Fine-tuning of Carbon nucleosynthesis, the basis of all life on earth
https://reasonandscience.catsboard.com/t1502-carbon-the-basis-of-all-life-on-earth
Carbon is the only element that can link up 4 other atoms, and also form unlimited chains made of links with itself. Carbon is therefore the glue that binds large and complex molecules together. Nearly every molecule with more than 5 atoms contains carbon. This includes essentially all biomolecules: carbohydrates, fats, proteins, RNA, DNA, amino acids, cell walls, hormones, and neurotransmitters. Without carbon, there could be no life as we know it. There could be no life based on molecules, since complex molecules are impossible without the glue of carbon to hold them together.
https://alwaysasking.com/is-the-universe-fine-tuned/
Hugh Ross:
Of the 112 known chemical elements, only carbon possesses a sufficiently complex chemical behaviour to sustain living systems.1 Carbon readily assembles into stable molecules comprised of individual and fused rings and linear and branched chains. It forms single, double, and triple bonds. Carbon also strongly bonds with itself as well as with oxygen, nitrogen, sulfur, and hydrogen. In other words, life molecules must be carbon-based.
At its basic level, however, chemical life must be able to carry the instructions for the construction of its progeny from basic atomic building blocks. These instructions, or “blueprints,” require, among other important things, a complex molecule as the carrier. This molecule must be stable enough to withstand significant chemical and thermal perturbations, but not so stable that it won’t react with other molecules at low temperatures. In other words, it must be metastable. Also, to allow for diverse chemistry, it must have an affinity for many other kinds of atoms comparable with the affinity it has for itself. Carbon excels in this regard, but silicon falls far short. Other elements aren’t even in the race. There are other arguments in favour of carbon, such as the fact that it forms gases when combined with oxygen (to make carbon dioxide) or hydrogen (to make methane), and both gases allow free exchange with the atmosphere and oceans. And most important, when other key atoms— hydrogen, nitrogen, oxygen, and phosphorus—are added to carbon, we get the informational backbones (DNA and RNA), and the building blocks (the amino acids and proteins) of life. Carbon gives these molecules an information-storage capacity vastly exceeding that of hypothetical alternatives. In fact, the half-dozen or so key chemical requirements for life discussed in the literature are rare or absent in other elements but are all present in carbon. (And in case you think there’s a loophole, it doesn’t work to try to create a carbon equivalent by combining several kinds of atoms.) 3
[The entire biological] process depends upon the unusual chemistry of carbon, which allows it to bond to itself, as well as other elements, creating highly complex molecules that are stable over prevailing terrestrial temperatures, and are capable of conveying genetic information (especially DNA). ... Whereas it might be argued that nature creates its own fine-tuning, this can only be done if the primordial constituents of the universe are such that an biological process can be initiated. The unique chemistry of carbon is the ultimate foundation of the capacity of nature to tune itself.
Synthesis of carbon
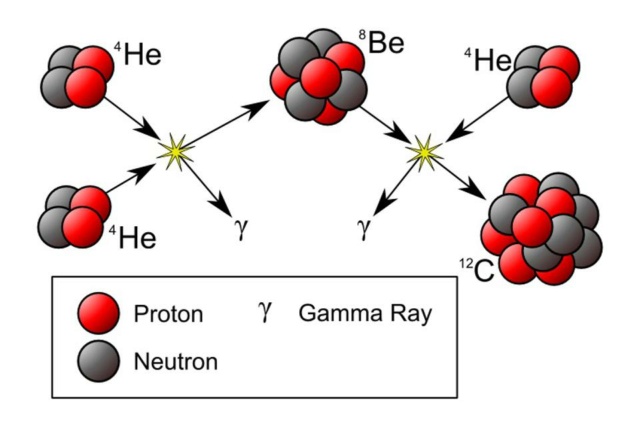
The synthesis of carbon is a two-step process. The first step is the formation of an 8Be nucleus out of two 4He nuclei (alpha particles), the second the formation of a 12C nucleus out of the 8Be nucleus and another 4He nucleus. The probability of this process would be extremely small, were it not for two “coincidences”: the 8Be ground state has almost exactly the energy of two alpha particles, and 8Be+4He has almost exactly the energy of an excited state of 12C. In other words, the 8Be ground state “resonates” with a system comprising two alpha particles, and the excited 12C state resonates with a systems comprising 8Be and 4He. The existence of the second resonance was predicted by Fred Hoyle before its actual observation, based on the observed abundance of carbon in the Universe and the necessity for it to be formed in stars. The energy at which this resonance occurs depends sensitively on the interplay between the strong and the weak nuclear interactions. If the strong force were slightly stronger or slightly weaker — by just 1% in either direction — then the binding energies of the nuclei would be different, and the requisite resonance would not exist. In that case, there would be no carbon or any heavier elements anywhere in the Universe. “I do not believe,” Hoyle concluded,[5] “that any scientist who examined the evidence would fail to draw the inference that the laws of nuclear physics have been deliberately designed with regard to the consequences they produce inside the stars.” 4
Attila Csoto: Fine-tuning the basic forces of nature through the triple-alpha process in red giant stars 17 Oct 2000]
A 0.5% change in the N-N interaction strength would lead to a Universe which does not contain an appreciable amount of carbon or oxygen. This would make the existence of carbon-based life highly unlikely. This very strong fine-tuning effect gives us a possibility to try to constrain the possible values of some fundamental constants in the Standard Model.
https://arxiv.org/abs/nucl-th/0010052v1
In 1932, John Cockcroft and Ernest Walton found that beryllium-8 is unstable. It lasts for less than a thousandth of a trillionth of a second. Their work won them the 1951 Nobel Prize in physics for transmuting the elements — realizing the long-held dream of alchemists.

There are no stable elements with a mass number of 5 or 8. “The mass gaps at 5 and 8 spelled the doom of Gamow’s hopes that all nuclear species could be produced in the big bang one unit of mass at a time.” — William Fowler
So yet another step was missing. With no known mechanism to get over the hurdle of the mass-5 and mass-8 roadblocks, there was no explanation for how elements necessary to life came to be. This problem led the cosmologist Fred Hoyle, in 1953, to make what’s described as “the most outrageous prediction” ever made in science. Hoyle’s outrageous prediction was the existence of a yet undiscovered excited energy state of the carbon-12 nucleus, which had somehow been missed by all the particle physicists in the world.
If this state existed, it would allow the triple-alpha process — the simultaneous collision of three helium-4 nuclei to yield carbon-12.
