4. Cell-cell adhesion and the Extra Cellular Matrix (ECM)
Cell-cell adhesion and the extracellular matrix (ECM) are fundamental components that play indispensable roles in upholding the structural integrity, proper function, and effective communication within tissues and organisms. These mechanisms encompass a intricate network of physical connections and interactions that occur between cells themselves and their surrounding microenvironment, exerting a profound influence on a wide array of biological processes. Cell-cell adhesion involves the establishment of robust connections between neighboring cells. These adhesion mechanisms are crucial for creating and maintaining tissue architecture, as they form the basis for the structural organization of multicellular organisms. Tight junctions, adherens junctions, desmosomes, and gap junctions are examples of cell-cell adhesion structures that not only anchor cells together but also enable the exchange of ions, nutrients, and signaling molecules. These connections are essential for proper tissue function, as they facilitate coordinated responses and allow cells to act as a synchronized unit. There are different types of cell-cell adhesion, including:
Tight Junctions: These create a barrier between cells, preventing substances from passing through the gaps between cells. They are essential in maintaining the integrity of epithelial and endothelial layers.
Desmosomes: Desmosomes provide mechanical strength to tissues, particularly in tissues subjected to mechanical stress, like skin and heart muscles. They consist of proteins that link the cytoskeletons of adjacent cells.
Gap Junctions: These allow direct communication between cells by forming channels that allow small molecules and ions to pass. They are crucial for coordinated cell activities, especially in excitable tissues like the heart.
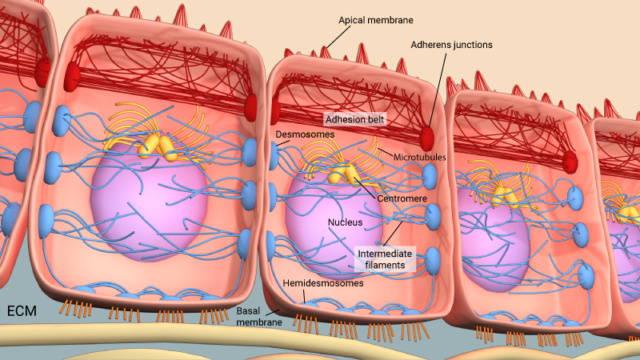
Adherens junctions play a crucial role in linking actin filaments between adjacent cells. The provided diagram illustrates adherens junctions depicted as red rectangles, effectively connecting actin filaments represented by red lines. In polarized epithelial cells, this interaction leads to the creation of contractile bundles comprising actin and myosin filaments in proximity to the apical surface. This unique arrangement gives rise to a distinct structure known as the adhesion belt, evident from the depicted arrows. Additionally, within these cells, there are other types of junctions known as desmosomes, indicated by larger blue rectangles, and hemi-desmosomes, depicted as smaller blue rectangles. These specialized junctions serve to link intermediate filaments, shown as blue lines, that extend between adjacent cells. This interconnected network of junctions not only contributes to the mechanical stability of tissues but also facilitates coordinated cell movements and supports the overall integrity of the epithelial layer. 1
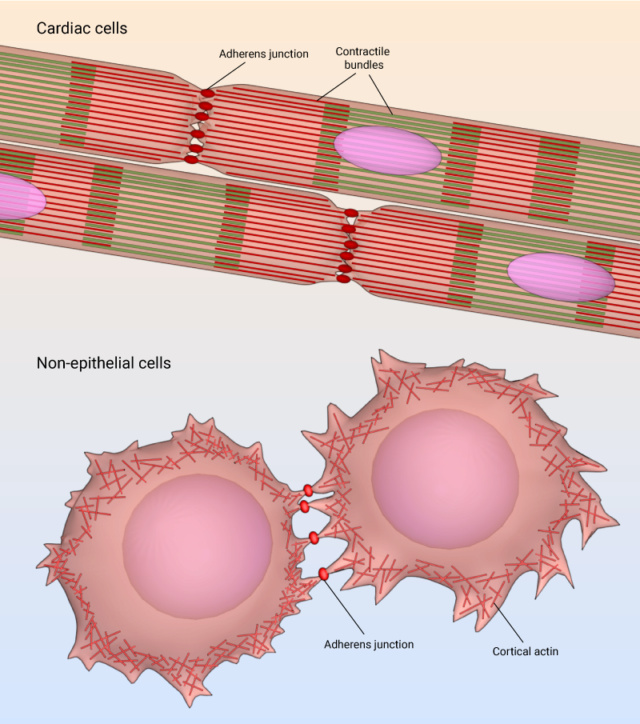
Adherens junctions, while often associated with epithelial cells, also hold vital roles in cardiac cells and various non-epithelial cell types. These specialized junctions are essential for maintaining tissue integrity, facilitating communication, and enabling coordinated actions in diverse cellular contexts. In cardiac cells, adherens junctions are particularly significant for the proper functioning of the heart. Cardiac tissue is composed of cardiomyocytes, which are the contractile cells responsible for generating the heart's rhythmic contractions. Adherens junctions in cardiac cells link adjacent cardiomyocytes through a protein called cadherin, specifically cardiac cadherin or N-cadherin. These junctions not only physically anchor cardiomyocytes together but also play a crucial role in transmitting mechanical forces during contraction. The intercalated discs in cardiac tissue represent specialized sites where adherens junctions are prominent. These discs consist of three main components: adherens junctions, desmosomes, and gap junctions. Adherens junctions provide mechanical stability by firmly attaching adjacent cardiomyocytes, which is vital for synchronized contractions. Additionally, they facilitate the transmission of signals between cells, enabling coordinated electrical impulses that regulate heartbeats. Beyond the realm of epithelial and cardiac cells, adherens junctions have been identified in various non-epithelial cell types as well. Neurons in the nervous system, for instance, utilize adherens junctions to establish connections at synapses, ensuring efficient communication between nerve cells. In vascular endothelial cells, these junctions contribute to the integrity of blood vessels and play a role in controlling vascular permeability.
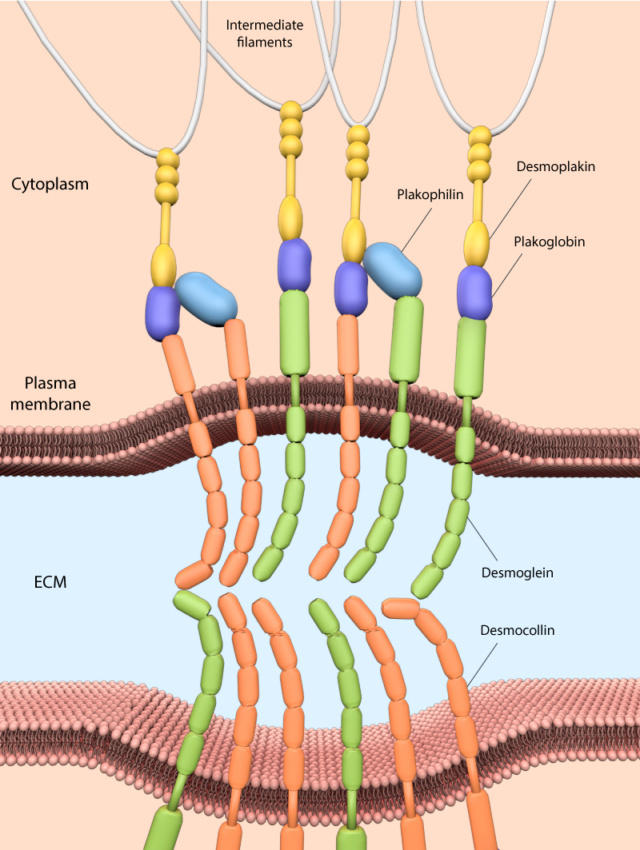
Desmosome junctions play a pivotal role in connecting intermediate filaments to the plasma membrane, contributing to the structural integrity and cohesion of tissues subjected to mechanical stress. These specialized junctions consist of a complex arrangement of molecular components that collectively ensure robust adhesion between adjacent cells. At the core of desmosomes are desmosomal cadherins, namely desmogleins and desmocollins. These transmembrane proteins span the plasma membrane and interact with their counterparts on neighboring cells, forming strong adhesive bonds. The extracellular domains of desmosomal cadherins create the adhesive interface that holds cells together. Inside the cell, desmosomal components further reinforce the connection. Cytoplasmic proteins like plakoglobin and plakophilins link the desmosomal cadherins to the network of intermediate filaments. Plakoglobin acts as an adaptor, bridging the cadherins to the intracellular machinery. Plakophilins, on the other hand, contribute to the stabilization of desmosomes by aiding in the interaction between desmosomal cadherins and intermediate filaments. The pivotal link between desmosomal cadherins and intermediate filaments is established by desmoplakin. This protein spans the cytoplasm and binds to the cytoplasmic domains of desmosomal cadherins on one end, while its other end associates with intermediate filaments. Desmoplakin serves as a molecular bridge, effectively tethering the cell-cell adhesion complex to the cell's internal structural framework. Collectively, desmosomes provide robust mechanical coupling between cells, especially in tissues subjected to stretching or shear forces, like the epidermis, cardiac muscle, and tissues lining internal cavities. This unique molecular assembly not only reinforces tissue integrity but also enables cells to withstand mechanical stress, ensuring the coherence and functionality of these specialized tissues.
