The Transport of Proteins into Mitochondria
Summary
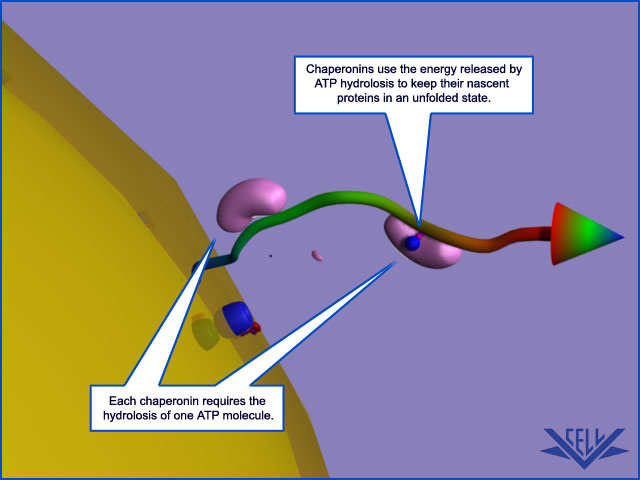
Although mitochondria have their own genetic systems, they produce only a small proportion of their own proteins. Instead, the two organelles import most of their proteins from the cytosol. Proteins are transported in an unfolded state across both outer and inner membranes simultaneously into the matrix space. Both ATP hydrolysis and a membrane potential across the inner membrane drive translocation into mitochondria. Chaperone proteins of the cytosolic hsp70 family maintain the precursor proteins in an unfolded state, and a second set of hsp70 proteins in the matrix space or stroma pulls the polypeptide chain into the organelle. Only proteins that contain a specific signal sequence are translocated. The signal sequence can either be located at the N-terminus and cleaved off after import or be internal and retained. Transport into the inner membrane sometimes uses a second, hydrophobic signal sequence that is unmasked when the first signal sequence is removed.
Proteomic studies have revealed that mitochondria contain more than 1,000 different proteins, more than 99% of which are encoded by nuclear DNA. Thus, these proteins are synthesized as precursors on cytosolic ribosomes, specifically targeted to mitochondria and sorted into one of the four mitochondrial subcompartments. Various protein translocation machineries of the inner and outer mitochondrial membranes accomplish the complicated task of selective protein sorting into and across lipid bilayers. 3
Mitochondria are double-membrane-enclosed organelles. They specialize in ATP synthesis, using energy derived from electron transport and oxidative phosphorylation in mitochondria and from photosynthesis in chloroplasts . Although both organelles contain their own DNA, ribosomes, and other components required for protein synthesis, most of their proteins are encoded in the cell nucleus and imported from the cytosol.
If the endosymbiosis theory were true, would the proteins not keep being encoded and produced inside mitochondria ?
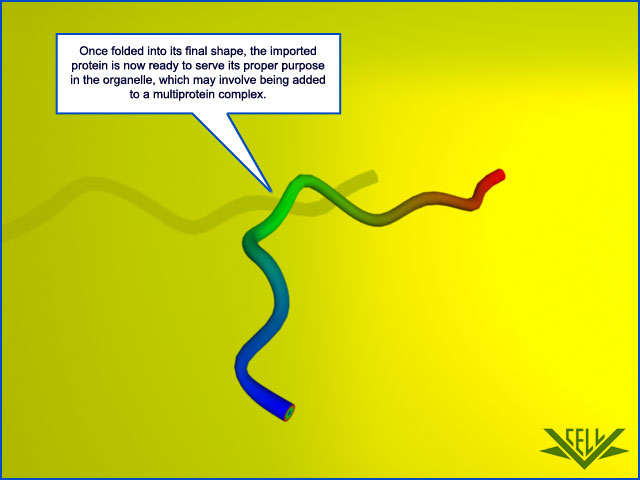
Each imported protein must reach the particular organelle subcompartment in which it functions. There are different subcompartments in mitochondria : the internal matrix space and the intermembrane space, which is continuous with the cristae space. These compartments are formed by the two concentric mitochondrial membranes: the inner membrane, which encloses the matrix space and forms extensive invaginations called cristae, and the outer membrane, which is in contact with the cytosol. Protein complexes provide boundaries at the junctions where the cristae invaginate and divide the inner membrane into two domains: one inner membrane domain surrounds the cristae space, and the otherdomain abuts the outer membrane. Chloroplasts also have an outer and inner membrane, which enclose an intermembrane space, and the stroma, which is the chloroplast equivalent of the mitochondrial matrix space . They have an additional subcompartment, the thylakoid space, which is surrounded by the thylakoid membrane. The thylakoid membrane derives from the inner membrane during plastid development and is pinched off to become discontinuous with it. Each of the subcompartments in mitochondria and chloroplasts contains a distinct set of proteins. New mitochondria and chloroplasts are produced by the growth of preexisting organelles, followed by fission . The growth depends mainly on theimport of proteins from the cytosol. The imported proteins must be transported across a number of membranes in succession and end up in the appropriate place. The process of protein movement across membranes is calledprotein translocation. This section explains how it occurs.
