Forces in Tissue Morphogenesis and Patterning 1
https://reasonandscience.catsboard.com/t2332-forces-in-tissue-morphogenesis-and-patterning
Integral to cell/tissue morphogenesis is the ability of cells to perceive mechanical forces and physical constraints modulating their specification and differentiation.Tissue morphogenesis describes the processes by which a tissue takes shape. Such processes typically involve changes in cell number, size, shape, and position.
When we talk about evolutionary novelties, the change of each of these must be taken into account, and be explained.
Changes in the number of cells within a tissue are achieved by cell proliferation and death. Proliferation of cells is driven by cell divisions,
How is the number of new cells produced regulated ? What determines the number of cell divisions ?
which distribute the two daughter cells along the orientation of division.
how is the right distribution achieved ?
Cell death usually results in the disappearance of the dying cell, vacating the position of the cell taken before its death.
How is cell death programmed and regulated to happen at the right time , and the right cells ?
Changes in cell size and shape can have manifold expressions—cells can increase their size, e.g., by metabolic growth or osmotic swelling.
How is the right cell shape, size , and expression of the right cell type achieved, aka programmed ?
Cell shape changes can range from large-scale changes, such as cell elongation, to local modulations in cell shape, such as the formation of specialized cell protrusions. Finally, changes in cell position are brought about by either cell migration or cellular rearrangements, such as cell intercalations and/or neighbor exchanges. Important for all these cellular processes to trigger tissue shape change is some form of force transmission between individual cells, commonly mediated by cell-cell adhesion. This will allow individual cell changes to be translated into more global changes in tissue morphology.
In order to get macroevolutionary novelties, new limbs, new body plans etc, in many cases tissue morphology must change, and new tissues and new cells must arise in a ordered coordinated fashion. How could this happen through mutations and natural selection , where a distant specific goal must be achieved ?
An example for coordinated changes in the shape of individual cells giving rise to global alterations in tissue morphology is the constriction of epithelial cells at their apical side, leading to local bending of epithelial cell sheets (reviewed in Pilot and Lecuit, 2005). Likewise, coordinated changes in the position of individual cells trigger tissue rotation (Aigouy et al., 2010; Suzanne et al., 2010) or simultaneous tissue narrowing and elongation due to cell intercalations (for review, see Keller, 2006). Finally, spatially controlled cell proliferation, cell division orientation, and cell death within multicellular tissues can give rise to global changes in tissue shape (reviewed in Hopyan et al., 2011). Thus, understanding tissue morphogenesis requires deciphering how forces are being generated on an individual cell basis, how those forces are being transmitted to neighboring cells, and how they are integrated within the tissue to trigger global changes in tissue shape.
Last not least, how is the origin of these forces explained ?
Although cells can generate forces via actin or microtubule polymerization and osmotic pressure, cellular force generation typically relies on the activities of motor proteins, such as myosins (reviewed in Howard, 2001).
These proteins interact with cytoskeletal structures such as actin fibers to change their organization (reviewed in Salbreux et al., 2012). Cytoskeletal changes are transmitted to neighboring cells and the extracellular environment by connecting the cytoskeleton to cell-cell and cell-matrix adhesion molecules such as cadherins and integrins, respectively.
How is the right connection achieved ? What mechanisms are acting to program the cytoskeleton to behave correctly , to connect to cell cell and cell matrix adhesion molecules at the right place ?
It is now well established that cell cortical tension due to actin-myosin contraction and cadherin-mediated cell-cell adhesion represent two fundamental and evolutionarily highly conserved force-generating and transmitting cell properties driving tissue self-organization (Dickinson et al., 2011).
To conceptualize how those properties drive tissue self-organization, various models have been developed. In most models, it is assumed that the tissue evolves via a succession of equilibrium states and that, therefore, the sum of the mechanical forces is in balance.
Equilibrium states are not the norm. They need to have a very specific complex configuration in order to reach this state. How was it achieved ?