Extracellular Matrix (ECM)
The ECM, on the other hand, is a complex network of proteins, glycoproteins, proteoglycans, and other molecules that provides a supportive scaffold for cells. This intricate matrix not only offers physical support but also participates in regulating various cellular activities. The ECM influences processes such as cell migration, differentiation, proliferation, and survival. It acts as a reservoir for growth factors, cytokines, and other signaling molecules, modulating cell behavior and orchestrating tissue development and repair. Furthermore, the ECM acts as a substrate for cell adhesion. Integrins, a family of transmembrane receptors, link the ECM to the cell's cytoskeleton, facilitating mechanical and biochemical communication between the two. This connection is vital for transmitting external cues into the cell and translating them into intracellular responses. As cells interact with the ECM, they can alter its composition through synthesis and degradation, thereby adapting to changing environmental conditions. Collectively, cell-cell adhesion and the ECM form a dynamic partnership that ensures tissue integrity and function. They not only create a structural framework but also regulate cellular behavior, enabling tissues to respond to physiological demands, developmental cues, and repair processes. Dysregulation of these mechanisms can lead to various diseases, underscoring the essential roles they play in maintaining the overall health and vitality of organisms. The ECM consists of various components:
Collagen: A fibrous protein that provides tensile strength to tissues like tendons, ligaments, and skin.
Elastin: A protein that imparts elasticity to tissues, such as blood vessels and lungs.
Proteoglycans: Large molecules that trap water, contributing to tissue hydration and resilience.
Fibronectin and Laminin: Adhesive proteins that facilitate cell attachment to the ECM and play a role in cell migration and differentiation.

Illustration of Extracellular Matrix (ECM) molecules. The extracellular matrix (ECM) is primarily comprised of glycosaminoglycans (GAGs) like hyaluronic acid (HA) and proteoglycans. These components form covalent bonds with GAGs, giving rise to a diverse range of protein complexes. Within cell membranes, integrins act as receptors that can bind to various ECM molecules including collagens, fibronectins, growth factors, and laminins, among others. The key receptors for HA are CD44 and CD168. The ECM's dynamic nature is maintained through processes like degradation by matrix metalloproteinases (MMPs), ensuring a responsive environment. The interactions between ECM molecules and cell receptors, including integrins and CD44/CD168, not only establish the extracellular framework but also trigger signals that set off downstream cellular changes. This interplay between ECM composition, receptor engagement, and subsequent signaling not only creates supportive cellular scaffolds but also initiates cascades of molecular events within the cells. 2
Importance in Biological Systems
Tissue Integrity: Cell-cell adhesion maintains tissue cohesion, preventing cells from detaching and maintaining the structural integrity of tissues.
Cell Communication: Gap junctions enable direct communication between cells, allowing ions, small molecules, and signaling molecules to pass. This is crucial for synchronized activities in tissues like the heart.
Embryonic Development: Cell adhesion and ECM play pivotal roles in embryonic development by guiding cell migration, tissue formation, and organogenesis.
Wound Healing and Repair: Proper cell adhesion and ECM are essential for wound healing, as cells migrate to close wounds and restore tissue integrity.
Cancer and Metastasis: Dysregulation of cell adhesion and ECM can contribute to cancer progression by promoting uncontrolled cell growth and metastasis.
Cell Differentiation: ECM components and adhesion molecules influence cell differentiation and specialization during development.
Mechanical Support: The ECM provides mechanical support to tissues and helps them withstand various physical forces.
Cell Migration: Cell adhesion and ECM interactions guide cell movement during processes like immune responses and tissue repair.
Cell-cell adhesion and the extracellular matrix are vital components of biological systems, contributing to tissue integrity, communication, development, repair, and disease progression. These mechanisms ensure the proper functioning of cells within tissues and organs, highlighting their importance in maintaining overall organism health.
How do cell-cell adhesion and interactions with the ECM contribute to tissue organization and morphogenesis?
Cell-cell adhesion and interactions with the extracellular matrix (ECM) play pivotal roles in tissue organization and morphogenesis during development. These processes involve complex molecular mechanisms that are highly interdependent, orchestrated, and precisely regulated, which suggests purposeful design.
Cell-Cell Adhesion: Cell-cell adhesion involves the binding of cells to each other through specific adhesion molecules, such as cadherins. This adhesion is crucial for the formation and maintenance of tissue structures. Key roles of cell-cell adhesion include:
Tissue Integrity: Adhesion molecules ensure that cells remain tightly connected within tissues. This integrity is essential for the formation of coherent tissues and organs.
Cell Sorting and Compartmentalization: Differential adhesion between different cell types contributes to the sorting of cells into specific regions and compartments. This process is vital for creating organized tissue architectures.
Cell Communication: Cell-cell adhesion molecules are often linked to signaling pathways that regulate cell behavior, including proliferation, differentiation, and migration. This communication contributes to the coordinated development of tissues.
Morphogenesis: During tissue remodeling and morphogenesis, cell-cell adhesion plays a role in shaping tissues and generating tissue-specific structures.
Interactions with the Extracellular Matrix (ECM)
The ECM is a complex network of proteins and molecules that provides structural support and guidance cues for cells. The interactions between cells and the ECM are critical for tissue organization and morphogenesis:
Cell Migration and Guidance: The ECM provides tracks and signals that guide cell migration during tissue development. Integrins, which are cell surface receptors, mediate these interactions and allow cells to move along ECM fibers.
Cell Differentiation: Cells receive signals from the ECM that influence their differentiation into specific cell types. Different regions of the ECM can provide different cues, directing cells to adopt particular fates.
Tissue Architecture: The ECM contributes to the formation of tissue architecture by providing mechanical support and defining the three-dimensional structure of tissues and organs.
Cell Survival and Apoptosis: Signals from the ECM can influence cell survival or apoptosis, contributing to tissue sculpting during development.
The complex interplay between cell-cell adhesion, interactions with the ECM, and various signaling pathways ensures that cells within tissues are organized, communicate effectively, and contribute to the formation of functional tissues and organs. The precision and coordination required for these processes suggest a purposeful design, where different elements of the system were intricately integrated to achieve specific developmental outcomes. The simultaneous presence of cell adhesion molecules, ECM components, and signaling pathways from the beginning becomes an essential feature, as a stepwise evolutionary process without all the components would likely result in non-functional or misshapen tissues.
What are the molecular components that mediate cell-ECM and cell-cell interactions during development?
Cell-ECM and cell-cell interactions during development involve a complex array of molecular components that allow cells to communicate, adhere, and respond to their environment. These components include:
Cell-ECM Interactions
Integrins: Transmembrane receptors that mediate the attachment of cells to the ECM components like fibronectin, collagen, and laminin. Integrins link the ECM to the cell's cytoskeleton, enabling mechanical signaling and migration.
Fibronectin: A glycoprotein present in the ECM that provides binding sites for integrins. It plays a crucial role in cell adhesion, migration, and tissue morphogenesis.
Collagen: A major component of the ECM, collagen fibers provide structural support to tissues. Different collagen types interact with different integrins and contribute to tissue-specific functions.
Laminin: A protein found in the basement membrane that interacts with integrins to mediate cell-ECM adhesion. It plays a role in cell differentiation, migration, and tissue development.
Proteoglycans: Molecules consisting of a core protein and glycosaminoglycan chains. They contribute to the ECM's hydrated gel-like properties and help regulate cell behavior.
Cell-Cell Interactions
Cadherins: Calcium-dependent adhesion molecules that mediate homophilic interactions between cells. Different types of cadherins are expressed in various tissues and contribute to tissue-specific cell sorting and organization.
Desmosomes: Cell-cell junctions that link intermediate filaments in adjacent cells, providing strong mechanical adhesion. They are particularly important in tissues subjected to mechanical stress.
Tight Junctions: Membrane junctions that seal adjacent cells together, preventing the passage of molecules between cells. They are essential for maintaining the barrier functions of epithelial tissues.
Gap Junctions: Channels that allow small molecules and ions to pass between adjacent cells. They enable direct communication and coordination between cells in tissues like the nervous system and cardiac muscle.
Notch Signaling: A cell-to-cell signaling mechanism involved in cell fate determination and differentiation. It plays a crucial role in processes such as tissue patterning and organ development.
These molecular components work together to regulate cell adhesion, migration, differentiation, and tissue organization during development. The specificity of these interactions, the diversity of molecules involved, and their coordinated functioning all point to a sophisticated design that ensures proper tissue formation and function. The intricate nature of these components suggests that they needed to be present and functional from the beginning to achieve the desired developmental outcomes, making stepwise evolution without all the components less plausible.