If carbon could be made this way, the mass-5 and mass-8 roadblocks could be cleared, and then other heavier elements could be built one hydrogen or helium nucleus at a time. Without this state, carbon would be many millions of times rarer, and we wouldn’t be here.
So, Hoyle reasoned, this state of carbon must exist.
In 1953, Hoyle traveled from Cambridge, England to visit William Fowler’s nuclear physics lab at Caltech. Hoyle asked that Fowler’s lab do the experiments to check for this state of the carbon-12 nucleus, which he predicted should be at an energy level of 7.68 million eV.
I was very skeptical that this steady state cosmologist, this theorist, should ask questions about the carbon-12 nucleus. […] Hoyle just insisted — remember, we didn’t know him all that well — here was this funny little man who thought that we should stop all this important work that we were doing otherwise and look for this state, and we kind of gave him the brush off.
William Fowler in interview (1973)
But Hoyle succeeded in convincing a junior physicist at the lab, Ward Whaling, to check for it. Five months later, Hoyle received word.
Whaling confirmed the existence of the excited state of carbon-12, and it was almost exactly where Hoyle predicted: at 7.655 million eV!
Hoyle’s prediction is remarkable because he used astrophysics (the physics of stars) to find unknown properties in nuclear physics (the physics of atoms and their nuclei). Fowler was an instant convert.
So it was really quite a tour de force, that a man who walked into the lab predicted the existence of an excited state of a nucleus, and when the appropriate experiment was performed it was found. And no nuclear theorist starting from basic nuclear theory could do that then, nor can they really do it now. So Hoyle’s prediction was a very striking one.
William Fowler in interview (1973)
Fowler took a year off from his post at Caltech to work with Hoyle in Cambridge. Together with two astronomers, Margaret and Geoffrey Burbidge they worked out a complete theory of element formation, showing how every element is produced and explaining the relative abundances of the elements as found in nature.
Their work was revolutionary and it made a name for the authors.
For this work, Fowler received the Nobel Prize in physics in 1983. Hoyle, however, did not share in the prize, creating controversy.
In any event, no one denied the significance of their accomplishment.
With their 1957 paper, humanity finally had an understanding of where all the matter, which makes up our world, our food, our shelter, our very bodies, came from — the innermost depths of long-dead stars.
And so, the world as we know it is owed to the carbon-12 nucleus having this chance property. Like the delicate balance of the density of the universe, the existence of this state hangs in a delicate balance.
As it happens, the energy level of this state is at 7.655 MeV. Had the energy level of this state been less than 7.596 MeV or greater than 7.716 MeV, there would be almost no carbon in the universe.
The minor miracle of the carbon-12 nucleus having this excited state and it being in exactly the right range did not go unnoticed.
Some super-calculating intellect must have designed the properties of the carbon atom, otherwise the chance of my finding such an atom through the blind forces of nature would be utterly minuscule.
[…]
A common sense interpretation of the facts suggests that a superintellect has monkeyed with physics, as well as with chemistry and biology, and that there are no blind forces worth speaking about in nature. The numbers one calculates from the facts seem to me so overwhelming as to put this conclusion almost beyond question.
Fred Hoyle in “The Universe: Past and Present Reflections” (1982)
The balancing of the universe’s density, and the fortune of the carbon-12 excited state were just the first of many “cosmic coincidences.”
The more scientists probed the inner workings of the universe, the more lucky coincidences they found. With each one, evidence gathered to support the idea that the laws of physics are finely-tuned to permit the emergence of complexity, and with that complexity, life. The excited state of carbon-12 is determined by electromagnetic and nuclear forces.
https://alwaysasking.com/is-the-universe-fine-tuned/
Siegel Ethan: Beyond the galaxy 2016
Steady-State proponent Fred Hoyle put forth a breathtaking paper is simply known as B2 FH (after its authors) — in 1957, detailing how nuclear fusion ought to work in the hearts of stars. Above a certain density and temperature threshold, protons from hydrogen nuclei in the core of massive enough stars — of at least 8% the mass of our Sun — could fuse together, first into deuterium and then quickly into helium-3 and then helium-4, releasing a tremendous amount of energy: 28 MeV for every helium-4 nucleus that is produced. And the release of this energy through fusion reactions in stellar cores would not only explain why the Sun shines, but it would also explain why all the stars on the main sequence shine as they do. In the core of a star like our Sun, the temperatures reach up to around 15 million K, with gravitational pressures so intense that the density of the plasma is 13 times greater than solid lead. As massive as our Sun is, it contains a total of around a whopping 10^57 protons, a little less than 10% of which are in the core at any given time. Under all that pressure and at such a high temperature, protons in the Sun’s core have tremendous amounts of kinetic energy, moving at a significant percentage of the speed of light. The collision rate is tremendous, as they bounce off of other protons and nuclei, with every particle undergoing billions of interactions every second. With all these collisions and interactions, you can then calculate how many protons have enough energy to take that first step in the nuclear chain reaction, and fuse into deuterium. The answer is exactly zero. Of all the proton pairs colliding in the sun’s core, not a single one has sufficient energy to fuse into anything heavier, meaning temperature and density can’t be telling the whole story. Then how does nuclear fusion occur? Through the power of quantum mechanics! The individual protons in a star’s core might not have enough energy to overcome the repulsive force caused by their electric charges, but there’s always a chance that these particles can undergo quantum tunneling, and wind up in a more stable bound state (e.g., deuterium) that causes the release of this fusion energy. Even though the probability of quantum tunneling is very small for any particular proton-proton interaction — somewhere on the order of 1-in-10^28, or the same as your odds of winning the Powerball lottery three times in a row with buying exactly three tickets — the fact that there are so many interactions in the core happening continuously means that a whopping 4 × 10^38 protons fuse into helium every second in our Sun. And this process, of nuclear fusion fueled by quantum physics, is what is responsible for powering all the main sequence stars in the Universe.
In a very low-mass star, the volume of the fusion-producing core is small, and so fusion proceeds at a leisurely pace, causing these stars to be cool, red in color, and dim, or low in luminosity. If your star is more massive, the volume of the core is going to be larger, with higher temperatures, densities, and rates (and probabilities) of fusion. The more massive your hydrogen-burning star is, the hotter, bluer, and brighter your star is going to be, explaining why bluer stars are also the more luminous ones. But, perhaps counterintuitively, the more massive, luminous and blue your star is, the shorter its lifetime will be. You see, a star that is twice as massive as another might have twice as much hydrogen fuel to burn, but it burns through the fuel in its core approximately eight times as fast, while one that is ten times as massive burns through its fuel around a thousand times as quickly. Over very long timescales (many tens of billions of years), the spent (helium) fuel in a star’s core can convect out while unburned hydrogen convects in, allowing the longest-lived stars to burn through 100% of their hydrogen fuel. But for higher-mass stars, including stars like the Sun, when the core runs out of hydrogen, that’s the end of their time on the main sequence. This epiphany led to a spectacular prediction on the part of Fred Hoyle.
