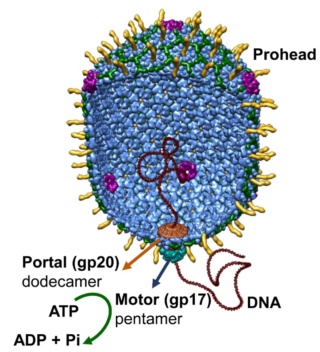
The dodecameric portal vertex acts as an initiator of head assembly. The major capsid protein assembles around a scaffolding core into a prohead. The core is removed by proteolysis to produce an empty unexpanded prohead.
(A) The packaging machine-DNA complex docks on portal and initiates packaging.
(B) The prohead expands after approximately 10–25% of the DNA is packaged.
(C) When the head is full, the packaging machine cuts the DNA and dissociates from the head.
(D) The neck proteins, gp13 and gp14, assemble on the portal to seal the DNA-full head.
(E) The accessory proteins, Hoc and Soc, bind to the head. The head is ready to be joined to the tail.
CrevInfo: Virus: Like DNA in a Hard Plastic Shell (2004): A European team of biophysicists studied the mechanical properties of a virus and found the shell, made of protein, to act like hard plastic. Writing in PNAS,1 they described the coat of a bacteriophage they studied:
I. L. Ivanovska (2004): The protective proteinaceous shells (capsids) of viruses are striking examples of biological materials engineering. These highly regular, self-assembled, nanometer-sized containers are minimalistic in design, but they combine complex passive and active functions. Besides chemical protection, they are involved in the selective packing and the injection of viral genetic material.
The capsids look like oblong, geometric shapes with pointy ends. The DNA is packed inside under pressure, and the coat can withstand indentations of 30%. “The measured Young’s modulus,” they found, “is comparable with that of hard plastic.” They seemed to admire the little cases: the bacteriophage capsid is remarkably dynamic yet resilient and tough enough to easily withstand the known packing pressure of DNA (~60 atmospheres). These capsids, thus, not only provide a chemical shield but also significant mechanical protection for their genetic contents. Viral shells are a remarkable example of nature’s solution to a challenging materials engineering problem: they self-assemble to form strong shells of precisely defined geometry by using a minimum amount of different proteins. 10
The team is looking at these miniaturized packages for inspiration in the burgeoning field of nanotechnology. Here is observational evidence that leads to interesting questions. It shows that living things need to overcome the same kinds of physics problems that engineers face. Yet viruses are not, by definition, alive; they rely on a host for replication. How could such precision bio-nanotechnology evolve? Why do viruses exist? Did they ever have a beneficial role, considering that the vast majority are harmless? We may never be able to explain such things completely, but we can marvel at the biophysics capabilities found in nature, and deduce that such things don’t just happen.
https://www.youtube.com/watch?v=dMvU6Eh4nlw
M.G. Rossmann (2011) How proteins and nucleic acids assemble, often spontaneously, into structurally well-defined three-dimensional objects is an intriguing question. The limited size of the phage genome and the multi-component composition of bacteriophages make them well suited for assembly investigations. Genetic manipulation of phages has made it easy to observe the effects of gene inactivation on protein-protein association, providing information on the sequence of assembly processes.

Packaging: refers to the viral genome placement inside a capsid or an envelope
Most viruses capsid spontaneously self-assembles around the viral genome in the cytoplasm, thus linking the assembly and packaging process.
-<u>Negatives stranded RNA viruses genome is concomitantly encapsidated during replication. The packaging of these viruses occurs prior budding at the plasma membrane.
Icosahedric capsids usually assemble by affinity around the viral genome.
Complex capsids need the help of scaffolding proteins to assemble into empty procapsids. The scaffolding proteins are removed from the empty capsid by maturation events before packaging.
Nucleo-Cytoplasmic Large DNA viruses (NCLDV) contain an internal membrane, consequently they have a complex and regulated assembly mechanism. Poxviridae capsid-like protein is removed before virion maturation and serves as a scaffolding protein.
"Reoviridae" and Totiviridae have their capsid assembled around messenger RNAs that are later replicated into genomic dsRNA, thereby hiding the dsRNA from cellular antiviral sensors.
