Phosphate, essential for life
http://reasonandscience.heavenforum.org/t2148-phosphate-essential-for-life
Hugh Ross:
The short supply of phosphorus poses a significant problem for a naturalistic origin of life because so much of this ingredient is required to make replicator molecules. Phosphates are part of the backbone of both DNA and RNA. A phosphate molecule must accompany every nucleoside in them. Possible precursors to DNA and RNA molecules would seem to require similar phosphate richness. Without life molecules (already assembled and operating), no known natural process can harvest the amounts of phosphorus necessary for life from the environment. All the phosphate-rich deposits on Earth are produced by life.
Biological Significance of Phosphorus 1
One problem regarding the prebiotic chemistry is the ―problem of phosphorus and phosphorylation
P is a key biogenic molecule which comprises of about 1% of the dry weight of cells . It serves in all life properties such as cellular metabolism (cellular respiration, involving sugar phosphates, P containing enzymes etc.), structure (phospholipids) and a necessary part of information storing molecules (RNA and DNA). Replication and metabolism are the two main biochemical processes that mainly rely on P as it constitutes 3% of the atomic composition of RNA, and about 1% of the atomic composition of the metabolomics . Most interestingly, about 44% of all metabolic compounds are biological P compounds. The phosphorylated biomolecules occurring in living organisms are classified into the following categories;
(1) Reactive organophosphates such as acetyl phosphate, phosphoenolpyruvate, phosphocreatine,
(2) stable phosphorylated biomolecules such as glycerol phosphates, ribose phosphate, and phosphoethanolamine and
(3) condensed phosphates such as adenosine-di-phosphates (ADP), and adenosine-tri-phosphates (ATP)
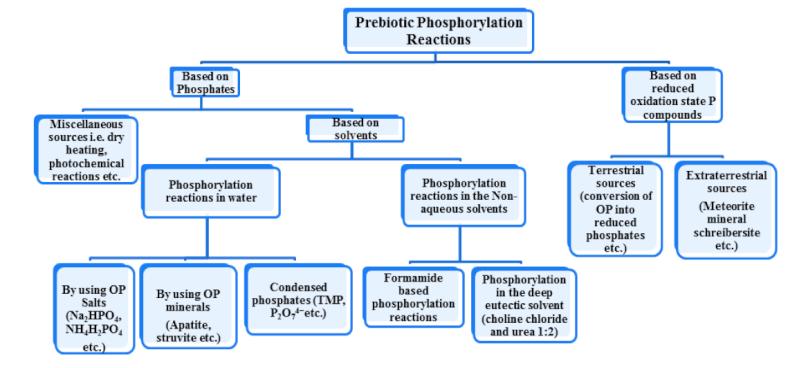
The chart above shows a generic scheme to show various routes that have been described so far in the prebiotic phosphorylation reactions where; OP stands for orthophosphate and TMP stands for trimetaphosphate.
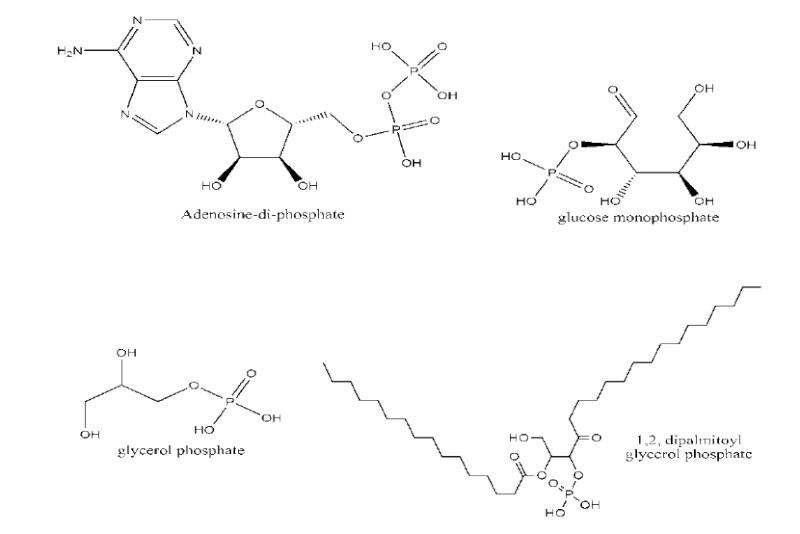
Some biological phosphates that are vital for cellular activities and hence for life. These include simple sugar phosphates such as glycerol phosphate (for respiration and cell structure) and highly complex molecules such as nucleotides (for storing information), and phospholipids (for structure).