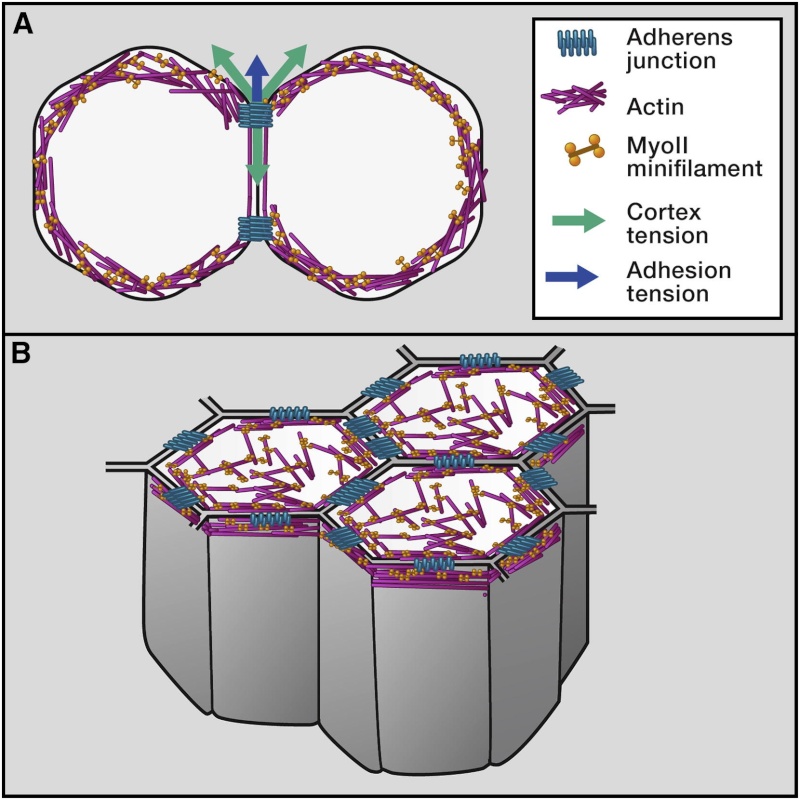
Mechanical equations can be written and solved either analytically or by using finite element methods to characterize tissue dynamics (Brodland et al., 2007; Ranft et al., 2010; Hannezo et al., 2012). Furthermore, assuming that adhesion and cortical tension are dominant determinants of cell/tissue shape and that cells/tissues have an inherent tendency to minimize their surface free energy, cell and tissue shapes can be described by their state of lowest energy (Steinberg, 1963; Foty et al., 1996). The nature of this energy relies on the binding of adhesion molecules causing cells to expand their cell-cell contacts and the contractile activity of the actin-myosin cell cortex inhibiting contact expansion at the contact and promoting it outside of the contact (reviewed in Amack and Manning, 2012; Figure 1A). A mathematical formulation of the concept of energy minimization to describe the organization of multicellular structures based on the combined activities of cortical tension and adhesion has been provided by the Cellular Potts Model (CPM), which has successfully been used to explain the outcome of various morphogenetic processes, such as cell positioning in the Drosophila ommatidium and in germ-layer progenitor cell segregation during vertebrate gastrulation (Graner and Glazier, 1992; Käfer et al., 2007; Krieg et al., 2008). Although those studies show that using the CPM is, in principle, sufficient to accurately describe how the combined activities of cortical tension and adhesion determine tissue organization, experimental tools to measure the input parameters, such as cell adhesion and cortex tension, are still sparse. One approach in this direction has been studies in zebrafish, in which experimentally determined values of cell adhesion (derived from the deadhesion forces of cell-cell contacts) and cortex tension have been used to show that cortical tension, rather than adhesion energy, drives progenitor cell-cell contact formation and segregation during zebrafish gastrulation (Krieg et al., 2008; Maître et al., 2012).
The principle of energy minimization has also been applied to various forms of epithelial morphogenesis in vertebrates and invertebrates. In Drosophila, the configuration of cell-cell junctions is thought to be driven by the interplay between the elasticity of the cell and cortical contractility and adhesion at the junctions (reviewed in Lecuit et al., 2011; Figure 1B).