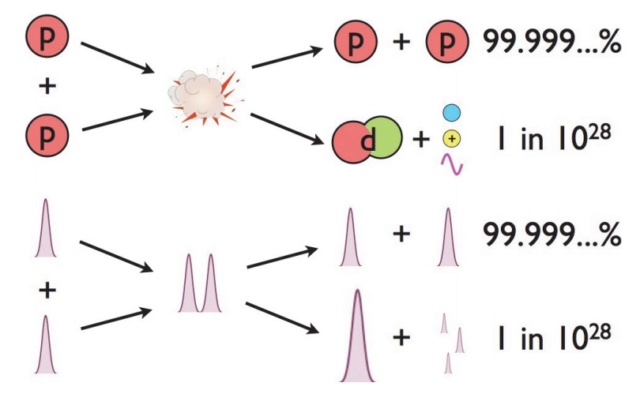
At the extreme temperatures in the core of our Sun, collisions between bare hydrogen nuclei (protons) are common, and occur at extremely high energies. However, these energies are insufficient to overcome the repulsive electric force on their own. It is only due to the laws of quantum physics, and the fact that each proton is a quantum particle with a probabilistic function describing its location, that enables the two wavefunctions to overlap ever so slightly. In virtually all the interactions, no fusion will occur, and the protons simply scatter off of one another. But in 1-in-1028 interactions, a fusion reaction does occur, forming a heavier element — like deuterium in this figure — and releasing energy in the process.
The only reason a hydrogen-burning star does not collapse under its own gravity is the incredible radiation pressure resulting from the nuclear fusion happening in the core. Yet, this process only creates the element helium, and judging from the sheer number and diversity of elements we see on Earth, there must, Hoyle reasoned, be a way to create heavier elements in these stars. At the temperatures and densities present in the core of a hydrogen-burning star, there would be no way to move up past helium-4; a proton could not fuse with it because there is no stable nucleus with a mass of 5, and another helium-4 could not fuse with it because there’s no stable nucleus of mass 8. Any nucleus temporarily formed with these masses decays back to helium-4 in a minuscule fraction of a second. But when you run out of hydrogen fuel in your core, the radiation pressure begins to drop, and suddenly the core of the star begins to collapse under its own gravity. When you have a very large number of particles together in a small space — like the core of a star — there is a lot of energy stored in the gravitational field between the particles. Unless you both collapse it very slowly and provide a very efficient way for that energy to escape, the process of collapse is going to cause a tremendous increase in both the temperature and the energies of the particles inside. This is the same principle behind how a diesel engine works — the rapid compression of the fuel inside causes it to ignite — only when the helium-4 reaches a certain threshold, it does not ignite, but rather begins fusing into beryllium-8! This isotope of beryllium is unstable, of course, and decays back to two helium-4 nuclei in approximately 10−17 seconds, but Hoyle recognized the importance of this isotope’s existence, even if it remained for only the briefest amounts of time. You see, the reason that nuclear fusion is so effective at releasing energy — the reason it can happen via quantum tunneling and the reason it can power so much — is because the rest mass of the products of a fusion reaction is significantly and measurably smaller than the mass of the reactants. Hydrogen eventually fuses into helium-4 because helium-4 has the mass equivalent (via the famous E = mc2 ) of 28 MeV less than the four hydrogen nuclei that went into it. Beryllium-8, on the other hand, has almost exactly the same mass as two helium-4 nuclei that go into it (the energy difference is less than 0.1 MeV), so there is no reason for it to be energetically favored, and that is why it decays back into helium-4 almost immediately. But that is an important almost, reasoned Hoyle, because if you could get a third helium-4 in there quickly enough, you could theoretically combine it with the beryllium-8 to form carbon-12, which is heavily energetically favored. But there was an important hurdle to overcome, which led Hoyle to make the most breathtaking prediction of his entire career. Just like atoms have excited states — where electrons can be in unstable, higher energy configurations that decay down to lower energy states, emitting a photon in the process — nuclei can also have excited states, or a spectrum of configurations where the lowest one, or the ground state, may be stable. The big difference between atomic excited states and nuclear excited states is that the latter are so significantly different in energy from one another that they have measurable mass differences (due to E = mc2 ) between them. Combining three helium-4 nuclei together would not be able to give you carbon-12, as the mass differences between the two systems are too great, with carbon-12 being significantly less massive. But if, Hoyle proposed, there were an excited state of carbon-12 that had strictly the same mass as three helium-4 nuclei combined, you could continue fusing elements in the hearts of stars even after they ran out of their hydrogen fuel. Since carbon exists, he reasoned, and is also required as a building block to form the plethora of even heavier elements present in stars, on Earth and throughout the Universe, this new excited state — which had never been observed — must exist, and must exist at precisely the same mass as three helium-4 nuclei combined!

The “ Hoyle state,” as its now known, was a proposed excited state of carbon-12. By reasoning that carbon must come to exist in great abundance, there must have been a way to form it in the core of stars. Beryllium-8 may be unstable, but at high enough densities and energies, it should be possible to get another helium-4 nucleus in there before it decays. If that happens, it can create the excited carbon-12 through what’s called the “triple-alpha” process, since a helium-4 nucleus is also an alpha-particle (emitted in some radioactive decays) and it takes three of them to make carbon-12. The excited carbon-12 then decays to normal, stable carbon-12 and emits a very high-energy photon, giving the core of the star an influx of new energy
This new hypothesized state was dubbed the Hoyle state, and the theoretical new process by which it was formed was known as the triple-alpha process since a helium-4 nucleus is also known as an alpha particle, emitted in some radioactive decays. Hoyle told his collaborator Willie Fowler about this hypothesis in 1952, and Fowler conceded that it was possible that such a state existed, and had been missed by nuclear physicists up until that point. It took five years of research but in 1957, the Hoyle state of carbon-12 was found and confirmed to have exactly the energy necessary to produce carbon in the cores of massive stars that had burned through their hydrogen! The key to unlocking the origin of all the heavy elements in the Universe had just been discovered
Ethan Siegel: What Can The Simple Fact That ‘We Exist’ Teach Us About The Universe? Oct 1, 2020
We know the mass of carbon-12, and the masses of the helium and hydrogen nuclei that are so abundant in the stars. The easiest way to get there would be to take three independent helium-4 nuclei and fuse them all together simultaneously. Helium-4 has two protons and two neutrons in its nucleus, so it’s easy to imagine that fusing three of them together would give you carbon-12, and hence could create the carbon we need in our Universe. But three helium nuclei, combined, are too massive to efficiently produce carbon-12. When two helium-4 nuclei fuse together, they produce beryllium-8 for just ~10-16 s, before it decays back to two helium nuclei. Although occasionally a third helium-4 nucleus could get in there if the temperatures are high enough, the energies are all wrong for producing carbon-12; there’s too much energy. The reaction just wouldn’t give us enough of the carbon our Universe needs. Fortunately, physicist Fred Hoyle realized that the Universe needed a pathway to make carbon from helium. He theorized that if there were an excited state of the carbon-12 nucleus, at a higher energy that was closer to the rest mass of three helium-4 nuclei combined, the reaction could occur. This nuclear state, known as the Hoyle State, was discovered just five years later by nuclear physicist Willie Fowler, who also discovered the triple-alpha process that formed it, just as Hoyle predicted.
