The dramatic cellular morphology of the Microvillar Cytoskeleton
https://reasonandscience.catsboard.com/t2141-the-dramatic-cellular-morphology-of-the-microvillar-cytoskeleton
Thousands of microvilli form a surface structure of some epithelial cells, such as the small intestines. 3 Microvilli are formed as cell extensions from the plasma membrane surface. They function as the primary surface of nutrient absorption in the gastrointestinal tract. Because of this vital function, the microvillar membrane is packed with enzymes that aid in the breakdown of complex nutrients into simpler compounds that are more easily absorbed. For example, enzymes that digest carbohydrates called glycosidases are present at high concentrations on the surface of enterocyte microvilli. Thus, microvilli not only increase the cellular surface area for absorption, they also increase the number of digestive enzymes that can be present on the cell surface.
Scientists have found there’s a lot more going on in the tips of these projections. 5 Science Daily reported on work at Vanderbilt that showed myosin is concentrated in the tips and appears actively involved in shedding membrane material at the tips.
Myosin is of the family of Kinseins motor proteins, which walk like post officers delivering cargo inside the cell

This process of vesicle formation and detachment may inject metabolic enzymes into the passing food material, as well as protect the lining of the intestine from invaders. It’s all done with motors: myosin 1a,“a protein with the potential to generate force and move cargo around in cells.” Matthew Tyska figured that there must be a reason these force-generating motors are concentrated in the microvilli, and sure enough, he found them at work: “It’s a little machine that can shed membrane from the tips,” he said. This could give a whole new dimension to the term bowel movement. Now his group is seeing if a similar mechanism operates in other cellular projections, like the hair cells of the inner ear.
Molecular motors may speed nutrient processing 4
Matthew Tyska, Ph.D., recalls being intrigued, from the first day of his postdoctoral fellowship in 1999, with a nearly 30-year-old photograph. It was an electron micrograph that showed the internal structures of an intestinal cell microvillus, a finger-like protrusion on the cell surface. Microvilli are common features on the epithelial cells that line the body's cavities.
At the time, Tyska knew that the core bundle traveling up the center of the microvillus was an array of the structural protein actin, and that the ladder-like "rungs" connecting the actin bundle to the cell membrane were composed of the motor protein myosin-1a. This myosin, though related to the myosin involved in muscle cell contraction, was thought to serve a purely structural role. "The textbook thinking for decades was that microvilli serve as a passive scaffold, a way to amplify the membrane surface area," said Tyska, assistant professor of Cell and Developmental Biology at Vanderbilt University.
In the intestines, an expanded cell surface increases the space for nutrient-processing enzymes and transporters, offering greater capacity for nutrient handling. But it didn't make sense to Tyska that a motor protein - a protein with the potential to generate force and move cargo around in cells - would play a passive structural role. "When I looked at that image, the near crystalline arrangement reminded me of actin and myosin in a muscle fiber," Tyska said. "I kept returning to the same question: why would the microvillus have this beautiful structure packed with motor proteins. The concentration of myosin motors in a single microvillus is very high; there's serious force-generating potential there."
Tyska and Russell McConnell, a student in his laboratory, tested the idea that these motor proteins are more than molecular glue binding the cell membrane to the actin bundle" The investigators purified the intestinal "brush border" - the layer of densely packed microvilli - from the intestines of rats or mice, and added ATP, the chemical fuel for myosin-1a. Through the microscope, they watched the cell membrane move toward the tips of the microvilli and pop off the ends in the form of vesicles, tiny bubble-like packets.
