HOW CELLS OBTAIN ENERGY FROM FOOD
The constant supply of energy that cells need to generate and maintain the biological order that keeps them alive comes from the chemical-bond energy in food molecules. The proteins, lipids, and polysaccharides that make up most of the food we eat must be broken down into smaller molecules before our cells can use them—either as a source of energy or as building blocks for other molecules. Enzymatic digestion breaks down the large polymeric molecules in food into their monomer subunits— proteins into amino acids, polysaccharides into sugars, and fats into fatty acids and glycerol. After digestion, the small organic molecules derived from food enter the cytosol of cells, where their gradual oxidation begins. Sugars are particularly important fuel molecules, and they are oxidized in small controlled steps to carbon dioxide (CO2) and water
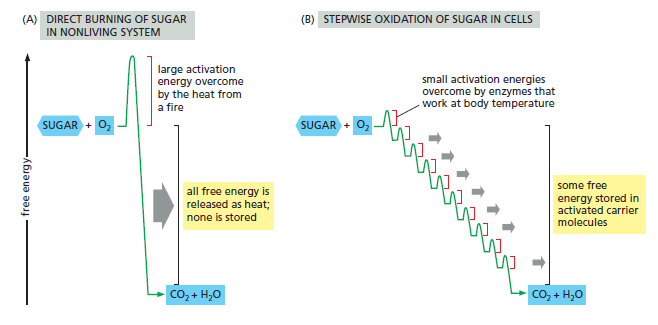
Schematic representation of the controlled stepwise oxidation of sugar in a cell, compared with ordinary burning. (A) If the sugar were oxidized to CO2 and H2O in a single step, it would release an amount of energy much larger than could be captured for useful purposes. (B) In the cell, enzymes catalyze oxidation via a series of small steps in which free energy is transferred in conveniently sized packets to carrier molecules—most often ATP and NADH. At each step, an enzyme controls the reaction by reducing the activation-energy barrier that has to be surmounted before the specific reaction can occur. The total free energy released is exactly the same in (A) and (B).
In this section, we trace the major steps in the breakdown, or catabolism, of sugars and show how they produce ATP, NADH, and other activated carrier molecules in animal cells. A very similar pathway also operates in plants, fungi, and many bacteria. As we shall see, the oxidation of fatty acids is equally important for cells. Other molecules, such as proteins, can also serve as energy sources when they are funneled through appropriate enzymatic pathways.
Glycolysis Is a Central ATP-Producing Pathway