The mathematical formulation of this concept in the form of a two-dimensional “vertex-model” and related models has been successfully applied to describe various types of morphogenetic processes in the Drosophila wing disc and germ-band epithelium (Farhadifar et al., 2007;Rauzi et al., 2008; Landsberg et al., 2009; Aegerter-Wilmsen et al., 2010; Aigouy et al., 2010; Schilling et al., 2011; Aliee et al., 2012). Examples for this are the formation of tissue compartment boundaries in Drosophila, in which anisotropic accumulation of myosin II (MyoII) at cell-cell junctions facing the boundary leads to enhanced contractility of the boundary, which, in turn, straightens the boundary and prevents cell mixing over it (Landsberg et al., 2009; Monier et al., 2010; Aliee et al., 2012). Furthermore, anisotropic MyoII accumulation at cell-cell junctions has been proposed to drive shortening of those junctions, which give rise to the cellular rearrangements underlying Drosophila germ-band extension and vertebrate neural tube folding (Rauzi et al., 2008; Nishimura et al., 2012). Finally, in ascidian gastrulation, reverse modeling to determine cell properties based on the morphogenetic process itself showed that increased cortical tension at the cell apex and along the lateral junctions promotes apical cell constriction and apical-basal cell shortening (Sherrard et al., 2010).
Cortical Tension Allocates the First Inner Cells of the Mammalian Embryo. 2
Every cell in our body originates from the pluripotent inner mass of the embryo, yet it is unknown how biomechanical forces allocate inner cells in vivo. Here we discover subcellular heterogeneities in tensile forces, generated by actomyosin cortical networks, which drive apical constriction to position the first inner cells of living mouse embryos. Myosin II accumulates specifically around constricting cells, and its disruption dysregulates constriction and cell fate. Laser ablations of actomyosin networks reveal that constricting cells have higher cortical tension, generate tension anisotropies and morphological changes in adjacent regions of neighboring cells, and require their neighbors to coordinate their own changes in shape. Thus, tensile forces determine the first spatial segregation of cells during mammalian development. We propose that, unlike more cohesive tissues, the early embryo dissipates tensile forces required by constricting cells via their neighbors, thereby allowing confined cell repositioning without jeopardizing global architecture.
Force transmission in epithelial tissues 3
In epithelial tissues, cells constantly generate and transmit forces between each other. Forces generated by the actomyosin cytoskeleton regulate tissue shape and structure and also provide signals that influence cells' decisions to divide, die, or differentiate.
Signals depend on information. Where did the information come from to instruct the cells to divide, die, or differentiate ?
Forces are transmitted across epithelia because cells are mechanically linked through junctional complexes, and forces can propagate through the cell cytoplasm. Here, we review some of the molecular mechanisms responsible for force generation, with a specific focus on the actomyosin cortex and adherens junctions. We then discuss evidence for how these mechanisms promote cell shape changes and force transmission in tissues.Taken together, various types of tissue self-organization can be explained by models based on the concept of energy minimization given by the combined activities of cell cortical tension and adhesion. However, the molecular and cellular mechanisms by which cortical tension and adhesion function together in these processes and the potential contribution of other fundamental cell properties, such as cell motility and directed migration, still need to be investigated.
First, the role of actin-myosin flow dynamics (pulsatile versus continuous) and direction (centripetal or anisotropic) for spatiotemporal variations in force generation have to be considered.
Second, for actin-myosin flows to result in cell/tissue shape changes, the flows need to be effectively coupled to adhesion complexes at the cell surface that transmit the forces resulting from those flows to other parts of the tissue.
Third, for processes in which cell/tissue deformations are transient due to pulsatile actin-myosin flows, for example, these deformations need to be stabilized in order to result in persistent cell shape changes. In the following, we will describe examples of developmental processes in which the above-mentioned aspects have been involved at varying degrees for describing the underlying dynamic changes in cell/tissue morphogenesis.
