http://reasonandscience.heavenforum.org/t2187-cell-junctions-and-the-extracellular-matrix
Of all the social interactions between cells in a multicellular organism, the most fundamental are those that hold the cells together. Cells may be linked by direct interactions, or they may be held together within the extracellular matrix, a complex network of proteins and polysaccharide chains that the cells secrete. By one means or another, cells must cohere if they are to form an organized multicellular structure that can withstand and respond to the various external forces that try to pull it apart. The mechanisms of cohesion govern the architecture of the body—its shape, its strength, and the arrangement of its different cell types. The making and breaking of the attachments between cells and the modeling of the extracellular matrix govern the way cells move within the organism, guiding them as the body grows, develops, and repairs itself. Attachments to other cells and to extracellular matrix control the orientation and behavior of the cell’s cytoskeleton, thereby allowing cells to sense and respond to changes in the mechanical features of their environment. Thus, the apparatus of cell junctions and the extracellular matrix is critical for every aspect of the organization, function, and dynamics of multicellular structures. Defects in this apparatus underlie an enormous variety of diseases. The key features of cell junctions and the extracellular matrix are best illustrated by considering two broad categories of tissues that are found in all animals.
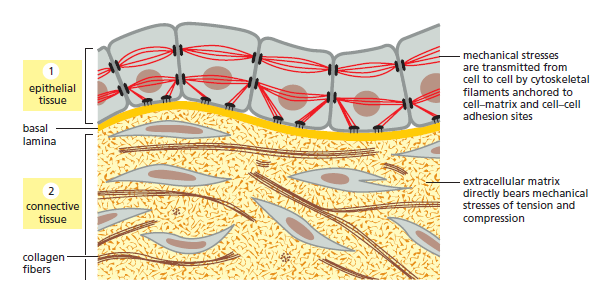
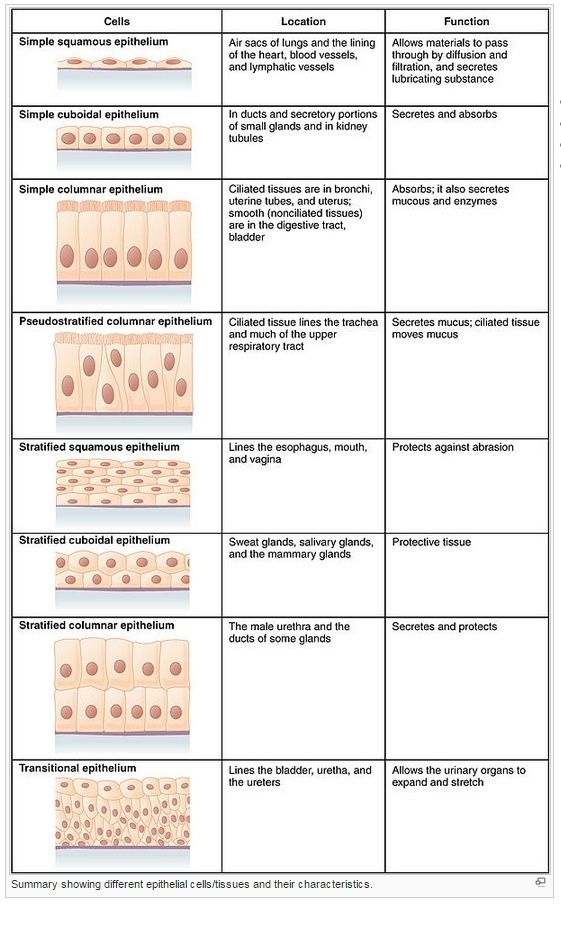
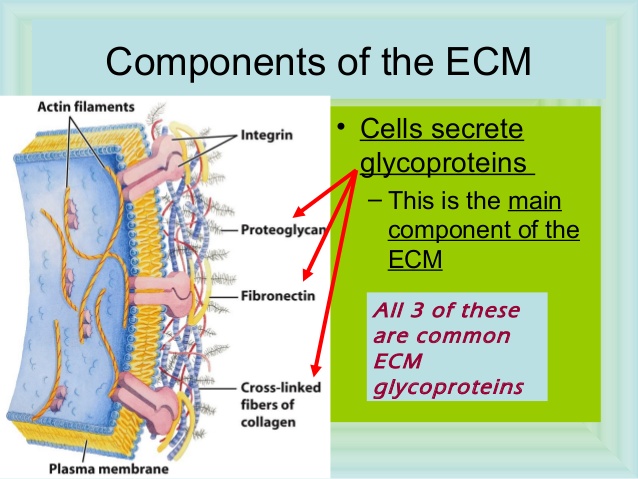
Connective tissues, such as bone or tendon, are formed from an extracellular matrix produced by cells that are distributed sparsely in the matrix. It is the matrix—rather than the cells—that bears most of the mechanical stress to which the tissue is subjected. Direct attachments between one cell and another are relatively rare, but the cells have important attachments to the matrix. These cell–matrix junctions link the cytoskeleton to the matrix, allowing