The major process for oxidizing sugars is the sequence of reactions known as glycolysis—from the Greek glukus, “sweet,” and lusis, “rupture.” Glycolysis produces ATP without the involvement of molecular oxygen (O2 gas). It occurs in the cytosol of most cells, including many anaerobic microorganisms. Glycolysis emerged early in the history of life. During glycolysis, a glucose molecule with six carbon atoms is converted into two molecules of pyruvate, each of which contains three carbon atoms. For each glucose molecule, two molecules of ATP are hydrolyzed to provide energy to drive the early steps, but four molecules of ATP are produced in the later steps. At the end of glycolysis, there is consequently a net gain of two molecules of ATP for each glucose molecule broken down. Two molecules of the activated carrier NADH are also produced. The glycolytic pathway is outlined below:
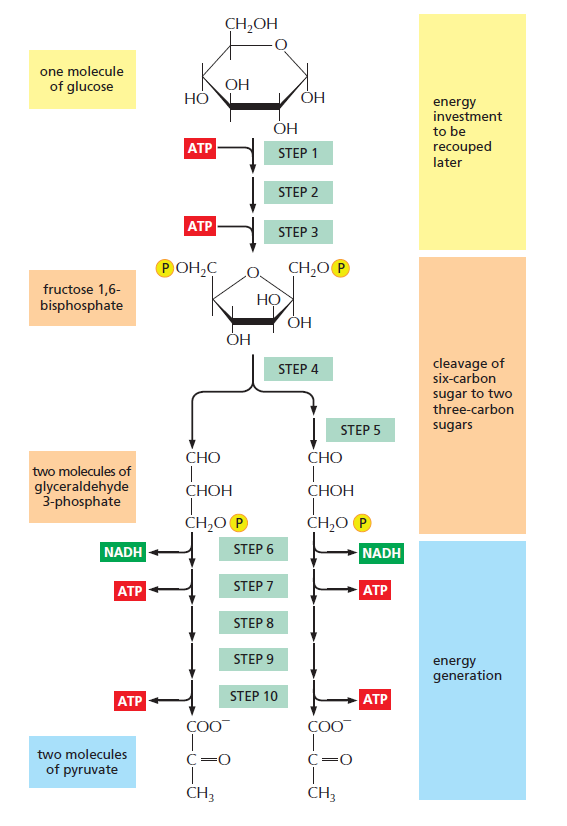
An outline of glycolysis. Each of the 10 steps shown is catalyzed by a different enzyme. Note that step 4 cleaves a six-carbon sugar into two threecarbon sugars, so that the number of molecules at every stage after this doubles. As indicated, step 6 begins the energy generation phase of glycolysis. Because two molecules of ATP are hydrolyzed in the early, energy-investment phase, glycolysis results in the net synthesis of 2 ATP and 2 NADH molecules per molecule of glucose
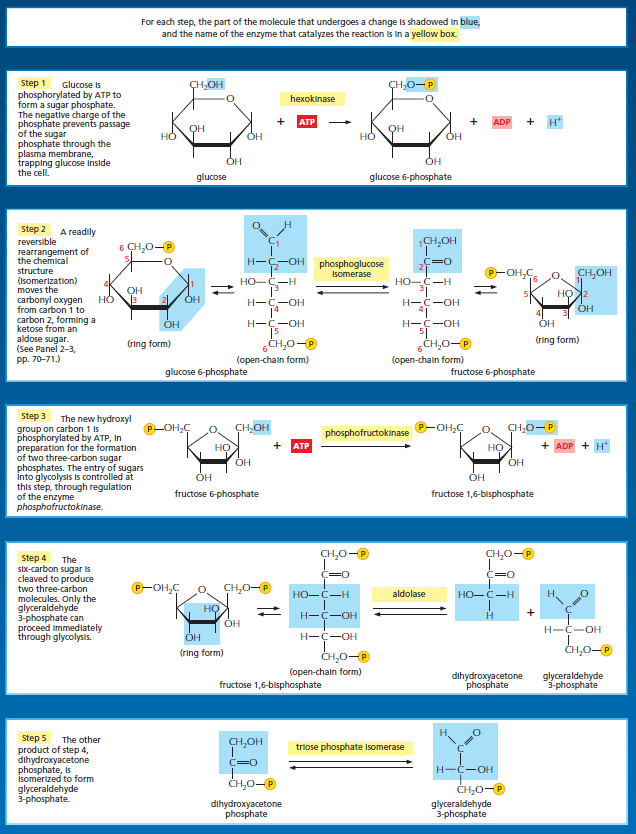
Glycolysis involves a sequence of 10 separate reactions, each producing a different sugar intermediate and each catalyzed by a different enzyme. Like most enzymes, these have names ending in ase—such as isomerase and dehydrogenase—to indicate the type of reaction they catalyze.Although no molecular oxygen is used in glycolysis, oxidation occurs, in that electrons are removed by NAD+ (producing NADH) from some of the carbons derived from the glucose molecule. The stepwise nature of the process releases the energy of oxidation in small packets, so that much of it can be stored in activated carrier molecules rather than all of it being released as heat. Thus, some of the energy released by oxidation drives the direct synthesis of ATP molecules from ADP and Pi, and some remains with the electrons in the electron carrier NADH. Two molecules of NADH are formed per molecule of glucose in the course of glycolysis. In aerobic organisms, these NADH molecules donate their electrons to the electron-transport chain, and the NAD+ formed from the NADH is used again for glycolysis.