Appearance of Cell-Cell Adhesion and ECM in the evolutionary timeline
The appearance and development of Cell-Cell Adhesion and the Extracellular Matrix (ECM) in the evolutionary timeline coincide with the emergence of multicellular organisms and the emergence of more complex body structures. As organisms supposedly transitioned from single-celled life forms to multicellular entities, the need for mechanisms that facilitate cell cohesion, communication, and tissue organization became increasingly important.
Early Single-Celled Organisms: In the early stages of life on Earth, single-celled organisms would have predominated. These organisms did not require elaborate cell-cell adhesion mechanisms or an extensive ECM, as their functions were largely individualistic, and they often lived in isolation.
Emergence of Multicellularity: With the supposed evolution of multicellularity, cells would have begun to cooperate and specialize in various functions. The transition from loose collections of cells to coordinated tissues required the development of adhesion mechanisms to keep cells together and form cohesive structures.
Evolution of Simple Tissues: The first multicellular organisms would have formed simple tissues where cells directly interacted with each other. Basic cell-cell adhesion proteins would have emerged to ensure these cells remained connected and functioned collectively.
Importance in Complex Tissues: As organisms would have emerged to have more complex body structures, the need for stronger cell-cell adhesion and ECM would have become evident. Tissues and organs with specific functions would have required a well-organized structure to maintain proper function.
Diversification of Adhesion Molecules: Over time, adhesion molecules and junctions like tight junctions, desmosomes, and gap junctions would have originated to provide different forms of adhesion and communication between cells. These mechanisms would have allowed cells to work together and coordinate activities.
Development of Extracellular Matrix: The ECM would have emerged as an intricate network of proteins and carbohydrates secreted by cells. It would have provided mechanical support, anchorage, and signaling cues for cells within tissues. Components like collagen, elastin, proteoglycans, and adhesive proteins would have gradually emerged.
Tissue Specialization: As tissues would have specialized for various functions, cell-cell adhesion and ECM components would have adapted to suit the needs of different tissues. For instance, connective tissues would have required strong collagen fibers, while epithelial tissues needed tight junctions for barriers.
Complex Organisms and Systems: In more complex organisms, cell-cell adhesion and the ECM would have become integral to the functioning of various systems, including nervous, circulatory, and immune systems. These mechanisms would have facilitated interactions and responses within tissues and organs.
Evolution of Integrins and ECM Proteins: Integrins, cell surface receptors that connect cells to the ECM, would have evolved to provide a dynamic link between cells and their environment. ECM proteins would have diversified and became more specialized in different tissues.
Importance in Development and Disease: Cell-cell adhesion and the ECM play critical roles in embryonic development, tissue repair, and disease processes like cancer metastasis. Their functions are tightly intertwined with the overall health and functionality of organisms.
The appearance of Cell-Cell Adhesion and the Extracellular Matrix is closely linked to the evolution of multicellularity and the development of more complex body structures. These mechanisms would have been essential for maintaining tissue cohesion, enabling communication between cells, and facilitating the emergence of specialized tissues and organs. As organisms would have diversified and specialized, the complexity of cell-cell adhesion and ECM systems increased, contributing to the intricate functioning of complex organisms.
De Novo Genetic Information necessary to instantiate Cell-cell adhesion and the extracellular matrix (ECM)
Cell-Cell Adhesion Genetic Information
Cadherin Genes: Cadherins are crucial molecules for cell-cell adhesion. New genetic information would be needed to code for various cadherin proteins that mediate adhesion specificity. Different tissues might require different types of cadherins.
Cytoskeletal Proteins: Genes encoding cytoskeletal proteins such as actin and myosin might need additional regulatory elements to be expressed in specific cell types for the formation of contractile structures.
Junctional Proteins: Genes coding for proteins like catenins, which link cadherins to the cytoskeleton, and proteins involved in gap junctions and tight junctions, would need to be introduced or modified.
Extracellular Matrix Genetic Information
Glycoproteins and Proteoglycans: New genes would need to be added or modified to code for various glycoproteins (e.g., fibronectin, laminin) and proteoglycans that compose the ECM.
Enzymes: Genes coding for enzymes responsible for synthesizing and modifying ECM components would need to be introduced. For example, genes for enzymes that create cross-links between collagen molecules.
Integrins and Receptors: New genetic information would be required to code for integrins and other cell surface receptors that interact with ECM components.
Matrix Metalloproteinases (MMPs): Genes for MMPs and their regulators would be needed to allow for controlled degradation of the ECM.
Developing these complex processes would also involve genetic changes related to cell signaling, tissue-specific expression, and regulatory elements. Creating a biological system as intricate as cell-cell adhesion and the ECM from scratch is an immensely complex process, involving numerous genetic alterations, regulatory mechanisms, and interactions.
Manufacturing codes and languages employed to instantiate Cell-cell adhesion and the extracellular matrix (ECM)
Blueprints and Design Changes
Imagine an organism without cell-cell adhesion and ECM as a basic structure with individual cells loosely interacting. To develop these features, genetic changes analogous to design blueprints would need to occur.
New genetic information (analogous to revised blueprints) would specify the production of adhesion molecules, such as cadherins and ECM components like fibronectin or collagen.
Cellular Communication Update
In manufacturing, design changes often require updates across departments. Similarly, cells need to "communicate" these changes to coordinate their actions. Signaling pathways within cells would need to be instantiated or be repurposed to trigger the expression of adhesion proteins and ECM components.
Protein Synthesis and Assembly Instructions
In manufacturing, new parts are manufactured based on updated designs. Similarly, cells must synthesize new proteins according to the updated genetic instructions. Cells would need the "instructions" to fold proteins correctly and assemble them into functional adhesion molecules and ECM components.
Quality Control and Integration
In manufacturing, quality control ensures new parts fit seamlessly. In biology, mechanisms akin to quality control would need to ensure newly synthesized adhesion molecules properly interact. Cells would need to integrate these new components into their existing structure while maintaining cohesion and stability.
Fine-Tuning and Adaptation
Manufacturing processes often require adjustments for optimal performance. Similarly, biological systems would require fine-tuning and adaptation.
Epigenetic Regulatory Mechanisms necessary to be instantiated for Cell-cell adhesion and the extracellular matrix (ECM)
The development of complex biological features like cell-cell adhesion and the extracellular matrix (ECM) involves intricate epigenetic regulations that control gene expression patterns. Epigenetic mechanisms play a crucial role in orchestrating the intricate processes required for these features to emerge and function effectively.
Epigenetic Regulation for Development
DNA Methylation: The addition of methyl groups to DNA can affect gene expression. In the development of cell-cell adhesion and the ECM, specific genes encoding adhesion proteins and ECM components would have to be regulated by DNA methylation.
Histone Modification: Chemical modifications to histone proteins can alter chromatin structure and gene accessibility. Histone acetylation and methylation could be involved in controlling the expression of genes related to adhesion and ECM.
Non-coding RNAs: MicroRNAs and long non-coding RNAs (lncRNAs) regulate gene expression post-transcriptionally. They fine-tune the expression of genes involved in adhesion and ECM formation.
Epigenetic Regulatory Mechanisms necessary to be instantiated to create Cell-Cell Adhesion and the ECM
DNA Methylation System: Enzymes such as DNA methyltransferases are responsible for adding methyl groups to DNA. Demethylases can remove these marks. The balance between these enzymes would determine the DNA methylation pattern.
Histone Modification System: Histone acetyltransferases (HATs) and histone deacetylases (HDACs) regulate histone acetylation levels. Similarly, histone methyltransferases and demethylases control histone methylation. The interplay of these enzymes maintains proper chromatin structure.
RNA-Mediated Regulation System: Enzymes like Dicer process miRNAs, which target specific mRNAs for degradation or translational repression. LncRNAs can also interact with chromatin-modifying complexes, influencing gene expression.
Maintaining Balance and Operation - Collaborative Systems
Transcription Factor Networks: Transcription factors play a role in establishing cell-specific gene expression patterns. They work in conjunction with epigenetic regulators to ensure precise gene activation or repression.
Cell Signaling Pathways: Signaling pathways can influence epigenetic marks and gene expression. For instance, growth factors or environmental signals can activate cascades that modulate DNA methylation or histone modifications.
Cell-Cell Communication: Cells within tissues communicate to establish coordinated gene expression. In the context of ECM and adhesion, cells might signal each other to ensure proper adhesion protein expression and ECM production.
Cell Cycle Control: The cell cycle influences epigenetic regulation. Cell division provides opportunities for resetting epigenetic marks, allowing cells to re-establish appropriate gene expression patterns during development.
Environmental Influences: External factors like diet, stress, and exposure to toxins can impact epigenetic marks, potentially influencing the development of adhesion and ECM systems.
