The Problems: Elucidating the Gap Between Non-Life and Life
X-ray Of Life:: Volume II: The Rise of Cellular Life: Link
X-ray Of Life: Volume III: Complexity and Integration in Early Life Link
1. Prebiotic Chemistry and Early Molecular Synthesis
II. The RNA world
III. Transition to RNA-Peptide World
IV. Formation of Proto-Cellular Structures
2. Prebiotic Chemistry
3. Prebiotic Nucleotide Synthesis
4. Prebiotic Carbohydrate Synthesis
5. Key Prebiotic Reactions and Processes
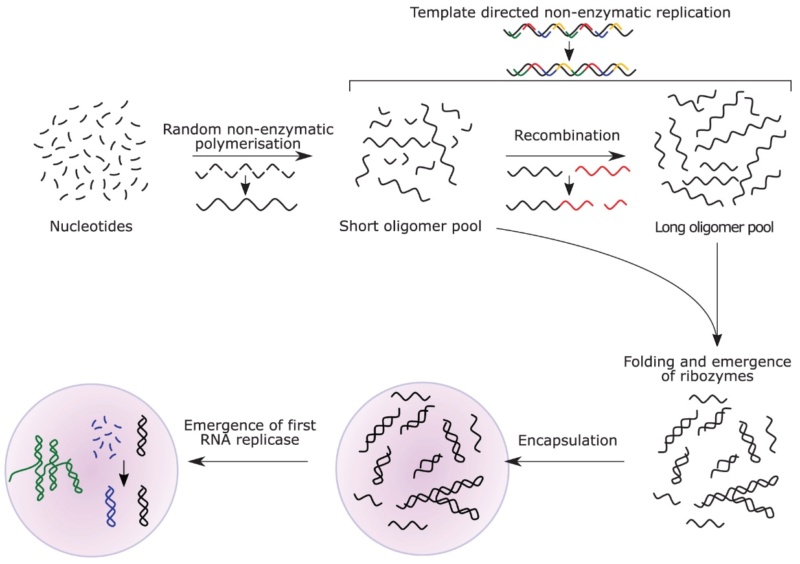
6. The RNA World Hypothesis: A Critical Examination
7. The RNA-Peptide World
9. Encapsulation in Vesicles
10. Life's Emergence and First Life Forms

Abstract for Volume 1
"X-ray Of Life: Volume I: From Prebiotic Chemistry to Cells" is the first installment in a groundbreaking three-volume series that meticulously examines the problems related to naturalistic hypotheses of the origin of life on Earth. This volume delves deep into the foundational challenges of life's emergence, offering a comprehensive analysis of the transition from simple chemical compounds to the first living cells. The book is divided into three main sections, each addressing critical aspects of prebiotic and early biotic emergence. In "Prebiotic Chemistry and Early Molecular Synthesis," readers are introduced to the primordial conditions of early Earth, exploring the formation of basic building blocks such as carbohydrates, phospholipids, and metal clusters. This section critically examines key prebiotic reactions and processes that would have been required to set the stage for life's emergence. "The RNA World and the Emergence of Genetic Information" takes readers on a journey through one of the most debated hypotheses in origin-of-life studies. It offers a critical examination of the RNA World hypothesis and its alleged transition to an RNA-peptide world, shedding light on how genetic information might have first emerged and been processed in early life forms. The final section, "Cellular Development and Early Cellular Life," bridges the gap between prebiotic chemistry and the first cellular entities. It explores the crucial steps of molecular encapsulation in vesicles and the characteristics of the first life forms, setting the stage for the more advanced cellular processes discussed in subsequent volumes. Throughout, the book maintains a rigorous scientific approach while questioning the plausibility of purely naturalistic explanations for life's origin. By presenting cutting-edge research and thought-provoking analyses, "X-ray Of Life: Volume I" invites readers to critically engage with one of science's most profound questions: how did life begin? This volume lays the groundwork for the subsequent books in the series, which will delve deeper into the rise of cellular life, the development of complex biological systems, and the integration of various cellular functions. Together, this trilogy promises to provide the most comprehensive and up-to-date examination of life's origins available to both scientists and interested laypersons alike.
1. Introduction: The Origin of Life - A Journey into Complexity
The quest to understand life's origins is among the most formidable challenges in modern science. Despite over seven decades of intensive investigation and thousands of studies, the transition from simple molecules to self-replicating cells remains poorly understood. Paradoxically, while human technological progress has been astounding during this time, our grasp of how life emerged has grown more complicated. The juxtaposition between advancing human ingenuity and persistent scientific uncertainty highlights a fascinating paradox.
Consider the trajectory of technological development from the 1950s to the present:
1950s
- Technology: First commercial computers (UNIVAC I, 1951), basic transistor electronics.
- Origin-of-Life Research (OoL): Miller-Urey experiment (1953) demonstrated the synthesis of simple amino acids, fostering optimism about solving life’s origins.
1960s
- Technology: Integrated circuits, early satellites, first human moon landing.
- OoL Research: Discovery of genetic code complexity introduces significant challenges to straightforward origins hypotheses.
1970s
- Technology: Personal computers, fiber optics, reusable space shuttles.
- OoL Research: Attempts at chemical evolution reveal significant barriers in prebiotic synthesis pathways.
1980s
- Technology: Internet foundations, advanced robotics, cell phones.
- OoL Research: Discovery of ribozymes initially seems promising, yet reveals new complexities.
1990s
- Technology: World Wide Web, GPS, advanced genetic engineering.
- OoL Research: Complete genome sequences uncover astonishing cellular complexity, complicating naturalistic explanations.
2000s
- Technology: Smartphones, social media, human genome sequencing.
