The electron transport chain

Electron Transport Chain
The electron transport chain is a series of protein complexes embedded in the mitochondrial membrane. Electrons captured from donor molecules are transferred through these complexes.
Coupled with this transfer is the pumping of hydrogen ions. This pumping generates the gradient used by the ATP synthase complex to synthesize ATP.
The following complexes are found in the electron transport chain: NADH dehydrogenase, cytochrome b-c1, cytochrome oxidase, and the complex that makes ATP, ATP synthase.
In addition to these complexes, two mobile carriers are also involved: ubiquinone, and cytochrome c.
Other key components in this process are NADH and the electrons from it, hydrogen ions, molecular oxygen, water, and ADP and Pi, which combine to form ATP.
At the start of the electron transport chain, two electrons are passed from NADH into the NADH dehydrogenase complex. Coupled with this transfer is the pumping of one hydrogen ion for each electron
Next, the two electrons are transfered to ubiquinone. Ubiquinone is called a mobile transfer molecule because it moves the electrons to the cytochrome b-c1 complex.
Each electron is then passed from the cytchrome b-c1 complex to cytochrome c. Cytochrome c accepts each electron one at a time. One hydrogen ion is pumped through the complex as each electron is transfered.
The next major step occurs in the cytochrome oxidase complex. This step requires four electrons. These four electrons interact with a molecular oxygen molecule and eight hydrogen ions. The four electrons, four of the hydrogen ions, and the molecular oxygen, are used to form two water molecules. The other four hydrogen ions are pumped across the membrane.
This series of hydrogen pumping steps creates a gradient. The potential energy in this gradient is used by ATP synthase to ATP from ADP and inorganic phosphate. The ATP synthesis steps you see here are discussed in greater detail in the ATP sythase gradients animation.
This animation illustrates two full cycles of electron donation. In biological systems, however, many electron transport cycles occur simultaneously--helping to ensure that the proton gradient is always maintained.

The electron transport chain is embedded in the inner membrane of the mitochondria. It consists of four large protein complexes, and two smaller mobile carrier proteins.

NADH is the electron donor in this system. It initiates the electron transport chain by donating electrons to NADH dehydrogenase (blue).

NADH donates two electrons to NADH dehydrogenase. At the same time, the complex also pumps two protons from the matrix space of the mitochondria into the intermembrane space.

The two electrons are now transferred to the mobile carrier protein known as ubiquinone. Ubiquinone transports the electrons, two at a time, to the next complex in the chain.

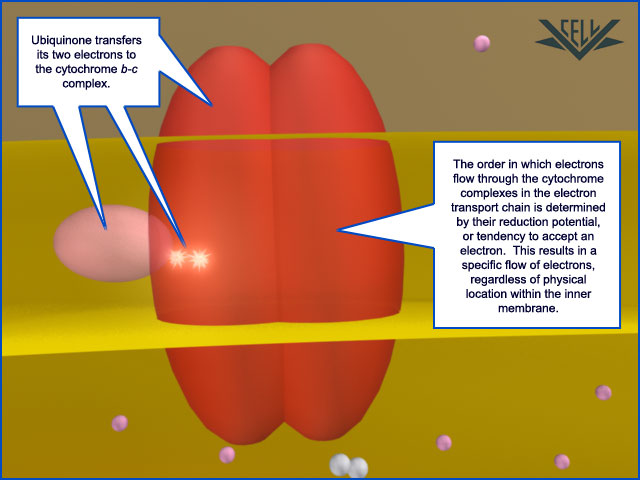
Ubiquinone (pink) delivers two electrons at a time to cytochrome b-c1 (red).