The prediction of the Hoyle State and the discovery of the triple-alpha process is perhaps the most stunningly successful use of anthropic reasoning in scientific history. This process is what explains the creation of the majority of carbon that's found in our modern-day Universe.
R.A.W. Bradford The Inevitability of Fine Tuning in a Complex Universe 2011
If the strength of the strong nuclear force, gs, were changed by 1% the rate of the triple-alpha reaction would be affected so markedly that the production of biophilic1 abundances of either carbon or oxygen would be prevented.
https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1081.137&rep=rep1&type=pdf
Elisabeth Vangioni Cosmic origin of the chemical elements rarety in nuclear astrophysics 23 November 2017
Carbon is a fascinating nucleus since it is the basis of life and it is the product of an extraordinary energy coincidence at the level of nuclei. It is striking that the process of complexification of matter has to overcome these different obstacles; one sees that the result is very inefficient since the present mass fraction of atoms heavier than helium is only about 2% of about 5% of the baryonic matter in the Universe. This shows the extraordinary quality of rarety regarding the ordinary matter.
https://www.tandfonline.com/doi/pdf/10.1080/21553769.2017.1411838?needAccess=true
Carbon is unique in its ability to combine with other atoms, forming a vast and unparalleled number of compounds in combination with hydrogen, oxygen, and nitrogen. This universe of organic chemistry— with its huge diversity of chemical and physical properties—is precisely what is needed for the assembling of complex chemical systems. Furthermore, the general ‘metastability’ of carbon bonds and the consequent relative ease with which they can be assembled and rearranged by living systems contributes greatly to the fitness of carbon chemistry for biochemical life. No other atom is nearly as fit as carbon for the formation of complex biochemistry. Today, one century later, no one doubts these claims. Indeed the peerless fitness of the carbon atom to build chemical complexity and to partake in biochemistry has been affirmed by a host of researchers. 2
One widely publicized coincidence is the ‘lucky’ fact that the nuclear resonances of the isotopes 12C and 16O are exactly what they need to be if carbon is to be synthesized and accumulate in any quantity in the interior of stars . The energy levels of these resonances ensure that 12C is first synthesized in stellar interiors from collisions between 8Be (beryllium) and He (helium) nuclei and that the carbon synthesized is not depleted later. Hoyle made this discovery in 1953 while working at Caltech with William Fowler. An intriguing aspect of the discovery is that Hoyle made it based on a prediction from the anthropic principle. Hoyle himself
famously commented:
If you wanted to produce carbon and oxygen in roughly equal quantities by stellar nucleosynthesis, these are the two levels you would have to fix, and your fixing would have to be just about where these levels are found to be ... A common sense interpretation of the facts suggests that a superintellect has monkeyed with physics, as well as chemistry and biology and that there are no blind forces worth speaking about in nature.
This discovery was acclaimed not only as a major scientific discovery but also as further evidence of the biocentricity of nature. Hoyle may have been one of the first to notice that the conditions necessary to permit carbon-based life require a very narrow range of basic physical constants, but the idea is now widely accepted. If those constants had been very slightly different, the universe would not have been conducive to the development of matter, astronomical structures, or elemental diversity, and thus the emergence of complex chemical systems.
Fine tuning of carbon
Tracey Peake: Foundations of Carbon-Based Life Leave Little Room for Error March 13, 2013
Life as we know it is based upon the elements of carbon and oxygen. Now a team of physicists, including one from North Carolina State University, is looking at the conditions necessary to the formation of those two elements in the universe. They’ve found that when it comes to supporting life, the universe leaves very little margin for error.
Both carbon and oxygen are produced when helium burns inside of giant red stars. Carbon-12, an essential element we’re all made of, can only form when three alpha particles, or helium-4 nuclei, combine in a very specific way. The key to formation is an excited state of carbon-12 known as the Hoyle state, and it has a very specific energy – measured at 379 keV (or 379,000 electron volts) above the energy of three alpha particles. Oxygen is produced by the combination of another alpha particle and carbon.
NC State physicist Dean Lee and German colleagues Evgeny Epelbaum, Hermann Krebs, Timo Laehde and Ulf-G. Meissner had previously confirmed the existence and structure of the Hoyle state with a numerical lattice that allowed the researchers to simulate how protons and neutrons interact. These protons and neutrons are made up of elementary particles called quarks. The light quark mass is one of the fundamental parameters of nature, and this mass affects particles’ energies.
In new lattice calculations done at the Juelich Supercomputer Centre the physicists found that just a slight variation in the light quark mass will change the energy of the Hoyle state, and this in turn would affect the production of carbon and oxygen in such a way that life as we know it wouldn’t exist.
“The Hoyle state of carbon is key,” Lee says. “If the Hoyle state energy was at 479 keV or more above the three alpha particles, then the amount of carbon produced would be too low for carbon-based life.
“The same holds true for oxygen,” he adds. “If the Hoyle state energy were instead within 279 keV of the three alphas, then there would be plenty of carbon. But the stars would burn their helium into carbon much earlier in their life cycle. As a consequence, the stars would not be hot enough to produce sufficient oxygen for life. In our lattice simulations, we find that more than a 2 or 3 percent change in the light quark mass would lead to problems with the abundance of either carbon or oxygen in the universe.”