- OoL Research: Systems biology reveals intricate, interdependent networks that challenge stepwise evolutionary models.
2010s
- Technology: CRISPR gene editing, artificial intelligence, quantum computing.
- OoL Research: Advanced analysis tools expose increasingly improbable scenarios for spontaneous biochemical organization.
2020s
- Technology: Advanced AI models, mRNA vaccines, quantum supremacy.
- OoL Research: Recognition grows regarding the astronomical improbability of spontaneous molecular self-assembly.
While human technology has progressed from room-sized computers to quantum processors, from basic rockets to Mars rovers, and from simple microscopes to atomic-resolution imaging, our understanding of life's origin has moved in the opposite direction. Each technological advance, rather than solving the puzzle, has revealed new layers of biological complexity that must be explained. Modern analytical tools show us that even the simplest cell requires thousands of precisely coordinated molecular machines, information processing systems, and regulatory networks - all of which must have somehow emerged together. This complexity paradox emerges from a simple pattern: as our analytical tools and techniques improve, they reveal ever-deeper layers of sophistication in even the simplest living systems. Each discovery, rather than simplifying the picture, has added new dimensions to the challenge. Consider Aquifex, one of the simplest known free-living organisms. Even this "minimal" cell requires thousands of precisely coordinated proteins, a sophisticated membrane system, and complex regulatory networks - all operating with remarkable precision.
Modern analysis reveals that even the simplest free-living cell requires:
- Precisely coordinated metabolic networks
- Sophisticated information processing systems
- Complex molecular machines operating with near-perfect efficiency
- Self-repairing and self-regulating mechanisms
- Remarkably precise quality control systems
The challenge begins with defining the problem itself. It extends far beyond identifying primitive cells or suitable environments. While we must certainly determine what the first living cells looked like and where they emerged, the deeper mystery lies in understanding the transformative processes that bridged non-living and living matter. How simple molecules organized into functional units capable of growth and replication. How energy from the environment - whether from hydrothermal vents, solar radiation, or chemical gradients - was captured and harnessed by emerging biological systems. Perhaps most important, we must unravel how molecules drove the transition from basic chemical reactions to the complex processes of metabolism and reproduction that characterizes even the simplest modern cells. Maybe the foremost problem is to elucidate the origin of biological information, and the genetic code. The challenge before us is not just identifying the components of early life, but understanding their integration. How did membranes, metabolic networks, and information-carrying molecules come together to form living, self-sustaining systems? What mechanisms guided molecules toward increasingly complex and life-like behaviors? The answers to these questions lie at the intersection of chemistry, physics, and biology, requiring us to think across traditional disciplinary boundaries. But that is not all. We need to visualize the problem in a integrative, holistic manner. We need to gain a wide understanding on systems level, that includes all relevant aspects, and an understanding of the entire trajectory - what is involved. This is an exceedingly difficult task, and has rarely - if ever - been done in an exhaustive way. This is what this book is about.
These fundamental questions resist simple answers. Without a time machine to observe early Earth directly, we must rely on careful analysis and inferences based on the evidence at hand. Yet, and this is what this book will unravel, each advance in our understanding of cellular complexity makes the spontaneous emergence of such systems seem more, not less, challenging to explain. The 3 volumes together will raise about 2000 unanswered questions. Hardly, before, did we know that so many questions find no answer based on a naturalistic framework. The sheer sophistication of these requirements has transformed our understanding of the challenge. Far from approaching a solution, we find ourselves facing an ever-expanding set of questions about how such precise and interdependent systems could have emerged through unguided processes. Yet this growing complexity should not discourage us - rather, it should inspire a more rigorous and comprehensive approach to the question. Understanding life's origin requires integrating insights from multiple fields: biochemistry, geology, information theory, systems biology, and engineering principles. Only by carefully analyzing all aspects of the transition from molecules to living cells can we begin to appreciate the true magnitude of the challenge. This volume attempts to systematically examine these requirements, beginning with a detailed analysis of what we know about the minimal requirements for life, based on our most detailed studies of simple existing cells. By establishing this baseline, we can better understand the gap that must be bridged between non-living chemistry and the first living systems.
Our exploration begins in the prebiotic world, examining the synthesis of organic compounds and the formation of autocatalytic reaction sets. We then move on into the proposed RNA World hypothesis, considering the challenges of achieving homochirality and the potential roles of RNA in early life. As we progress, we venture into the formation of more complex systems, including protein synthesis and the encapsulation of these components within vesicles. We carefully examine the requirements for the first enzyme-mediated cells and the development of sophisticated cellular functions. Throughout this journey, we pay close attention to the information content required in the genome to specify these complex systems. We consider the interplay between nucleic acids, proteins, and metabolic processes, and how these would have had to co-emerge to produce the first living cellular entities. By the end of this trilogy, readers will gain a comprehensive understanding of the immense complexity involved in the origin of life, appreciating the gigantic leap from non-living chemistry to biology.
As Eugene V. Koonin aptly states in The Logic of Chance (2012):
"Despite many interesting results to its credit, when judged by the straightforward criterion of reaching (or even approaching) the ultimate goal, the origin of life field is a failure—we still do not have even a plausible coherent model, let alone a validated scenario, for the emergence of life on Earth. Certainly, this is due not to a lack of experimental and theoretical effort, but to the extraordinary intrinsic difficulty and complexity of the problem. A succession of exceedingly unlikely steps is essential for the origin of life, from the synthesis and accumulation of nucleotides to the origin of translation; through the multiplication of probabilities, these make the final outcome seem almost like a miracle." 1
We will trace the evolution of thought in this field, from the broad concepts of early pioneers to the more specific chemical scenarios of modern researchers:
- Alexander Oparin (1930s): Proposed the formation of simple organic compounds from atmospheric gases as the first step.