Fermentations Produce ATP in the Absence of Oxygen
For most animal and plant cells, glycolysis is only a prelude to the final stage of the breakdown of food molecules. In these cells, the pyruvate formed by glycolysis is rapidly transported into the mitochondria, where it is converted into CO2 plus acetyl CoA, whose acetyl group is then completely oxidized to CO2 and H2O. In contrast, for many anaerobic organisms—which do not utilize molecular oxygen and can grow and divide without it—glycolysis is the principal source of the cell’s ATP. Certain animal tissues, such as skeletal muscle, can also continue to function when molecular oxygen is limited. In these anaerobic conditions, the pyruvate and the NADH electrons stay in the cytosol. The pyruvate is converted into products excreted from the cell—for example, into ethanol and CO2 in the yeasts used in brewing and breadmaking, or into lactate in muscle. In this process, the NADH gives up its electrons and is converted back into NAD+. This regeneration of NAD+ is required to maintain the reactions of glycolysis. Energy-yielding pathways like these, in which organic molecules both donate and accept electrons (and which are often, as in these cases, anaerobic), are called fermentations.

Studies of the commercially important fermentations carried out by yeasts inspired much of early biochemistry. Work in the nineteenth century led in 1896 to the then startling recognition that these processes could be studied outside living organisms, in cell extracts. This revolutionary discovery eventually made it possible to dissect out and study each of the individual reactions in the fermentation process. The piecing together of the complete glycolytic pathway in the 1930s was a major triumph of biochemistry, and it was quickly followed by the recognition of the central role of ATP in cell processes.
Glycolysis Illustrates How Enzymes Couple Oxidation to Energy Storage
The formation of ATP during glycolysis provides a particularly clear demonstration of how enzymes couple energetically unfavorable reactions with favorable ones, thereby driving the many chemical reactions that make life possible. Two central reactions in glycolysis (steps 6 and 7) convert the three-carbon sugar intermediate glyceraldehyde 3-phosphate (an aldehyde) into 3-phosphoglycerate (a carboxylic acid), thus oxidizing an aldehyde group to a carboxylic acid group. The overall reaction releases enough free energy to convert a molecule of ADP to ATP and to transfer two electrons (and a proton) from the aldehyde to NAD+ to form NADH, while still liberating enough heat to the environment to make the overall reaction energetically favorable .

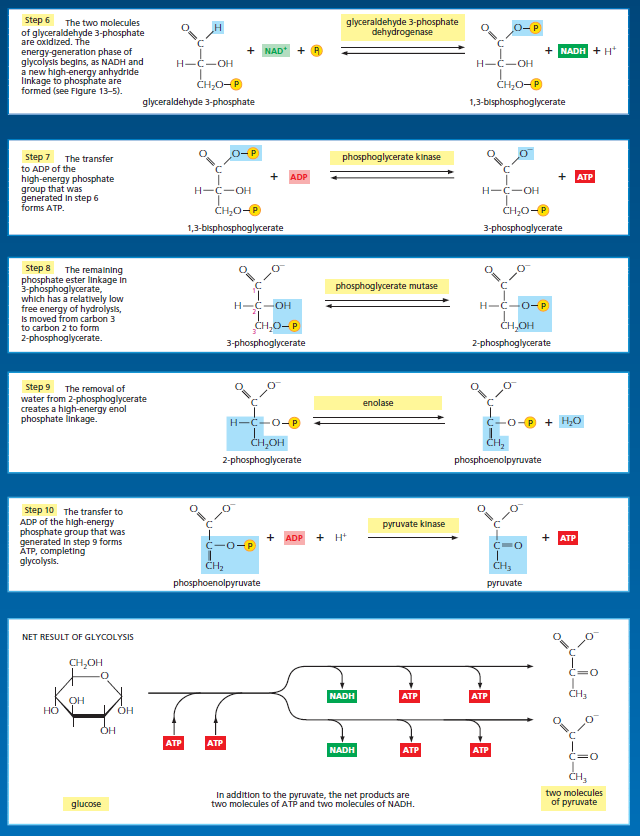
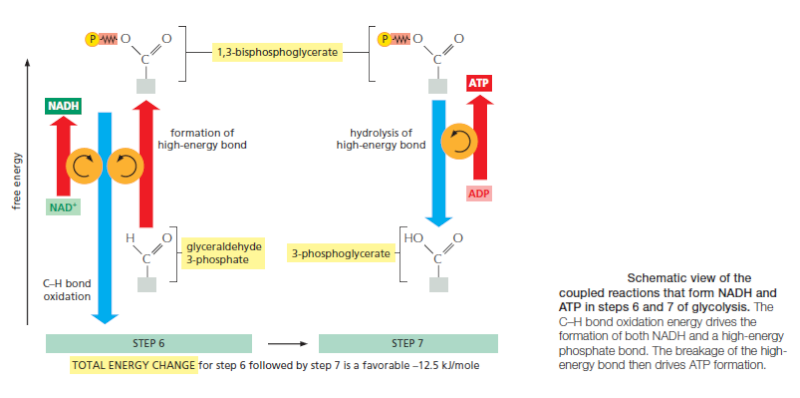
Energy storage in steps 6 and 7 of glycolysis. (A) In step 6, the enzyme glyceraldehyde 3-phosphate dehydrogenase couples the energetically favorable oxidation of an aldehyde to the energetically unfavorable formation of a high-energy phosphate bond. At the same time, it enables energy to be stored in NADH. The formation of the high-energy phosphate bond is driven by the oxidation reaction, and the enzyme thereby acts like the “paddle wheel” coupler, see below :