The collaboration of these systems ensures the precise activation and repression of genes required for cell-cell adhesion and ECM formation. Epigenetic mechanisms act as a dynamic regulatory layer, responding to cues from both the internal cellular environment and external signals to ensure proper development, function, and maintenance of these complex biological features.
Signaling Pathways necessary to create, and maintain Cell-Cell Adhesion and the ECM
The emergence of complex biological features like cell-cell adhesion and the extracellular matrix (ECM) involves intricate signaling pathways that coordinate various cellular processes.
Wnt Signaling Pathway
Wnt signaling plays a critical role in tissue development, stem cell maintenance, and cell adhesion. It can influence the expression of cadherins and other adhesion molecules, affecting cell-cell interactions.
Interconnection: Wnt signaling crosstalks with other pathways, such as the Notch and Hedgehog pathways, enhancing regulatory complexity.
Transforming Growth Factor-Beta (TGF-β) Pathway
TGF-β is involved in various processes, including ECM synthesis and remodeling. It stimulates the expression of ECM components like collagen and fibronectin.
Interconnection: TGF-β signaling interacts with other pathways, like MAPK and BMP, to regulate diverse cellular functions.
Integrin-Mediated Signaling
Integrins connect ECM components to the cell's cytoskeleton and activate signaling pathways upon ligand binding. Integrin signaling influences cell adhesion, migration, and ECM remodeling.
Interconnection: Integrin signaling cross-communicates with growth factor pathways, modulating cellular responses.
Notch Signaling Pathway
Notch signaling is involved in cell fate determination and tissue development. It can influence cell adhesion through the regulation of cadherin expression.
Interconnection: Notch crosstalks with Wnt and other pathways to coordinate developmental decisions.
MAPK/ERK Pathway
MAPK/ERK pathway controls cell proliferation, differentiation, and migration. It can impact ECM synthesis and cell adhesion molecule expression.
Interconnection: MAPK/ERK crosstalks with integrin and growth factor pathways to modulate cell behavior.
Hedgehog Signaling Pathway
Hedgehog signaling regulates tissue patterning and development. It may influence ECM production and remodeling processes.
Interconnection: Hedgehog pathway crosstalks with Wnt and TGF-β pathways for coordinated effects.
PI3K/AKT Pathway
PI3K/AKT pathway controls cell survival, growth, and migration. It can impact integrin-mediated cell adhesion and ECM interactions.
Interconnection: PI3K/AKT crosstalks with multiple pathways, including growth factor pathways.
These pathways are highly interconnected and interdependent. Crosstalk between them allows for intricate regulation and response to various stimuli. Additionally, these pathways communicate with other biological systems, such as developmental pathways, immune responses, and cellular metabolism. The interconnectedness allows cells to integrate signals from different sources, ensuring coordinated responses and adaptive behaviors, ultimately contributing to the emergence, maintenance, and function of cell-cell adhesion and the ECM in complex organisms.
Regulatory codes necessary for maintenance and operation
The maintenance and operation of complex biological systems like cell-cell adhesion and the extracellular matrix (ECM) involve intricate regulatory codes and languages that ensure proper function, adaptation, and balance.
Feedback Loops and Homeostasis
Just as in a control system, biological systems employ feedback loops to maintain stability. Regulatory mechanisms ensure that cell-cell adhesion and ECM components are produced in appropriate amounts. Cells might sense the density of adhesion molecules or ECM components and adjust their expression accordingly.
Signal Integration and Cross-Communication
Cells integrate various signals from their environment, translating them into appropriate responses. Regulatory pathways like MAPK, PI3K/AKT, and others act as interpreters, relaying information from growth factors, hormones, and mechanical cues to regulate adhesion and ECM-related gene expression.
Epigenetic Regulation for Memory and Plasticity
Epigenetic modifications, like DNA methylation and histone modifications, can serve as "memory" marks. These marks maintain stable gene expression patterns over time, ensuring that adhesion and ECM components are consistently produced in the right contexts.
Cell-cell communication and Quorum Sensing
Cells in tissues communicate with each other to synchronize behavior. Cells might employ mechanisms similar to quorum sensing to determine the presence of neighboring cells and adjust adhesion and ECM production accordingly.
Dynamic Remodeling and ECM Degradation
Just as a construction site adapts to changing needs, cells can modify the ECM based on requirements. Regulatory pathways control matrix metalloproteinases (MMPs) that degrade and remodel ECM components, ensuring dynamic adaptation.
Cell Differentiation and Specialization
Regulatory codes guide stem cells to differentiate into specific cell types, some of which contribute to cell adhesion and ECM production.Signaling pathways like Wnt, Notch, and BMP play roles in determining cell fate.
Tissue-Specific Regulation
Different tissues require different levels of adhesion and ECM components. Regulatory mechanisms ensure tissue-specific expression patterns, maintaining the uniqueness of each tissue type.
Feedback from Cellular Mechanics
Just as a machine's performance feedback affects its operation, cells can sense mechanical forces and adjust adhesion and ECM accordingly. Mechanotransduction pathways translate mechanical cues into biochemical responses.
These regulatory mechanisms collaborate in a language of molecular interactions to maintain the operation of cell-cell adhesion and the ECM. The intricate orchestration of these codes ensures that cells can adhere, communicate, adapt, and contribute to tissue integrity and function in the dynamic environment of a living organism.
How did the molecular machinery for cell-cell adhesion and ECM interactions emerge to support multicellular organisms?
The emergence of the molecular machinery for cell-cell adhesion and extracellular matrix (ECM) interactions is a complex process that likely involved the coordination of various molecular components. While the specific details of this process are still a subject of scientific investigation, there are several hypotheses and mechanisms that would explain the origin of these essential cellular processes:
Cooption of Pre-existing Molecules: It's claimed that some of the molecules involved in cell-cell adhesion and ECM interactions had pre-existing functions in single-celled organisms. These functions would have included interactions with the external environment or with other cells. Through genetic changes and adaptations, these molecules would have been coopted for cell-cell and cell-ECM interactions in multicellular contexts.
Gene Duplication and Divergence: Gene duplication events followed by divergence would have played a role in creating new molecules with adhesion-related functions. Over time, these duplicated genes would have evolved distinct roles in cell-cell and cell-ECM interactions.
Horizontal Gene Transfer: Genetic material can sometimes be transferred between different species or organisms. Horizontal gene transfer would have introduced novel genes or gene variants into multicellular organisms, providing the molecular basis for adhesion and ECM interactions.
Emergence of Protein Domains: Some protein domains have inherent adhesive properties. Through gene duplication, recombination, and mutation, these domains would have been integrated into larger proteins that facilitated adhesion and ECM interactions.
Evolution of Cell Signaling: Cell adhesion and ECM interactions are closely linked to cell signaling pathways. It is claimed that the evolution of these pathways would have enabled cells to communicate and respond to each other and their environment, promoting coordinated multicellular behavior.
Symbiotic Relationships: Multicellularity would have evolved from symbiotic relationships between different types of cells. These cells would need to adhere to each other and establish cooperative interactions for survival, leading to the development of adhesion and ECM-related mechanisms.
Once Cell-Cell Adhesion and the Extra Cellular Matrix (ECM) are operational, what other intra and extracellular systems is it interdependent with?
Once Cell-Cell Adhesion and the Extracellular Matrix (ECM) are instantiated and operational, they become interdependent with various intra and extracellular systems to ensure proper tissue structure, function, and communication. Here are some of the key systems with which Cell-Cell Adhesion and the ECM are interconnected:
Intracellular Systems
Cytoskeleton and Cell Shape: Cell-cell adhesion and ECM interactions influence cytoskeletal organization, which in turn affects cell shape, migration, and mechanical stability.
Cell Signaling Pathways: Interactions with Cell-Cell Adhesion and the ECM can activate signaling pathways that regulate cell survival, proliferation, differentiation, and migration.
Cellular Transport and Communication: Cell-cell adhesion and the ECM affect the localization of membrane proteins involved in cellular transport and communication, influencing nutrient uptake, waste removal, and intercellular signaling.
Gene Expression and Differentiation: Cell-cell adhesion and ECM interactions can impact gene expression patterns that drive cell differentiation and tissue-specific functions.
Extracellular Systems
Immune Response: Cell-cell adhesion and the ECM influence immune cell trafficking, recruitment, and interactions with target cells during immune responses.
Blood Circulation and Oxygen Delivery: The ECM provides structural support for blood vessels, while Cell-Cell Adhesion guides the organization of endothelial cells lining vessels, affecting blood flow and oxygen/nutrient delivery to tissues.
Extracellular Signaling Molecules: The ECM can store and release signaling molecules that regulate cell behavior, tissue repair, and immune responses.
Nervous System and Neurodevelopment: The ECM contributes to neural development and synapse formation, while Cell-Cell Adhesion guides neuronal migration and connectivity in the developing nervous system.