Translocation into Mitochondria Depends on Signal Sequences and Protein Translocators
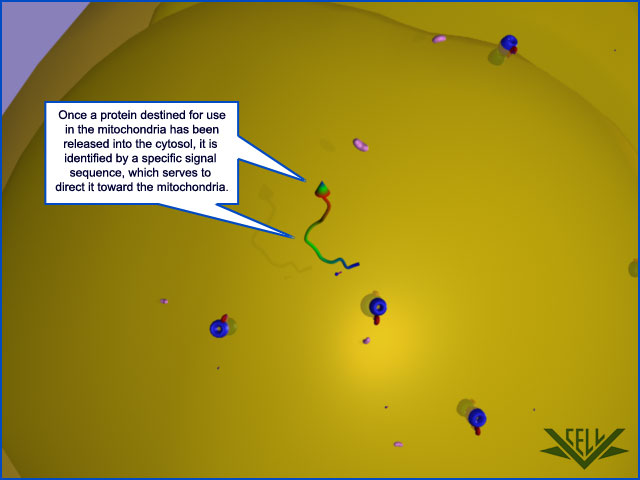
Proteins imported into mitochondria are usually taken up from the cytosol within seconds or minutes of their release from ribosomes. Thus, in contrast to protein translocation into the ER, which often takes place simultaneously with translation by a ribosome docked on the rough ER membrane , mitochondrial proteins are first fully synthesized as mitochondrial precursor proteins in the cytosol and then translocated into mitochondria by a post-translational mechanism. One or more signal sequences direct all mitochondrial precursor proteins to their appropriate mitochondrial subcompartment. Many proteins entering the matrix space contain a signal sequence at their N-terminus that a signal peptidase rapidly removes after import. Other imported proteins, including all outer membrane and many inner membrane and intermembrane space proteins, have internal signal sequences that are not removed. The signal sequences are both necessary and sufficient for the import and correct localization of the proteins: when genetic engineering techniques are used to link these signals to a cytosolic protein, the signals direct the protein to the correct mitochondrial subcompartment.
Question: had this signalling not have to be fully developed and functioning right from the beginning ?
The signal sequences that direct precursor proteins into the mitochondrial matrix space are best understood. They all form an amphiphilic α helix, in which positively charged residues cluster on one side of the helix, while uncharged hydrophobic residues cluster on the opposite side. Specific receptor proteins that initiate protein translocation recognize this configuration rather than the precise amino acid sequence of the signal sequence

A signal sequence for mitochondrial protein import. Cytochrome oxidase is a large multiprotein complex located in the inner mitochondrial membrane, where it functions as the terminal enzyme in the electron-transport chain. (A) The first 18 amino acids of the precursor to subunit IV of this enzyme serve as a signal sequence for import of the subunit into the mitochondrion. (B) When the signal sequence is folded as an α helix, the positively charged amino acids (red) are clustered on one face of the helix, while the nonpolar ones (green) are clustered primarily on the opposite face. Uncharged polar amino acids are shaded orange; nitrogen atoms on the side chains of Arg and Gln are colored blue. Signal sequences that direct proteins into the matrix space always have the potential to form such an amphiphilic α helix, which is recognized by specific receptor proteins on the mitochondrial surface. (C) The structure of a signal sequence (of alcohol dehydrogenase, another mitochondrial matrix enzyme), bound to an import receptor (gray), as determined by nuclear magnetic resonance. The amphiphilic α helix binds with its hydrophobic face to a hydrophobic groove in the receptor
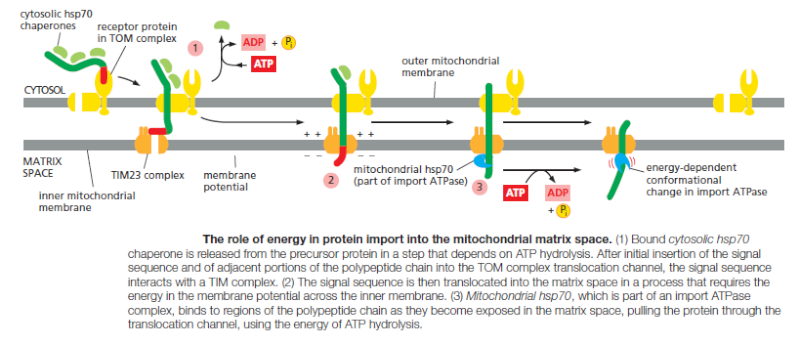
Multisubunit protein complexes that function as protein translocators mediate protein movement across mitochondrial membranes. The TOM complex transfers proteins across the outer membrane, and two TIM complexes (TIM23 and TIM22) transfer proteins across the inner membrane


These complexes contain some components that act as receptors for mitochondrial precursor proteins, and other components that form the translocation channels. The TOM complex is required for the import of all nucleus-encoded mitochondrial proteins.
This is another reason why the endosymbiosis theory makes no sense. The mechanism of synthesis of all proteins INSIDE the bacteria would change, and a new mechanism where the proteins are synthesised in the nucleus would have to emerge. And so the import mechanism with all related proteins and signalling mechanisms. Not a easy task.