In step 7, the newly formed high-energy phosphate bond in 1,3-bisphosphoglycerate is transferred to ADP, forming a molecule of ATP and leaving a free carboxylic acid group on the oxidized sugar. The part of the molecule that undergoes a change is shaded in blue; the rest of the molecule remains unchanged throughout all these reactions. (B) Summary of the overall chemical change produced by reactions 6 and 7.
The figure above outlines this remarkable feat of energy harvesting. The chemical reactions are precisely guided by two enzymes to which the sugar intermediates are tightly bound. The first enzyme (glyceraldehyde 3-phosphate dehydrogenase) forms a short-lived covalent bond to the aldehyde through a reactive –SH group on the enzyme, and catalyzes its oxidation by NAD+ in this attached state. The reactive enzyme–substrate bond is then displaced by an inorganic phosphate ion to produce a high-energy phosphate intermediate, which is released from the enzyme. This intermediate binds to the second enzyme (phosphoglycerate kinase), which catalyzes the energetically favorable transfer of the high-energy phosphate just created to ADP, forming ATP and completing the process of oxidizing an aldehyde to a carboxylic acid. Note that the C–H bond oxidation energy in step 6 drives the formation of both NADH and a high-energy phosphate bond. The breakage of the high-energy bond then drives ATP formation. We have shown this particular oxidation process in some detail because it provides a clear example of enzyme-mediated energy storage through coupled reactions
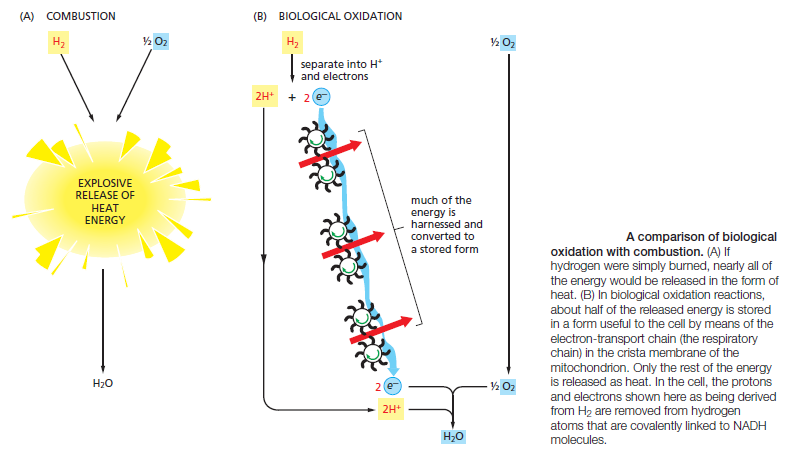
The constant supply of energy that cells need to generate and maintain the biological order that keeps them alive comes from the chemical-bond energy in food molecules. The proteins, lipids, and polysaccharides that make up most of the food we eat must be broken down into smaller molecules before our cells can use them—either as a source of energy or as building blocks for other molecules. Enzymatic digestion breaks down the large polymeric molecules in food into their monomer subunits— proteins into amino acids, polysaccharides into sugars, and fats into fatty acids and glycerol. After digestion, the small organic molecules derived from food enter the cytosol of cells, where their gradual oxidation begins. Sugars are particularly important fuel molecules, and they are oxidized in small controlled steps to carbon dioxide (CO2) and water

Schematic representation of the controlled stepwise oxidation of sugar in a cell, compared with ordinary burning. (A) If the sugar were oxidized to CO2 and H2O in a single step, it would release an amount of energy much larger than could be captured for useful purposes. (B) In the cell, enzymes catalyze oxidation via a series of small steps in which free energy is transferred in conveniently sized packets to carrier molecules—most often ATP and NADH. At each step, an enzyme controls the reaction by reducing the activation-energy barrier that has to be surmounted before the specific reaction can occur. The total free energy released is exactly the same in (A) and (B).
