11.10. Complex IV: Cytochrome c oxidaseCytochrome c oxidase, also known as Complex IV plays a pivotal role in the final step of the electron transport chain. The significance of Complex IV represents the culmination of a sophisticated energy production system that has allowed organisms to thrive in oxygen-rich environments. At its core, cytochrome c oxidase's primary function is to catalyze the reduction of oxygen to water, coupled with the translocation of protons across the membrane. This process is essential for the generation of the electrochemical gradient that drives ATP synthesis, the universal energy currency of cells. The complex structure of cytochrome c oxidase, comprising multiple subunits and cofactors, allows for the efficient coupling of electron transfer to proton pumping, a critical feature for energy production in living organisms. What's particularly noteworthy is the existence of alternative terminal oxidases in various organisms, such as the bd-type oxidases found in some bacteria and archaea. These alternative systems perform similar functions but show striking structural and mechanistic differences from the typical aa3-type cytochrome c oxidase. The diversity of these terminal oxidases raises questions about the origins of life on Earth. The lack of clear homology between cytochrome c oxidase and these alternative oxidases challenges the notion of a single, universal common ancestor for all life forms. The apparent independence in design and function of these diverse terminal oxidases points towards the possibility of separate trajectories in the origins of these crucial life-sustaining mechanisms.Key subunits involved:
Cytochrome c oxidase subunit 1 (EC 1.9.3.1): Smallest known: 514 amino acids (Thermus thermophilus): Central to the catalytic activity of the enzyme, plays a crucial role in electron transfer to oxygen. This subunit contains the heme a and heme a3-CuB binuclear center, which is the site of oxygen reduction.
Cytochrome c oxidase subunit 2 (EC 1.9.3.1): Smallest known: 195 amino acids (Paracoccus denitrificans): Integral component for electron transfer from cytochrome c to the active site of the complex. This subunit contains the CuA center, which is the initial electron acceptor from cytochrome c.
Cytochrome c oxidase subunit 3 (EC 1.9.3.1): Smallest known: 261 amino acids (Paracoccus denitrificans): Critical for maintaining the structural integrity of the complex. While not directly involved in electron transfer, this subunit is essential for the assembly and stability of the enzyme complex.
The Cytochrome c oxidase Complex IV essential enzyme group consists of 3 subunits. The total number of amino acids for the smallest known versions of these subunits is 970.
Information on metal clusters or cofactors:
Cytochrome c oxidase Complex IV (EC 1.9.3.1): Contains multiple metal-containing prosthetic groups essential for its function:
- Cytochrome c oxidase subunit 1:
- Heme a: A six-coordinated heme group involved in electron transfer
- Heme a3-CuB binuclear center: Consists of a five-coordinated heme a3 and a copper ion (CuB) in close proximity. This is the site of oxygen binding and reduction.
- Cytochrome c oxidase subunit 2:
- CuA center: A binuclear copper center that serves as the initial electron acceptor from cytochrome c
- Additional cofactors:
- Magnesium ion (Mg2+): Located at the interface of subunits 1 and 2, it plays a structural role
- Zinc ion (Zn2+): Found in some bacterial cytochrome c oxidases, its exact function is not fully understood
The arrangement of these metal centers allows for efficient electron transfer from cytochrome c to molecular oxygen:
1. Electrons are first accepted by the CuA center in subunit 2
2. They are then transferred to heme a in subunit 1
3. Finally, they reach the heme a3-CuB binuclear center, where oxygen is reduced to water
This electron transfer is coupled to proton pumping across the membrane, contributing to the proton motive force used for ATP synthesis. The complex pumps approximately one proton per electron transferred, demonstrating remarkable efficiency in energy transduction. The cytochrome c oxidase complex showcases the sophisticated evolution of biological electron transfer systems. Its ability to catalyze the four-electron reduction of oxygen to water without releasing partially reduced, potentially harmful intermediates is a testament to nature's engineering prowess. The conservation of this complex across diverse aerobic organisms underscores its fundamental importance in biological energy production and its role in the adaptation of life to an oxygen-rich atmosphere.