Similar to the situation in Drosophila gastrulation, ingression of endodermal precursors in C. elegans gastrulation is triggered by pulsatile, isotropic, and centripetal actin-myosin flows at the apex of these cells. Interestingly, these pulsatile apical actin-myosin flows do not initially produce significant apical cell constrictions, suggesting that the actin-myosin network is not yet efficiently coupled to the apical junctions of the endodermal cells. Eventually, the pulsatile actin-myosin flows are translated into apical cell constrictions due to junctional coupling of the actin-myosin network, which stepwise reduces the size of the cell apex.
During cell intercalation, junction shortening is followed by the formation and extension of new junctions oriented perpendicular to the shortened junctions. In the Drosophila pupal wing, elongation and stabilization of these newly formed junctions is dependent on the activity of the PTEN tumor suppressor, which reduces MyoII level at the newly formed junction. This illustrates that junction lengthening can also be an active process and explains how MyoII homogenous cortical distribution can be restored upon intercalation to control tissue organization (Bardet et al., 2013).
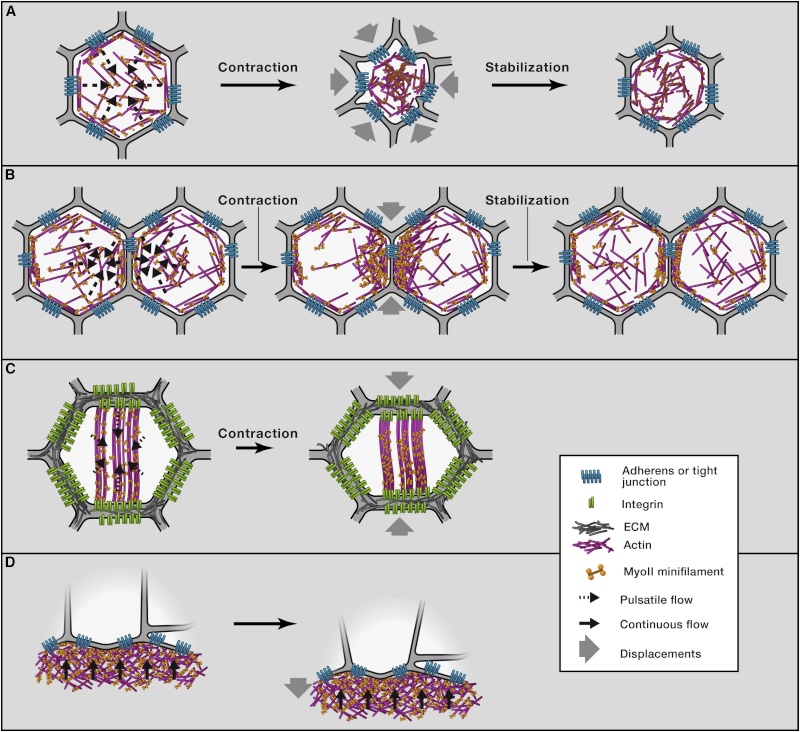
(A) Once coupled to adhesive contacts, pulsatile and centripetal flow of the apical actin-myosin network promotes apical cell constriction. In Drosophila mesodermal cells, the accumulation of apical actin-myosin is thought to stabilize cell shape changes between each pulse, leading to incremental reductions of the cell apex area.
(B) Pulsatile anisotropic flow induces junction shortening during cell intercalation. Resultant enrichment of actin-myosin at the junction stabilizes junction length reduction.
(C) Basal myosin flow on a static-oriented actin network produces anisotropic deformation of the base of the Drosophila follicular cells.
(D) Continuous actin-myosin flow in the zebrafish yolk cell produces the mechanical force necessary for EVL spreading over the yolk cell during early zebrafish development.