In this section, we trace the major steps in the breakdown, or catabolism, of sugars and show how they produce ATP, NADH, and other activated carrier molecules in animal cells. A very similar pathway also operates in plants, fungi, and many bacteria. As we shall see, the oxidation of fatty acids is equally important for cells. Other molecules, such as proteins, can also serve as energy sources when they are funneled through appropriate enzymatic pathways.
Glycolysis Is a Central ATP-Producing Pathway
The major process for oxidizing sugars is the sequence of reactions known as glycolysis—from the Greek glukus, “sweet,” and lusis, “rupture.” Glycolysis produces ATP without the involvement of molecular oxygen (O2 gas). It occurs in the cytosol of most cells, including many anaerobic microorganisms. Glycolysis emerged early in the history of life. During glycolysis, a glucose molecule with six carbon atoms is converted into two molecules of pyruvate, each of which contains three carbon atoms. For each glucose molecule, two molecules of ATP are hydrolyzed to provide energy to drive the early steps, but four molecules of ATP are produced in the later steps. At the end of glycolysis, there is consequently a net gain of two molecules of ATP for each glucose molecule broken down. Two molecules of the activated carrier NADH are also produced. The glycolytic pathway is outlined below:

An outline of glycolysis. Each of the 10 steps shown is catalyzed by a different enzyme. Note that step 4 cleaves a six-carbon sugar into two threecarbon sugars, so that the number of molecules at every stage after this doubles. As indicated, step 6 begins the energy generation phase of glycolysis. Because two molecules of ATP are hydrolyzed in the early, energy-investment phase, glycolysis results in the net synthesis of 2 ATP and 2 NADH molecules per molecule of glucose


Glycolysis involves a sequence of 10 separate reactions, each producing a different sugar intermediate and each catalyzed by a different enzyme. Like most enzymes, these have names ending in ase—such as isomerase and dehydrogenase—to indicate the type of reaction they catalyze.Although no molecular oxygen is used in glycolysis, oxidation occurs, in that electrons are removed by NAD+ (producing NADH) from some of the carbons derived from the glucose molecule. The stepwise nature of the process releases the energy of oxidation in small packets, so that much of it can be stored in activated carrier molecules rather than all of it being released as heat. Thus, some of the energy released by oxidation drives the direct synthesis of ATP molecules from ADP and Pi, and some remains with the electrons in the electron carrier NADH. Two molecules of NADH are formed per molecule of glucose in the course of glycolysis. In aerobic organisms, these NADH molecules donate their electrons to the electron-transport chain, and the NAD+ formed from the NADH is used again for glycolysis.