Hormonal Regulation: Cell-cell adhesion and ECM interactions can affect hormone receptor availability and signaling, influencing physiological responses.
Tissue Regeneration and Repair: Proper Cell-Cell Adhesion and ECM are crucial for tissue regeneration and wound healing, providing the structural support needed for new tissue growth.
Mechanical Integrity: The ECM provides mechanical support to tissues and organs, ensuring their structural integrity and protection.
Cell Differentiation and Organization: Cell-cell adhesion and the ECM play roles in organizing cells into functional tissues, allowing for cooperative interactions and specialized functions.
These interconnected systems highlight how Cell-Cell Adhesion and the ECM, once instantiated and operational, are integral components of the overall physiological framework. Their interactions with various cellular and extracellular processes contribute to tissue homeostasis, communication, and the proper functioning of diverse biological systems.
1. The intricate interdependence observed between Cell-Cell Adhesion, the Extracellular Matrix (ECM), and various intra and extracellular systems, including cytoskeleton, cell signaling, immune response, blood circulation, nervous system, and more, forms a tightly integrated network crucial for proper tissue structure, function, and communication.
2. These interdependent systems exhibit a level of coordinated complexity that suggests a designed setup rather than a random accumulation of components over time. The immediate functionality and seamless interaction among these systems imply a purposeful arrangement to achieve optimal biological function.
3. The simultaneous emergence and functional integration of Cell-Cell Adhesion, the ECM, and multiple interconnected systems highlight a coherent and intentional design that enables effective tissue organization, communication, and response to environmental cues.
Conclusion: The evident interconnectedness and functional reliance on Cell-Cell Adhesion, the ECM, and diverse biological systems provide strong indications of a designed framework. The intricate coordination, immediate functionality, and harmonious collaboration between these systems point toward an intelligently orchestrated setup that supports the intricate physiological requirements of organisms.
1. What are cell-cell adhesions?
2. M.Karlinski Unfolding the Folds: How the Biomechanics of the Extracellular Matrix contributes to Cortical Gyrification September 2018
Cell-cell adhesion and the extracellular matrix (ECM) are fundamental components that play indispensable roles in upholding the structural integrity, proper function, and effective communication within tissues and organisms. These mechanisms encompass a intricate network of physical connections and interactions that occur between cells themselves and their surrounding microenvironment, exerting a profound influence on a wide array of biological processes. Cell-cell adhesion involves the establishment of robust connections between neighboring cells. These adhesion mechanisms are crucial for creating and maintaining tissue architecture, as they form the basis for the structural organization of multicellular organisms. Tight junctions, adherens junctions, desmosomes, and gap junctions are examples of cell-cell adhesion structures that not only anchor cells together but also enable the exchange of ions, nutrients, and signaling molecules. These connections are essential for proper tissue function, as they facilitate coordinated responses and allow cells to act as a synchronized unit. There are different types of cell-cell adhesion, including:
Tight Junctions: These create a barrier between cells, preventing substances from passing through the gaps between cells. They are essential in maintaining the integrity of epithelial and endothelial layers.
Desmosomes: Desmosomes provide mechanical strength to tissues, particularly in tissues subjected to mechanical stress, like skin and heart muscles. They consist of proteins that link the cytoskeletons of adjacent cells.
Gap Junctions: These allow direct communication between cells by forming channels that allow small molecules and ions to pass. They are crucial for coordinated cell activities, especially in excitable tissues like the heart.

Adherens junctions play a crucial role in linking actin filaments between adjacent cells. The provided diagram illustrates adherens junctions depicted as red rectangles, effectively connecting actin filaments represented by red lines. In polarized epithelial cells, this interaction leads to the creation of contractile bundles comprising actin and myosin filaments in proximity to the apical surface. This unique arrangement gives rise to a distinct structure known as the adhesion belt, evident from the depicted arrows. Additionally, within these cells, there are other types of junctions known as desmosomes, indicated by larger blue rectangles, and hemi-desmosomes, depicted as smaller blue rectangles. These specialized junctions serve to link intermediate filaments, shown as blue lines, that extend between adjacent cells. This interconnected network of junctions not only contributes to the mechanical stability of tissues but also facilitates coordinated cell movements and supports the overall integrity of the epithelial layer. 1

Adherens junctions, while often associated with epithelial cells, also hold vital roles in cardiac cells and various non-epithelial cell types. These specialized junctions are essential for maintaining tissue integrity, facilitating communication, and enabling coordinated actions in diverse cellular contexts. In cardiac cells, adherens junctions are particularly significant for the proper functioning of the heart. Cardiac tissue is composed of cardiomyocytes, which are the contractile cells responsible for generating the heart's rhythmic contractions. Adherens junctions in cardiac cells link adjacent cardiomyocytes through a protein called cadherin, specifically cardiac cadherin or N-cadherin. These junctions not only physically anchor cardiomyocytes together but also play a crucial role in transmitting mechanical forces during contraction. The intercalated discs in cardiac tissue represent specialized sites where adherens junctions are prominent. These discs consist of three main components: adherens junctions, desmosomes, and gap junctions. Adherens junctions provide mechanical stability by firmly attaching adjacent cardiomyocytes, which is vital for synchronized contractions. Additionally, they facilitate the transmission of signals between cells, enabling coordinated electrical impulses that regulate heartbeats. Beyond the realm of epithelial and cardiac cells, adherens junctions have been identified in various non-epithelial cell types as well. Neurons in the nervous system, for instance, utilize adherens junctions to establish connections at synapses, ensuring efficient communication between nerve cells. In vascular endothelial cells, these junctions contribute to the integrity of blood vessels and play a role in controlling vascular permeability.

Desmosome junctions play a pivotal role in connecting intermediate filaments to the plasma membrane, contributing to the structural integrity and cohesion of tissues subjected to mechanical stress. These specialized junctions consist of a complex arrangement of molecular components that collectively ensure robust adhesion between adjacent cells. At the core of desmosomes are desmosomal cadherins, namely desmogleins and desmocollins. These transmembrane proteins span the plasma membrane and interact with their counterparts on neighboring cells, forming strong adhesive bonds. The extracellular domains of desmosomal cadherins create the adhesive interface that holds cells together. Inside the cell, desmosomal components further reinforce the connection. Cytoplasmic proteins like plakoglobin and plakophilins link the desmosomal cadherins to the network of intermediate filaments. Plakoglobin acts as an adaptor, bridging the cadherins to the intracellular machinery. Plakophilins, on the other hand, contribute to the stabilization of desmosomes by aiding in the interaction between desmosomal cadherins and intermediate filaments. The pivotal link between desmosomal cadherins and intermediate filaments is established by desmoplakin. This protein spans the cytoplasm and binds to the cytoplasmic domains of desmosomal cadherins on one end, while its other end associates with intermediate filaments. Desmoplakin serves as a molecular bridge, effectively tethering the cell-cell adhesion complex to the cell's internal structural framework. Collectively, desmosomes provide robust mechanical coupling between cells, especially in tissues subjected to stretching or shear forces, like the epidermis, cardiac muscle, and tissues lining internal cavities. This unique molecular assembly not only reinforces tissue integrity but also enables cells to withstand mechanical stress, ensuring the coherence and functionality of these specialized tissues.
Extracellular Matrix (ECM)
The ECM, on the other hand, is a complex network of proteins, glycoproteins, proteoglycans, and other molecules that provides a supportive scaffold for cells. This intricate matrix not only offers physical support but also participates in regulating various cellular activities. The ECM influences processes such as cell migration, differentiation, proliferation, and survival. It acts as a reservoir for growth factors, cytokines, and other signaling molecules, modulating cell behavior and orchestrating tissue development and repair. Furthermore, the ECM acts as a substrate for cell adhesion. Integrins, a family of transmembrane receptors, link the ECM to the cell's cytoskeleton, facilitating mechanical and biochemical communication between the two. This connection is vital for transmitting external cues into the cell and translating them into intracellular responses. As cells interact with the ECM, they can alter its composition through synthesis and degradation, thereby adapting to changing environmental conditions. Collectively, cell-cell adhesion and the ECM form a dynamic partnership that ensures tissue integrity and function. They not only create a structural framework but also regulate cellular behavior, enabling tissues to respond to physiological demands, developmental cues, and repair processes. Dysregulation of these mechanisms can lead to various diseases, underscoring the essential roles they play in maintaining the overall health and vitality of organisms. The ECM consists of various components:
Collagen: A fibrous protein that provides tensile strength to tissues like tendons, ligaments, and skin.
Elastin: A protein that imparts elasticity to tissues, such as blood vessels and lungs.
Proteoglycans: Large molecules that trap water, contributing to tissue hydration and resilience.
Fibronectin and Laminin: Adhesive proteins that facilitate cell attachment to the ECM and play a role in cell migration and differentiation.