1) http://www.cell.com/cell/fulltext/S0092-8674(13)00573-4
2) http://www.ncbi.nlm.nih.gov/pubmed/26279486
3) http://www.ncbi.nlm.nih.gov/pubmed/26756938
4) https://en.wikipedia.org/wiki/Apical_constriction
https://reasonandscience.catsboard.com/t2332-forces-in-tissue-morphogenesis-and-patterning
Integral to cell/tissue morphogenesis is the ability of cells to perceive mechanical forces and physical constraints modulating their specification and differentiation.Tissue morphogenesis describes the processes by which a tissue takes shape. Such processes typically involve changes in cell number, size, shape, and position.
When we talk about evolutionary novelties, the change of each of these must be taken into account, and be explained.
Changes in the number of cells within a tissue are achieved by cell proliferation and death. Proliferation of cells is driven by cell divisions,
How is the number of new cells produced regulated ? What determines the number of cell divisions ?
which distribute the two daughter cells along the orientation of division.
how is the right distribution achieved ?
Cell death usually results in the disappearance of the dying cell, vacating the position of the cell taken before its death.
How is cell death programmed and regulated to happen at the right time , and the right cells ?
Changes in cell size and shape can have manifold expressions—cells can increase their size, e.g., by metabolic growth or osmotic swelling.
How is the right cell shape, size , and expression of the right cell type achieved, aka programmed ?
Cell shape changes can range from large-scale changes, such as cell elongation, to local modulations in cell shape, such as the formation of specialized cell protrusions. Finally, changes in cell position are brought about by either cell migration or cellular rearrangements, such as cell intercalations and/or neighbor exchanges. Important for all these cellular processes to trigger tissue shape change is some form of force transmission between individual cells, commonly mediated by cell-cell adhesion. This will allow individual cell changes to be translated into more global changes in tissue morphology.
In order to get macroevolutionary novelties, new limbs, new body plans etc, in many cases tissue morphology must change, and new tissues and new cells must arise in a ordered coordinated fashion. How could this happen through mutations and natural selection , where a distant specific goal must be achieved ?
An example for coordinated changes in the shape of individual cells giving rise to global alterations in tissue morphology is the constriction of epithelial cells at their apical side, leading to local bending of epithelial cell sheets (reviewed in Pilot and Lecuit, 2005). Likewise, coordinated changes in the position of individual cells trigger tissue rotation (Aigouy et al., 2010; Suzanne et al., 2010) or simultaneous tissue narrowing and elongation due to cell intercalations (for review, see Keller, 2006). Finally, spatially controlled cell proliferation, cell division orientation, and cell death within multicellular tissues can give rise to global changes in tissue shape (reviewed in Hopyan et al., 2011). Thus, understanding tissue morphogenesis requires deciphering how forces are being generated on an individual cell basis, how those forces are being transmitted to neighboring cells, and how they are integrated within the tissue to trigger global changes in tissue shape.
Last not least, how is the origin of these forces explained ?
Although cells can generate forces via actin or microtubule polymerization and osmotic pressure, cellular force generation typically relies on the activities of motor proteins, such as myosins (reviewed in Howard, 2001).
These proteins interact with cytoskeletal structures such as actin fibers to change their organization (reviewed in Salbreux et al., 2012). Cytoskeletal changes are transmitted to neighboring cells and the extracellular environment by connecting the cytoskeleton to cell-cell and cell-matrix adhesion molecules such as cadherins and integrins, respectively.
How is the right connection achieved ? What mechanisms are acting to program the cytoskeleton to behave correctly , to connect to cell cell and cell matrix adhesion molecules at the right place ?
It is now well established that cell cortical tension due to actin-myosin contraction and cadherin-mediated cell-cell adhesion represent two fundamental and evolutionarily highly conserved force-generating and transmitting cell properties driving tissue self-organization (Dickinson et al., 2011).
To conceptualize how those properties drive tissue self-organization, various models have been developed. In most models, it is assumed that the tissue evolves via a succession of equilibrium states and that, therefore, the sum of the mechanical forces is in balance.
Equilibrium states are not the norm. They need to have a very specific complex configuration in order to reach this state. How was it achieved ?