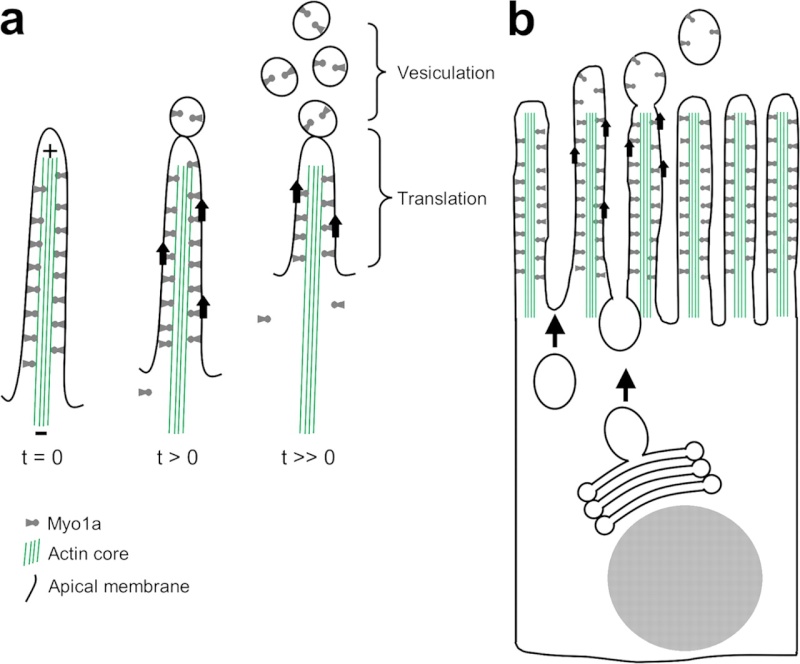
membrane shedding model. (A) In isolated BBs, Myo1a motor activity powers the translation of apical membrane toward the actin bundle plus-end at the microvillus tip. Upon reaching the tip, membrane vesiculates and is shed from the BB. (B) In the context of an intact enterocyte, Myo1a-powered membrane translation may underlie the regulated turnover of BB membrane. New membrane is continually delivered to the terminal web, while older membrane is translated along microvillar actin bundles and eventually released from tips, into the intestinal lumen.
Their findings, reported in the May 21 Journal of Cell Biology with one of their images featured on the issue cover, have implications for nutrient processing and other aspects of gastrointestinal physiology. Tyska is excited about the group's unexpected discovery. "What we're showing is that the microvillus is more than just a scaffold to increase the amount of cell membrane," Tyska said. "It's a little machine that can shed membrane from the tips." The team confirmed that myosin-1a is the motor that moves membrane up the microvillus. Brush borders isolated from knockout mice lacking the myosin-1a gene shed membrane at only five percent of the level of brush borders from wild-type animals.
The investigators are working now to understand why intestinal cells might launch vesicles from their microvilli. They know from ongoing vesicle sorting and mass spectrometry studies that the vesicles contain nutrient-processing enzymes and transporters, like the microvillar membrane. "One idea is that these vesicles operate remotely to speed nutrient processing, before the nutrients even get to the brush border to be absorbed by the (intestinal epithelial cell)," Tyska said.
The team is also exploring other possibilities for the role of membrane shedding: that it offers protection against microbes and pathogens by expelling them from the surface before they can enter the cell; that it provides a mechanism for altering the composition of the microvillar surface to handle changes in "what comes down the pipe;" and that it serves a role in cell-cell communication by launching vesicles that contain signaling proteins. Tyska and his team also plan to explore whether myosin-1a is serving a similar membrane-moving role in its other known location: the hair cells of the inner ear, and if other microvilli also use myosin motors to jettison vesicles from their tips.
The dramatic cellular morphology of the Microvillar Cytoskeleton 1
In order to facilitate exchange between the extracellular milieu and the intracellular cytosol, the absorptive epithelium of the gastrointestinal tract and the renal proximal convoluted tubule have developed a highly specialized apical membrane, termed the brush border, which provides a ~30-fold increase in surface area over a similarly sized planar surface. This brush border is composed of a hexagonal array of uniformly sized, finger-like projections, called microvilli.
this large macromolecular complex is primarily composed of only six protein components, which were later identified as actin, fimbrin, villin, brush border myosin (Myo1A), calmodulin, and a non-erythrocytic spectrin (Reviewed by Mooseker [1]). Briefly, ~19 actin filaments, cross-linked by fimbrin and villin, serve as the “core bundle,” which is laterally tethered to the adjacent membrane through myosin1A:calmodulin cross-bridges.
Although individual microvilli are amotile, persistent, uniformly sized structures, their underlying cytoskeleton is highly dynamic. The entire macromolecular complex is turned over every ~20 minutes
Remarkable Symmetry of the Microvillus and Brush Border
The majority of both soluble and membrane bound proteins form homo- and heteromeric macromolecular complexes, which confer genetic, allosteric, and several physicochemical advantages over a similarly large structure formed from a single peptide chain (reviewed in ). However, the brush border is an extreme example of a symmetrical apparatus in both the paracrystalline order exhibited by the actin core bundle and the immense size of the complex, which encompasses the entire apical membrane of the enterocyte and therefore the vast majority of the small intestine.