Illustration of Extracellular Matrix (ECM) molecules. The extracellular matrix (ECM) is primarily comprised of glycosaminoglycans (GAGs) like hyaluronic acid (HA) and proteoglycans. These components form covalent bonds with GAGs, giving rise to a diverse range of protein complexes. Within cell membranes, integrins act as receptors that can bind to various ECM molecules including collagens, fibronectins, growth factors, and laminins, among others. The key receptors for HA are CD44 and CD168. The ECM's dynamic nature is maintained through processes like degradation by matrix metalloproteinases (MMPs), ensuring a responsive environment. The interactions between ECM molecules and cell receptors, including integrins and CD44/CD168, not only establish the extracellular framework but also trigger signals that set off downstream cellular changes. This interplay between ECM composition, receptor engagement, and subsequent signaling not only creates supportive cellular scaffolds but also initiates cascades of molecular events within the cells. 2
Importance in Biological Systems
Tissue Integrity: Cell-cell adhesion maintains tissue cohesion, preventing cells from detaching and maintaining the structural integrity of tissues.
Cell Communication: Gap junctions enable direct communication between cells, allowing ions, small molecules, and signaling molecules to pass. This is crucial for synchronized activities in tissues like the heart.
Embryonic Development: Cell adhesion and ECM play pivotal roles in embryonic development by guiding cell migration, tissue formation, and organogenesis.
Wound Healing and Repair: Proper cell adhesion and ECM are essential for wound healing, as cells migrate to close wounds and restore tissue integrity.
Cancer and Metastasis: Dysregulation of cell adhesion and ECM can contribute to cancer progression by promoting uncontrolled cell growth and metastasis.
Cell Differentiation: ECM components and adhesion molecules influence cell differentiation and specialization during development.
Mechanical Support: The ECM provides mechanical support to tissues and helps them withstand various physical forces.
Cell Migration: Cell adhesion and ECM interactions guide cell movement during processes like immune responses and tissue repair.
Cell-cell adhesion and the extracellular matrix are vital components of biological systems, contributing to tissue integrity, communication, development, repair, and disease progression. These mechanisms ensure the proper functioning of cells within tissues and organs, highlighting their importance in maintaining overall organism health.
How do cell-cell adhesion and interactions with the ECM contribute to tissue organization and morphogenesis?
Cell-cell adhesion and interactions with the extracellular matrix (ECM) play pivotal roles in tissue organization and morphogenesis during development. These processes involve complex molecular mechanisms that are highly interdependent, orchestrated, and precisely regulated, which suggests purposeful design.
Cell-Cell Adhesion: Cell-cell adhesion involves the binding of cells to each other through specific adhesion molecules, such as cadherins. This adhesion is crucial for the formation and maintenance of tissue structures. Key roles of cell-cell adhesion include:
Tissue Integrity: Adhesion molecules ensure that cells remain tightly connected within tissues. This integrity is essential for the formation of coherent tissues and organs.
Cell Sorting and Compartmentalization: Differential adhesion between different cell types contributes to the sorting of cells into specific regions and compartments. This process is vital for creating organized tissue architectures.
Cell Communication: Cell-cell adhesion molecules are often linked to signaling pathways that regulate cell behavior, including proliferation, differentiation, and migration. This communication contributes to the coordinated development of tissues.
Morphogenesis: During tissue remodeling and morphogenesis, cell-cell adhesion plays a role in shaping tissues and generating tissue-specific structures.
Interactions with the Extracellular Matrix (ECM)
The ECM is a complex network of proteins and molecules that provides structural support and guidance cues for cells. The interactions between cells and the ECM are critical for tissue organization and morphogenesis:
Cell Migration and Guidance: The ECM provides tracks and signals that guide cell migration during tissue development. Integrins, which are cell surface receptors, mediate these interactions and allow cells to move along ECM fibers.
Cell Differentiation: Cells receive signals from the ECM that influence their differentiation into specific cell types. Different regions of the ECM can provide different cues, directing cells to adopt particular fates.
Tissue Architecture: The ECM contributes to the formation of tissue architecture by providing mechanical support and defining the three-dimensional structure of tissues and organs.
Cell Survival and Apoptosis: Signals from the ECM can influence cell survival or apoptosis, contributing to tissue sculpting during development.
The complex interplay between cell-cell adhesion, interactions with the ECM, and various signaling pathways ensures that cells within tissues are organized, communicate effectively, and contribute to the formation of functional tissues and organs. The precision and coordination required for these processes suggest a purposeful design, where different elements of the system were intricately integrated to achieve specific developmental outcomes. The simultaneous presence of cell adhesion molecules, ECM components, and signaling pathways from the beginning becomes an essential feature, as a stepwise evolutionary process without all the components would likely result in non-functional or misshapen tissues.
What are the molecular components that mediate cell-ECM and cell-cell interactions during development?
Cell-ECM and cell-cell interactions during development involve a complex array of molecular components that allow cells to communicate, adhere, and respond to their environment. These components include:
Cell-ECM Interactions
Integrins: Transmembrane receptors that mediate the attachment of cells to the ECM components like fibronectin, collagen, and laminin. Integrins link the ECM to the cell's cytoskeleton, enabling mechanical signaling and migration.
Fibronectin: A glycoprotein present in the ECM that provides binding sites for integrins. It plays a crucial role in cell adhesion, migration, and tissue morphogenesis.
Collagen: A major component of the ECM, collagen fibers provide structural support to tissues. Different collagen types interact with different integrins and contribute to tissue-specific functions.
Laminin: A protein found in the basement membrane that interacts with integrins to mediate cell-ECM adhesion. It plays a role in cell differentiation, migration, and tissue development.
Proteoglycans: Molecules consisting of a core protein and glycosaminoglycan chains. They contribute to the ECM's hydrated gel-like properties and help regulate cell behavior.
Cell-Cell Interactions
Cadherins: Calcium-dependent adhesion molecules that mediate homophilic interactions between cells. Different types of cadherins are expressed in various tissues and contribute to tissue-specific cell sorting and organization.
Desmosomes: Cell-cell junctions that link intermediate filaments in adjacent cells, providing strong mechanical adhesion. They are particularly important in tissues subjected to mechanical stress.
Tight Junctions: Membrane junctions that seal adjacent cells together, preventing the passage of molecules between cells. They are essential for maintaining the barrier functions of epithelial tissues.
Gap Junctions: Channels that allow small molecules and ions to pass between adjacent cells. They enable direct communication and coordination between cells in tissues like the nervous system and cardiac muscle.
Notch Signaling: A cell-to-cell signaling mechanism involved in cell fate determination and differentiation. It plays a crucial role in processes such as tissue patterning and organ development.
These molecular components work together to regulate cell adhesion, migration, differentiation, and tissue organization during development. The specificity of these interactions, the diversity of molecules involved, and their coordinated functioning all point to a sophisticated design that ensures proper tissue formation and function. The intricate nature of these components suggests that they needed to be present and functional from the beginning to achieve the desired developmental outcomes, making stepwise evolution without all the components less plausible.
Appearance of Cell-Cell Adhesion and ECM in the evolutionary timeline
The appearance and development of Cell-Cell Adhesion and the Extracellular Matrix (ECM) in the evolutionary timeline coincide with the emergence of multicellular organisms and the emergence of more complex body structures. As organisms supposedly transitioned from single-celled life forms to multicellular entities, the need for mechanisms that facilitate cell cohesion, communication, and tissue organization became increasingly important.
Early Single-Celled Organisms: In the early stages of life on Earth, single-celled organisms would have predominated. These organisms did not require elaborate cell-cell adhesion mechanisms or an extensive ECM, as their functions were largely individualistic, and they often lived in isolation.
Emergence of Multicellularity: With the supposed evolution of multicellularity, cells would have begun to cooperate and specialize in various functions. The transition from loose collections of cells to coordinated tissues required the development of adhesion mechanisms to keep cells together and form cohesive structures.
Evolution of Simple Tissues: The first multicellular organisms would have formed simple tissues where cells directly interacted with each other. Basic cell-cell adhesion proteins would have emerged to ensure these cells remained connected and functioned collectively.
Importance in Complex Tissues: As organisms would have emerged to have more complex body structures, the need for stronger cell-cell adhesion and ECM would have become evident. Tissues and organs with specific functions would have required a well-organized structure to maintain proper function.
Diversification of Adhesion Molecules: Over time, adhesion molecules and junctions like tight junctions, desmosomes, and gap junctions would have originated to provide different forms of adhesion and communication between cells. These mechanisms would have allowed cells to work together and coordinate activities.
Development of Extracellular Matrix: The ECM would have emerged as an intricate network of proteins and carbohydrates secreted by cells. It would have provided mechanical support, anchorage, and signaling cues for cells within tissues. Components like collagen, elastin, proteoglycans, and adhesive proteins would have gradually emerged.
