31. Neuronal Pruning and Synaptogenesis
How do neuronal pruning and synaptogenesis regulate neural circuits during development and in response to experience?
Neuronal pruning and synaptogenesis are critical processes that regulate neural circuits during development and in response to experience. These processes shape the intricate network of connections within the brain, allowing it to efficiently process information and adapt to changing environments. Here's how neuronal pruning and synaptogenesis contribute to the regulation of neural circuits:
Neuronal Pruning
Overproduction of Neurons and Connections: During early brain development, there is an overproduction of neurons and synapses. This abundance of connections is important to ensure that the brain has the potential to establish a wide range of circuits.
Competition for Resources: Neurons and synapses compete for limited resources, such as nutrients and trophic factors. This competition leads to the selective survival of the fittest neurons and synapses while eliminating weaker ones.
Synaptic Elimination: Neuronal pruning involves the selective elimination of excess synapses. This process is often guided by neural activity; synapses that are less active are more likely to be eliminated. This activity-dependent pruning helps refine and strengthen the most relevant connections.
Role of Apoptosis: In some cases, the elimination of excess neurons occurs through programmed cell death, or apoptosis. This controlled cell death is a natural part of neural development and helps sculpt the brain's architecture.
Synaptogenesis
Formation of New Synapses: Synaptogenesis is the process by which new synapses are formed between neurons. This process begins early in development and continues throughout life, allowing the brain to adapt to new experiences and learn new information.
Activity-Dependent Wiring: Neural activity plays a crucial role in synaptogenesis. Neurons that fire together establish connections, leading to the strengthening of synapses and the creation of functional circuits. This process is a basis for learning and memory.
Structural and Functional Plasticity: Synaptogenesis contributes to the brain's plasticity – its ability to reorganize itself in response to experience. New synapses can form in response to learning, environmental changes, or sensory input.
Critical Periods: During certain developmental stages, such as critical periods, the brain is particularly sensitive to experience, and synaptogenesis is highly active. These periods are essential for the proper wiring of sensory systems and the development of complex skills.
Neuronal pruning and synaptogenesis work in concert to refine neural circuits by eliminating unnecessary connections and strengthening relevant ones. This dynamic interplay between elimination and formation of synapses is crucial for the development, plasticity, and adaptability of the brain's neural circuits in both early development and throughout life.
How do these processes contribute to the overall functionality and plasticity of the nervous system?
Neuronal pruning and synaptogenesis play pivotal roles in shaping the functionality and plasticity of the nervous system. These processes collectively contribute to the refinement, efficiency, and adaptability of neural circuits, allowing the brain to process information, learn, and respond to experiences in a dynamic manner.
Overall Functionality:
Elimination of Redundant Connections: Neuronal pruning ensures that only the most relevant and effective connections are retained in the neural network. By eliminating redundant or weaker connections, the brain optimizes the transmission of signals and reduces noise, leading to more efficient information processing.
Circuit Specialization: Pruning and synaptogenesis help neural circuits become specialized for specific functions. As connections are refined, distinct circuits dedicated to sensory processing, motor control, memory, and other cognitive functions emerge. This specialization enhances the overall functionality of the nervous system.
Network Balance: Neuronal pruning prevents circuits from becoming overly complex and unwieldy. This maintains a balance between different neuronal populations, preventing an excessive number of connections that could impede efficient information flow.
Plasticity and Adaptability:
Experience-Dependent Changes: Synaptogenesis allows the nervous system to adapt to changing environments and experiences. New synapses can form as a response to learning or exposure to novel stimuli, enabling the brain to incorporate new information into existing networks.
Learning and Memory: The ability of synapses to strengthen or weaken in response to activity, known as synaptic plasticity, underlies learning and memory processes. Long-term potentiation (LTP) and long-term depression (LTD) are forms of synaptic plasticity that contribute to the encoding and retention of information.
Sensory Development: During critical periods in development, synaptogenesis is particularly active, allowing sensory systems to wire themselves in response to specific experiences. This is crucial for the proper development of sensory perception.
Recovery from Injury: Neuronal pruning and synaptogenesis also play roles in recovery after neural injury. The brain can rewire itself to some extent by forming new connections around damaged areas, aiding in functional recovery.
Neurodevelopmental Disorders and Plasticity:
Imbalance and Disorders: Disruptions in neuronal pruning and synaptogenesis can lead to neurodevelopmental disorders. For instance, conditions like autism spectrum disorder (ASD) are associated with altered synapse formation and connectivity.
Therapeutic Potential: Understanding these processes is essential for developing therapies for neurodevelopmental disorders. Promoting adaptive synaptogenesis and modifying pruning patterns could potentially help treat certain conditions.
Neuronal pruning and synaptogenesis are crucial mechanisms that not only refine neural circuits for optimal functionality but also provide the nervous system with the capacity to adapt, learn, and respond to experiences throughout life. These processes are foundational to the brain's remarkable ability to process information, form memories, and continuously reshape itself in response to the world around it.
How do neuronal pruning and synaptogenesis contribute to the overall functionality and plasticity of the nervous system?
Neuronal pruning and synaptogenesis are fundamental processes that play crucial roles in shaping the functionality and plasticity of the nervous system. These processes involve the refinement of neural connections, leading to more efficient neural circuits and adaptive responses. Here's how neuronal pruning and synaptogenesis contribute to the overall functionality and plasticity of the nervous system:
Neuronal Pruning
Exuberant Connection Formation: During early development, neurons form an excessive number of connections, resulting in a dense network of synapses. This exuberant connectivity allows the nervous system to establish a wide range of potential pathways and interactions.