It initially transports their signal sequences into the intermembrane space and helps to insert transmembrane proteins into the outer membrane. β-barrel proteins, which are particularly abundant in the outer membrane, are then passed on to an additional translocator, the SAM complex, which helps them to fold properly in the outer membrane. The TIM23 complex transports some soluble proteins into the matrix space and helps to insert transmembrane proteins into the inner membrane. The TIM22 complex mediates the insertion of a subclass of inner membrane proteins, including the transporter that moves ADP, ATP, and phosphate in and out of mitochondria.
This strongly suggests that the whole mechanism, and so the proteins, had to emerge all at once, and be fully operational right from the start. How did the TIM23 complex " learn " how to transport the soluble proteins into the matrix space, and help to insert transmembrane proteins into the inner membrane ? trial and error ?
Yet another protein translocator in the inner mitochondrial membrane, the OXA complex, mediates the insertion of those inner membrane proteins that are synthesized within mitochondria. It also helps to insert some imported inner membrane proteins that are initially transported into the matrix space by the other complexes
Only 13 proteins necessary for a mitochondrion are actually coded in mitochondrial DNA. The vast majority of proteins destined for the mitochondria are encoded in the nucleus and synthesized in the cytoplasm. These are tagged by an N-terminal signal sequence. Following transport through the cytosol from the nucleus, the signal sequence is recognized by a receptor protein in the transporter outer membrane (TOM) complex. 1
This reinforces the evidence that the endosymbiosis theory is false.

Thats what precisely was to be expected: some wishy washy pseudo scientific speculation about " proto " mitochondrion, and a primitive setup of protein translocases. The problem with this line of reasoning is always, its baseless speculation , where words like " might be ", " it has been suggested ", " would have ", " demonstrates the feasability " are nothing else than assertions without a shred of evidence, just in order to provide a framework, where the evolutionary paradigm might fit. Thats not science. Thats pseudo science at its best.
1) https://en.wikipedia.org/wiki/TIM/TOM_complex
2) https://books.google.com.br/books?id=4D-5BAAAQBAJ&pg=PA25&lpg=PA25&dq=tom+complex,+endosymbiosis&source=bl&ots=O0go8qur_w&sig=2vhcA2_y2XHIgbu2Q9DhSiFnKRw&hl=en&sa=X&ved=0CCIQ6AEwAGoVChMIuLeQwKadxwIVxyCQCh1eXQ9Z#v=onepage&q&f=false
3) https://www.biochemie.uni-freiburg.de/ag/vanderLaan/research?set_language=en
Summary
Although mitochondria have their own genetic systems, they produce only a small proportion of their own proteins. Instead, the two organelles import most of their proteins from the cytosol. Proteins are transported in an unfolded state across both outer and inner membranes simultaneously into the matrix space. Both ATP hydrolysis and a membrane potential across the inner membrane drive translocation into mitochondria. Chaperone proteins of the cytosolic hsp70 family maintain the precursor proteins in an unfolded state, and a second set of hsp70 proteins in the matrix space or stroma pulls the polypeptide chain into the organelle. Only proteins that contain a specific signal sequence are translocated. The signal sequence can either be located at the N-terminus and cleaved off after import or be internal and retained. Transport into the inner membrane sometimes uses a second, hydrophobic signal sequence that is unmasked when the first signal sequence is removed.