As each electron makes its way through the complex, a hydrogen ion, or proton, is pumped from the matrix space of the mitochondria into the intermembrane space, helping to maintain the proton gradient.
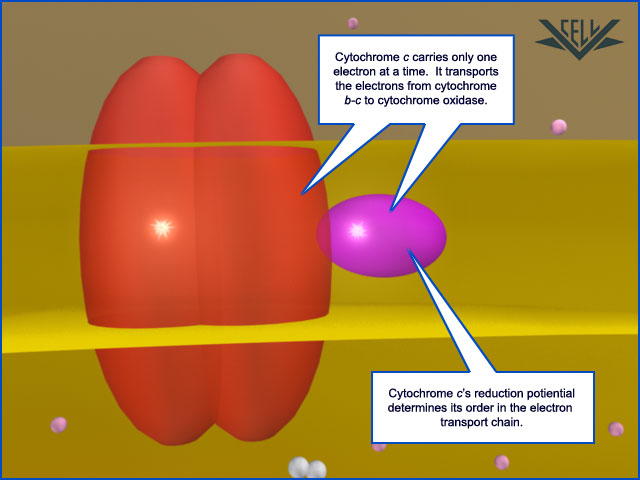
After affecting the pumping of a proton across the membrane, the electron leaves cytochrome b-c1 and enters the mobile carrier protein, cytochrome c (purple).
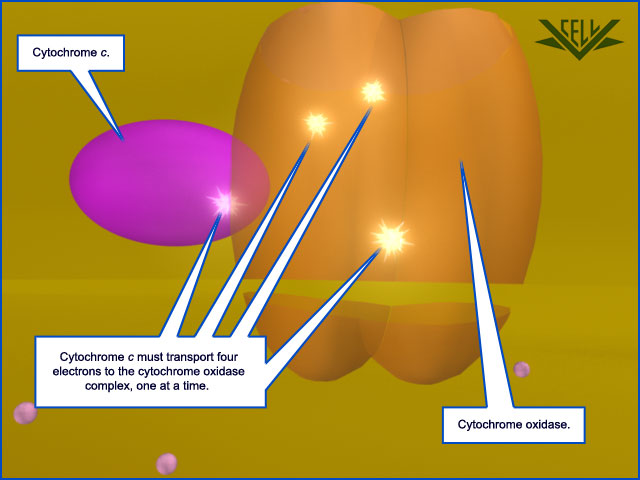
The mobile carrier protein cytochrome c (purple) transfers electrons, one at a time, to cytochrome oxidase (orange). Four electrons must be transferred to the oxidase comples in order for the next major reaction to occur.

The next major event is the reaction of the four electrons, a molecule of O2 (oxygen), and eight protons.

The reaction results in the pumping of four hydrogen ions across the inner membrane into the intermembrane space, and the release of two H20 (water) molecules into the matrix space.

ATP synthase accepts one proton from the intermembrane space and releases a different proton into the matrix space to create the energy it needs to synthesize ATP. It must do this three times to synthesize one ATP from the substrates ADP and Pi (inorganic phosphate).
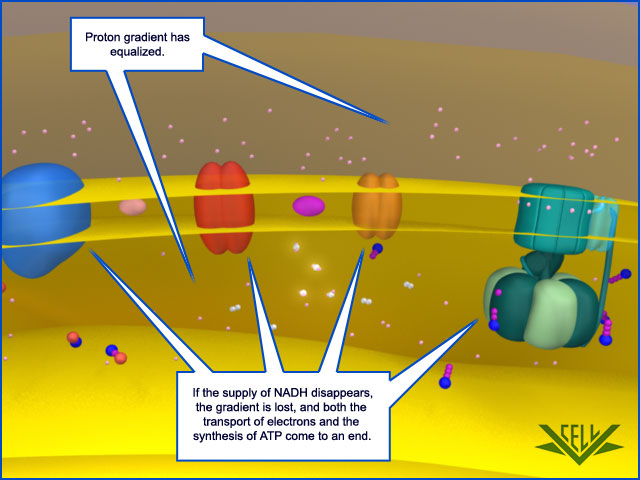
With the supply of NADH exhausted, the electron transport chain can no longer maintain the proton gradient that powers ATP synthase, and ATP synthesis comes to a stop.