There is no physical law to explain it. And neither PHYSICAL NEED. What remains, is chance, or design......
https://news.ncsu.edu/2013/03/tpleeanthropic/
1. http://news.ncsu.edu/2013/03/tpleeanthropic/
2. http://bio-complexity.org/ojs/index.php/main/article/view/BIO-C.2013.1/BIO-C.2013.1
3. Gonzalez, Privileged Planet, page 33
4. https://thisquantumworld.com/a-critique-of-quantum-mechanics/fine-tuning/
https://reasonandscience.catsboard.com/t1502-carbon-the-basis-of-all-life-on-earth
Carbon is the only element that can link up 4 other atoms, and also form unlimited chains made of links with itself. Carbon is therefore the glue that binds large and complex molecules together. Nearly every molecule with more than 5 atoms contains carbon. This includes essentially all biomolecules: carbohydrates, fats, proteins, RNA, DNA, amino acids, cell walls, hormones, and neurotransmitters. Without carbon, there could be no life as we know it. There could be no life based on molecules, since complex molecules are impossible without the glue of carbon to hold them together.
https://alwaysasking.com/is-the-universe-fine-tuned/
Hugh Ross:
Of the 112 known chemical elements, only carbon possesses a sufficiently complex chemical behaviour to sustain living systems.1 Carbon readily assembles into stable molecules comprised of individual and fused rings and linear and branched chains. It forms single, double, and triple bonds. Carbon also strongly bonds with itself as well as with oxygen, nitrogen, sulfur, and hydrogen. In other words, life molecules must be carbon-based.
At its basic level, however, chemical life must be able to carry the instructions for the construction of its progeny from basic atomic building blocks. These instructions, or “blueprints,” require, among other important things, a complex molecule as the carrier. This molecule must be stable enough to withstand significant chemical and thermal perturbations, but not so stable that it won’t react with other molecules at low temperatures. In other words, it must be metastable. Also, to allow for diverse chemistry, it must have an affinity for many other kinds of atoms comparable with the affinity it has for itself. Carbon excels in this regard, but silicon falls far short. Other elements aren’t even in the race. There are other arguments in favour of carbon, such as the fact that it forms gases when combined with oxygen (to make carbon dioxide) or hydrogen (to make methane), and both gases allow free exchange with the atmosphere and oceans. And most important, when other key atoms— hydrogen, nitrogen, oxygen, and phosphorus—are added to carbon, we get the informational backbones (DNA and RNA), and the building blocks (the amino acids and proteins) of life. Carbon gives these molecules an information-storage capacity vastly exceeding that of hypothetical alternatives. In fact, the half-dozen or so key chemical requirements for life discussed in the literature are rare or absent in other elements but are all present in carbon. (And in case you think there’s a loophole, it doesn’t work to try to create a carbon equivalent by combining several kinds of atoms.) 3
[The entire biological] process depends upon the unusual chemistry of carbon, which allows it to bond to itself, as well as other elements, creating highly complex molecules that are stable over prevailing terrestrial temperatures, and are capable of conveying genetic information (especially DNA). ... Whereas it might be argued that nature creates its own fine-tuning, this can only be done if the primordial constituents of the universe are such that an biological process can be initiated. The unique chemistry of carbon is the ultimate foundation of the capacity of nature to tune itself.
Synthesis of carbon
The synthesis of carbon is a two-step process. The first step is the formation of an 8Be nucleus out of two 4He nuclei (alpha particles), the second the formation of a 12C nucleus out of the 8Be nucleus and another 4He nucleus. The probability of this process would be extremely small, were it not for two “coincidences”: the 8Be ground state has almost exactly the energy of two alpha particles, and 8Be+4He has almost exactly the energy of an excited state of 12C. In other words, the 8Be ground state “resonates” with a system comprising two alpha particles, and the excited 12C state resonates with a systems comprising 8Be and 4He. The existence of the second resonance was predicted by Fred Hoyle before its actual observation, based on the observed abundance of carbon in the Universe and the necessity for it to be formed in stars. The energy at which this resonance occurs depends sensitively on the interplay between the strong and the weak nuclear interactions. If the strong force were slightly stronger or slightly weaker — by just 1% in either direction — then the binding energies of the nuclei would be different, and the requisite resonance would not exist. In that case, there would be no carbon or any heavier elements anywhere in the Universe. “I do not believe,” Hoyle concluded,[5] “that any scientist who examined the evidence would fail to draw the inference that the laws of nuclear physics have been deliberately designed with regard to the consequences they produce inside the stars.” 4
Attila Csoto: Fine-tuning the basic forces of nature through the triple-alpha process in red giant stars 17 Oct 2000]
A 0.5% change in the N-N interaction strength would lead to a Universe which does not contain an appreciable amount of carbon or oxygen. This would make the existence of carbon-based life highly unlikely. This very strong fine-tuning effect gives us a possibility to try to constrain the possible values of some fundamental constants in the Standard Model.
https://arxiv.org/abs/nucl-th/0010052v1
In 1932, John Cockcroft and Ernest Walton found that beryllium-8 is unstable. It lasts for less than a thousandth of a trillionth of a second. Their work won them the 1951 Nobel Prize in physics for transmuting the elements — realizing the long-held dream of alchemists.

There are no stable elements with a mass number of 5 or 8. “The mass gaps at 5 and 8 spelled the doom of Gamow’s hopes that all nuclear species could be produced in the big bang one unit of mass at a time.” — William Fowler
So yet another step was missing. With no known mechanism to get over the hurdle of the mass-5 and mass-8 roadblocks, there was no explanation for how elements necessary to life came to be. This problem led the cosmologist Fred Hoyle, in 1953, to make what’s described as “the most outrageous prediction” ever made in science. Hoyle’s outrageous prediction was the existence of a yet undiscovered excited energy state of the carbon-12 nucleus, which had somehow been missed by all the particle physicists in the world.
If this state existed, it would allow the triple-alpha process — the simultaneous collision of three helium-4 nuclei to yield carbon-12.
If carbon could be made this way, the mass-5 and mass-8 roadblocks could be cleared, and then other heavier elements could be built one hydrogen or helium nucleus at a time. Without this state, carbon would be many millions of times rarer, and we wouldn’t be here.
So, Hoyle reasoned, this state of carbon must exist.
In 1953, Hoyle traveled from Cambridge, England to visit William Fowler’s nuclear physics lab at Caltech. Hoyle asked that Fowler’s lab do the experiments to check for this state of the carbon-12 nucleus, which he predicted should be at an energy level of 7.68 million eV.
I was very skeptical that this steady state cosmologist, this theorist, should ask questions about the carbon-12 nucleus. […] Hoyle just insisted — remember, we didn’t know him all that well — here was this funny little man who thought that we should stop all this important work that we were doing otherwise and look for this state, and we kind of gave him the brush off.