- J.B.S. Haldane (1940s): Suggested that the origin of life was essentially a chemical process.
- Stanley Miller (1950s): Demonstrated amino acid synthesis under simulated early Earth conditions.
- Francis Crick (1960s): Noted that the origin of life appeared "almost a miracle" due to the required conditions.
- Richard Dawkins (1980s): Argued that evolutionary theory explains the illusion of design without requiring a designer.
Despite decades of research, we find ourselves paradoxically further from solving this puzzle than we were 70 years ago. As our knowledge of life's complexity has grown, so too has the challenge of explaining its emergence. The transition from non-life to life, once thought to be a small step, now appears to be a quantum leap of staggering proportions.
This trilogy will elucidate why Lynn Margulis's observation is so profound:
"The smallest bacterium is so much more like people than Stanley Miller's mixtures of chemicals, because it already has these system properties. So to go from a bacterium to people is less of a step than to go from a mixture of amino acids to that bacterium." 2
We will explore recent experimental findings that have both expanded our knowledge and highlighted the complexity of the origin of life problem. For instance, the discovery of ribozymes lent support to the RNA World hypothesis, but challenges in RNA synthesis under prebiotic conditions have led to alternative scenarios like the "RNA-peptide world" proposed by Loren Williams 3.
Theoretical models, such as Jeremy England's "dissipative adaptation," suggest new ways to understand the emergence of complex, life-like behaviors 4. However, these theories remain hotly debated and require further validation.
We will also delve into current controversies, including debates about early Earth conditions, the viability of the RNA World hypothesis, and alternative "metabolism first" scenarios proposed by researchers like Harold Morowitz 5. The role of information in life's origin, as explored by Paul Davies, shifts our focus to information processing in physical systems, raising profound questions about how biological information could emerge from non-life 6.
As we look to the future, we will explore promising directions in origin of life research, including systems chemistry and the exploration of alternative chemistries that could support life, such as Steven Benner's work on "weird life" 7.
This trilogy does not presuppose any particular framework for the origin of life. Instead, it presents empirical data and theoretical models, encouraging readers to critically evaluate the evidence. We will consider whether the cumulative evidence supports the emergence of life through unguided, naturalistic processes on the early Earth, or whether it points to alternative explanations.
By the end of this journey, readers will appreciate the sophistication of even the simplest living cell and understand why the transition from non-life to life represents a more formidable challenge than the subsequent transition from simple cells to complex organisms like humans. This exploration invites us to marvel at one of science's most enduring enigmas and challenges us to grapple with fundamental questions about the nature of life itself.
1.1. What is Life? Understanding the Origin Challenge
Before we can meaningfully investigate the origin of life, we must first comprehend what makes living systems uniquely different from non-living matter. This understanding is crucial because any naturalistic explanation for life's origin must account for how each essential characteristic emerged from purely chemical processes - a challenge that becomes more formidable as we better understand life's complexity.
Paul Davies suggests that life can be understood as the combination of chemistry and information, emphasizing that both components are essential to define and explain living systems. This dual nature - the interplay between physical structure and encoded information - presents one of the fundamental challenges in explaining life's origin through natural processes. The question becomes not just how complex chemicals formed, but how they became organized into information-processing systems.
Living systems exhibit several defining characteristics, each of which presents specific challenges that must be addressed by any viable origin of life hypothesis:
Information Content and Processing
The information aspect of life presents perhaps the most significant challenge to naturalistic origin scenarios. Living systems demonstrate:
- Highly specified genetic information encoded within DNA/RNA
- Complex information processing systems for reading and implementing genetic instructions
- Error detection and correction mechanisms
- The capacity to transmit information across generations with high fidelity
This raises fundamental questions addressed in later sections: How did meaningful genetic information first arise? What mechanisms could generate and preserve complex coded instructions through purely chemical processes?
Hardware/Software Integration
The interdependence between nucleic acids (information storage) and proteins (functional machinery) creates what appears to be an irreducible system:
- DNA/RNA store instructions for making proteins
- Proteins are required for reading and copying DNA/RNA
- This mutual dependency must be explained in any origin scenario
- The genetic code itself requires explanation
This chicken-and-egg paradox becomes central to our later examination of the RNA World hypothesis and its alternatives.
Organized Complexity
Unlike the random complexity found in non-living systems (such as hurricanes or crystals), life exhibits:
- Purposeful organization at multiple levels
- Hierarchical structure from molecules to organisms
- Coordinated interactions between components
- Integration of multiple subsystems
- Maintenance of order against entropy
These characteristics raise crucial questions about how such organized complexity could emerge spontaneously, a topic we'll explore in our analysis of various origin hypotheses.
Metabolic Systems
Living systems require sophisticated chemical networks:
- Complex webs of interdependent chemical reactions
- Energy capture and transformation mechanisms
- Waste management and material cycling
- Precise regulation of chemical processes
- The ability to maintain far-from-equilibrium states
The emergence of integrated metabolic systems presents unique challenges that will be examined in our discussion of metabolism-first hypotheses.
Reproduction and Self-Maintenance
Life's reproductive capabilities present multiple challenges:
- The need for high-fidelity information copying
- Replication of both information and the machinery to process it
- Development of complex regulatory systems
- Error correction mechanisms
- Growth and repair processes
These requirements become particularly relevant when we examine hypotheses about the first self-replicating systems.