Proteomic studies have revealed that mitochondria contain more than 1,000 different proteins, more than 99% of which are encoded by nuclear DNA. Thus, these proteins are synthesized as precursors on cytosolic ribosomes, specifically targeted to mitochondria and sorted into one of the four mitochondrial subcompartments. Various protein translocation machineries of the inner and outer mitochondrial membranes accomplish the complicated task of selective protein sorting into and across lipid bilayers. 3
Mitochondria are double-membrane-enclosed organelles. They specialize in ATP synthesis, using energy derived from electron transport and oxidative phosphorylation in mitochondria and from photosynthesis in chloroplasts . Although both organelles contain their own DNA, ribosomes, and other components required for protein synthesis, most of their proteins are encoded in the cell nucleus and imported from the cytosol.
If the endosymbiosis theory were true, would the proteins not keep being encoded and produced inside mitochondria ?
Each imported protein must reach the particular organelle subcompartment in which it functions. There are different subcompartments in mitochondria : the internal matrix space and the intermembrane space, which is continuous with the cristae space. These compartments are formed by the two concentric mitochondrial membranes: the inner membrane, which encloses the matrix space and forms extensive invaginations called cristae, and the outer membrane, which is in contact with the cytosol. Protein complexes provide boundaries at the junctions where the cristae invaginate and divide the inner membrane into two domains: one inner membrane domain surrounds the cristae space, and the otherdomain abuts the outer membrane. Chloroplasts also have an outer and inner membrane, which enclose an intermembrane space, and the stroma, which is the chloroplast equivalent of the mitochondrial matrix space . They have an additional subcompartment, the thylakoid space, which is surrounded by the thylakoid membrane. The thylakoid membrane derives from the inner membrane during plastid development and is pinched off to become discontinuous with it. Each of the subcompartments in mitochondria and chloroplasts contains a distinct set of proteins. New mitochondria and chloroplasts are produced by the growth of preexisting organelles, followed by fission . The growth depends mainly on theimport of proteins from the cytosol. The imported proteins must be transported across a number of membranes in succession and end up in the appropriate place. The process of protein movement across membranes is calledprotein translocation. This section explains how it occurs.
Translocation into Mitochondria Depends on Signal Sequences and Protein Translocators
Proteins imported into mitochondria are usually taken up from the cytosol within seconds or minutes of their release from ribosomes. Thus, in contrast to protein translocation into the ER, which often takes place simultaneously with translation by a ribosome docked on the rough ER membrane , mitochondrial proteins are first fully synthesized as mitochondrial precursor proteins in the cytosol and then translocated into mitochondria by a post-translational mechanism. One or more signal sequences direct all mitochondrial precursor proteins to their appropriate mitochondrial subcompartment. Many proteins entering the matrix space contain a signal sequence at their N-terminus that a signal peptidase rapidly removes after import. Other imported proteins, including all outer membrane and many inner membrane and intermembrane space proteins, have internal signal sequences that are not removed. The signal sequences are both necessary and sufficient for the import and correct localization of the proteins: when genetic engineering techniques are used to link these signals to a cytosolic protein, the signals direct the protein to the correct mitochondrial subcompartment.
Question: had this signalling not have to be fully developed and functioning right from the beginning ?