Large dsDNA bacteriophages, herpesviruses, adenoviruses and microviruses encode a powerful DNA-translocating machinenery that encapsidates a viral genome into a preassembled capsid or procapsid. The packaging machine is often composed of a portal structure, which provides a gate for DNA entry, and an ATP-driven motor. This motor is composed of the large subunit whose ATPase activity fuels DNA translocation, and most frequently, a small subunit that binds to the viral packaging site. DNA cleavage can be coupled to genome packaging.
Dong-Hua Chen (2010): Molecular Mechanism for Capsid Assembly, Scaffolding Protein Release, and Capsid Maturation: To form a functional P22 procapsid, at least four types of proteins are required: coat, scaffolding, ejection, and portal proteins, in which the portal proteins form a unique 12-fold portal complex at a fivefold vertex. The lack of scaffolding proteins results in the failure to incorporate the portal and can lead to incomplete particles.
Based on our density maps, we propose that the formation of a unique portal complex with the requisite scaffolding and coat proteins is likely the key for initiating proper procapsid assembly

Pathway for capsid assembly, scaffolding protein release and capsid maturation.
(A and B) The portal (gray) associates with scaffolding (red) and coat (cyan) proteins to initiate procapsid assembly. The assembly continues with the addition of scaffolding and coat subunits to the growing shell
(C) until the full procapsid is assembled
(D). DNA is then packaged into the procapsid shell through the channel of the portal by the terminase motor. The scaffolding proteins are released by the electrostatic forces from DNA being packaged and exit through the central large openings of hexamers
(E). During the release of scaffolding proteins, conformational changes associated with the maturation transition occur. After capsid expansion and DNA packaging, the tail hub, needle, and tail spikes are attached to the portal to form an infectious virion (magenta)
(F). In B and C, the insets show the side views. In D and F, the insets show one hexamer and the adjacent pentamer rotated from the side view to the end-on view.
Scaffolding proteins may play a critical role during the capsid assembly nucleation because the portal would not be incorporated into the procapsid when the scaffolding proteins are absent. Once nucleated, procapsid assembly proceeds by the addition of scaffolding and coat subunits to the growing shell until the full procapsid is assembled with the proper size and shape as directed by the scaffolding proteins (Fig. 5 C and D). Coat proteins are likely added as monomers or dimers, with the mediation of scaffolding proteins, because our structures show that each scaffolding protein’s C-terminal helix-loop-helix motif interacts with the N arm of the corresponding coat protein. Though the exact timing is not known, ejection proteins are also incorporated and possibly interact with the scaffolding and portal proteins before procapsid assembly is completed. Once the capsid shell forms (Fig. 5D), DNA packaging can begin. In the early stages of DNA packaging, the terminase complex (gp2 and gp3) docks against the portal and hydrolyzes ATP to drive DNA into the procapsid shell through the portal.
Siyu Li ( 2018): More than 50 y ago, Caspar and Klug made the striking observation that the capsids of most spherical viruses display icosahedral order (IO), defined by 12 five-coordinated units (disclinations or pentamers) occupying the vertices of an icosahedron surrounded by hexameric units. A nonspecific template not only selects the radius of the capsid, but also leads to the error-free assembly of protein subunits into capsids with universal icosahedral order(IO). Under many circumstances, small icosahedral capsids assemble spontaneously around their genetic material. Larg double-stranded (ds) RNA or DNA viruses require what we generically denote as the template: scaffolding proteins (SPs) or an inner core. A “generic” template provides a robust path to self-assembly of large shells with IO. For large shells successful assembly into IO requires a nonspecific attractive interaction between protein subunits and a template. 8
Viruses: An Intelligent Design Perspective
“Our study shows that if a messy shell forms because of the high protein concentration or strong attractive interaction, then, as the shell grows larger, the cost of elastic energy becomes so high that several bonds can get broken, resulting in the disassembly and subsequent reassembly of a symmetric shell.”
The paper by Zandi’s team, published in ACS Nano, describes how the shells, even if disordered, can break apart and reassemble into symmetrical forms by physical forces like elastic energy. As the proteins attract,
the key for the disorder–order transition in both en masse and nucleation and growth pathways lies in the strength of elastic energy compared to the other forces in the system including protein–protein interactions and the chemical potential of free subunits. Our findings explain, at least in part, why perfect virions with icosahedral order form under different conditions including physiological ones.