Figure above. The fimbrin cross-linked core bundle
A. Ribbon diagram of fimbrin (blue) cross-linking two actin filaments (orange surfaces). B. When viewed down the long axis of the bundle, fimbrin cross-links exist between every adjacent pair of microfilaments. C. A side view, rotated 90° with respect to B, displays the three distinct vertical levels (d, e, and f) of fimbrin cross-links corresponding to the three different directions of fimbrin cross-links (D, E, and F, respectively). The slight irregularity in the vertical orientation of d, e, and f is a consequence of cross-linking actin's 13/6 symmetry within a hexagonal lattice.
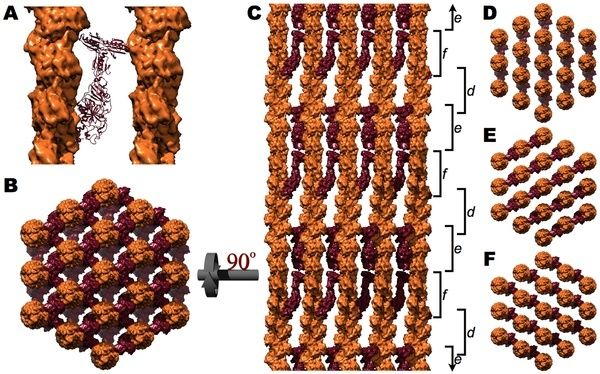
Figure above. The villin cross-linked core bundle .
A. Ribbon diagram of villin (maroon) cross-linking two actin filaments (orange surfaces). B. When viewed down the long axis of the bundle, a villin cross-link exists between every adjacent pair of microfilaments. C. A side view, rotated 90° with respect to B, displays the three distinct vertical levels (d, e, and f) of villin cross-links corresponding to the three different directions of the villin cross-links (D, E, and F, respectively). The slight irregularity in the vertical orientation of d, e, and f is a consequence of cross-linking actin's 13/6 symmetry within a hexagonal lattice.

Figure above. Structure of the myosin 1A, calmodulin cross-bridges.
A. Ribbon diagram of brush border myosin (green) [78] in a near rigor conformation with its three associated calmodulin light chains (purple) [38] bound to actin (orange surface). B. When viewed down the long axis, two to three Myo1A:CaM cross-bridges radially extend out from each outer filament in the core bundle. C, D. When viewed from the side one may appreciate the barber-pole like motif of Myo1A:Calmodulin cross-bridges about the actin core bundle (depicted as orange molecular surfaces in C and as a transparent orange cylinder in D).

Figure above. The microvillar cytoskeleton in situ.
A. When our modeled cytoskeleton is enveloped with a membrane of appropriate dimensions, it is apparent that the proposed model of the microvillar cytoskeleton precisely spans the ~100 nm diameter required to establish a circumferential connection to the membrane. The color irregularity of membrane is an “Artistic License” employed to emphasize the importance of lipid raft domains within the brush border membrane . B. The relative size of the microvillar cytoskeleton with respect to the brush border may be appreciated when the microvilli are hexagonally arranged as they exist within the brush border . C. A schematic representation of the terminal web, in which multiple spectrin tetramers (α-spectrin, brown; β-spectrin, yellow) cross-link and hexagonally arrange the microvillar core bundles (depicted here as orange molecular surfaces) as they enter the apical cytoplasm. Within the microvillus, the barbed end of actin is positioned towards the apex, and therefore the vertical orientation of actin in A and B is reversed relative to how actin is traditionally viewed (pointed end up; Figures 1–4).
Here a great video:
https://vimeo.com/123920402
 2
2
1) http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0009406
2) http://www.uni-koeln.de/med-fak/biochemie/staff/rivero/06_project03.shtml
3) https://en.wikipedia.org/wiki/Microvillus
4) http://www.eurekalert.org/pub_releases/2007-05/vumc-mmm053007.php
5) http://creationsafaris.com/crev200705.htm
https://reasonandscience.catsboard.com/t2141-the-dramatic-cellular-morphology-of-the-microvillar-cytoskeleton
Thousands of microvilli form a surface structure of some epithelial cells, such as the small intestines. 3 Microvilli are formed as cell extensions from the plasma membrane surface. They function as the primary surface of nutrient absorption in the gastrointestinal tract. Because of this vital function, the microvillar membrane is packed with enzymes that aid in the breakdown of complex nutrients into simpler compounds that are more easily absorbed. For example, enzymes that digest carbohydrates called glycosidases are present at high concentrations on the surface of enterocyte microvilli. Thus, microvilli not only increase the cellular surface area for absorption, they also increase the number of digestive enzymes that can be present on the cell surface.
Scientists have found there’s a lot more going on in the tips of these projections. 5 Science Daily reported on work at Vanderbilt that showed myosin is concentrated in the tips and appears actively involved in shedding membrane material at the tips.
Myosin is of the family of Kinseins motor proteins, which walk like post officers delivering cargo inside the cell