The signal sequences that direct precursor proteins into the mitochondrial matrix space are best understood. They all form an amphiphilic α helix, in which positively charged residues cluster on one side of the helix, while uncharged hydrophobic residues cluster on the opposite side. Specific receptor proteins that initiate protein translocation recognize this configuration rather than the precise amino acid sequence of the signal sequence

A signal sequence for mitochondrial protein import. Cytochrome oxidase is a large multiprotein complex located in the inner mitochondrial membrane, where it functions as the terminal enzyme in the electron-transport chain. (A) The first 18 amino acids of the precursor to subunit IV of this enzyme serve as a signal sequence for import of the subunit into the mitochondrion. (B) When the signal sequence is folded as an α helix, the positively charged amino acids (red) are clustered on one face of the helix, while the nonpolar ones (green) are clustered primarily on the opposite face. Uncharged polar amino acids are shaded orange; nitrogen atoms on the side chains of Arg and Gln are colored blue. Signal sequences that direct proteins into the matrix space always have the potential to form such an amphiphilic α helix, which is recognized by specific receptor proteins on the mitochondrial surface. (C) The structure of a signal sequence (of alcohol dehydrogenase, another mitochondrial matrix enzyme), bound to an import receptor (gray), as determined by nuclear magnetic resonance. The amphiphilic α helix binds with its hydrophobic face to a hydrophobic groove in the receptor
Multisubunit protein complexes that function as protein translocators mediate protein movement across mitochondrial membranes. The TOM complex transfers proteins across the outer membrane, and two TIM complexes (TIM23 and TIM22) transfer proteins across the inner membrane


These complexes contain some components that act as receptors for mitochondrial precursor proteins, and other components that form the translocation channels. The TOM complex is required for the import of all nucleus-encoded mitochondrial proteins.
This is another reason why the endosymbiosis theory makes no sense. The mechanism of synthesis of all proteins INSIDE the bacteria would change, and a new mechanism where the proteins are synthesised in the nucleus would have to emerge. And so the import mechanism with all related proteins and signalling mechanisms. Not a easy task.
It initially transports their signal sequences into the intermembrane space and helps to insert transmembrane proteins into the outer membrane. β-barrel proteins, which are particularly abundant in the outer membrane, are then passed on to an additional translocator, the SAM complex, which helps them to fold properly in the outer membrane. The TIM23 complex transports some soluble proteins into the matrix space and helps to insert transmembrane proteins into the inner membrane. The TIM22 complex mediates the insertion of a subclass of inner membrane proteins, including the transporter that moves ADP, ATP, and phosphate in and out of mitochondria.
This strongly suggests that the whole mechanism, and so the proteins, had to emerge all at once, and be fully operational right from the start. How did the TIM23 complex " learn " how to transport the soluble proteins into the matrix space, and help to insert transmembrane proteins into the inner membrane ? trial and error ?
Yet another protein translocator in the inner mitochondrial membrane, the OXA complex, mediates the insertion of those inner membrane proteins that are synthesized within mitochondria. It also helps to insert some imported inner membrane proteins that are initially transported into the matrix space by the other complexes
Only 13 proteins necessary for a mitochondrion are actually coded in mitochondrial DNA. The vast majority of proteins destined for the mitochondria are encoded in the nucleus and synthesized in the cytoplasm. These are tagged by an N-terminal signal sequence. Following transport through the cytosol from the nucleus, the signal sequence is recognized by a receptor protein in the transporter outer membrane (TOM) complex. 1
This reinforces the evidence that the endosymbiosis theory is false.

Thats what precisely was to be expected: some wishy washy pseudo scientific speculation about " proto " mitochondrion, and a primitive setup of protein translocases. The problem with this line of reasoning is always, its baseless speculation , where words like " might be ", " it has been suggested ", " would have ", " demonstrates the feasability " are nothing else than assertions without a shred of evidence, just in order to provide a framework, where the evolutionary paradigm might fit. Thats not science. Thats pseudo science at its best.
1) https://en.wikipedia.org/wiki/TIM/TOM_complex
2) https://books.google.com.br/books?id=4D-5BAAAQBAJ&pg=PA25&lpg=PA25&dq=tom+complex,+endosymbiosis&source=bl&ots=O0go8qur_w&sig=2vhcA2_y2XHIgbu2Q9DhSiFnKRw&hl=en&sa=X&ved=0CCIQ6AEwAGoVChMIuLeQwKadxwIVxyCQCh1eXQ9Z#v=onepage&q&f=false
3) https://www.biochemie.uni-freiburg.de/ag/vanderLaan/research?set_language=en
Last edited by Admin on Mon Aug 10, 2015 5:00 pm; edited 8 times in total