Homeostasis and Adaptation
Living systems maintain internal stability while responding to environmental changes:
- Complex feedback mechanisms
- Environmental sensing systems
- Regulatory networks
- Adaptive responses
- Stress resistance mechanisms
The origin of these capabilities must be explained by any comprehensive theory of life's emergence.
As we proceed to examine various origin of life hypotheses in subsequent sections, we must evaluate how effectively each proposal addresses the emergence of these essential characteristics. The challenge is not simply to explain how complex molecules formed, but how they became organized into systems displaying all these attributes of life. This framework will guide our analysis of competing theories and help identify the key problems that any successful explanation must resolve. In the following sections, we will examine how various hypotheses attempt to bridge the gap between non-living chemistry and these essential characteristics of life. We will see that while individual processes might be explained, accounting for the simultaneous emergence of all these features through naturalistic mechanisms presents a formidable challenge.
1.2. The Journey from Non-Life to Life: A Scientific Perspective from Half a Century Ago
Starting about 100 years ago, scientists and researchers envisioned the transition from non-living matter to life, and started in the attempt of explaining how natural processes could have transformed simple carbon and nitrogen compounds in ancient oceans into living entities. Pioneers like A. I. Oparin (1924) and J. B. S. Haldane (1929) were the first to seriously tackle this enigma. Their work inspired numerous scientists to conduct laboratory experiments and formulate theories based on their findings. Notable contributors included J. D. Bernal, M. Calvin, S. W. Fox, S. L. Miller, L. E. Orgel, J. Oró, C. A. Ponnamperuma, and H. C. Urey. Researchers proposed that the chemical evolution leading to life occurred in three distinct phases. The first phase involved the creation of simple building blocks monomers. The second phase saw the development of complex molecules or polymers. The final phase encompassed the organization of these polymers into self-regulating systems that we recognize as living cells. Scientists theorized that the formation of amino acids and nitrogen bases required energy. They identified several potential energy sources present on Earth over supposedly three billion years ago, including lightning, radiation from radioactive elements and cosmic rays, solar ultraviolet rays, meteorite impacts, and heat. In 1953, S. L. Miller conducted a groundbreaking experiment. He created a mixture simulating Earth's early atmosphere and subjected it to electrical discharges, mimicking lightning. This process successfully produced amino acids, demonstrating that primordial energy sources could have generated these essential building blocks. Subsequent experiments showed that all theorized energy sources from the early Earth could potentially form amino acids in both atmospheric and oceanic conditions. Additionally, chemical analysis of meteorites suggested that amino acids could have formed in interplanetary dust and arrived on Earth via meteorite impacts. Researchers proposed various mechanisms for concentrating monomers to facilitate their combination into biologically significant polymers. Oparin suggested coacervate formation, while Bernal proposed adsorption onto clay, which then catalyzed monomer joining. Fox hypothesized concentration through evaporation in tidal pools, followed by heat-induced joining. Calvin and Oró suggested that certain cyanogen-derived compounds could enable direct polymer formation in seawater without prior concentration. Laboratory experiments confirmed the viability of all these proposed methods, indicating multiple potential pathways for natural processes to combine amino acids into protein-like chains and to form nucleic acids from nitrogen bases, sugars, and phosphates. Researchers also noted that spontaneous amino acid combinations in laboratories showed preferences for certain pairings, mirroring patterns observed in cellular proteins. This similarity suggested that protein molecule amino acid sequences evolved from natural affinities between amino acids. Scientists theorized that the accidental joining of metal molecules (like iron) to amino acids might have initiated enzyme evolution. They also proposed that the combination of phosphates with nitrogen bases and sugars could have led to ATP formation. Additionally, experiments showed that phospholipids and proteins could spontaneously form sheets resembling cell membranes under specific conditions.
This final phase remained the most mysterious, with limited experimental evidence to support theories. Scientists were unsure whether it occurred after or concurrently with polymer formation. Despite the lack of concrete evidence, these theories were valuable in guiding future experiments and refining hypotheses. Oparin's coacervate theory provided the most detailed scenario for cell evolution. He envisioned the primordial sea as a vast "organic soup" where increasingly complex molecules formed through natural processes. Amino acids, capable of diverse combinations, produced a variety of protein molecules large enough to form colloidal particles, gradually transforming the sea into a colloidal solution. These colloidal particles sometimes formed clusters known as coacervates, separated from the surrounding sea by a thin water molecule skin. This development was seen as a crucial step towards the formation of protoplasm. The coacervate's skin acted as both a barrier and a passageway, allowing a steady flow of molecules in and out of the cluster. Over time, some coacervates developed a balance between breakdown and buildup processes, leading to stability and growth. This balance was seen as a precursor to cellular metabolism. The need for a continuous supply of molecules for the building-up process was likened to the "feeding" behavior of living organisms. Scientists proposed that a form of natural selection occurred among these coacervates, with the more stable ones persisting and evolving. They suggested that some coacervates developed catalytic processes that enhanced their efficiency, leading to the eventual evolution of enzyme systems. As organic compounds in the sea were increasingly absorbed by coacervates, competition for resources intensified. This "struggle for existence" was thought to have accelerated the process of natural selection, favoring coacervates with the most stable metabolism, efficient enzyme systems, and effective reproduction mechanisms. Through countless small changes over millions of years, researchers believed that coacervates eventually developed into the first primitive living organisms. These early life forms were imagined to be even simpler than bacteria or blue-green algae, obtaining energy through anaerobic fermentation processes. Despite their simplicity, scientists speculated that these organisms must have possessed some mechanism for energy storage and transfer, possibly involving ATP or a similar compound. This narrative represents the scientific community's best understanding of life's origins up to approximately half a century ago. It laid the groundwork for further research and continues to influence our quest to unravel the mysteries of life's beginnings.