the cells to move through the matrix and monitor changes in its mechanical properties. In epithelial tissues, such as the lining of the gut or the epidermal covering of the skin, cells are tightly bound together into sheets called epithelia. The extracellular matrix is less pronounced, consisting mainly of a thin mat called the basal lamina (or basement membrane) underlying the sheet. Within the epithelium, cells are attached to each other directly by cell–cell junctions, where cytoskeletal filaments are anchored, transmitting stresses across the interiors of the cells, from adhesion site to adhesion site. The cytoskeleton of epithelial cells is also linked to the basal lamina through cell–matrix junctions
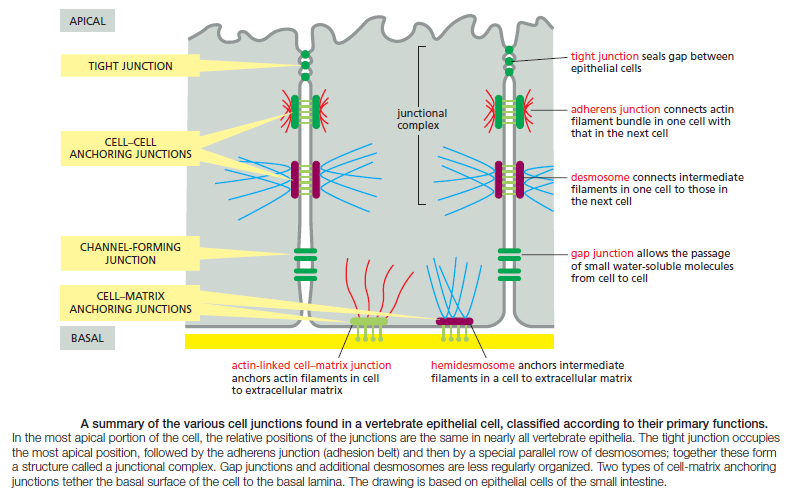
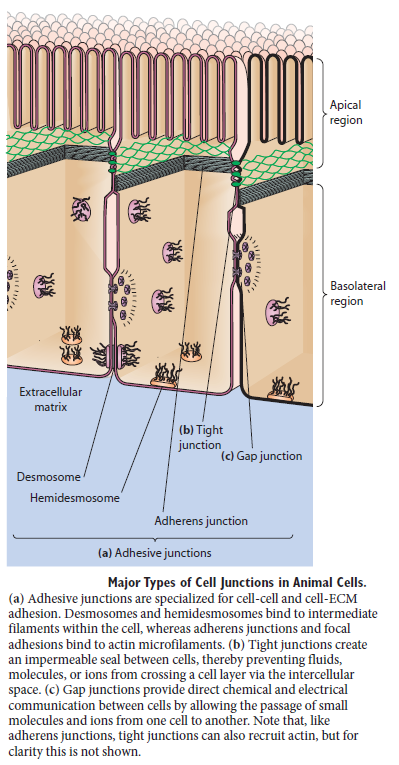
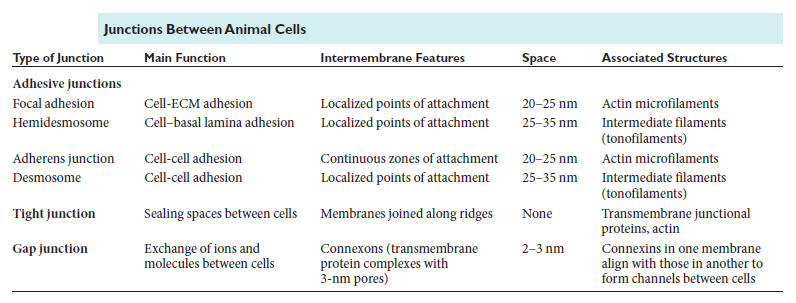
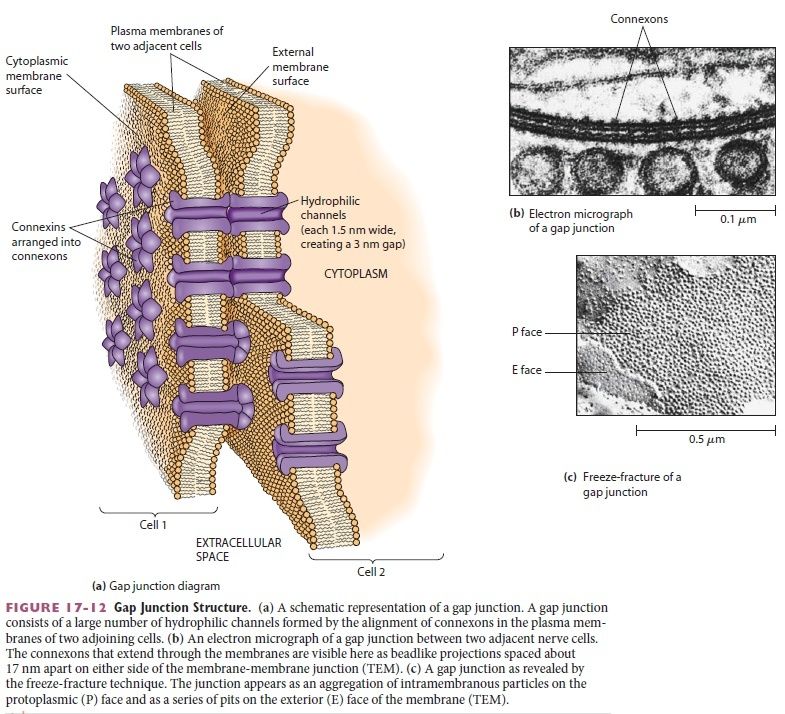
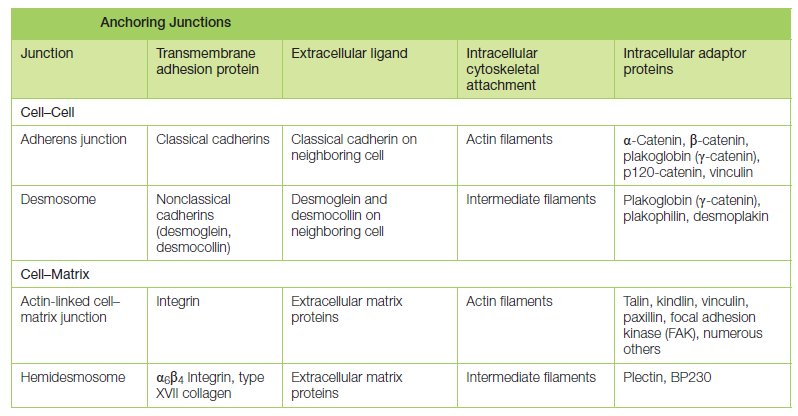
Figure above provides a closer view of epithelial cells to illustrate the major types of cell–cell and cell–matrix junctions. The diagram shows the typical arrangement of junctions in a simple columnar epithelium such as the lining of the small intestine of a vertebrate. Here, a single layer of tall cells stands on a basal lamina, with the cells’ uppermost surface, or apex, free and exposed to the extracellular medium. On their sides, or lateral surfaces, the cells make junctions with one another. Two types of anchoring junctions link the cytoskeletons of adjacent cells: adherens junctions are anchorage sites for actin filaments; desmosomes are anchorage sites for intermediate filaments. Two additional types of anchoring junctions link the cytoskeleton of the epithelial cells to the basal lamina: actin-linked cell–matrix junctions anchor actin filaments to the matrix, while hemidesmosomes anchor intermediate filaments to it.
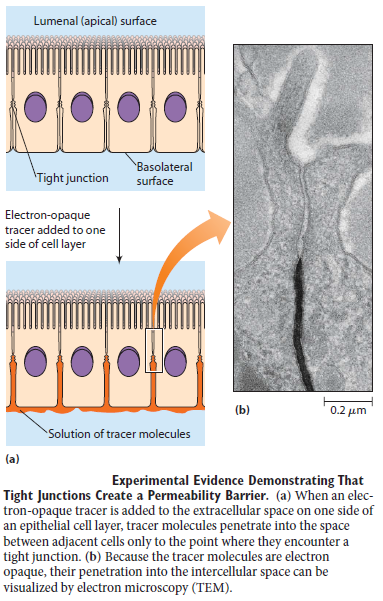