Tissue Specialization: As tissues would have specialized for various functions, cell-cell adhesion and ECM components would have adapted to suit the needs of different tissues. For instance, connective tissues would have required strong collagen fibers, while epithelial tissues needed tight junctions for barriers.
Complex Organisms and Systems: In more complex organisms, cell-cell adhesion and the ECM would have become integral to the functioning of various systems, including nervous, circulatory, and immune systems. These mechanisms would have facilitated interactions and responses within tissues and organs.
Evolution of Integrins and ECM Proteins: Integrins, cell surface receptors that connect cells to the ECM, would have evolved to provide a dynamic link between cells and their environment. ECM proteins would have diversified and became more specialized in different tissues.
Importance in Development and Disease: Cell-cell adhesion and the ECM play critical roles in embryonic development, tissue repair, and disease processes like cancer metastasis. Their functions are tightly intertwined with the overall health and functionality of organisms.
The appearance of Cell-Cell Adhesion and the Extracellular Matrix is closely linked to the evolution of multicellularity and the development of more complex body structures. These mechanisms would have been essential for maintaining tissue cohesion, enabling communication between cells, and facilitating the emergence of specialized tissues and organs. As organisms would have diversified and specialized, the complexity of cell-cell adhesion and ECM systems increased, contributing to the intricate functioning of complex organisms.
De Novo Genetic Information necessary to instantiate Cell-cell adhesion and the extracellular matrix (ECM)
Cell-Cell Adhesion Genetic Information
Cadherin Genes: Cadherins are crucial molecules for cell-cell adhesion. New genetic information would be needed to code for various cadherin proteins that mediate adhesion specificity. Different tissues might require different types of cadherins.
Cytoskeletal Proteins: Genes encoding cytoskeletal proteins such as actin and myosin might need additional regulatory elements to be expressed in specific cell types for the formation of contractile structures.
Junctional Proteins: Genes coding for proteins like catenins, which link cadherins to the cytoskeleton, and proteins involved in gap junctions and tight junctions, would need to be introduced or modified.
Extracellular Matrix Genetic Information
Glycoproteins and Proteoglycans: New genes would need to be added or modified to code for various glycoproteins (e.g., fibronectin, laminin) and proteoglycans that compose the ECM.
Enzymes: Genes coding for enzymes responsible for synthesizing and modifying ECM components would need to be introduced. For example, genes for enzymes that create cross-links between collagen molecules.
Integrins and Receptors: New genetic information would be required to code for integrins and other cell surface receptors that interact with ECM components.
Matrix Metalloproteinases (MMPs): Genes for MMPs and their regulators would be needed to allow for controlled degradation of the ECM.
Developing these complex processes would also involve genetic changes related to cell signaling, tissue-specific expression, and regulatory elements. Creating a biological system as intricate as cell-cell adhesion and the ECM from scratch is an immensely complex process, involving numerous genetic alterations, regulatory mechanisms, and interactions.
Manufacturing codes and languages employed to instantiate Cell-cell adhesion and the extracellular matrix (ECM)
Blueprints and Design Changes
Imagine an organism without cell-cell adhesion and ECM as a basic structure with individual cells loosely interacting. To develop these features, genetic changes analogous to design blueprints would need to occur.
New genetic information (analogous to revised blueprints) would specify the production of adhesion molecules, such as cadherins and ECM components like fibronectin or collagen.
Cellular Communication Update
In manufacturing, design changes often require updates across departments. Similarly, cells need to "communicate" these changes to coordinate their actions. Signaling pathways within cells would need to be instantiated or be repurposed to trigger the expression of adhesion proteins and ECM components.
Protein Synthesis and Assembly Instructions
In manufacturing, new parts are manufactured based on updated designs. Similarly, cells must synthesize new proteins according to the updated genetic instructions. Cells would need the "instructions" to fold proteins correctly and assemble them into functional adhesion molecules and ECM components.
Quality Control and Integration
In manufacturing, quality control ensures new parts fit seamlessly. In biology, mechanisms akin to quality control would need to ensure newly synthesized adhesion molecules properly interact. Cells would need to integrate these new components into their existing structure while maintaining cohesion and stability.
Fine-Tuning and Adaptation
Manufacturing processes often require adjustments for optimal performance. Similarly, biological systems would require fine-tuning and adaptation.
Epigenetic Regulatory Mechanisms necessary to be instantiated for Cell-cell adhesion and the extracellular matrix (ECM)
The development of complex biological features like cell-cell adhesion and the extracellular matrix (ECM) involves intricate epigenetic regulations that control gene expression patterns. Epigenetic mechanisms play a crucial role in orchestrating the intricate processes required for these features to emerge and function effectively.
Epigenetic Regulation for Development
DNA Methylation: The addition of methyl groups to DNA can affect gene expression. In the development of cell-cell adhesion and the ECM, specific genes encoding adhesion proteins and ECM components would have to be regulated by DNA methylation.
Histone Modification: Chemical modifications to histone proteins can alter chromatin structure and gene accessibility. Histone acetylation and methylation could be involved in controlling the expression of genes related to adhesion and ECM.
Non-coding RNAs: MicroRNAs and long non-coding RNAs (lncRNAs) regulate gene expression post-transcriptionally. They fine-tune the expression of genes involved in adhesion and ECM formation.
Epigenetic Regulatory Mechanisms necessary to be instantiated to create Cell-Cell Adhesion and the ECM
DNA Methylation System: Enzymes such as DNA methyltransferases are responsible for adding methyl groups to DNA. Demethylases can remove these marks. The balance between these enzymes would determine the DNA methylation pattern.
Histone Modification System: Histone acetyltransferases (HATs) and histone deacetylases (HDACs) regulate histone acetylation levels. Similarly, histone methyltransferases and demethylases control histone methylation. The interplay of these enzymes maintains proper chromatin structure.
RNA-Mediated Regulation System: Enzymes like Dicer process miRNAs, which target specific mRNAs for degradation or translational repression. LncRNAs can also interact with chromatin-modifying complexes, influencing gene expression.
Maintaining Balance and Operation - Collaborative Systems
Transcription Factor Networks: Transcription factors play a role in establishing cell-specific gene expression patterns. They work in conjunction with epigenetic regulators to ensure precise gene activation or repression.
Cell Signaling Pathways: Signaling pathways can influence epigenetic marks and gene expression. For instance, growth factors or environmental signals can activate cascades that modulate DNA methylation or histone modifications.
Cell-Cell Communication: Cells within tissues communicate to establish coordinated gene expression. In the context of ECM and adhesion, cells might signal each other to ensure proper adhesion protein expression and ECM production.
Cell Cycle Control: The cell cycle influences epigenetic regulation. Cell division provides opportunities for resetting epigenetic marks, allowing cells to re-establish appropriate gene expression patterns during development.
Environmental Influences: External factors like diet, stress, and exposure to toxins can impact epigenetic marks, potentially influencing the development of adhesion and ECM systems.
The collaboration of these systems ensures the precise activation and repression of genes required for cell-cell adhesion and ECM formation. Epigenetic mechanisms act as a dynamic regulatory layer, responding to cues from both the internal cellular environment and external signals to ensure proper development, function, and maintenance of these complex biological features.
Signaling Pathways necessary to create, and maintain Cell-Cell Adhesion and the ECM
The emergence of complex biological features like cell-cell adhesion and the extracellular matrix (ECM) involves intricate signaling pathways that coordinate various cellular processes.
Wnt Signaling Pathway
Wnt signaling plays a critical role in tissue development, stem cell maintenance, and cell adhesion. It can influence the expression of cadherins and other adhesion molecules, affecting cell-cell interactions.
Interconnection: Wnt signaling crosstalks with other pathways, such as the Notch and Hedgehog pathways, enhancing regulatory complexity.
Transforming Growth Factor-Beta (TGF-β) Pathway
TGF-β is involved in various processes, including ECM synthesis and remodeling. It stimulates the expression of ECM components like collagen and fibronectin.
Interconnection: TGF-β signaling interacts with other pathways, like MAPK and BMP, to regulate diverse cellular functions.
Integrin-Mediated Signaling
Integrins connect ECM components to the cell's cytoskeleton and activate signaling pathways upon ligand binding. Integrin signaling influences cell adhesion, migration, and ECM remodeling.
Interconnection: Integrin signaling cross-communicates with growth factor pathways, modulating cellular responses.
Notch Signaling Pathway
Notch signaling is involved in cell fate determination and tissue development. It can influence cell adhesion through the regulation of cadherin expression.
Interconnection: Notch crosstalks with Wnt and other pathways to coordinate developmental decisions.
MAPK/ERK Pathway
MAPK/ERK pathway controls cell proliferation, differentiation, and migration. It can impact ECM synthesis and cell adhesion molecule expression.
Interconnection: MAPK/ERK crosstalks with integrin and growth factor pathways to modulate cell behavior.
Hedgehog Signaling Pathway
Hedgehog signaling regulates tissue patterning and development. It may influence ECM production and remodeling processes.