The scientific understanding of life's origins has evolved significantly over the past half-century. In the 1980s, the discovery of ribozymes (RNA molecules with catalytic properties) by Thomas Cech and Sidney Altman led to the formulation of the RNA World hypothesis. This theory proposed that RNA could have served as both a genetic material and a catalyst in early life forms, potentially bridging the gap between prebiotic chemistry and cellular life. Researchers like Stanley Miller continued to refine prebiotic synthesis experiments, exploring a wider range of potential early Earth conditions. The discovery of extraterrestrial organic compounds in meteorites and comets strengthened the idea that some of life's building blocks might have an cosmic origin. The field of systems chemistry emerged, focusing on how complex networks of chemical reactions could lead to self-sustaining, self-replicating systems. Jack Szostak and others made significant progress in creating socalled protocells - simple membrane-enclosed structures capable of growth and division. Hydrothermal vents, particularly alkaline vents, gained attention as potential cradles of life. Researchers like Nick Lane proposed that the chemical and energy gradients in these environments could have driven the formation of early metabolic processes.
1.3. The Origin of Life: A Puzzle with Many Attempts to Solve It
Numerous hypotheses attempt to unravel the processes that led to the emergence of life from non-living matter. Each theory brings its perspective, from the role of chemical reactions in primordial environments to the formation of self-replicating molecules. The journey through these ideas reveals not only the ingenuity of scientific thought but also the immense challenge of piecing together the puzzle of life's beginnings. While each hypothesis offers valuable insights, they collectively underline the fundamental issue: can naturalistic, unguided processes sufficiently account for the origin of life?
1.3.1. Charles Darwin and the Origin of Life
Darwin's perspective on life's origins underwent significant changes throughout his career. Initially, he was quite reserved about the subject. In the 3rd edition of "The Origin of Species" (1861), he maintained a cautious stance, stating that "it is no valid objection that science as yet throws no light on the far higher problem of the essence or origin of life." This skepticism was reinforced in his 1863 letter to Joseph Dalton Hooker, where he dismissed the entire question as premature: "it is mere rubbish thinking, at present, of origin of life; one might as well think of the origin of matter."
However, by 1871, his views had evolved considerably. In a famous letter to Hooker, he proposed what would become one of the first scientific hypotheses about life's origins - the "warm little pond" scenario. He wrote: "it is often said that all the conditions for the first production of a living being are now present, which could ever have been present. But if (and oh what a big if) we could conceive in some warm little pond with all sort of ammonia and phosphoric salts,—light, heat, electricity present, that a protein compound was chemically formed, ready to undergo still more complex changes..." He notably added that in the present day such compounds "would be instantly devoured, or absorbed, which would not have been the case before living creatures were formed."
This intellectual progression is also reflected in his treatment of the topic in "On the Origin of Species." From the 2nd edition onwards, he included the famous closing sentence about life "having been originally breathed by the Creator into a few forms or into one," adding "by the Creator" to the original text. While historians debate whether this addition was a genuine theological statement or a strategic concession to religious sensibilities of the time, it demonstrates Darwin's careful navigation of the complex questions surrounding life's origins. Let's analyze this progression:
1. Initial Skepticism (1861-1863):
In the early 1860s, Darwin appeared to view the question of life's origin as beyond the scope of contemporary scientific inquiry. His statement in the 3rd edition of "The Origin of Species" and his 1863 letter to Hooker both express a clear reluctance to speculate on this topic. This attitude likely stemmed from:
a) The limited scientific knowledge of the time regarding cellular and molecular biology.
b) Darwin's focus on explaining the diversity of life through natural selection, rather than its initial emergence.
c) A desire to avoid controversy in areas where he felt scientific evidence was lacking.
2. Shift in Perspective (1871):
By 1871, Darwin's view had noticeably shifted. His famous "warm little pond" hypothesis represents a significant departure from his earlier stance. This change might be attributed to:
a) Advancements in scientific understanding during the intervening years.
b) Darwin's own continued contemplation of the subject.
c) A growing recognition that the origin of life was a logical extension of his work on evolution.
3. The "Warm Little Pond" Hypothesis:
This hypothesis is remarkable for several reasons:
a) It proposes a naturalistic explanation for the origin of life, consistent with Darwin's overall scientific approach.
b) It anticipates several key elements of modern abiogenesis theories, including the importance of a primordial soup of chemicals and energy sources.
c) It recognizes the difference between prebiotic and biotic environments, noting that early organic compounds would not have been consumed by existing life forms.
4. Implications:
Darwin's evolving thoughts on this matter demonstrate:
a) His willingness to reconsider and update his views based on new information or insights.
b) The interconnectedness of evolutionary theory and questions about life's origins.
c) The early stages of what would become a major field of scientific inquiry in the 20th and 21st centuries.
5. Historical Context:
Darwin was speculating well before the discovery of DNA, the understanding of protein synthesis, or the development of sophisticated geochemical analyses. His "warm little pond" idea was thus remarkably prescient, laying groundwork for future research directions.