Two other types of cell–cell junction are shown in Figure above. Tight junctions hold the cells closely together near the apex, sealing the gap between the cells and thereby preventing molecules from leaking across the epithelium. Near the basal end of the cells are channel-forming junctions, called gap junctions, that create passageways linking the cytoplasms of adjacent cells. Each of the four major anchoring junction types depends on transmembrane adhesion proteins that span the plasma membrane, with one end linking to the cytoskeleton inside the cell and the other end linking to other structures outside it
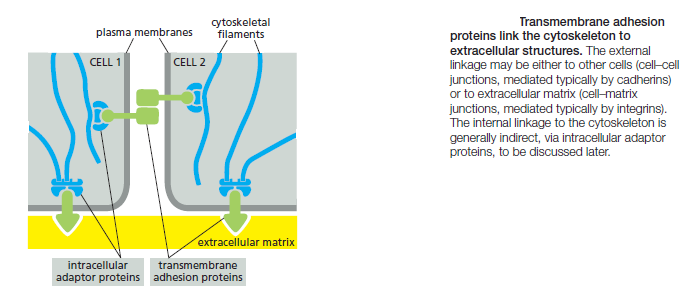
These cytoskeleton-linked transmembrane proteins fall neatly into two superfamilies, corresponding to the two basic kinds of external attachment. Proteins of the cadherin superfamily chiefly mediate attachment of cell to cell
Proteins of the integrin superfamily chiefly mediate attachment of cells to matrix. There is specialization within each family: some cadherins link to actin and form adherens junctions, while others link to intermediate filaments and form desmosomes; likewise, some integrins link to actin and form actinlinked cell–matrix junctions, while others link to intermediate filaments and form hemidesmosomes
There are some exceptions to these rules. Some integrins, for example, mediate cell–cell rather than cell–matrix attachment. Moreover, there are other types of cell adhesion molecules that can provide transient cell–cell attachments more flimsy than anchoring junctions, but sufficient to stick cells together in special circumstances. We begin the chapter with a discussion of the major forms of cell–cell junctions. We then consider in turn the extracellular matrix of animals, the structure and function of integrin-mediated cell–matrix junctions, and, finally, the plant cell wall, a special form of extracellular matrix (ECM)
CELL–CELL JUNCTIONS
Cell–cell junctions come in many forms and can be regulated by a variety of mechanisms. The best understood and most common are the two types of cell– cell anchoring junctions, which employ cadherins to link the cytoskeleton of one cell with that of its neighbor. Their primary function is to resist the external forces that pull cells apart. The epithelial cells of your skin, for example, must remain tightly linked when they are stretched, pinched, or poked. Cell–cell