This process of vesicle formation and detachment may inject metabolic enzymes into the passing food material, as well as protect the lining of the intestine from invaders. It’s all done with motors: myosin 1a,“a protein with the potential to generate force and move cargo around in cells.” Matthew Tyska figured that there must be a reason these force-generating motors are concentrated in the microvilli, and sure enough, he found them at work: “It’s a little machine that can shed membrane from the tips,” he said. This could give a whole new dimension to the term bowel movement. Now his group is seeing if a similar mechanism operates in other cellular projections, like the hair cells of the inner ear.
Molecular motors may speed nutrient processing 4
Matthew Tyska, Ph.D., recalls being intrigued, from the first day of his postdoctoral fellowship in 1999, with a nearly 30-year-old photograph. It was an electron micrograph that showed the internal structures of an intestinal cell microvillus, a finger-like protrusion on the cell surface. Microvilli are common features on the epithelial cells that line the body's cavities.
At the time, Tyska knew that the core bundle traveling up the center of the microvillus was an array of the structural protein actin, and that the ladder-like "rungs" connecting the actin bundle to the cell membrane were composed of the motor protein myosin-1a. This myosin, though related to the myosin involved in muscle cell contraction, was thought to serve a purely structural role. "The textbook thinking for decades was that microvilli serve as a passive scaffold, a way to amplify the membrane surface area," said Tyska, assistant professor of Cell and Developmental Biology at Vanderbilt University.
In the intestines, an expanded cell surface increases the space for nutrient-processing enzymes and transporters, offering greater capacity for nutrient handling. But it didn't make sense to Tyska that a motor protein - a protein with the potential to generate force and move cargo around in cells - would play a passive structural role. "When I looked at that image, the near crystalline arrangement reminded me of actin and myosin in a muscle fiber," Tyska said. "I kept returning to the same question: why would the microvillus have this beautiful structure packed with motor proteins. The concentration of myosin motors in a single microvillus is very high; there's serious force-generating potential there."
Tyska and Russell McConnell, a student in his laboratory, tested the idea that these motor proteins are more than molecular glue binding the cell membrane to the actin bundle" The investigators purified the intestinal "brush border" - the layer of densely packed microvilli - from the intestines of rats or mice, and added ATP, the chemical fuel for myosin-1a. Through the microscope, they watched the cell membrane move toward the tips of the microvilli and pop off the ends in the form of vesicles, tiny bubble-like packets.

membrane shedding model. (A) In isolated BBs, Myo1a motor activity powers the translation of apical membrane toward the actin bundle plus-end at the microvillus tip. Upon reaching the tip, membrane vesiculates and is shed from the BB. (B) In the context of an intact enterocyte, Myo1a-powered membrane translation may underlie the regulated turnover of BB membrane. New membrane is continually delivered to the terminal web, while older membrane is translated along microvillar actin bundles and eventually released from tips, into the intestinal lumen.
Their findings, reported in the May 21 Journal of Cell Biology with one of their images featured on the issue cover, have implications for nutrient processing and other aspects of gastrointestinal physiology. Tyska is excited about the group's unexpected discovery. "What we're showing is that the microvillus is more than just a scaffold to increase the amount of cell membrane," Tyska said. "It's a little machine that can shed membrane from the tips." The team confirmed that myosin-1a is the motor that moves membrane up the microvillus. Brush borders isolated from knockout mice lacking the myosin-1a gene shed membrane at only five percent of the level of brush borders from wild-type animals.
The investigators are working now to understand why intestinal cells might launch vesicles from their microvilli. They know from ongoing vesicle sorting and mass spectrometry studies that the vesicles contain nutrient-processing enzymes and transporters, like the microvillar membrane. "One idea is that these vesicles operate remotely to speed nutrient processing, before the nutrients even get to the brush border to be absorbed by the (intestinal epithelial cell)," Tyska said.
The team is also exploring other possibilities for the role of membrane shedding: that it offers protection against microbes and pathogens by expelling them from the surface before they can enter the cell; that it provides a mechanism for altering the composition of the microvillar surface to handle changes in "what comes down the pipe;" and that it serves a role in cell-cell communication by launching vesicles that contain signaling proteins. Tyska and his team also plan to explore whether myosin-1a is serving a similar membrane-moving role in its other known location: the hair cells of the inner ear, and if other microvilli also use myosin motors to jettison vesicles from their tips.
The dramatic cellular morphology of the Microvillar Cytoskeleton 1
In order to facilitate exchange between the extracellular milieu and the intracellular cytosol, the absorptive epithelium of the gastrointestinal tract and the renal proximal convoluted tubule have developed a highly specialized apical membrane, termed the brush border, which provides a ~30-fold increase in surface area over a similarly sized planar surface. This brush border is composed of a hexagonal array of uniformly sized, finger-like projections, called microvilli.
this large macromolecular complex is primarily composed of only six protein components, which were later identified as actin, fimbrin, villin, brush border myosin (Myo1A), calmodulin, and a non-erythrocytic spectrin (Reviewed by Mooseker [1]). Briefly, ~19 actin filaments, cross-linked by fimbrin and villin, serve as the “core bundle,” which is laterally tethered to the adjacent membrane through myosin1A:calmodulin cross-bridges.
Although individual microvilli are amotile, persistent, uniformly sized structures, their underlying cytoskeleton is highly dynamic. The entire macromolecular complex is turned over every ~20 minutes
Remarkable Symmetry of the Microvillus and Brush Border
The majority of both soluble and membrane bound proteins form homo- and heteromeric macromolecular complexes, which confer genetic, allosteric, and several physicochemical advantages over a similarly large structure formed from a single peptide chain (reviewed in ). However, the brush border is an extreme example of a symmetrical apparatus in both the paracrystalline order exhibited by the actin core bundle and the immense size of the complex, which encompasses the entire apical membrane of the enterocyte and therefore the vast majority of the small intestine.