William Fowler in interview (1973)
But Hoyle succeeded in convincing a junior physicist at the lab, Ward Whaling, to check for it. Five months later, Hoyle received word.
Whaling confirmed the existence of the excited state of carbon-12, and it was almost exactly where Hoyle predicted: at 7.655 million eV!
Hoyle’s prediction is remarkable because he used astrophysics (the physics of stars) to find unknown properties in nuclear physics (the physics of atoms and their nuclei). Fowler was an instant convert.
So it was really quite a tour de force, that a man who walked into the lab predicted the existence of an excited state of a nucleus, and when the appropriate experiment was performed it was found. And no nuclear theorist starting from basic nuclear theory could do that then, nor can they really do it now. So Hoyle’s prediction was a very striking one.
William Fowler in interview (1973)
Fowler took a year off from his post at Caltech to work with Hoyle in Cambridge. Together with two astronomers, Margaret and Geoffrey Burbidge they worked out a complete theory of element formation, showing how every element is produced and explaining the relative abundances of the elements as found in nature.
Their work was revolutionary and it made a name for the authors.
For this work, Fowler received the Nobel Prize in physics in 1983. Hoyle, however, did not share in the prize, creating controversy.
In any event, no one denied the significance of their accomplishment.
With their 1957 paper, humanity finally had an understanding of where all the matter, which makes up our world, our food, our shelter, our very bodies, came from — the innermost depths of long-dead stars.
And so, the world as we know it is owed to the carbon-12 nucleus having this chance property. Like the delicate balance of the density of the universe, the existence of this state hangs in a delicate balance.
As it happens, the energy level of this state is at 7.655 MeV. Had the energy level of this state been less than 7.596 MeV or greater than 7.716 MeV, there would be almost no carbon in the universe.
The minor miracle of the carbon-12 nucleus having this excited state and it being in exactly the right range did not go unnoticed.
Some super-calculating intellect must have designed the properties of the carbon atom, otherwise the chance of my finding such an atom through the blind forces of nature would be utterly minuscule.
[…]
A common sense interpretation of the facts suggests that a superintellect has monkeyed with physics, as well as with chemistry and biology, and that there are no blind forces worth speaking about in nature. The numbers one calculates from the facts seem to me so overwhelming as to put this conclusion almost beyond question.
Fred Hoyle in “The Universe: Past and Present Reflections” (1982)
The balancing of the universe’s density, and the fortune of the carbon-12 excited state were just the first of many “cosmic coincidences.”
The more scientists probed the inner workings of the universe, the more lucky coincidences they found. With each one, evidence gathered to support the idea that the laws of physics are finely-tuned to permit the emergence of complexity, and with that complexity, life. The excited state of carbon-12 is determined by electromagnetic and nuclear forces.
https://alwaysasking.com/is-the-universe-fine-tuned/
Siegel Ethan: Beyond the galaxy 2016
Steady-State proponent Fred Hoyle put forth a breathtaking paper is simply known as B2 FH (after its authors) — in 1957, detailing how nuclear fusion ought to work in the hearts of stars. Above a certain density and temperature threshold, protons from hydrogen nuclei in the core of massive enough stars — of at least 8% the mass of our Sun — could fuse together, first into deuterium and then quickly into helium-3 and then helium-4, releasing a tremendous amount of energy: 28 MeV for every helium-4 nucleus that is produced. And the release of this energy through fusion reactions in stellar cores would not only explain why the Sun shines, but it would also explain why all the stars on the main sequence shine as they do. In the core of a star like our Sun, the temperatures reach up to around 15 million K, with gravitational pressures so intense that the density of the plasma is 13 times greater than solid lead. As massive as our Sun is, it contains a total of around a whopping 10^57 protons, a little less than 10% of which are in the core at any given time. Under all that pressure and at such a high temperature, protons in the Sun’s core have tremendous amounts of kinetic energy, moving at a significant percentage of the speed of light. The collision rate is tremendous, as they bounce off of other protons and nuclei, with every particle undergoing billions of interactions every second. With all these collisions and interactions, you can then calculate how many protons have enough energy to take that first step in the nuclear chain reaction, and fuse into deuterium. The answer is exactly zero. Of all the proton pairs colliding in the sun’s core, not a single one has sufficient energy to fuse into anything heavier, meaning temperature and density can’t be telling the whole story. Then how does nuclear fusion occur? Through the power of quantum mechanics! The individual protons in a star’s core might not have enough energy to overcome the repulsive force caused by their electric charges, but there’s always a chance that these particles can undergo quantum tunneling, and wind up in a more stable bound state (e.g., deuterium) that causes the release of this fusion energy. Even though the probability of quantum tunneling is very small for any particular proton-proton interaction — somewhere on the order of 1-in-10^28, or the same as your odds of winning the Powerball lottery three times in a row with buying exactly three tickets — the fact that there are so many interactions in the core happening continuously means that a whopping 4 × 10^38 protons fuse into helium every second in our Sun. And this process, of nuclear fusion fueled by quantum physics, is what is responsible for powering all the main sequence stars in the Universe.
In a very low-mass star, the volume of the fusion-producing core is small, and so fusion proceeds at a leisurely pace, causing these stars to be cool, red in color, and dim, or low in luminosity. If your star is more massive, the volume of the core is going to be larger, with higher temperatures, densities, and rates (and probabilities) of fusion. The more massive your hydrogen-burning star is, the hotter, bluer, and brighter your star is going to be, explaining why bluer stars are also the more luminous ones. But, perhaps counterintuitively, the more massive, luminous and blue your star is, the shorter its lifetime will be. You see, a star that is twice as massive as another might have twice as much hydrogen fuel to burn, but it burns through the fuel in its core approximately eight times as fast, while one that is ten times as massive burns through its fuel around a thousand times as quickly. Over very long timescales (many tens of billions of years), the spent (helium) fuel in a star’s core can convect out while unburned hydrogen convects in, allowing the longest-lived stars to burn through 100% of their hydrogen fuel. But for higher-mass stars, including stars like the Sun, when the core runs out of hydrogen, that’s the end of their time on the main sequence. This epiphany led to a spectacular prediction on the part of Fred Hoyle.

At the extreme temperatures in the core of our Sun, collisions between bare hydrogen nuclei (protons) are common, and occur at extremely high energies. However, these energies are insufficient to overcome the repulsive electric force on their own. It is only due to the laws of quantum physics, and the fact that each proton is a quantum particle with a probabilistic function describing its location, that enables the two wavefunctions to overlap ever so slightly. In virtually all the interactions, no fusion will occur, and the protons simply scatter off of one another. But in 1-in-1028 interactions, a fusion reaction does occur, forming a heavier element — like deuterium in this figure — and releasing energy in the process.