anchoring junctions must also be dynamic and adaptable, so that they can be altered or rearranged when tissues are remodeled or repaired, or when there are changes in the forces acting on them. In this section, we focus primarily on the cadherin-based anchoring junctions. We then briefly describe tight junctions and gap junctions. Finally, we consider the more transient cell–cell adhesion mechanisms employed by some cells in the bloodstream.
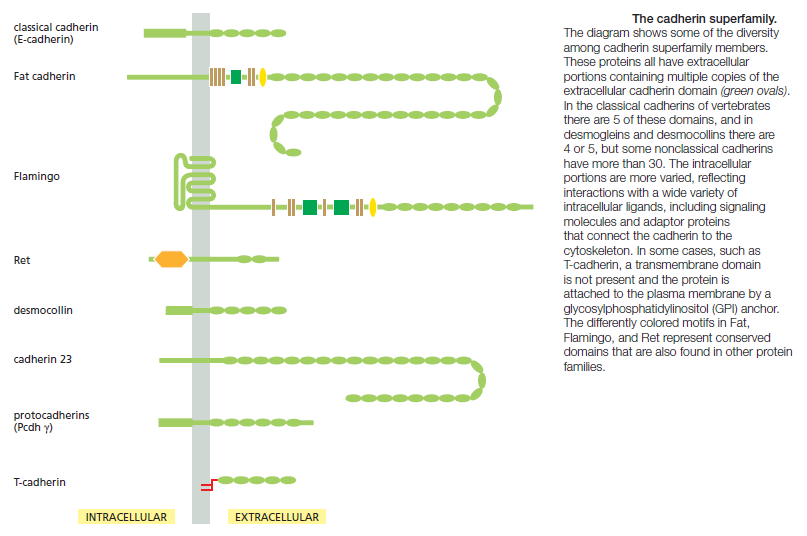
Cadherins Form a Diverse Family of Adhesion Molecules
Cadherins are present in all multicellular animals whose genomes have been analyzed. They are also present in the choanoflagellates, which can exist either as free-living unicellular organisms or as multicellular colonies and are thought to be representatives of the group of protists . Other eukaryotes, including fungi and plants, lack cadherins, and they are also absent from bacteria and archaea. Cadherins therefore seem to be part of the essence of what it is to be an animal. The cadherins take their name from their dependence on Ca2+ ions: removing Ca2+ from the extracellular medium causes adhesions mediated by cadherins to come apart. The first three cadherins to be discovered were named according to the main tissues in which they were found: E-cadherin is present on many types of epithelial cells; N-cadherin on nerve, muscle, and lens cells; and P-cadherin on cells in the placenta and epidermis. All are also found in other tissues. These and other classical cadherins are closely related in sequence throughout their extracellular and intracellular domains. There are also a large number of nonclassical cadherins that are more distantly related in sequence, with more than 50 expressed in the brain alone. The nonclassical cadherins include proteins with known adhesive function, such as the diverse protocadherins found in the brain, and the desmocollins and desmogleins that form desmosomes . Other family members are involved primarily in signaling. Together, the classical and nonclassical cadherin proteins constitute the cadherin superfamily , with more than 180 members
in humans.