Figure above. The fimbrin cross-linked core bundle
A. Ribbon diagram of fimbrin (blue) cross-linking two actin filaments (orange surfaces). B. When viewed down the long axis of the bundle, fimbrin cross-links exist between every adjacent pair of microfilaments. C. A side view, rotated 90° with respect to B, displays the three distinct vertical levels (d, e, and f) of fimbrin cross-links corresponding to the three different directions of fimbrin cross-links (D, E, and F, respectively). The slight irregularity in the vertical orientation of d, e, and f is a consequence of cross-linking actin's 13/6 symmetry within a hexagonal lattice.

Figure above. The villin cross-linked core bundle .
A. Ribbon diagram of villin (maroon) cross-linking two actin filaments (orange surfaces). B. When viewed down the long axis of the bundle, a villin cross-link exists between every adjacent pair of microfilaments. C. A side view, rotated 90° with respect to B, displays the three distinct vertical levels (d, e, and f) of villin cross-links corresponding to the three different directions of the villin cross-links (D, E, and F, respectively). The slight irregularity in the vertical orientation of d, e, and f is a consequence of cross-linking actin's 13/6 symmetry within a hexagonal lattice.

Figure above. Structure of the myosin 1A, calmodulin cross-bridges.
A. Ribbon diagram of brush border myosin (green) [78] in a near rigor conformation with its three associated calmodulin light chains (purple) [38] bound to actin (orange surface). B. When viewed down the long axis, two to three Myo1A:CaM cross-bridges radially extend out from each outer filament in the core bundle. C, D. When viewed from the side one may appreciate the barber-pole like motif of Myo1A:Calmodulin cross-bridges about the actin core bundle (depicted as orange molecular surfaces in C and as a transparent orange cylinder in D).

Figure above. The microvillar cytoskeleton in situ.
A. When our modeled cytoskeleton is enveloped with a membrane of appropriate dimensions, it is apparent that the proposed model of the microvillar cytoskeleton precisely spans the ~100 nm diameter required to establish a circumferential connection to the membrane. The color irregularity of membrane is an “Artistic License” employed to emphasize the importance of lipid raft domains within the brush border membrane . B. The relative size of the microvillar cytoskeleton with respect to the brush border may be appreciated when the microvilli are hexagonally arranged as they exist within the brush border . C. A schematic representation of the terminal web, in which multiple spectrin tetramers (α-spectrin, brown; β-spectrin, yellow) cross-link and hexagonally arrange the microvillar core bundles (depicted here as orange molecular surfaces) as they enter the apical cytoplasm. Within the microvillus, the barbed end of actin is positioned towards the apex, and therefore the vertical orientation of actin in A and B is reversed relative to how actin is traditionally viewed (pointed end up; Figures 1–4).
Here a great video:
https://vimeo.com/123920402
 2
21) http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0009406
2) http://www.uni-koeln.de/med-fak/biochemie/staff/rivero/06_project03.shtml
3) https://en.wikipedia.org/wiki/Microvillus
4) http://www.eurekalert.org/pub_releases/2007-05/vumc-mmm053007.php
5) http://creationsafaris.com/crev200705.htm