Peter E. Prevelige (2011): Scaffolding proteins mediate, catalyze, and promote proper virus assembly. For many smaller viruses, all the information required for high fidelity assembly can be encoded, or self-contained, entirely in the coat protein subunits. However, larger viruses or small viruses frequently require additional proteins to insure robust assembly. Among those proteins are the “scaffolding” proteins, a class of auxiliary proteins that are present transiently during assembly and are not part of the final structure. Although common, scaffolding proteins are not ubiquitous. As coat proteins (the capsid are called capsid proteins or viral coat proteins (VCP).) perform additional functions, efficient assembly may have become compromised. In some systems, best represented by the parvoviruses, maximal infectivity and/or fitness most likely requires constructing capsids with two or three-coat protein variants. The P = 3 picornavirus capsids represent a more complex example: capsids contain three unique coat proteins. Most coat proteins will form aberrant capsid-like structures if left to their own devices. Therefore, a mechanism to ensure morphogenetic fidelity, vis-à-vis proper capsid size and shape formation is required, and this mechanism includes scaffolding proteins. There is a need to rapidly assemble capsids before cell death and/or programmed cell lysis. This contribution of this factor is most apparent with the microviruses, ostensibly simple viruses that accomplish an almost unimaginable fast replication cycle by employing two-scaffolding proteins.
Phage-encoded molecular chaperones
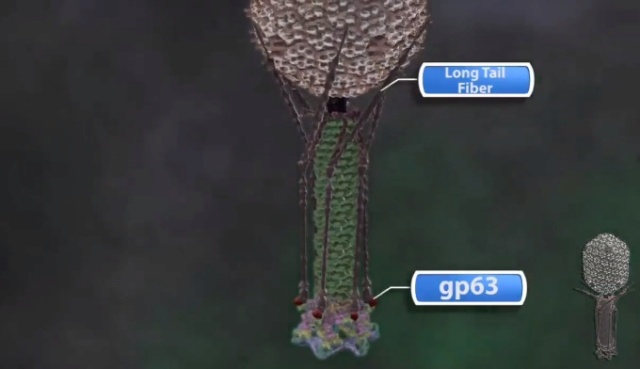
F.Arisaka (2005): A molecular chaperone is a group of proteins which facilitate protein folding in the cell. GroE of E. coli was originally discovered as an essential host factor for the growth of phages. The name “GroE” stems from the fact that the growth of phage lambda requires a host factor and that the factor is required for the proper folding of the major capsid protein gpE. It was subsequently found that phage T4 also requires GroE for its growth and that groE consists of two genes: groEL and groES. Later, it was reported that they are heat shock proteins and molecular chaperones. GroEL, which is now known as Hsp60, together with GroES or Hsp10, is called chaperonin. T4 phage requires GroEL for the folding of its major capsid protein gp23. The fact that T4 does not require GroES had been a mystery, but recent investigations have established that gp31 of phage T4 functions as GroES in the T4-infected cells. Gp31 forms a heptameric ring, as does GroES. The high-resolution structure of gp31 and GroES complexes revealed that their three-dimensional shapes are very similar, except that the former has an extra loop that made the “Anfinsen cage” of the GroEL/gp31 complex larger than that of GroEL/GroES. “Anfinsen cage” denotes a hole inside the chaperonin complex, where the intermediate folding protein is eventually completely folded. The reason that GroEL/gp31 is required but not the Gro EL/GroES system for gp23 folding is unknown, but recent investigation indicated that the GroEL/gp31 complex, but not GroEL/GroES, has the affinity to the folding intermediate of gp23, the major capsid protein. There are a number of other phage-encoded chaperone or chaperone-like proteins. Gp40 is known to be essential for head formation and is a membrane protein, but its precise function is not known. Gp57A is essential for the fiber formation of both long and short tail fibers. This is a small protein with 79 amino acid residues. It is a helix-rich fibrous protein and hexamer appears to be the main oligomer species, which reversibly dissociates into trimers and then monomers. The mechanism of chaperone function is not known. Gp38 is also essential for the fiber formation of the distal half-fibers. Gp51 has long been known to function catalytically for the formation of the hub of the baseplate. On the other hand, gp63 is known to facilitate the binding of tail fibers to the baseplate. In the absence of gp63, the tail fiber attachment is much slower, but the mechanism is not known. Gp63 has an RNA ligase activity, but that activity is not related to the tail attachment function.