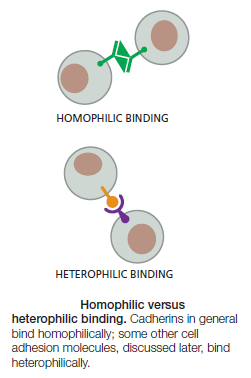
Cadherins Mediate Homophilic Adhesion
Anchoring junctions between cells are usually symmetrical: if the linkage is to actin in the cell on one side of the junction, it will be to actin in the cell on the other side. In fact, the binding between cadherins is generally homophilic (like to- like ): cadherin molecules of a specific subtype on one cell bind to cadherin molecules of the same or closely related subtype on adjacent cells.
The spacing between the cell membranes at an anchoring junction is precisely defined and depends on the structure of the participating cadherin molecules. All the members of the superfamily, by definition, have an extracellular portion consisting of several copies of the extracellular cadherin (EC) domain. Homophilic binding occurs at the N-terminal tips of the cadherin molecules—the cadherin domains that lie furthest from the membrane. These terminal domains each form a knob and a nearby pocket, and the cadherin molecules protruding from opposite cell membranes bind by insertion of the knob of one domain into the pocket of the other (Figure A below).
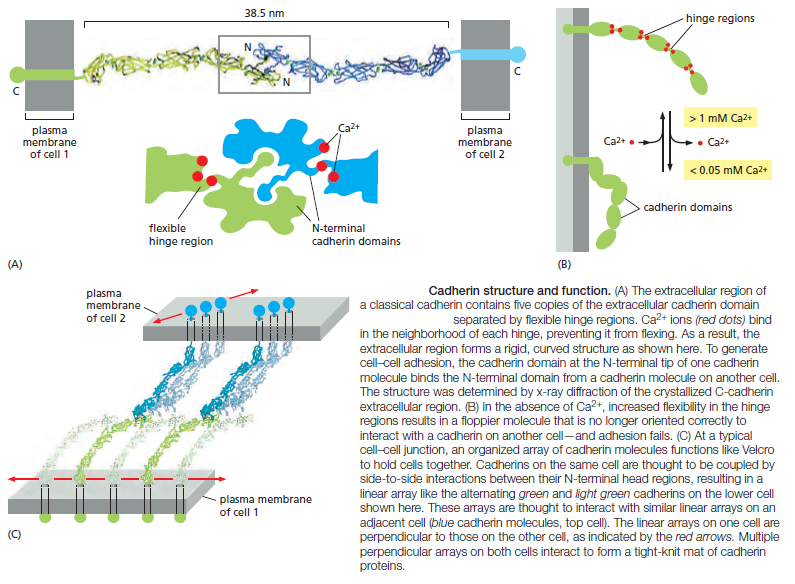
Each cadherin domain forms a more-or-less rigid unit, joined to the next cadherin domain by a hinge. Ca2+ ions bind to sites near each hinge and prevent it from flexing, so that the whole string of cadherin domains behaves as a rigid and slightly curved rod. When Ca2+ is removed, the hinges can flex, and the structure becomes floppy (Figure B above ). At the same time, the conformation at the N-terminus
is thought to change slightly, weakening the binding affinity for the matching cadherin molecule on the opposite cell. Unlike receptors for soluble signal molecules, which bind their specific ligand with high affinity, cadherins (and most other cell–cell adhesion proteins) typically bind to their partners with relatively low affinity. Strong attachments result from the formation of many such weak bonds in parallel. When binding to oppositely oriented partners on another cell, cadherin molecules are often clustered side-to-side with many other cadherin molecules on the same cell (Figure C). The strength of this junction is far greater than that of any individual intermolecular bond, and yet regulatory mechanisms can easily disassemble the junction by separating the molecules sequentially, just as two pieces of fabric can be joined strongly by Velcro and yet easily peeled apart from the sides. A similar “Velcro principle” also operates at cell–cell and cell–matrix adhesions formed by other types of transmembrane adhesion proteins.