Mechanical equations can be written and solved either analytically or by using finite element methods to characterize tissue dynamics (Brodland et al., 2007; Ranft et al., 2010; Hannezo et al., 2012). Furthermore, assuming that adhesion and cortical tension are dominant determinants of cell/tissue shape and that cells/tissues have an inherent tendency to minimize their surface free energy, cell and tissue shapes can be described by their state of lowest energy (Steinberg, 1963; Foty et al., 1996). The nature of this energy relies on the binding of adhesion molecules causing cells to expand their cell-cell contacts and the contractile activity of the actin-myosin cell cortex inhibiting contact expansion at the contact and promoting it outside of the contact (reviewed in Amack and Manning, 2012; Figure 1A). A mathematical formulation of the concept of energy minimization to describe the organization of multicellular structures based on the combined activities of cortical tension and adhesion has been provided by the Cellular Potts Model (CPM), which has successfully been used to explain the outcome of various morphogenetic processes, such as cell positioning in the Drosophila ommatidium and in germ-layer progenitor cell segregation during vertebrate gastrulation (Graner and Glazier, 1992; Käfer et al., 2007; Krieg et al., 2008). Although those studies show that using the CPM is, in principle, sufficient to accurately describe how the combined activities of cortical tension and adhesion determine tissue organization, experimental tools to measure the input parameters, such as cell adhesion and cortex tension, are still sparse. One approach in this direction has been studies in zebrafish, in which experimentally determined values of cell adhesion (derived from the deadhesion forces of cell-cell contacts) and cortex tension have been used to show that cortical tension, rather than adhesion energy, drives progenitor cell-cell contact formation and segregation during zebrafish gastrulation (Krieg et al., 2008; Maître et al., 2012).
The principle of energy minimization has also been applied to various forms of epithelial morphogenesis in vertebrates and invertebrates. In Drosophila, the configuration of cell-cell junctions is thought to be driven by the interplay between the elasticity of the cell and cortical contractility and adhesion at the junctions (reviewed in Lecuit et al., 2011; Figure 1B).

Self-Organization of Cells at Steady State Determined by Actin-Myosin Contractility and Cell Adhesion
(A) Upon cell-cell contact, the contacting cells change their shape in response to mechanical forces associated with actin-myosin contractility (green arrow) and adhesion (blue arrow).
(B) In epithelial tissues, adhesive contacts and the actin-myosin network are organized in belt-like structures at the apical domain of the cell. At steady state, the arrangement of epithelial cells at their apex is determined by actin-myosin contractility and cell-cell adhesion.
The mathematical formulation of this concept in the form of a two-dimensional “vertex-model” and related models has been successfully applied to describe various types of morphogenetic processes in the Drosophila wing disc and germ-band epithelium (Farhadifar et al., 2007;Rauzi et al., 2008; Landsberg et al., 2009; Aegerter-Wilmsen et al., 2010; Aigouy et al., 2010; Schilling et al., 2011; Aliee et al., 2012). Examples for this are the formation of tissue compartment boundaries in Drosophila, in which anisotropic accumulation of myosin II (MyoII) at cell-cell junctions facing the boundary leads to enhanced contractility of the boundary, which, in turn, straightens the boundary and prevents cell mixing over it (Landsberg et al., 2009; Monier et al., 2010; Aliee et al., 2012). Furthermore, anisotropic MyoII accumulation at cell-cell junctions has been proposed to drive shortening of those junctions, which give rise to the cellular rearrangements underlying Drosophila germ-band extension and vertebrate neural tube folding (Rauzi et al., 2008; Nishimura et al., 2012). Finally, in ascidian gastrulation, reverse modeling to determine cell properties based on the morphogenetic process itself showed that increased cortical tension at the cell apex and along the lateral junctions promotes apical cell constriction and apical-basal cell shortening (Sherrard et al., 2010).