Interconnection: Hedgehog pathway crosstalks with Wnt and TGF-β pathways for coordinated effects.
PI3K/AKT Pathway
PI3K/AKT pathway controls cell survival, growth, and migration. It can impact integrin-mediated cell adhesion and ECM interactions.
Interconnection: PI3K/AKT crosstalks with multiple pathways, including growth factor pathways.
These pathways are highly interconnected and interdependent. Crosstalk between them allows for intricate regulation and response to various stimuli. Additionally, these pathways communicate with other biological systems, such as developmental pathways, immune responses, and cellular metabolism. The interconnectedness allows cells to integrate signals from different sources, ensuring coordinated responses and adaptive behaviors, ultimately contributing to the emergence, maintenance, and function of cell-cell adhesion and the ECM in complex organisms.
Regulatory codes necessary for maintenance and operation
The maintenance and operation of complex biological systems like cell-cell adhesion and the extracellular matrix (ECM) involve intricate regulatory codes and languages that ensure proper function, adaptation, and balance.
Feedback Loops and Homeostasis
Just as in a control system, biological systems employ feedback loops to maintain stability. Regulatory mechanisms ensure that cell-cell adhesion and ECM components are produced in appropriate amounts. Cells might sense the density of adhesion molecules or ECM components and adjust their expression accordingly.
Signal Integration and Cross-Communication
Cells integrate various signals from their environment, translating them into appropriate responses. Regulatory pathways like MAPK, PI3K/AKT, and others act as interpreters, relaying information from growth factors, hormones, and mechanical cues to regulate adhesion and ECM-related gene expression.
Epigenetic Regulation for Memory and Plasticity
Epigenetic modifications, like DNA methylation and histone modifications, can serve as "memory" marks. These marks maintain stable gene expression patterns over time, ensuring that adhesion and ECM components are consistently produced in the right contexts.
Cell-cell communication and Quorum Sensing
Cells in tissues communicate with each other to synchronize behavior. Cells might employ mechanisms similar to quorum sensing to determine the presence of neighboring cells and adjust adhesion and ECM production accordingly.
Dynamic Remodeling and ECM Degradation
Just as a construction site adapts to changing needs, cells can modify the ECM based on requirements. Regulatory pathways control matrix metalloproteinases (MMPs) that degrade and remodel ECM components, ensuring dynamic adaptation.
Cell Differentiation and Specialization
Regulatory codes guide stem cells to differentiate into specific cell types, some of which contribute to cell adhesion and ECM production.Signaling pathways like Wnt, Notch, and BMP play roles in determining cell fate.
Tissue-Specific Regulation
Different tissues require different levels of adhesion and ECM components. Regulatory mechanisms ensure tissue-specific expression patterns, maintaining the uniqueness of each tissue type.
Feedback from Cellular Mechanics
Just as a machine's performance feedback affects its operation, cells can sense mechanical forces and adjust adhesion and ECM accordingly. Mechanotransduction pathways translate mechanical cues into biochemical responses.
These regulatory mechanisms collaborate in a language of molecular interactions to maintain the operation of cell-cell adhesion and the ECM. The intricate orchestration of these codes ensures that cells can adhere, communicate, adapt, and contribute to tissue integrity and function in the dynamic environment of a living organism.
How did the molecular machinery for cell-cell adhesion and ECM interactions emerge to support multicellular organisms?
The emergence of the molecular machinery for cell-cell adhesion and extracellular matrix (ECM) interactions is a complex process that likely involved the coordination of various molecular components. While the specific details of this process are still a subject of scientific investigation, there are several hypotheses and mechanisms that would explain the origin of these essential cellular processes:
Cooption of Pre-existing Molecules: It's claimed that some of the molecules involved in cell-cell adhesion and ECM interactions had pre-existing functions in single-celled organisms. These functions would have included interactions with the external environment or with other cells. Through genetic changes and adaptations, these molecules would have been coopted for cell-cell and cell-ECM interactions in multicellular contexts.
Gene Duplication and Divergence: Gene duplication events followed by divergence would have played a role in creating new molecules with adhesion-related functions. Over time, these duplicated genes would have evolved distinct roles in cell-cell and cell-ECM interactions.
Horizontal Gene Transfer: Genetic material can sometimes be transferred between different species or organisms. Horizontal gene transfer would have introduced novel genes or gene variants into multicellular organisms, providing the molecular basis for adhesion and ECM interactions.
Emergence of Protein Domains: Some protein domains have inherent adhesive properties. Through gene duplication, recombination, and mutation, these domains would have been integrated into larger proteins that facilitated adhesion and ECM interactions.
Evolution of Cell Signaling: Cell adhesion and ECM interactions are closely linked to cell signaling pathways. It is claimed that the evolution of these pathways would have enabled cells to communicate and respond to each other and their environment, promoting coordinated multicellular behavior.
Symbiotic Relationships: Multicellularity would have evolved from symbiotic relationships between different types of cells. These cells would need to adhere to each other and establish cooperative interactions for survival, leading to the development of adhesion and ECM-related mechanisms.
Once Cell-Cell Adhesion and the Extra Cellular Matrix (ECM) are operational, what other intra and extracellular systems is it interdependent with?
Once Cell-Cell Adhesion and the Extracellular Matrix (ECM) are instantiated and operational, they become interdependent with various intra and extracellular systems to ensure proper tissue structure, function, and communication. Here are some of the key systems with which Cell-Cell Adhesion and the ECM are interconnected:
Intracellular Systems
Cytoskeleton and Cell Shape: Cell-cell adhesion and ECM interactions influence cytoskeletal organization, which in turn affects cell shape, migration, and mechanical stability.
Cell Signaling Pathways: Interactions with Cell-Cell Adhesion and the ECM can activate signaling pathways that regulate cell survival, proliferation, differentiation, and migration.
Cellular Transport and Communication: Cell-cell adhesion and the ECM affect the localization of membrane proteins involved in cellular transport and communication, influencing nutrient uptake, waste removal, and intercellular signaling.
Gene Expression and Differentiation: Cell-cell adhesion and ECM interactions can impact gene expression patterns that drive cell differentiation and tissue-specific functions.
Extracellular Systems
Immune Response: Cell-cell adhesion and the ECM influence immune cell trafficking, recruitment, and interactions with target cells during immune responses.
Blood Circulation and Oxygen Delivery: The ECM provides structural support for blood vessels, while Cell-Cell Adhesion guides the organization of endothelial cells lining vessels, affecting blood flow and oxygen/nutrient delivery to tissues.
Extracellular Signaling Molecules: The ECM can store and release signaling molecules that regulate cell behavior, tissue repair, and immune responses.
Nervous System and Neurodevelopment: The ECM contributes to neural development and synapse formation, while Cell-Cell Adhesion guides neuronal migration and connectivity in the developing nervous system.
Hormonal Regulation: Cell-cell adhesion and ECM interactions can affect hormone receptor availability and signaling, influencing physiological responses.
Tissue Regeneration and Repair: Proper Cell-Cell Adhesion and ECM are crucial for tissue regeneration and wound healing, providing the structural support needed for new tissue growth.
Mechanical Integrity: The ECM provides mechanical support to tissues and organs, ensuring their structural integrity and protection.
Cell Differentiation and Organization: Cell-cell adhesion and the ECM play roles in organizing cells into functional tissues, allowing for cooperative interactions and specialized functions.
These interconnected systems highlight how Cell-Cell Adhesion and the ECM, once instantiated and operational, are integral components of the overall physiological framework. Their interactions with various cellular and extracellular processes contribute to tissue homeostasis, communication, and the proper functioning of diverse biological systems.
1. The intricate interdependence observed between Cell-Cell Adhesion, the Extracellular Matrix (ECM), and various intra and extracellular systems, including cytoskeleton, cell signaling, immune response, blood circulation, nervous system, and more, forms a tightly integrated network crucial for proper tissue structure, function, and communication.
2. These interdependent systems exhibit a level of coordinated complexity that suggests a designed setup rather than a random accumulation of components over time. The immediate functionality and seamless interaction among these systems imply a purposeful arrangement to achieve optimal biological function.
3. The simultaneous emergence and functional integration of Cell-Cell Adhesion, the ECM, and multiple interconnected systems highlight a coherent and intentional design that enables effective tissue organization, communication, and response to environmental cues.
Conclusion: The evident interconnectedness and functional reliance on Cell-Cell Adhesion, the ECM, and diverse biological systems provide strong indications of a designed framework. The intricate coordination, immediate functionality, and harmonious collaboration between these systems point toward an intelligently orchestrated setup that supports the intricate physiological requirements of organisms.
1. What are cell-cell adhesions?
2. M.Karlinski Unfolding the Folds: How the Biomechanics of the Extracellular Matrix contributes to Cortical Gyrification September 2018
Last edited by Otangelo on Sun Sep 03, 2023 3:18 pm; edited 1 time in total