Cadherin-Dependent Cell–Cell Adhesion Guides the Organization of Developing Tissues
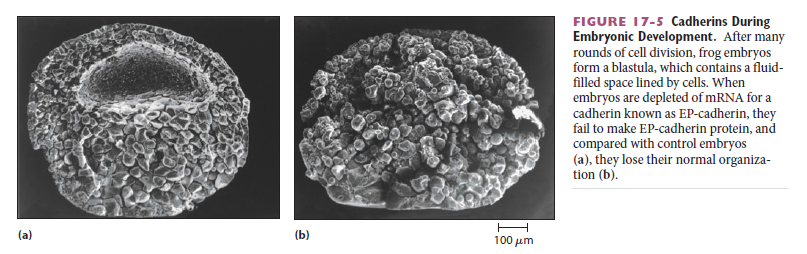
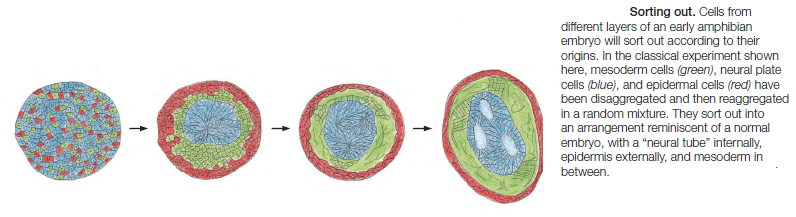
Cadherins form specific homophilic attachments, explaining why there are so many different family members. Cadherins are not like glue, making cell surfaces generally sticky. Rather, they mediate highly selective recognition, enabling cells of a similar type to stick together and to stay segregated from other types of cells. Selectivity in the way that animal cells consort with one another was first demonstrated
in the 1950s, long before the discovery of cadherins, in experiments in which amphibian embryos were dissociated into single cells. These cells were then mixed up and allowed to reassociate. Remarkably, the dissociated cells often reassembled into structures resembling those of the original embryo.These experiments, together with numerous more recent experiments, reveal that selective cell–cell recognition systems make cells of the same differentiated tissue preferentially adhere to one another
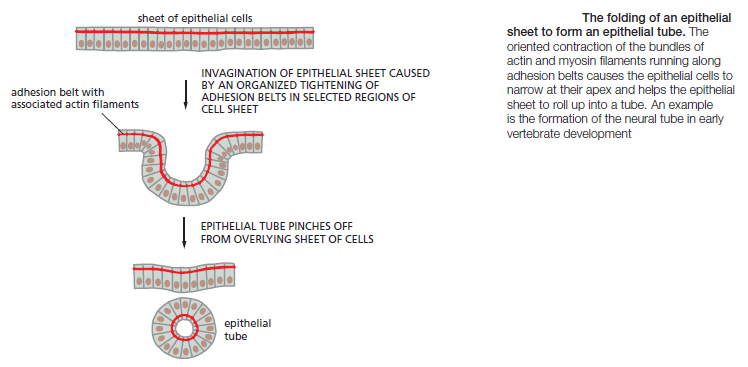
Cadherins play a crucial part in these cell-sorting processes during development. The appearance and disappearance of specific cadherins correlate with steps in embryonic development where cells regroup and change their contacts to create new tissue structures. In the vertebrate embryo, for example, changes in cadherin expression are seen when the neural tube forms and pinches off from the overlying ectoderm: neural tube cells lose E-cadherin and acquire other cadherins, including N-cadherin, while the cells in the overlying ectoderm continue to express E-cadherin (Figure A and B below).
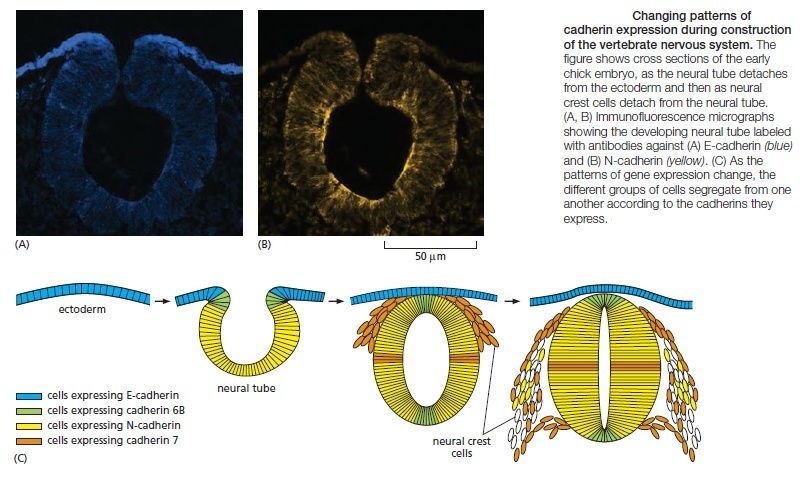
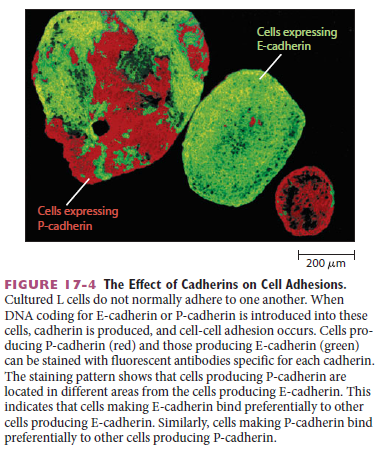
Then, when the neural crest cells migrate away from the neural tube, these cadherins become scarcely detectable, and another cadherin (cadherin 7) appears that helps hold the migrating cells together as loosely associated cell groups (Figure C). Finally, when the cells aggregate to form a ganglion, they switch on expression of N-cadherin again. If N-cadherin is artificially overexpressed in the emerging neural crest cells, the cells fail to escape from the neural tube. Studies with cultured cells further support the idea that the homophilic binding of cadherins controls these processes of tissue segregation. In a line of cultured fibroblasts called L cells, for example, cadherins are not expressed and the cells do not adhere to one another. When these cells are transfected with DNA encoding E-cadherin, E-cadherins on one cell bind to E-cadherins on another, resulting in cell–cell adhesion. If L cells expressing different cadherins are mixed together, they sort out and aggregate separately, indicating that different cadherins preferentially bind to their own type (Figure A below)
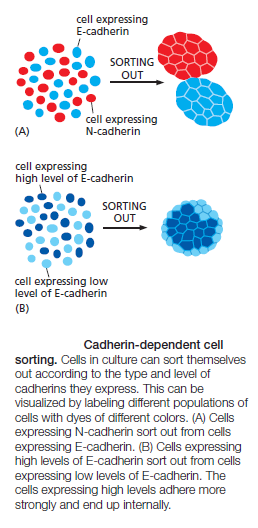
mimicking what happens when cells derived from tissues that express different cadherins are mixed together. A similar segregation of cells occurs if L cells expressing different amounts of the same cadherin are mixed together (Figure B above). It therefore seems likely that both qualitative and quantitative differences in the expression of cadherins have a role in organizing tissues.
Epithelial–Mesenchymal Transitions Depend on Control of Cadherins