Prebiotic Phosphorylation by Phosphates
phosphorylation reaction means a dehydration reaction between an OP and organic molecule.
R-OH + HO-PO32− R-O-PO32− + H2O [2]
The reaction given above is thermodynamically unfavorable. This is because this process is trying to ―pull out water into the aqueous environment, thus making phosphorylation extremely challenging and with low efficiency. This is a major reason why the phosphorylation process has been a challenge in prebiotic chemistry. The major challenging aspects include: a common route to synthesize significant biological P compounds with good yields and with conditions relevant to the Hadean Earth and in the presence of water.
Phosphorus in prebiotic chemistry 2
A variety of pathways now are available as possible solutions for what this author used to refer to as the ‘phosphate problem’. The ‘problem’ seems to have evolved gradually into the less perplexing one of having to decide which of several possible mechanisms are most likely to have operated on the primitive Earth.
1) file:///E:/Downloads/challenges-05-00193%20(3).pdf
2) http://rstb.royalsocietypublishing.org/content/361/1474/1743
http://reasonandscience.heavenforum.org/t2148-phosphate-essential-for-life
Hugh Ross:
The short supply of phosphorus poses a significant problem for a naturalistic origin of life because so much of this ingredient is required to make replicator molecules. Phosphates are part of the backbone of both DNA and RNA. A phosphate molecule must accompany every nucleoside in them. Possible precursors to DNA and RNA molecules would seem to require similar phosphate richness. Without life molecules (already assembled and operating), no known natural process can harvest the amounts of phosphorus necessary for life from the environment. All the phosphate-rich deposits on Earth are produced by life.
Biological Significance of Phosphorus 1
One problem regarding the prebiotic chemistry is the ―problem of phosphorus and phosphorylation
P is a key biogenic molecule which comprises of about 1% of the dry weight of cells . It serves in all life properties such as cellular metabolism (cellular respiration, involving sugar phosphates, P containing enzymes etc.), structure (phospholipids) and a necessary part of information storing molecules (RNA and DNA). Replication and metabolism are the two main biochemical processes that mainly rely on P as it constitutes 3% of the atomic composition of RNA, and about 1% of the atomic composition of the metabolomics . Most interestingly, about 44% of all metabolic compounds are biological P compounds. The phosphorylated biomolecules occurring in living organisms are classified into the following categories;
(1) Reactive organophosphates such as acetyl phosphate, phosphoenolpyruvate, phosphocreatine,
(2) stable phosphorylated biomolecules such as glycerol phosphates, ribose phosphate, and phosphoethanolamine and
(3) condensed phosphates such as adenosine-di-phosphates (ADP), and adenosine-tri-phosphates (ATP)

The chart above shows a generic scheme to show various routes that have been described so far in the prebiotic phosphorylation reactions where; OP stands for orthophosphate and TMP stands for trimetaphosphate.

Some biological phosphates that are vital for cellular activities and hence for life. These include simple sugar phosphates such as glycerol phosphate (for respiration and cell structure) and highly complex molecules such as nucleotides (for storing information), and phospholipids (for structure).
Prebiotic Phosphorylation by Phosphates
phosphorylation reaction means a dehydration reaction between an OP and organic molecule.
R-OH + HO-PO32− R-O-PO32− + H2O [2]
The reaction given above is thermodynamically unfavorable. This is because this process is trying to ―pull out water into the aqueous environment, thus making phosphorylation extremely challenging and with low efficiency. This is a major reason why the phosphorylation process has been a challenge in prebiotic chemistry. The major challenging aspects include: a common route to synthesize significant biological P compounds with good yields and with conditions relevant to the Hadean Earth and in the presence of water.
Phosphorus in prebiotic chemistry 2
A variety of pathways now are available as possible solutions for what this author used to refer to as the ‘phosphate problem’. The ‘problem’ seems to have evolved gradually into the less perplexing one of having to decide which of several possible mechanisms are most likely to have operated on the primitive Earth.
1) file:///E:/Downloads/challenges-05-00193%20(3).pdf
2) http://rstb.royalsocietypublishing.org/content/361/1474/1743