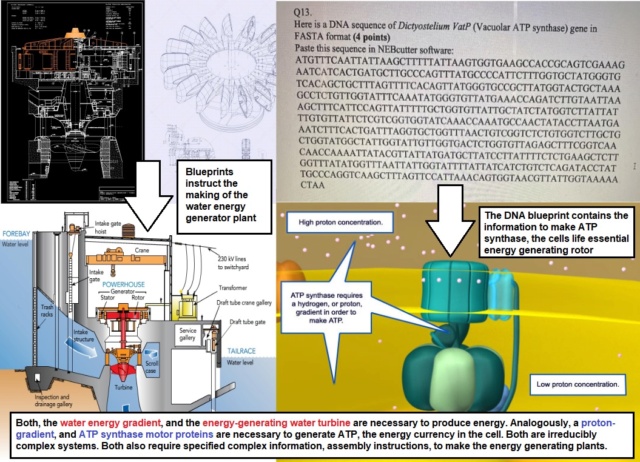
Unresolved Challenges in Cytochrome c Oxidase1. Structural Complexity and SpecificityCytochrome c oxidase is a remarkably complex enzyme, consisting of multiple subunits with intricate structures. The precise arrangement of these subunits, particularly the catalytic core (subunits 1-3), poses a significant challenge to naturalistic explanations. For instance, subunit 1, central to the enzyme's catalytic activity, requires a specific configuration to facilitate electron transfer. The origin of such a precisely structured protein without a guiding mechanism remains unexplained.Conceptual problem: Spontaneous Structural Precision- No known mechanism for generating highly specific, multi-subunit enzyme complexes spontaneously- Difficulty explaining the origin of precise spatial arrangements necessary for electron transfer2. Cofactor IntegrationCytochrome c oxidase incorporates several metal cofactors, including heme groups and copper centers, crucial for its function. The integration of these cofactors into the protein structure with exact positioning represents a significant challenge. For example, the CuA center in subunit 2 requires precise coordination for efficient electron transfer from cytochrome c. Explaining the spontaneous incorporation of these cofactors in their correct positions lacks a plausible naturalistic mechanism.Conceptual problem: Cofactor-Protein Coordination- Absence of explanations for the precise integration of metal cofactors into protein structures- Challenge in accounting for the specific spatial arrangements of multiple cofactors3. Proton Pumping MechanismThe proton pumping function of cytochrome c oxidase is fundamental to its role in energy production. This process requires a sophisticated mechanism to couple electron transfer with proton translocation across the membrane. The origin of this intricate coupling mechanism, involving specific proton channels and conformational changes, presents a significant hurdle for naturalistic explanations.Conceptual problem: Emergence of Coupled Processes- Lack of explanation for the development of coordinated electron transfer and proton pumping- Difficulty in accounting for the precise structural features required for proton channeling4. Alternative Oxidases and Lack of HomologyThe existence of alternative terminal oxidases, such as bd-type oxidases, that perform similar functions but lack structural homology with cytochrome c oxidase presents a significant challenge. This diversity suggests independent origins, contradicting the concept of a single, universal ancestor. Explaining the emergence of functionally similar yet structurally distinct enzymes through unguided processes remains an unresolved issue.Conceptual problem: Convergent Functionality- Difficulty in explaining the independent origin of functionally similar but structurally diverse enzymes- Challenge to account for the development of alternative oxidases without invoking guided processes5. Interdependence with Electron Transport ChainCytochrome c oxidase functions as part of the larger electron transport chain. Its effectiveness depends on the presence and proper functioning of other complexes in this chain. This interdependence raises questions about how such a coordinated system could have arisen through unguided processes. The challenge lies in explaining the simultaneous emergence of multiple, intricately linked enzyme complexes.Conceptual problem: System-Level Coordination- No clear explanation for the concurrent development of interdependent enzyme complexes- Difficulty in accounting for the precise matching of electron donors and acceptors in the chain6. Oxygen Utilization SpecificityThe ability of cytochrome c oxidase to specifically utilize oxygen as the final electron acceptor requires a highly tuned active site. This specificity is crucial for efficient energy production and avoiding harmful side reactions. Explaining the origin of such precise substrate specificity through unguided processes remains a significant challenge in understanding the enzyme's emergence.Conceptual problem: Specialized Substrate Recognition- Lack of explanation for the development of highly specific oxygen-binding sites- Difficulty in accounting for the evolution of mechanisms to prevent harmful side reactions11.11. Complex V ATP Synthesis and Cellular EnergyATP synthase is responsible for producing adenosine triphosphate (ATP), often referred to as the "energy currency" of cells. This molecule is utilized in countless cellular processes, from protein synthesis to muscle contraction. The importance of ATP synthase in maintaining