Darwin's changing perspective on the origin of life reflects both the rapid advancement of scientific thought during his lifetime and his own intellectual courage in tackling one of the most fundamental questions in biology. His final stance, while speculative, opened the door for future generations of scientists to explore this critical area of inquiry. In 1871, Ernst Haeckel published in Nature Magazine an article on the origin of life, describing Biological cells as essentially and nothing more than a bit of structureless, simple " Protoplasm "- and their other vital properties can therefore simply and entirely brought about by the entirely by the peculiar and complex manner in which carbon under certain conditions can combine with the other elements further down, the author writes: Abiogenesis is, in fact, a necessary and integral part of the universal evolution theory. 1
This 1871 article by Ernst Haeckel in Nature Magazine represents a significant moment in the history of origin of life theories. Let's analyze its key points and implications:
1. Simplification of cellular structure:
Haeckel's description of cells as "essentially and nothing more than a bit of structureless, simple 'Protoplasm'" reflects the limited understanding of cellular complexity at the time. This view:
a) Aligned with the prevailing "protoplasm theory" of the late 19th century.
b) Greatly underestimated the intricate organization within cells, which we now know includes numerous organelles and complex molecular machinery.
c) Provided a seemingly simpler target for explaining life's origin, as it reduced the problem to the formation of this "simple" protoplasm.
2. Chemical basis of life:
Haeckel's assertion that vital properties can be "brought about entirely by the peculiar and complex manner in which carbon under certain conditions can combine with the other elements" is noteworthy because:
a) It recognizes the central role of carbon in organic chemistry and life.
b) It suggests a purely materialistic explanation for life's properties, without invoking vitalism or supernatural forces.
c) It anticipates later research into the chemical basis of life and the importance of carbon's unique bonding properties.
3. Abiogenesis as part of evolution:
Haeckel's statement that "Abiogenesis is, in fact, a necessary and integral part of the universal evolution theory" is particularly significant:
a) It explicitly links the origin of life to the broader theory of evolution, seeing them as part of a continuous process.
b) It challenges the idea of a fundamental divide between living and non-living matter.
c) It sets the stage for later research that would attempt to bridge the gap between chemistry and biology.
4. Historical context and impact:
Haeckel's views should be understood within the scientific and philosophical context of his time:
a) They reflect the growing materialist and naturalistic approach to biology in the late 19th century.
b) They contributed to the ongoing debates about spontaneous generation and the nature of life.
c) They helped to establish the origin of life as a legitimate scientific question, rather than a purely philosophical or religious one.
5. Limitations and later developments:
While groundbreaking for its time, Haeckel's view had significant limitations:
a) The oversimplification of cellular structure would later be revealed by advances in microscopy and cell biology.
b) The ease with which he assumed life could arise from non-life underestimated the complexity of even the simplest living systems.
c) Later research would reveal the immense challenges in explaining the origin of life, leading to more sophisticated theories and experiments.
Haeckel's 1871 article represents an important step in the scientific approach to the origin of life. By framing abiogenesis as a natural process and an extension of evolutionary theory, he helped to establish it as a field of scientific inquiry. While many of his specific ideas have been superseded, his general approach – seeking naturalistic explanations for life's origins – continues to guide research in this area today. His work, along with Darwin's speculations from the same year, marks a pivotal moment in the history of origin of life studies, setting the stage for the more detailed chemical and biological investigations that would follow in the 20th and 21st centuries.
1.3.2. Development of Origin of Life Hypotheses: A Theoretical Evolution
The quest to understand life's origins has produced numerous hypotheses over the past 150 years. These theories reflect not only advancing scientific knowledge but also changing perspectives on life's fundamental nature. Below, we organize these hypotheses into major theoretical frameworks, showing how different approaches to the origin problem have evolved over time.
A. Early Conceptual Frameworks (1866-1950s)
The earliest hypotheses were largely conceptual, attempting to bridge the gap between simple and complex matter:
1866: Haeckel's Monera Hypothesis
- Proposed simple, homogeneous substances as life's precursors
- First attempt to explain life's emergence through natural processes
- Influenced later theories about simple-to-complex progression 1
1920s: Heterotroph Hypothesis
- Suggested first organisms were consumers rather than producers
- Addressed the metabolic aspect of early life
- Influenced later metabolic theories 2
B. Compartmentalization Theories (1930s-1970s)
These hypotheses focused on how biological materials might have become organized into cell-like structures:
1930s: Coacervate Hypothesis
- Proposed droplets as primitive cellular structures
- First serious attempt to explain cellular compartmentalization
- Influenced later theories about protocells 3
1950s: Fox's Microsphere Hypothesis
- Refined coacervate concept with specific chemical mechanisms
- Demonstrated experimental formation of cell-like structures
- Connected structure formation with protein chemistry 4
2017: Droplet Hypothesis
- Modern revival of compartmentalization theories
- Incorporates current understanding of phase separation
- Links to contemporary cell biology 5
C. Self-Organization and Complexity Theories (1970s-2000s)
These hypotheses explore how complex systems could emerge from simpler components:
1970s: Eigen's Hypercycle Hypothesis
- Introduced mathematical framework for self-organization
- Addressed information transfer and replication
- Influenced modern complexity theories 6
1970s: Autocatalytic Networks Hypothesis
- Proposed self-sustaining chemical networks
- Connected metabolism with self-organization
- Influenced systems chemistry approaches 7
2017: Modular Hierarchy Hypothesis
- Modern synthesis of self-organization principles
- Emphasizes hierarchical structure development
- Incorporates contemporary systems biology 8
D. Contemporary Molecular Approaches (2000s-Present)
Recent hypotheses incorporate modern understanding of molecular biology and chemistry:
2017: Chemically Driven RNA Hypothesis
- Focuses on prebiotic RNA synthesis
- Addresses chemical plausibility
- Links to RNA World hypothesis 9
2016: Minimotif Synthesis Hypothesis
- Proposes peptide-first scenario
- Challenges RNA World paradigm
- Integrates protein and genetic systems 10
2017: Foldamer Hypothesis
- Combines structural and informational aspects
- Addresses polymer evolution
- Links to modern protein science 11
E. Environmental and Energy-Based Approaches (2000s-Present)
These hypotheses focus on energy sources and environmental conditions:
2004: Organic Aerosols Hypothesis
- Considers atmospheric chemistry
- Addresses molecule concentration problem
- Links to environmental conditions 12
2019: Photochemical Origin of Life Hypothesis
- Focuses on UV light as energy source
- Addresses prebiotic synthesis
- Considers environmental context 13
1.3.2.1 Recent Developments in Origin of Life Research (2020-2024)
Recent years have seen significant advances in both theoretical frameworks and experimental techniques for studying life's origins. These developments have been driven by new analytical tools, computational methods, and interdisciplinary approaches.