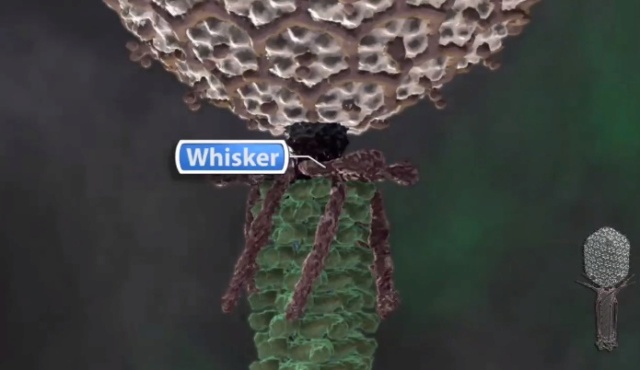
E. Fibritin „whisker… and foldon, an internal molecular chaperone
Another protein that also facilitates the binding of long tail fibers to the baseplate is a structural protein called “whiskers” or “fibritin.” They are trimeric fibrous protein with coiled-coil structure, and six of them stick out from the neck. They are not essential, but without them, the tail fiber attachment takes a much longer time. The distal end of the fibritin, the C-terminal domain, binds to the kink region of the long tail fiber i.e., the junction between the distal and proximal tail fibers and orients the fibers so that the proximal end of the tail fiber approaches the baseplate, which facilitates the binding of the tail fibers to the baseplate. The C-terminal domain, consisting of 27 amino acid residues, is essential for trimerization of the fibritin. It is called “foldon.” When the foldon part was cloned and overexpressed, it spontaneously formed a trimer. Apparently, the foldon domain facilitates the alignment of the other part of the three polypeptide chains and functions as an internal chaperonin. The foldon has been fused to the C-termini of several other proteins including collagen peptides and has been shown to successfully make the homogeneous trimeric collagen helix. 19
Assembly of the baseplate
Moh Lan Yap (2016): Bacteriophage T4 consists of a head for protecting its genome and a sheathed tail for inserting its genome into a host. The tail terminates with a multiprotein baseplate that changes its conformation from a “high-energy” dome-shaped to a “low-energy” star-shaped structure during infection. Although these two structures represent different minima in the total energy landscape of the baseplate assembly, as the dome-shaped structure readily changes to the star-shaped structure when the virus infects a host bacterium, the dome-shaped structure must have more energy than the star-shaped structure. This structure, together with other genetic and structural data, shows why the high-energy baseplate is formed in the presence of the central hub and how the baseplate changes to the low-energy structure, via two steps during infection. Thus, the presence of the central hub is required to initiate the assembly of metastable, high-energy structures. If the high-energy structure is formed and stabilized faster than the low-energy structure, there will be insufficient components to assemble the low-energy structure. Most bacteriophages have a tail. At the distal end of the tail there is usually a baseplate that is decorated by some fibers. The baseplate initiates infection when the tail fibers bind to a host cell. Signals are transmitted from the tail fibers via the baseplate to the tail that then trigger the ejection of the phage genome from the head into the host cell through the tail tube. Two evolutionary-related structures, of pyocin and of the type VI secretion system, are found in bacteria as defense systems to kill competing bacteria. These structures are remarkably similar to the tail baseplate structure of bacteriophages, suggesting that tail baseplate-like structures are effective organelles for infecting bacteria. T4 is a member of the Myoviridae family of bacteriophages. These phages have a sheath around the tail tube that contracts during infection (Figure below).
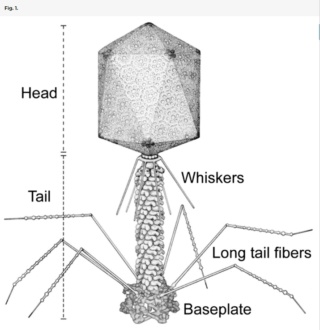
Schematic diagram of bacteriophage T4.