The assembly of cells into an epithelium is a reversible process. By switching on expression of adhesion molecules, dispersed unattached mesenchymal cells, such as fibroblasts, can come together to form an epithelium. Conversely, epithelial cells can change their character, disassemble, and migrate away from their parent epithelium as separate cells. Such epithelial–mesenchymal transitions play an important part in normal embryonic development; the origin of the neural crest is one example. These transitions depend in part on transcription regulatory proteins called Slug, Snail, and Twist. Increased expression of Twist, for example, converts epithelial cells to a mesenchymal character, and switching it off does the opposite. Twist exerts its effects, in part, by inhibiting expression of cadherins, including E-cadherin, that hold epithelial cells together. Epithelial–mesenchymal transitions also occur as pathological events during adult life, in cancer. Most cancers originate in epithelia, but become dangerously prone to spread—that is, malignant—only when the cancer cells escape from the epithelium of origin and invade other tissues. Experiments with malignant breast cancer cells in culture show that blocking expression of Twist can convert the cells back toward a nonmalignant character. Conversely, by forcing Twist expression, one can make normal epithelial cells undergo an epithelial–mesenchymal transition and behave like malignant cells. Mutations that disrupt the production or function of E-cadherin are often found in cancer cells and are thought to help make them malignant.
Catenins Link Classical Cadherins to the Actin Cytoskeleton
The extracellular domains of cadherins mediate homophilic binding at adherens junctions. The intracellular domains of typical cadherins, including all classical and some nonclassical ones, interact with filaments of the cytoskeleton: actin at adherens junctions and intermediate filaments at desmosomes. These cytoskeletal linkages are essential for efficient cell–cell adhesion, as cadherins that lack their cytoplasmic domains cannot stably hold cells together. The linkage of cadherins to the cytoskeleton is indirect and depends on adaptor proteins that assemble on the cytoplasmic tail of the cadherin. At adherens junctions, the cadherin tail binds two such proteins: β-catenin and a distant relative called p120-catenin; a third protein called α-catenin interacts with β-catenin and recruits a variety of other proteins to provide a dynamic linkage to actin filaments
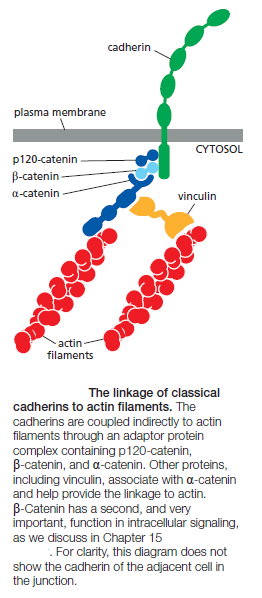
At desmosomes, cadherins are linked to intermediate filaments through other adaptor proteins, including a β-catenin-related protein called plakoglobin. In their mature form, adherens junctions are enormous protein complexes containing hundreds to thousands of cadherin molecules, packed into dense, regular arrays that are linked on the extracellular side by lateral interactions between cadherin domains, On the cytoplasmic side, a complex network of catenins, actin regulators, and contractile actin bundles holds the cluster of cadherins together and links it to the actin cytoskeleton. Assembling a structure of this complexity is not a simple task, and it involves a complex sequence of events controlled by the actin-regulatory proteins. The general features of the assembly process are summarized below:
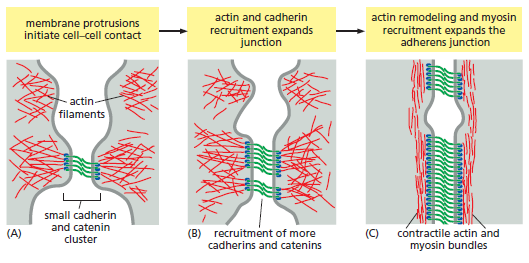
Assembly of an adherens junction.