A. Recent Theoretical Advances
1. 2020: Dynamic Protocell Theory
- Proposes that early protocells were highly dynamic systems
- Emphasizes role of non-equilibrium processes
- Integrates membrane dynamics with metabolic networks 1
2. 2022: Quantum-Classical Transition Model
- Examines quantum effects in prebiotic chemistry
- Proposes role of quantum coherence in early molecular evolution
- Bridges quantum chemistry and origin of life studies 2
3. 2023: Assembly Theory Model:
- Quantifies molecular complexity through assembly steps
- Provides experimental measure of biosignatures
- Bridges chemistry and biosignature detection
Assembly Theory, developed by Lee Cronin's team and extended with Sara Imari Walker, offers a framework for measuring molecular complexity based on the minimum number of assembly steps required to build molecules from basic components. The theory:
- Assigns an "assembly index" to molecules based on construction steps
- Higher index values (>15) strongly indicate biological origin
- Can be verified experimentally via mass spectrometry
- Defines probability boundaries between abiotic and biotic molecules
- Allows quantitative measurement of selection processes
This approach introduces mathematical rigor to biosignature detection through the formula:
A = ∑(i=1 to N) e^ai(ni-1/NT)
Where:
- NT represents total objects
- N represents unique objects
- ni denotes copy numbers
- ai represents assembly index
Key implications for origin of life research:
- Provides quantifiable distinction between biological and non-biological molecules
- Offers experimental method to identify biosignatures
- Helps map chemical space complexity
- Applicable to astrobiology and drug discovery 3
B. Advanced Analytical Techniques (2020-2024)
1. High-Resolution Mass Spectrometry
- Enables detection of prebiotic molecules at unprecedented sensitivity
- Allows tracking of complex reaction networks
- Identifies intermediate compounds in prebiotic reactions
2. Microfluidic Systems
- Permits study of protocell formation in controlled conditions
- Enables high-throughput screening of prebiotic reactions
- Allows precise manipulation of chemical environments
3. Cryo-Electron Microscopy
- Reveals structure of protocell assemblies
- Provides insights into membrane organization
- Shows molecular-level interactions
4. Advanced Computational Modeling
- Simulates prebiotic reaction networks
- Models protocell behavior
- Predicts stable molecular configurations
C. Key Research Trends (2020-2024)
1. Integration of Multiple Approaches
- Combining experimental and computational methods
- Merging chemistry with information theory
- Linking quantum and classical descriptions
2. Focus on System Dynamics
- Emphasis on non-equilibrium processes
- Study of self-organizing systems
- Investigation of energy flows
3. Environmental Context
- Consideration of early Earth conditions
- Role of specific minerals and catalysts
- Influence of physical parameters
D. Emerging Challenges and Questions
Recent research has highlighted several key challenges:
1. Information Problem
- How did meaningful information emerge from chemical systems?
- What role did early information processing play?
- How did coding systems develop?
2. Integration Challenge
- How did different subsystems combine?
- What coordinated multiple chemical processes?
- How did complexity increase systematically?
3. Energy Coupling
- How were energy sources harnessed?
- What drove early metabolic processes?
- How was energy storage achieved?
These contemporary studies continue to reveal the extraordinary complexity of life's origin while highlighting the significant challenges facing naturalistic explanations.
E. Implications for Origin of Life Research
Recent developments (2020-2024) have:
1. Increased our understanding of the complexity involved
2. Revealed new layers of integrated systems
3. Highlighted the need for multiple, synchronized processes
4. Demonstrated the inadequacy of simple, linear explanations
5. Shown the requirement for precise, coordinated mechanisms
While new techniques provide deeper insights into prebiotic chemistry, they also reveal additional layers of complexity that must be explained in any naturalistic origin scenario.
Key Theoretical Developments Over Time:
1. Progression from simple descriptive models to detailed molecular mechanisms
2. Increasing integration of multiple disciplines and approaches
3. Growing emphasis on experimental validation
4. Shift from linear progression models to complex system dynamics
5. Greater attention to chemical and physical plausibility
6. Integration of information theory and complexity science
7. Recognition of the multiple, interconnected challenges in life's origin
This theoretical evolution reflects both advancing scientific knowledge and growing appreciation for the complexity of the origin of life problem. Each new hypothesis has contributed additional insights while also revealing new challenges that must be addressed.
1.3.3. Primordial Soup and Pond Hypotheses
The concept of a "primordial soup" has long been a cornerstone in origin of life theories. This section examines hypotheses that focus on Earth's early aqueous environments as potential cradles of life. From the classic Oparin-Haldane hypothesis to more recent ideas involving wet-dry cycles, these theories explore how Earth's early chemistry might have given rise to the first organic molecules and protocells. These hypotheses paint a picture of early Earth as a vast chemical laboratory, where the ingredients for life slowly came together in primordial waters.