Cortical Tension Allocates the First Inner Cells of the Mammalian Embryo. 2
Every cell in our body originates from the pluripotent inner mass of the embryo, yet it is unknown how biomechanical forces allocate inner cells in vivo. Here we discover subcellular heterogeneities in tensile forces, generated by actomyosin cortical networks, which drive apical constriction to position the first inner cells of living mouse embryos. Myosin II accumulates specifically around constricting cells, and its disruption dysregulates constriction and cell fate. Laser ablations of actomyosin networks reveal that constricting cells have higher cortical tension, generate tension anisotropies and morphological changes in adjacent regions of neighboring cells, and require their neighbors to coordinate their own changes in shape. Thus, tensile forces determine the first spatial segregation of cells during mammalian development. We propose that, unlike more cohesive tissues, the early embryo dissipates tensile forces required by constricting cells via their neighbors, thereby allowing confined cell repositioning without jeopardizing global architecture.
Force transmission in epithelial tissues 3
In epithelial tissues, cells constantly generate and transmit forces between each other. Forces generated by the actomyosin cytoskeleton regulate tissue shape and structure and also provide signals that influence cells' decisions to divide, die, or differentiate.
Signals depend on information. Where did the information come from to instruct the cells to divide, die, or differentiate ?
Forces are transmitted across epithelia because cells are mechanically linked through junctional complexes, and forces can propagate through the cell cytoplasm. Here, we review some of the molecular mechanisms responsible for force generation, with a specific focus on the actomyosin cortex and adherens junctions. We then discuss evidence for how these mechanisms promote cell shape changes and force transmission in tissues.Taken together, various types of tissue self-organization can be explained by models based on the concept of energy minimization given by the combined activities of cell cortical tension and adhesion. However, the molecular and cellular mechanisms by which cortical tension and adhesion function together in these processes and the potential contribution of other fundamental cell properties, such as cell motility and directed migration, still need to be investigated.
Force Generation and Transmission at Cell Scale
The concept of energy minimization based on the activities of adhesion and cortical contractility provides valuable insights into how the distribution of molecules determining the adhesive and contractile cell properties dictate cell and tissue shape at equilibrium. To account for the inherent dynamics in cell and tissue morphogenesis, several studies have begun to analyze how dynamic changes in the subcellular distribution of cytoskeletal and adhesive components drive tissue morphogenesis. Most prominently, intracellular flows of actin and/or myosin have been involved in various key morphogenetic processes in embryogenesis. Flows of actin and myosin have been extensively studied on a single-cell level in processes such as cell migration, cytokinesis, and zygote polarization (Bray and White, 1988; Munro et al., 2004; Mayer et al., 2010). In order to understand how those single-cell flows give rise to changes in tissue morphogenesis, several important aspects related to force generation by actin-myosin flows need to be taken into account.First, the role of actin-myosin flow dynamics (pulsatile versus continuous) and direction (centripetal or anisotropic) for spatiotemporal variations in force generation have to be considered.
Second, for actin-myosin flows to result in cell/tissue shape changes, the flows need to be effectively coupled to adhesion complexes at the cell surface that transmit the forces resulting from those flows to other parts of the tissue.
Third, for processes in which cell/tissue deformations are transient due to pulsatile actin-myosin flows, for example, these deformations need to be stabilized in order to result in persistent cell shape changes. In the following, we will describe examples of developmental processes in which the above-mentioned aspects have been involved at varying degrees for describing the underlying dynamic changes in cell/tissue morphogenesis.
Gastrulation
In Drosophila gastrulation, mesoderm invagination is driven by the coordinated apical constriction of mesodermal cells (reviewed in Leptin, 1995). Apical constriction 4 of invaginating mesodermal cells again is triggered by the formation of MyoII spots and fibers at their apical cortex (Martin et al., 2010). These apical MyoII structures are dynamic, repeatedly increase in intensity, and move toward the center of the cell apex, resulting in pulsatile centripetal actin-myosin flows. Pulsatile flows translate into periodic apical constrictions of mesodermal cells due to the inward movement of the apical cell-cell junctions to which the actin-myosin network is coupled (Martin et al., 2009; Roh-Johnson et al., 2012; Figure 2A and ). Apical constrictions are eventually stabilized by the maintenance of higher levels of MyoII at the apex of the cell. Actin-myosin network coupling to apical junctions also leads to apical MyoII organizing into a supracellular network that connects each cell to transmit forces across the tissue (Martin et al., 2010).Similar to the situation in Drosophila gastrulation, ingression of endodermal precursors in C. elegans gastrulation is triggered by pulsatile, isotropic, and centripetal actin-myosin flows at the apex of these cells. Interestingly, these pulsatile apical actin-myosin flows do not initially produce significant apical cell constrictions, suggesting that the actin-myosin network is not yet efficiently coupled to the apical junctions of the endodermal cells. Eventually, the pulsatile actin-myosin flows are translated into apical cell constrictions due to junctional coupling of the actin-myosin network, which stepwise reduces the size of the cell apex.