life cannot be overstated, as it is present in all known organisms, from bacteria to humans. The complexity of ATP synthase is astounding. It consists of multiple subunits working in precise coordination to convert the energy stored in proton gradients into chemical energy in the form of ATP. This process, known as chemiosmotic coupling, requires a delicate balance of components, including a rotor, stator, and various other precisely arranged parts. When considering the origin of life, the presence of such a sophisticated energy-producing system poses a significant challenge. For life to begin, a method of energy production would have been necessary. However, the nature of ATP synthase suggests that it is unlikely to have emerged spontaneously in its current form. Interestingly, while ATP synthase is ubiquitous in modern life, there are alternative energy-producing pathways in some organisms. These include substrate-level phosphorylation and other less common mechanisms. Substrate-level phosphorylation is an alternative ATP production method that doesn't rely on ATP synthase or the electron transport chain. It involves the direct transfer of a phosphate group from a high-energy compound to ADP, forming ATP. This process occurs in glycolysis, the citric acid cycle, and fermentation. Unlike ATP synthase-driven oxidative phosphorylation, substrate-level phosphorylation doesn't require oxygen or complex machinery, making it suitable for anaerobic conditions. It's faster but less efficient in ATP yield per glucose molecule. Several organisms rely solely or predominantly on this method, including obligate anaerobic bacteria like Clostridium species, some archaeal species such as methanogenic archaea, anaerobic protozoans like Giardia lamblia and Trichomonas vaginalis, certain anaerobic fungi like Neocallimastigomycota, some unicellular eukaryotes under anaerobic conditions, and certain parasitic helminths in their adult stages. These organisms have adapted to thrive in oxygen-free environments, demonstrating the diversity of life's energy production strategies. The existence of this simpler ATP production method provides insights into potential early forms of energy metabolism in the emergence of life, predating more complex systems like oxidative phosphorylation. The existence of these diverse energy-producing systems, which often share little to no homology with ATP synthase, points to the possibility of multiple independent origins of energy production in early life. This diversity of energy-producing mechanisms, coupled with their lack of shared ancestry, challenges the notion of a single, universal common ancestor for all life. Instead, it suggests a polyphyletic origin of life, where different lineages may have emerged independently with distinct energy-producing systems. The precise arrangement and coordination of multiple components required for ATP synthase to function effectively seem to defy explanation by random, unguided processes. Given the existence of simpler energy-producing mechanisms like substrate-level phosphorylation, it becomes clear that ATP synthase is not essential for the origin of life. However, ATP synthase remains as the most probable player in early life forms for several reasons. Its widespread presence across all domains of life suggests it confers significant advantages. The efficiency of ATP production through chemiosmotic coupling far surpasses that of substrate-level phosphorylation, potentially providing early adopters with a substantial energetic edge. Additionally, the ability of ATP synthase to work in reverse as an ATP-powered proton pump could have been crucial for maintaining ion gradients in primitive cells, aiding in processes like nutrient uptake or pH regulation. Thus, while not necessary for life's origin, ATP synthase likely played a pivotal role in the diversification and complexity of early life forms. 3D view at the molecular level of ATP synthase: nano power plant vital to life on this planet.
3D view at the molecular level of ATP synthase: nano power plant vital to life on this planet.Key subunits involved:
ATP synthase subunit alpha (EC 7.1.2.2): Smallest known: 502 amino acids (Escherichia coli): Plays a key role in ATP synthesis by rotational catalysis. This subunit forms part of the catalytic F1 domain and undergoes conformational changes during ATP synthesis.
ATP synthase subunit beta (EC 7.1.2.2): Smallest known: 459 amino acids (Aquifex aeolicus): Essential for cellular energy production, containing the binding site for ATP synthesis. This subunit, along with the alpha subunit, forms the catalytic core of the F1 domain.