Electron Transport Chain
The electron transport chain is a series of protein complexes embedded in the mitochondrial membrane. Electrons captured from donor molecules are transferred through these complexes.
Coupled with this transfer is the pumping of hydrogen ions. This pumping generates the gradient used by the ATP synthase complex to synthesize ATP.
The following complexes are found in the electron transport chain: NADH dehydrogenase, cytochrome b-c1, cytochrome oxidase, and the complex that makes ATP, ATP synthase.
In addition to these complexes, two mobile carriers are also involved: ubiquinone, and cytochrome c.
Other key components in this process are NADH and the electrons from it, hydrogen ions, molecular oxygen, water, and ADP and Pi, which combine to form ATP.
At the start of the electron transport chain, two electrons are passed from NADH into the NADH dehydrogenase complex. Coupled with this transfer is the pumping of one hydrogen ion for each electron
Next, the two electrons are transfered to ubiquinone. Ubiquinone is called a mobile transfer molecule because it moves the electrons to the cytochrome b-c1 complex.
Each electron is then passed from the cytchrome b-c1 complex to cytochrome c. Cytochrome c accepts each electron one at a time. One hydrogen ion is pumped through the complex as each electron is transfered.
The next major step occurs in the cytochrome oxidase complex. This step requires four electrons. These four electrons interact with a molecular oxygen molecule and eight hydrogen ions. The four electrons, four of the hydrogen ions, and the molecular oxygen, are used to form two water molecules. The other four hydrogen ions are pumped across the membrane.
This series of hydrogen pumping steps creates a gradient. The potential energy in this gradient is used by ATP synthase to ATP from ADP and inorganic phosphate. The ATP synthesis steps you see here are discussed in greater detail in the ATP sythase gradients animation.
This animation illustrates two full cycles of electron donation. In biological systems, however, many electron transport cycles occur simultaneously--helping to ensure that the proton gradient is always maintained.

The electron transport chain is embedded in the inner membrane of the mitochondria. It consists of four large protein complexes, and two smaller mobile carrier proteins.

NADH is the electron donor in this system. It initiates the electron transport chain by donating electrons to NADH dehydrogenase (blue).

NADH donates two electrons to NADH dehydrogenase. At the same time, the complex also pumps two protons from the matrix space of the mitochondria into the intermembrane space.

The two electrons are now transferred to the mobile carrier protein known as ubiquinone. Ubiquinone transports the electrons, two at a time, to the next complex in the chain.


Ubiquinone (pink) delivers two electrons at a time to cytochrome b-c1 (red).

As each electron makes its way through the complex, a hydrogen ion, or proton, is pumped from the matrix space of the mitochondria into the intermembrane space, helping to maintain the proton gradient.

After affecting the pumping of a proton across the membrane, the electron leaves cytochrome b-c1 and enters the mobile carrier protein, cytochrome c (purple).

The mobile carrier protein cytochrome c (purple) transfers electrons, one at a time, to cytochrome oxidase (orange). Four electrons must be transferred to the oxidase comples in order for the next major reaction to occur.

The next major event is the reaction of the four electrons, a molecule of O2 (oxygen), and eight protons.

The reaction results in the pumping of four hydrogen ions across the inner membrane into the intermembrane space, and the release of two H20 (water) molecules into the matrix space.

ATP synthase accepts one proton from the intermembrane space and releases a different proton into the matrix space to create the energy it needs to synthesize ATP. It must do this three times to synthesize one ATP from the substrates ADP and Pi (inorganic phosphate).

With the supply of NADH exhausted, the electron transport chain can no longer maintain the proton gradient that powers ATP synthase, and ATP synthesis comes to a stop.
Last edited by Admin on Sun Aug 09, 2015 2:21 am; edited 3 times in total