The only reason a hydrogen-burning star does not collapse under its own gravity is the incredible radiation pressure resulting from the nuclear fusion happening in the core. Yet, this process only creates the element helium, and judging from the sheer number and diversity of elements we see on Earth, there must, Hoyle reasoned, be a way to create heavier elements in these stars. At the temperatures and densities present in the core of a hydrogen-burning star, there would be no way to move up past helium-4; a proton could not fuse with it because there is no stable nucleus with a mass of 5, and another helium-4 could not fuse with it because there’s no stable nucleus of mass 8. Any nucleus temporarily formed with these masses decays back to helium-4 in a minuscule fraction of a second. But when you run out of hydrogen fuel in your core, the radiation pressure begins to drop, and suddenly the core of the star begins to collapse under its own gravity. When you have a very large number of particles together in a small space — like the core of a star — there is a lot of energy stored in the gravitational field between the particles. Unless you both collapse it very slowly and provide a very efficient way for that energy to escape, the process of collapse is going to cause a tremendous increase in both the temperature and the energies of the particles inside. This is the same principle behind how a diesel engine works — the rapid compression of the fuel inside causes it to ignite — only when the helium-4 reaches a certain threshold, it does not ignite, but rather begins fusing into beryllium-8! This isotope of beryllium is unstable, of course, and decays back to two helium-4 nuclei in approximately 10−17 seconds, but Hoyle recognized the importance of this isotope’s existence, even if it remained for only the briefest amounts of time. You see, the reason that nuclear fusion is so effective at releasing energy — the reason it can happen via quantum tunneling and the reason it can power so much — is because the rest mass of the products of a fusion reaction is significantly and measurably smaller than the mass of the reactants. Hydrogen eventually fuses into helium-4 because helium-4 has the mass equivalent (via the famous E = mc2 ) of 28 MeV less than the four hydrogen nuclei that went into it. Beryllium-8, on the other hand, has almost exactly the same mass as two helium-4 nuclei that go into it (the energy difference is less than 0.1 MeV), so there is no reason for it to be energetically favored, and that is why it decays back into helium-4 almost immediately. But that is an important almost, reasoned Hoyle, because if you could get a third helium-4 in there quickly enough, you could theoretically combine it with the beryllium-8 to form carbon-12, which is heavily energetically favored. But there was an important hurdle to overcome, which led Hoyle to make the most breathtaking prediction of his entire career. Just like atoms have excited states — where electrons can be in unstable, higher energy configurations that decay down to lower energy states, emitting a photon in the process — nuclei can also have excited states, or a spectrum of configurations where the lowest one, or the ground state, may be stable. The big difference between atomic excited states and nuclear excited states is that the latter are so significantly different in energy from one another that they have measurable mass differences (due to E = mc2 ) between them. Combining three helium-4 nuclei together would not be able to give you carbon-12, as the mass differences between the two systems are too great, with carbon-12 being significantly less massive. But if, Hoyle proposed, there were an excited state of carbon-12 that had strictly the same mass as three helium-4 nuclei combined, you could continue fusing elements in the hearts of stars even after they ran out of their hydrogen fuel. Since carbon exists, he reasoned, and is also required as a building block to form the plethora of even heavier elements present in stars, on Earth and throughout the Universe, this new excited state — which had never been observed — must exist, and must exist at precisely the same mass as three helium-4 nuclei combined!

The “ Hoyle state,” as its now known, was a proposed excited state of carbon-12. By reasoning that carbon must come to exist in great abundance, there must have been a way to form it in the core of stars. Beryllium-8 may be unstable, but at high enough densities and energies, it should be possible to get another helium-4 nucleus in there before it decays. If that happens, it can create the excited carbon-12 through what’s called the “triple-alpha” process, since a helium-4 nucleus is also an alpha-particle (emitted in some radioactive decays) and it takes three of them to make carbon-12. The excited carbon-12 then decays to normal, stable carbon-12 and emits a very high-energy photon, giving the core of the star an influx of new energy
This new hypothesized state was dubbed the Hoyle state, and the theoretical new process by which it was formed was known as the triple-alpha process since a helium-4 nucleus is also known as an alpha particle, emitted in some radioactive decays. Hoyle told his collaborator Willie Fowler about this hypothesis in 1952, and Fowler conceded that it was possible that such a state existed, and had been missed by nuclear physicists up until that point. It took five years of research but in 1957, the Hoyle state of carbon-12 was found and confirmed to have exactly the energy necessary to produce carbon in the cores of massive stars that had burned through their hydrogen! The key to unlocking the origin of all the heavy elements in the Universe had just been discovered
Ethan Siegel: What Can The Simple Fact That ‘We Exist’ Teach Us About The Universe? Oct 1, 2020
We know the mass of carbon-12, and the masses of the helium and hydrogen nuclei that are so abundant in the stars. The easiest way to get there would be to take three independent helium-4 nuclei and fuse them all together simultaneously. Helium-4 has two protons and two neutrons in its nucleus, so it’s easy to imagine that fusing three of them together would give you carbon-12, and hence could create the carbon we need in our Universe. But three helium nuclei, combined, are too massive to efficiently produce carbon-12. When two helium-4 nuclei fuse together, they produce beryllium-8 for just ~10-16 s, before it decays back to two helium nuclei. Although occasionally a third helium-4 nucleus could get in there if the temperatures are high enough, the energies are all wrong for producing carbon-12; there’s too much energy. The reaction just wouldn’t give us enough of the carbon our Universe needs. Fortunately, physicist Fred Hoyle realized that the Universe needed a pathway to make carbon from helium. He theorized that if there were an excited state of the carbon-12 nucleus, at a higher energy that was closer to the rest mass of three helium-4 nuclei combined, the reaction could occur. This nuclear state, known as the Hoyle State, was discovered just five years later by nuclear physicist Willie Fowler, who also discovered the triple-alpha process that formed it, just as Hoyle predicted.

The prediction of the Hoyle State and the discovery of the triple-alpha process is perhaps the most stunningly successful use of anthropic reasoning in scientific history. This process is what explains the creation of the majority of carbon that's found in our modern-day Universe.