Bacteriophage T4 has a contractile tail and a complex baseplate. Six long-tail fibers are attached to the upper part of the baseplate and six short-tail fibers are folded under the baseplate before infection.
T4 has a complex baseplate that is essential for assuring a highly efficient infection mechanism. After recognition of an Escherichia coli host cell by some of the six long-tail fibers (LTF), the short-tail fibers (STF) that are a part of the baseplate, bind irreversibly to the cell. This process is accompanied by a large conformational change in the baseplate from a “high-energy” dome- to a “low-energy” star-shaped structure, although each of these structures represent an energy minimum in the energy landscape of the baseplate assembly. This change triggers contraction of the tail sheath, driving the tail tube into the outer host cell membrane and further across the periplasmic space to the inner membrane. The genomic DNA is then ejected into the host’s cytoplasm. Hence, the baseplate serves as the nerve center for transmitting signals from the tail fibers to the head for the release of DNA into the host.
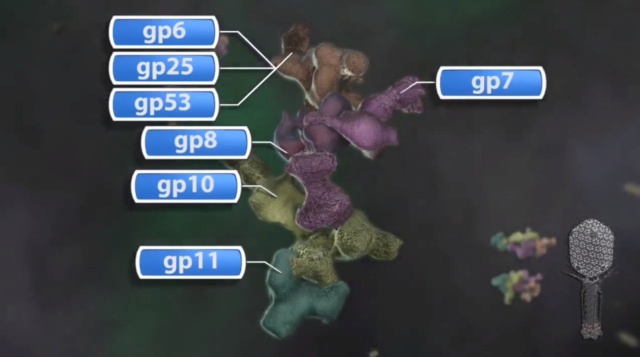
The hexagonal dome-shaped T4 baseplate assembles from six wedges surrounding a central hub. A total of 134 protein subunits from 15 different proteins form the ∼6.5-MDa baseplate. The assembly of a wedge had been shown to follow a strictly ordered sequence. First, an initial complex is formed by a monomer of gp7 and a trimer of gp10, followed sequentially by binding of a dimer of gp8 and a dimer of gp6 to the complex. In the absence of a central hub, at least five proteins (gp7, gp10, gp8, gp6, and gp53) are required for assembly of wedges in vitro into a star-shaped, low-energy baseplate-like structure. Assembly of the high-energy, dome-shaped structure requires the presence of the central hub. However, how the sequential wedge assembly events are regulated remained unknown. In particular, the question remained how the high-energy dome-shaped baseplate could assemble.
How is it possible to assemble the high-energy dome-shaped baseplate initially as opposed to the low-energy star-shaped baseplate? After the individual wedges have assembled, six wedges assemble around the central hub to form the dome-shaped baseplate (Fig. 4B and Movie S2). The present structure has shown the complete structure of gp6, which when fitted into the cryo-EM density of the dome-shaped baseplate shows that gp6 binds tightly around the central hub protein gp27 (Fig. 5A).
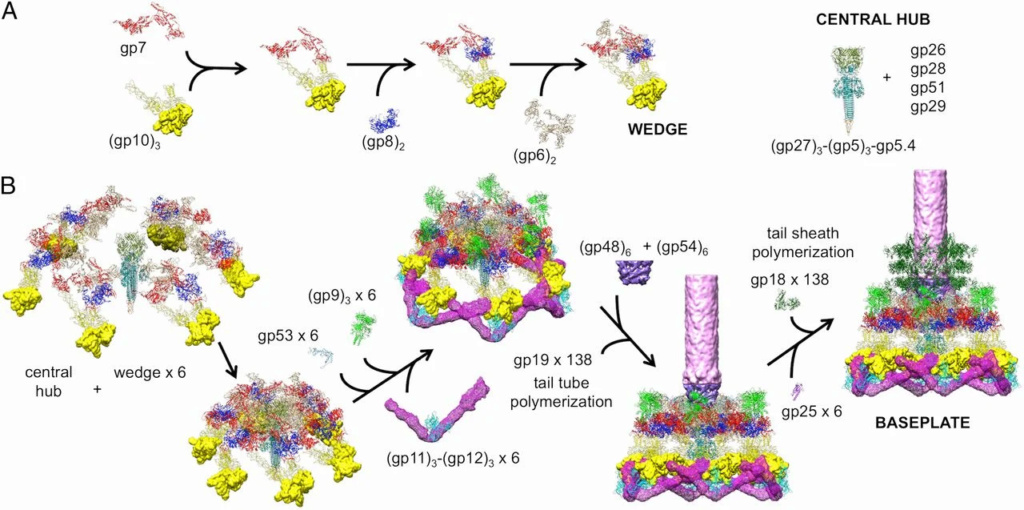
Assembly of a baseplate based on present and earlier results.