ATP synthase subunit c (EC 7.1.2.2): Smallest known: 69 amino acids (Aquifex aeolicus): Forms the transmembrane channels that permit hydrogen ion flow. Multiple copies of this subunit form the c-ring, which rotates as protons pass through the Fo domain.
ATP synthase subunit a (EC 7.1.2.2): Smallest known: 271 amino acids (Escherichia coli): Forms part of the stator stalk, linking F1 and Fo domains. This subunit is crucial for proton translocation and the generation of rotary torque.
ATP synthase gamma chain (EC 7.1.2.2): Smallest known: 291 amino acids (Bacillus PS3): Central rotor axis of the ATP synthase complex. This subunit transmits the rotational energy from the c-ring to the catalytic F1 domain.
ATP synthase subunit A (F0F1 ATP synthase subunit A) (EC 7.1.2.2): Smallest known: 46 amino acids (Methanothermobacter thermautotrophicus): Helps in the proton transfer within the ATP synthase complex. This small subunit is found in some archaeal ATP synthases.
ATP synthase subunit b (EC 7.1.2.2): Smallest known: 156 amino acids (Escherichia coli): Integral part of the stator stalk, providing stability to the complex. This subunit helps to prevent the F1 domain from rotating with the central stalk.
ATP synthase subunit delta (EC 7.1.2.2): Smallest known: 177 amino acids (Escherichia coli): Aids in the coupling efficiency of the enzyme. This subunit forms part of the stator stalk and helps to connect the F1 and Fo domains.
ATP synthase subunit epsilon (EC 7.1.2.2): Smallest known: 138 amino acids (Escherichia coli): Modulates ATP synthase activity in response to cellular conditions. This subunit can act as an inhibitor of ATP hydrolysis when ATP levels are low.
The ATP Synthase Complex V essential enzyme group consists of
9 subunits. The total number of amino acids for the smallest known versions of these subunits is 2,109.
Information on metal clusters or cofactors:
ATP Synthase Complex V (EC 7.1.2.2): While ATP Synthase doesn't contain metal clusters in the same way as other respiratory complexes, it does require specific ions and molecules for its function:
- Magnesium ions (Mg2+): Essential for ATP synthesis and hydrolysis. Mg2+ forms a complex with ATP and ADP, facilitating their binding to the catalytic sites.
- Phosphate (Pi): Inorganic phosphate is a substrate for ATP synthesis.
- Protons (H+): The flow of protons through the Fo domain drives the rotation of the c-ring and central stalk.
- ATP/ADP: The substrates and products of the reaction catalyzed by ATP Synthase.
The ATP Synthase complex is a marvel of biological engineering, demonstrating nature's ability to create nanoscale rotary motors. Its structure and function showcase several key features:
1.
Rotary Catalysis: The enzyme uses a unique rotary mechanism to couple proton flow to ATP synthesis.
2.
Conformational Changes: The beta subunits undergo significant conformational changes during catalysis, alternating between open, loose, and tight states.
3.
Energy Transduction: The complex efficiently converts the energy stored in the proton gradient into the chemical energy of ATP.
4.
Reversibility: Under certain conditions, ATP Synthase can run in reverse, hydrolyzing ATP to pump protons against their concentration gradient.
5.
Regulatory Mechanisms: Subunits like epsilon can modulate the enzyme's activity in response to cellular energy states.
The ATP Synthase complex represents a pinnacle of biochemical refinement in energy metabolism. Its presence across all domains of life underscores its fundamental importance in biological energy production. The enzyme's ability to produce about 100 ATP molecules per second under optimal conditions highlights its remarkable efficiency and its critical role in sustaining life.
Unresolved Challenges in ATP Synthesis and Cellular Energy Production
1. The Complexity of ATP Synthase
ATP synthase, often referred to as Complex V, is a marvel of biological engineering, functioning as a nano-scale power plant essential for life on Earth. Its intricate structure consists of multiple subunits, including a rotor, stator, and catalytic core, each playing a precise role in the conversion of a proton gradient into chemical energy in the form of ATP. The synthesis of ATP via chemiosmotic coupling is a highly efficient process, but the complexity of this mechanism poses a significant challenge. The precise arrangement and coordination of the ATP synthase components appear dauntingly improbable to have emerged through random, unguided processes. Each subunit is not only necessary but must be arranged and operate in perfect synchrony for the complex to function. The challenge is in explaining how such an integrated and highly specialized molecular machine could have arisen spontaneously in early life forms.