1. 1920s: The Oparin-Haldane Hypothesis: Proposed by Aleksandr Oparin and J.B.S. Haldane. This theory posits that life originated from organic compounds synthesized in a reducing atmosphere, with energy from lightning or ultraviolet light. 1
2. 1950s: Electric Spark Hypothesis: Based on the idea that lightning could have sparked chemical reactions in the Earth's early atmosphere, producing organic compounds from simple molecules, as demonstrated by the Miller-Urey experiment. 2
3. 1953: Miller-Urey Experiment: Conducted by Stanley Miller and Harold Urey, this experiment demonstrated that amino acids could form under early Earth conditions, supporting the Oparin-Haldane Hypothesis. 3
4. 1993: Bubbles Hypothesis: Suggests that bubbles on the surface of the primordial seas could have concentrated and catalyzed organic molecules, eventually leading to the first living cells. 4
5. 2016: Primordial Soup and Shocks Hypothesis: Proposes that shocks from meteorite impacts or lightning could have contributed to the synthesis of organic molecules in the primordial soup. 5
6. 2020: Wet-Dry Cycle Hypothesis: Suggests that life began in environments experiencing wet-dry cycles, such as tidal pools or ponds. These cycles could have driven the formation of complex polymers like RNA and proteins. 6
1.3.4. Hydrothermal Vent and Submarine Hypotheses
The depths of Earth's oceans have led to a group of hypotheses that explore the potential role of hydrothermal vents and submarine environments in fostering the conditions necessary for abiogenesis. These theories suggest that the unique chemical and energy dynamics found in these underwater realms may have provided the perfect crucible for life's emergence. From the discovery of submarine hot springs to recent models proposing near-inevitable life formation, these hypotheses highlight the ocean depths as a promising cradle for early life.
1. 1977: Submarine Hot Springs Hypothesis: Proposed after the discovery of hydrothermal vents. This hypothesis suggests that the energy and chemical conditions at oceanic ridge crests may have initiated life. 1
2. 1980s: Deep Sea Vent Hypothesis: Posits that life originated around hydrothermal vents deep in the ocean, where superheated water rich in minerals provided the energy and chemical conditions necessary for early life. 2
3. 1988: Iron-Sulfur World Hypothesis: Proposed by Günter Wächtershäuser. This theory posits that life originated on iron and nickel sulfide surfaces near hydrothermal vents, where organic molecules were synthesized through catalysis. 3
4. 2016: Hydrothermal Vent Models (Near-Inevitable Life): Posits that life was a near-inevitable consequence of chemical conditions at hydrothermal vents, rather than a miraculous event. 4
5. 2016: LUCA Near Underwater Volcanoes Hypothesis: Suggests that the Last Universal Common Ancestor (LUCA) lived near hydrothermal vents and metabolized hydrogen. 5
6. 2020: Hydrothermal Cliff Hypothesis: Proposes that life originated near underwater cliffs or porous rock formations, where minerals and hydrothermal fluids created ideal microenvironments for the development of early metabolic systems and the formation of cell-like structures. 6
7. 2021: Metabolism-First Hypothesis (Updated): Suggests that self-sustaining metabolic pathways could have formed in deep-sea hydrothermal vents, predating the emergence of genetic materials like RNA or DNA. 7
1.3.5. Volcano-Related Hypotheses
[size=13]Volcanic activity, with its intense heat and unique chemical processes, offers another intriguing avenue for exploring life's origins. The hypotheses in this section examine how volcanic environments might have contributed to the formation of early organic molecules and metabolic processes. These ideas leverage the extreme conditions found in volcanic settings to explain the emergence of life's building blocks. From pyrite formation to electrochemical origins, these theories demonstrate how volcanic environments could have provided the energy and chemical complexity necessary for life's inception.
1. 1988: Pyrite Formation Hypothesis: Proposed by Wächtershäuser. He claimed that the formation of pyrite (FeS2) from hydrogen sulfide and iron provided an energy source for early autotrophic life forms. 1
2. 1995: Thermoreduction Hypothesis: This theory posits that life originated from thermophiles in extreme heat environments, possibly linked to the Last Universal Common Ancestor (LUCA). 2
3. 2022: Electrochemical Origin Hypothesis: Suggests that electric fields, particularly in environments near volcanoes or within the Earth’s crust, might have helped concentrate key ions and organic compounds, kickstarting metabolic and replicative systems. 3
1.3.6. From Space Hypotheses (Panspermia, Meteorites, Solar Wind)
Looking beyond our planet, these hypotheses consider the possibility that life's origins may have extraterrestrial roots. From the concept of panspermia to the role of meteorites and solar wind, these theories explore how cosmic factors might have influenced or even initiated the development of life on Earth. They broaden our perspective on abiogenesis to include the vast expanse of the universe. These hypotheses challenge us to consider how interplanetary or even interstellar processes might have contributed to the emergence of life on our planet.
1. 2011: Asteroids and Formamide Hypothesis: Researchers showed that the combination of meteorite material and formamide could produce nucleic acids and other biomolecules under prebiotic conditions. 1. (Researchers in Italy found that mixing formamide with meteorite material could produce nucleic acids, amino acids, and other biomolecules under prebiotic conditions.)
2. 2015: Meteorite and Solar Wind Hypothesis: Italian researchers suggest that solar wind interacting with meteorite material could have created life's building blocks before they arrived on Earth. 2.(Suggests that meteorite material and solar wind could have synthesized prebiotic compounds in space, which were later delivered to Earth.)
3. 2022: Chemical Evolution of Exoplanets Hypothesis: Proposes that life could have originated on other planets under extreme chemical and environmental conditions, and could have been transported to Earth via panspermia. 3. (Proposes that life could have originated under extreme conditions on other planets, and was transported to Earth via pansper
Last edited by Otangelo on Sun Nov 17, 2024 11:05 am; edited 32 times in total