Fermentations Produce ATP in the Absence of Oxygen
For most animal and plant cells, glycolysis is only a prelude to the final stage of the breakdown of food molecules. In these cells, the pyruvate formed by glycolysis is rapidly transported into the mitochondria, where it is converted into CO2 plus acetyl CoA, whose acetyl group is then completely oxidized to CO2 and H2O. In contrast, for many anaerobic organisms—which do not utilize molecular oxygen and can grow and divide without it—glycolysis is the principal source of the cell’s ATP. Certain animal tissues, such as skeletal muscle, can also continue to function when molecular oxygen is limited. In these anaerobic conditions, the pyruvate and the NADH electrons stay in the cytosol. The pyruvate is converted into products excreted from the cell—for example, into ethanol and CO2 in the yeasts used in brewing and breadmaking, or into lactate in muscle. In this process, the NADH gives up its electrons and is converted back into NAD+. This regeneration of NAD+ is required to maintain the reactions of glycolysis. Energy-yielding pathways like these, in which organic molecules both donate and accept electrons (and which are often, as in these cases, anaerobic), are called fermentations.

Studies of the commercially important fermentations carried out by yeasts inspired much of early biochemistry. Work in the nineteenth century led in 1896 to the then startling recognition that these processes could be studied outside living organisms, in cell extracts. This revolutionary discovery eventually made it possible to dissect out and study each of the individual reactions in the fermentation process. The piecing together of the complete glycolytic pathway in the 1930s was a major triumph of biochemistry, and it was quickly followed by the recognition of the central role of ATP in cell processes.
Glycolysis Illustrates How Enzymes Couple Oxidation to Energy Storage
The formation of ATP during glycolysis provides a particularly clear demonstration of how enzymes couple energetically unfavorable reactions with favorable ones, thereby driving the many chemical reactions that make life possible. Two central reactions in glycolysis (steps 6 and 7) convert the three-carbon sugar intermediate glyceraldehyde 3-phosphate (an aldehyde) into 3-phosphoglycerate (a carboxylic acid), thus oxidizing an aldehyde group to a carboxylic acid group. The overall reaction releases enough free energy to convert a molecule of ADP to ATP and to transfer two electrons (and a proton) from the aldehyde to NAD+ to form NADH, while still liberating enough heat to the environment to make the overall reaction energetically favorable .

Energy storage in steps 6 and 7 of glycolysis. (A) In step 6, the enzyme glyceraldehyde 3-phosphate dehydrogenase couples the energetically favorable oxidation of an aldehyde to the energetically unfavorable formation of a high-energy phosphate bond. At the same time, it enables energy to be stored in NADH. The formation of the high-energy phosphate bond is driven by the oxidation reaction, and the enzyme thereby acts like the “paddle wheel” coupler, see below :

In step 7, the newly formed high-energy phosphate bond in 1,3-bisphosphoglycerate is transferred to ADP, forming a molecule of ATP and leaving a free carboxylic acid group on the oxidized sugar. The part of the molecule that undergoes a change is shaded in blue; the rest of the molecule remains unchanged throughout all these reactions. (B) Summary of the overall chemical change produced by reactions 6 and 7.
The figure above outlines this remarkable feat of energy harvesting. The chemical reactions are precisely guided by two enzymes to which the sugar intermediates are tightly bound. The first enzyme (glyceraldehyde 3-phosphate dehydrogenase) forms a short-lived covalent bond to the aldehyde through a reactive –SH group on the enzyme, and catalyzes its oxidation by NAD+ in this attached state. The reactive enzyme–substrate bond is then displaced by an inorganic phosphate ion to produce a high-energy phosphate intermediate, which is released from the enzyme. This intermediate binds to the second enzyme (phosphoglycerate kinase), which catalyzes the energetically favorable transfer of the high-energy phosphate just created to ADP, forming ATP and completing the process of oxidizing an aldehyde to a carboxylic acid. Note that the C–H bond oxidation energy in step 6 drives the formation of both NADH and a high-energy phosphate bond. The breakage of the high-energy bond then drives ATP formation. We have shown this particular oxidation process in some detail because it provides a clear example of enzyme-mediated energy storage through coupled reactions