R.A.W. Bradford The Inevitability of Fine Tuning in a Complex Universe 2011
If the strength of the strong nuclear force, gs, were changed by 1% the rate of the triple-alpha reaction would be affected so markedly that the production of biophilic1 abundances of either carbon or oxygen would be prevented.
https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1081.137&rep=rep1&type=pdf
Elisabeth Vangioni Cosmic origin of the chemical elements rarety in nuclear astrophysics 23 November 2017
Carbon is a fascinating nucleus since it is the basis of life and it is the product of an extraordinary energy coincidence at the level of nuclei. It is striking that the process of complexification of matter has to overcome these different obstacles; one sees that the result is very inefficient since the present mass fraction of atoms heavier than helium is only about 2% of about 5% of the baryonic matter in the Universe. This shows the extraordinary quality of rarety regarding the ordinary matter.
https://www.tandfonline.com/doi/pdf/10.1080/21553769.2017.1411838?needAccess=true
Carbon is unique in its ability to combine with other atoms, forming a vast and unparalleled number of compounds in combination with hydrogen, oxygen, and nitrogen. This universe of organic chemistry— with its huge diversity of chemical and physical properties—is precisely what is needed for the assembling of complex chemical systems. Furthermore, the general ‘metastability’ of carbon bonds and the consequent relative ease with which they can be assembled and rearranged by living systems contributes greatly to the fitness of carbon chemistry for biochemical life. No other atom is nearly as fit as carbon for the formation of complex biochemistry. Today, one century later, no one doubts these claims. Indeed the peerless fitness of the carbon atom to build chemical complexity and to partake in biochemistry has been affirmed by a host of researchers. 2
One widely publicized coincidence is the ‘lucky’ fact that the nuclear resonances of the isotopes 12C and 16O are exactly what they need to be if carbon is to be synthesized and accumulate in any quantity in the interior of stars . The energy levels of these resonances ensure that 12C is first synthesized in stellar interiors from collisions between 8Be (beryllium) and He (helium) nuclei and that the carbon synthesized is not depleted later. Hoyle made this discovery in 1953 while working at Caltech with William Fowler. An intriguing aspect of the discovery is that Hoyle made it based on a prediction from the anthropic principle. Hoyle himself
famously commented:
If you wanted to produce carbon and oxygen in roughly equal quantities by stellar nucleosynthesis, these are the two levels you would have to fix, and your fixing would have to be just about where these levels are found to be ... A common sense interpretation of the facts suggests that a superintellect has monkeyed with physics, as well as chemistry and biology and that there are no blind forces worth speaking about in nature.
This discovery was acclaimed not only as a major scientific discovery but also as further evidence of the biocentricity of nature. Hoyle may have been one of the first to notice that the conditions necessary to permit carbon-based life require a very narrow range of basic physical constants, but the idea is now widely accepted. If those constants had been very slightly different, the universe would not have been conducive to the development of matter, astronomical structures, or elemental diversity, and thus the emergence of complex chemical systems.
Fine tuning of carbon
The synthesis of carbon is a two- step process. The first step is the formation of an 8Be nucleus out of two 4He nuclei (alpha particles), the second the formation of a 12C nucleus out of the 8Be nucleus and another 4He nucleus. The probability of this process would be extremely small, were it not for two “coincidences”: the 8Be ground state has almost exactly the energy of two alpha particles, and 8Be+4He has almost exactly the energy of an excited state of 12C. In other words, the 8Be ground state “resonates” with a system comprising two alpha particles, and the excited 12C state resonates with a systems comprising 8Be and 4He. The existence of the second resonance was predicted by Fred Hoyle before its actual observation, based on the observed abundance of carbon in the Universe and the necessity for it to be formed in stars. The energy at which this resonance occurs depends sensitively on the interplay between the strong and the weak nuclear interactions. If the strong force were slightly stronger or slightly weaker — by just 1% in either direction — then the binding energies of the nuclei would be different, and the requisite resonance would not exist. In that case, there would be no carbon or any heavier elements anywhere in the Universe. “I do not believe,” Hoyle concluded, “that any scientist who examined the evidence would fail to draw the inference that the laws of nuclear physics have been deliberately designed with regard to the consequences they produce inside the stars.”
http://thisquantumworld.com/wp/a-critique-of-quantum-mechanics/fine-tuning/Tracey Peake: Foundations of Carbon-Based Life Leave Little Room for Error March 13, 2013
Life as we know it is based upon the elements of carbon and oxygen. Now a team of physicists, including one from North Carolina State University, is looking at the conditions necessary to the formation of those two elements in the universe. They’ve found that when it comes to supporting life, the universe leaves very little margin for error.
Both carbon and oxygen are produced when helium burns inside of giant red stars. Carbon-12, an essential element we’re all made of, can only form when three alpha particles, or helium-4 nuclei, combine in a very specific way. The key to formation is an excited state of carbon-12 known as the Hoyle state, and it has a very specific energy – measured at 379 keV (or 379,000 electron volts) above the energy of three alpha particles. Oxygen is produced by the combination of another alpha particle and carbon.
NC State physicist Dean Lee and German colleagues Evgeny Epelbaum, Hermann Krebs, Timo Laehde and Ulf-G. Meissner had previously confirmed the existence and structure of the Hoyle state with a numerical lattice that allowed the researchers to simulate how protons and neutrons interact. These protons and neutrons are made up of elementary particles called quarks. The light quark mass is one of the fundamental parameters of nature, and this mass affects particles’ energies.
In new lattice calculations done at the Juelich Supercomputer Centre the physicists found that just a slight variation in the light quark mass will change the energy of the Hoyle state, and this in turn would affect the production of carbon and oxygen in such a way that life as we know it wouldn’t exist.
“The Hoyle state of carbon is key,” Lee says. “If the Hoyle state energy was at 479 keV or more above the three alpha particles, then the amount of carbon produced would be too low for carbon-based life.
“The same holds true for oxygen,” he adds. “If the Hoyle state energy were instead within 279 keV of the three alphas, then there would be plenty of carbon. But the stars would burn their helium into carbon much earlier in their life cycle. As a consequence, the stars would not be hot enough to produce sufficient oxygen for life. In our lattice simulations, we find that more than a 2 or 3 percent change in the light quark mass would lead to problems with the abundance of either carbon or oxygen in the universe.”
There is no physical law to explain it. And neither PHYSICAL NEED. What remains, is chance, or design......
https://news.ncsu.edu/2013/03/tpleeanthropic/
1. http://news.ncsu.edu/2013/03/tpleeanthropic/
2. http://bio-complexity.org/ojs/index.php/main/article/view/BIO-C.2013.1/BIO-C.2013.1
3. Gonzalez, Privileged Planet, page 33
4. https://thisquantumworld.com/a-critique-of-quantum-mechanics/fine-tuning/
Last edited by Admin on Wed Jan 25, 2017 4:04 pm; edited 4 times in total