(A) Assembly begins when two unattached epithelial cell precursors explore their surroundings with membrane protrusions, generated by local nucleation of actin networks. When the cells make contact, small cadherin and catenin clusters take shape at the contact sites and associate with actin, leading to activation of the small monomeric GTPase Rac (not shown), an important actin regulator.
(B) Rac promotes additional actin protrusions in the vicinity, expanding the size of the contact zone and thereby promoting further recruitment of cadherins and their associated catenin proteins.
(C) Eventually, Rac is inactivated and replaced by the related GTPase Rho (not shown), which shifts actin remodeling toward the assembly of linear, contractile filament bundles. Rho also promotes
the assembly of myosin II filaments that associate with bundles of actin filaments to generate contractile activity. This contractile activity generates tension that stimulates further actin recruitment and expansion of the junction, in part through the mechanisms illustrated in Figure below.
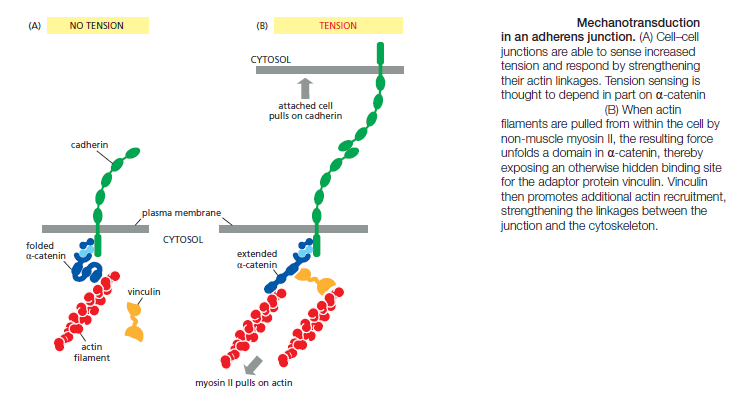
Adherens Junctions Respond to Forces Generated by the Actin Cytoskeleton
Most adherens junctions are linked to contractile bundles of actin filaments and non-muscle myosin II. These junctions are therefore subjected to pulling forces generated by the attached actin. The pulling forces are important for junction assembly and maintenance: disruption of myosin activity, for example, results in the disassembly of many adherens junctions. Furthermore, the contractile forces acting on a junction in one cell are balanced by contractile forces at the junction of the opposite cell, so that no cell pulls others toward it and thereby disrupts the uniform distribution of cells in the tissue. We do not understand the mechanisms responsible for maintaining this balance. Adherens junctions seem to sense the forces acting on them and modify local actin and myosin behavior to balance the forces on both sides of the junction. Evidence for these mechanisms comes from studies of pairs of cultured mammalian cells connected by adherens junctions. If contractile activity in one cell is increased experimentally, the adherens junctions linking the two cells increase in size, and the contractile activity of the second cell increases to match that of the first—resulting in a balance of forces across the junction. These and other experiments suggest that adherens junctions are not simply passive sites of protein–protein binding but are dynamic tension sensors that regulate their behavior in response to changing mechanical conditions. This ability to transduce a mechanical signal into a change in junctional behavior is an example of mechanotransduction. We will see later that it is also important at cell–matrix junctions. The mechanotransduction at cell–cell junctions is thought to depend, at least in part, on proteins in the cadherin complex that alter their shape when stretched by tension. The protein α-catenin, for example, is stretched from a folded to an extended conformation when contractile activity increases at the junction. The unfolding exposes a cryptic binding site for another protein, vinculin, which promotes the recruitment of more actin to the junction (Figure above).
By mechanisms such as this, pulling on a junction makes it stronger. Furthermore, as noted above, pulling on a junction in one cell will increase the contractile force generated in the attached cell. In some cell types, actin contractility reduces cell–cell adhesion, particularly if large forces are involved. Large actin-based contractile forces might, in some tissues, pull sufficiently hard on the edges of cell–cell adhesions to peel them apart, particularly if contraction is coupled to additional regulatory mechanisms that weaken the adhesion. This mechanism might be important in certain forms of tissue remodeling during development.
Last edited by Admin on Mon Apr 24, 2017 9:54 pm; edited 12 times in total