Conceptual problem: Coordinated Emergence of Complex Machinery
- The spontaneous formation of such a complex and highly coordinated molecular machine without any guiding influence is difficult to explain.
- The requirement for all components to be present and functional from the onset challenges the idea of a gradual, unguided origin.
2. Existence of Simpler ATP Production Pathways
The presence of simpler energy production mechanisms, such as substrate-level phosphorylation, further complicates the understanding of ATP synthase's origin. Substrate-level phosphorylation, which occurs in processes like glycolysis and fermentation, does not require the sophisticated machinery of ATP synthase or an electron transport chain. This method is simpler, faster, and operates effectively under anaerobic conditions, suggesting it could have been a viable energy production method in early life. The existence of these alternative pathways raises the question: if simpler methods for ATP production were available, why did such a complex system as ATP synthase emerge? The fact that many organisms rely solely on substrate-level phosphorylation for their energy needs suggests that ATP synthase was not essential for the origin of life. Yet, its ubiquity and efficiency suggest it provided a significant evolutionary advantage, raising questions about how and why it came to dominate as the primary energy-producing mechanism in most life forms.
Conceptual problem: Necessity and Emergence of Complexity
- The emergence of ATP synthase as the dominant energy-producing mechanism, despite the availability of simpler alternatives, is challenging to explain.
- The question remains as to why such a complex system would emerge if simpler, less demanding pathways were sufficient for early life.
3. The Polyphyletic Origins of Energy Production Systems
The diversity of energy-producing systems across different life forms suggests the possibility of multiple independent origins of these mechanisms. While ATP synthase is ubiquitous, various organisms utilize alternative pathways that show little to no homology with ATP synthase. This diversity challenges the notion of a single, universal common ancestor, as it suggests that distinct lineages may have emerged with their own unique energy production strategies. The lack of shared ancestry among these systems implies that they arose independently, a hypothesis that raises significant questions about the origins of life. How could such diverse and complex systems emerge independently, each perfectly suited to its environment, without some form of guiding influence?
Conceptual problem: Independent Emergence of Complex Systems
- The independent emergence of complex, functionally distinct energy production systems challenges the idea of a single origin for life.
- The lack of shared ancestry or clear evolutionary pathways among these systems suggests a more complex origin story than traditionally assumed.
4. Functional Advantage of ATP Synthase
Despite its complexity, ATP synthase likely conferred significant advantages to early life forms. The efficiency of ATP production through chemiosmotic coupling far exceeds that of substrate-level phosphorylation, providing organisms that utilized ATP synthase with a substantial energetic edge. Additionally, ATP synthase's ability to operate in reverse as an ATP-powered proton pump could have been crucial in maintaining ion gradients, which are essential for various cellular processes, including nutrient uptake and pH regulation. This suggests that while ATP synthase was not necessary for the origin of life, it played a pivotal role in the diversification and complexity of early life forms. However, this raises further questions: how did such a complex system become so widely adopted, and why did it not simply coexist with simpler systems in more life forms?
Conceptual problem: Widespread Adoption and Functional Integration
- The widespread adoption of ATP synthase as the primary energy production mechanism suggests a strong selective advantage, yet its complexity is difficult to account for in a purely unguided origin scenario.
- The question remains as to why ATP synthase became so integral to life, while simpler systems remained confined to a limited range of organisms.
Conclusion
ATP synthase represents one of the most complex and essential molecular machines in living organisms. Its emergence, alongside the existence of simpler energy-producing systems and the apparent polyphyletic origins of these mechanisms, presents significant challenges to the concept of a natural, unguided origin of life. The questions surrounding the necessity, emergence, and widespread adoption of such a complex system as ATP synthase highlight the need for a deeper understanding of life's origins, one that may require reconsideration of traditional assumptions about the nature of early life and the mechanisms that led to its diversity and complexity.