(A) Wedge assembly. Gp10, gp8, and gp6 bind sequentially to the gp7 backbone protein. The central hub of the baseplate is assembled independently.
(B) Baseplate and tail assembly. Six wedges assemble around the central hub to form a baseplate. Gp53 binds adjacent wedges together. Subsequently, gp9 and the gp11–gp12 complex bind to the baseplate, further stabilizing the dome-shaped configuration. Then, gp48 and gp54 bind to the top of the central hub and initiate polymerization of the tail tube. Gp25 attaches to the gp48–gp54 complex, initiating polymerization of the tail sheath. For clarity, only three rings of the tail sheath are shown.
Thus, the nucleation of the baseplate assembly from individual wedges is dependent on the interaction of gp6 with gp27. However, in the star-shaped baseplate structure the tight ring of six gp6 dimers around gp27 has expanded and lost contacts with gp27. Thus, the initiation of baseplate assembly is the association of gp6 with gp27, from wedges that are probably assembled as in the dome-shaped baseplate. In the absence of gp27, as was the case for the in vitro-assembled baseplate reported here, gp6 can assemble only as a star-shaped baseplate. Thus, the route to the low-energy star-shaped wedge is via the initially assembled dome-shaped wedge.
Infection Mechanism.
Phage T4 uses the LTFs to recognize a LPS and/or OmpC receptor on the surface of the host cell. Because phages have been observed that have baseplates, which have changed to star-shaped structure, but that still have extended sheaths, presumably, the initial step of infection is the change of the dome-shaped structure to an intermediate structure in which the STFs are released. Subsequently, attachment of the STFs to cell-surface molecules triggers the baseplate to change its conformation to the final star shape, which in turn causes the sheath to contract and the tail tube to puncture the outer cell membrane. Thus, it is likely that the conformational changes of the baseplate occur in two steps—namely, first from the dome-shaped structure to an intermediate star-shaped structure and then from the intermediate structure to the final star-shaped structure.
The LTFs are attached to the baseplate via an adaptor protein gp9. The fit of the gp7 and gp9 structures into the 16-Å resolution cryo-EM density of the dome-shaped baseplate shows that domain III of gp7 interacts with density that represents the N-terminal part of gp9 (Movie S2). Thus, a flexible region of gp9 contacts gp7, allowing the LTFs to have multiple conformations in solution. When the LTFs attach to the cell, the Brownian motion of the phage particle may cause these flexible regions (31) to pull on domain III of gp7. This domain has extensive interactions with the gp8 dimer in the adjacent wedge, and with the C-terminal domain IV of gp10 in the same wedge. The rigid gp8 dimer in turn interacts extensively with the C-terminal domain VI of gp7, which is attached to the N-terminal domain I of gp10. Therefore, the interaction of gp9 with domain III of gp7 is likely to disturb interactions among gp7, gp8, and gp10 at the vertices of the dome-shaped baseplate and trigger the periphery of the baseplate to change to a star-shaped conformation (Movie S3). As a result, the STFs attached to gp10 might unfold from the baseplate and point toward the cell surface with their C-terminal domain, whereas the N-terminal part of the STFs remain attached to domain II of gp10, after release of gp11 (Fig. 6). During the conformational change from dome-shaped to a star-shaped intermediate structure, the gp11 molecules, attached to domain III of the gp10 molecules, move closer to the phage head and push on the end of the LTFs close to the baseplate. The interaction of gp11 with the LTFs probably causes the parts of the LTFs closest to the baseplate to rotate with respect to the baseplate as seen in the star-shaped baseplate. Thus, LTFs attached to the cell surface are used as levers to bring the phage closer to the cell surface. On completion of these movements, the baseplate will have the structure of the intermediate state that has a star-shaped periphery (gp7, gp8, gp9, gp10, gp11, and gp12), although the inner ring of gp6-gp53-gp25 molecules probably remains attached to the hub (Fig. 6).