Coordinated Cell Intercalation
Actin-myosin flows have also been observed during epithelial tissue elongation driven by coordinated cell intercalations (Skoglund et al., 2008; Rauzi et al., 2010;Sawyer et al., 2011). Examples for this are the pulsatile actin-myosin flows found at the apex of epithelial cells during cell intercalation along the dorsal-ventral (DV) axis of the Drosophila germ band, leading to germ-band elongation along its anterior-posterior (AP) axis (Rauzi et al., 2010; Sawyer et al., 2011; Figure 2B ). These flows are both centripetal, leading to the formation of local actin-myosin accumulations, and are anisotropically oriented toward the DV junctions of the cells, leading to MyoII accumulation there. Anisotropic flow of MyoII toward the DV junctions causes shortening of these junctions, which is an important step in cell intercalation during germ-band elongation. Accumulation of MyoII at the DV junction coincides with the shortening of DV junctions and is thought to be required for stabilization of the shortened junction. The coupling and/or orientation of the actin-myosin flow to the DV junction require the activity of α-catenin, E-cadherin, and Canoe/Afadin (Rauzi et al., 2010; Sawyer et al., 2011). Notably, the concentrations of catenins and E-Cadherin are lower at DV junctions compared to AP junctions (Simões et al., 2010; Rauzi et al., 2010;Tamada et al., 2012). The E-Cadherin concentration is regulated by the Frizzled planar cell polarity pathway via RhoGEF2 (Warrington et al., 2013). Such lower concentration is hypothesized to more loosely anchor the actin-myosin network between the two DV junctions of the cell and thus allow the actin-myosin network to more freely move between these two junctions (Rauzi et al., 2010).During cell intercalation, junction shortening is followed by the formation and extension of new junctions oriented perpendicular to the shortened junctions. In the Drosophila pupal wing, elongation and stabilization of these newly formed junctions is dependent on the activity of the PTEN tumor suppressor, which reduces MyoII level at the newly formed junction. This illustrates that junction lengthening can also be an active process and explains how MyoII homogenous cortical distribution can be restored upon intercalation to control tissue organization (Bardet et al., 2013).

Figure 2
Actin-Myosin Network Dynamics and Force Generation(A) Once coupled to adhesive contacts, pulsatile and centripetal flow of the apical actin-myosin network promotes apical cell constriction. In Drosophila mesodermal cells, the accumulation of apical actin-myosin is thought to stabilize cell shape changes between each pulse, leading to incremental reductions of the cell apex area.
(B) Pulsatile anisotropic flow induces junction shortening during cell intercalation. Resultant enrichment of actin-myosin at the junction stabilizes junction length reduction.
(C) Basal myosin flow on a static-oriented actin network produces anisotropic deformation of the base of the Drosophila follicular cells.
(D) Continuous actin-myosin flow in the zebrafish yolk cell produces the mechanical force necessary for EVL spreading over the yolk cell during early zebrafish development.
1) http://www.cell.com/cell/fulltext/S0092-8674(13)00573-4
2) http://www.ncbi.nlm.nih.gov/pubmed/26279486
3) http://www.ncbi.nlm.nih.gov/pubmed/26756938
4) https://en.wikipedia.org/wiki/Apical_constriction