Assembly of the tail
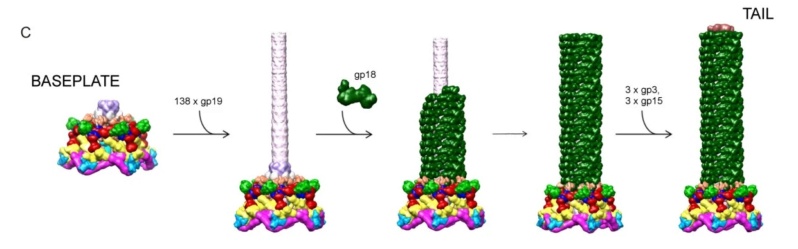
Assembly of the tail. Rows A, B and C show the assembly of the wedge; the baseplate and the tail tube with the sheath, respectively.
M. G. Rossmann et.al (2010): The fully assembled baseplate is a prerequisite for the assembly of the tail tube and the sheath both of which polymerize into the extended structure using the baseplate as the assembly nucleus (Figure 2). The baseplate is comprised of about 140 polypeptide chains of at least 16 proteins. Two gene products, gp51 and gp57A, are required for assembly, but are not present in the final particle. The baseplate has sixfold symmetry and is assembled from 6 wedges and the central hub. The only known enzyme associated with the phage particle, the T4 tail lysozyme, is a baseplate component. It is encoded by gene 5 (gp5). 17
1. http://www.nature.com/ncomms/2015/150706/ncomms8548/full/ncomms8548.html
full text pdf: http://www.nature.com/ncomms/2015/150706/ncomms8548/pdf/ncomms8548.pdf
2. Amy D. Migliori: Evidence for an electrostatic mechanism of force generation by the bacteriophage T4 DNA packaging motor 17 June 2014
3. http://mmbr.asm.org/content/75/3/423.full.pdf
4. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1208326/
5. http://www.uncommondescent.com/intelligent-design/in-other-words-phylogenetic-reconstruction-is-sheer-fantasy/
6. http://physicsworld.com/cws/article/news/2014/jun/25/relaxation-and-repulsion-helps-viruses-pack-dna
7. Dong-Hua Chen: Structural basis for scaffolding-mediated assembly and maturation of a dsDNA virus October 22, 2010
8.Siyu Li: Why large icosahedral viruses need scaffolding proteins October 9, 2018
9. Peter E. Prevelige: Building the Machines: Scaffolding Protein Functions During Bacteriophage Morphogenesis 01 January 2011
10. I. L. Ivanovska: Bacteriophage capsids: Tough nanoshells with complex elastic properties May 7, 2004
11. Lei Sun et al.,: Cryo-EM structure of the bacteriophage T4 portal protein assembly at near-atomic resolution 06 July 2015
12. Zhihong Zhang: A Promiscuous DNA Packaging Machine from Bacteriophage T4 2011 Feb 15
13. John E. Johnson: The Structure of an Infectious P22 Virion Shows the Signal for Headful DNA Packaging (2006)
14. Peter E. Prevelige: Building the Machines: Scaffolding Protein Functions During Bacteriophage Morphogenesis 01 January 2011
15. Moh Lan Yap: Role of bacteriophage T4 baseplate in regulating assembly and infection February 29, 2016
16. M. G. Rossmann et.al: Structure and morphogenesis of bacteriophage T4 November 2003
17. M. G. Rossmann et.al: Morphogenesis of the T4 tail and tail fibers 03 December 2010
18. Moh Lan Yap: Structure and function of bacteriophage T4 2015 Aug 1
19. Fumio Arisaka: Assembly and infection process of bacteriophage T4 03 November 2005
20. Andreas Kuhn: The Beauty of Bacteriophage T4 Research: Lindsay W. Black and the T4 Head Assembly 28 March 2022