Competition and Refinement: Neuronal activity plays a crucial role in determining which synapses are strengthened and which are weakened. Synapses that are frequently activated are reinforced, while those that are less active are eliminated through a process called synaptic pruning. This competitive process refines neural connections, eliminating unnecessary or weak synapses and enhancing the efficiency of information transmission.
Sculpting Circuitry: Neuronal pruning is responsible for sculpting neural circuits into more precise and functional configurations. This fine-tuning of connections enhances the specificity of neural pathways, allowing for more accurate and efficient signal processing.
Synaptogenesis
Formation of New Synapses: Synaptogenesis involves the formation of new synapses between neurons. This process occurs throughout life, not just during development, and it contributes to learning, memory, and adaptive responses to environmental changes.
Experience-Dependent Plasticity: Synaptogenesis is influenced by experiences and environmental factors. Learning new skills or adapting to new situations often involves the creation of new synapses or the strengthening of existing ones. This experience-dependent plasticity allows the nervous system to adapt and learn from its surroundings.
Neuroplasticity and Recovery: Following injuries or changes in sensory input, synaptogenesis can contribute to the brain's ability to rewire itself and recover lost function. Neurons can establish new connections or alter existing ones to compensate for damage or changes in input.
Neuronal pruning and synaptogenesis are essential processes that optimize the structure and function of the nervous system. Neuronal pruning refines neural connections, while synaptogenesis allows for the formation of new synapses, enabling learning, memory, and adaptive responses. These processes together contribute to the remarkable plasticity and adaptability of the nervous system throughout life.
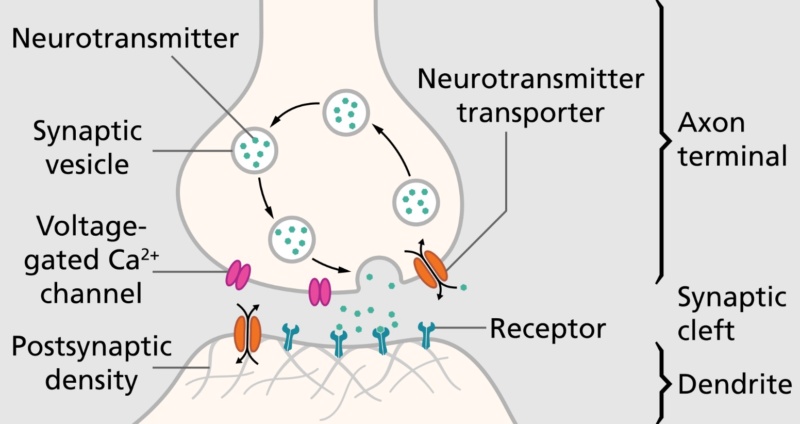
A model view of the synapse 1
At what point in the evolutionary timeline did neuronal pruning and synaptogenesis first appear?
Neuronal pruning and synaptogenesis are complex processes that are intimately linked to the development and functionality of the nervous system. While the exact point in the evolutionary timeline when these processes first appeared is not definitively known, it's supposed that they emerged gradually as nervous systems became more sophisticated.
The evolution of nervous systems would have been a gradual process that spans millions of years, making it challenging to pinpoint precise stages in which specific mechanisms like neuronal pruning and synaptogenesis emerged.
Early Nervous System Evolution: In the earliest multicellular organisms, nerve cells (neurons) would have started to form basic networks, allowing for simple sensory and motor responses. These early networks would have lacked the complex pruning and refinement mechanisms seen in more advanced nervous systems.
Emergence of Synaptic Connections: As nervous systems would have become more complex, the formation of synaptic connections would have became more important. Synapses, the junctions between neurons, would have allowed for communication and signal transmission between nerve cells. Over time, mechanisms that promoted the strengthening or weakening of synapses would have emerged to enhance the efficiency of signal transmission.
Refinement and Pruning: As nervous systems would have continued to evolve, mechanisms of neuronal pruning probably would have developed as a way to fine-tune neural connections. This would have been driven by the need for more efficient neural circuits, as well as the optimization of limited resources in the developing organisms.
Adaptation and Plasticity: The ability to form new synapses and adapt existing ones, which is a hallmark of synaptogenesis, would have provided significant evolutionary advantages. Organisms with the ability to adjust their neural circuits based on experiences and environmental changes would have been better equipped to survive and thrive in changing conditions.
What de novo genetic information is thought to have been necessary to instantiate neuronal pruning and synaptogenesis?
The mechanisms underlying neuronal pruning and synaptogenesis involve intricate genetic and molecular processes that regulate the formation, refinement, and elimination of neural connections. While it's not necessarily the case that entirely new genetic information was required to instantiate these processes, the proper orchestration of existing genetic information would have been crucial. Here are some key aspects of genetic information and molecular mechanisms thought to be involved:
Gene Expression and Regulation: Existing genes in an organism's genome are responsible for producing the proteins and molecules necessary for neuronal development and plasticity. The activation or repression of specific genes during different developmental stages is critical for initiating and guiding processes like synaptogenesis and neuronal pruning.
Signaling Pathways: Various signaling pathways, involving proteins and molecules such as growth factors, neurotransmitters, and their receptors, play essential roles in regulating neuronal development and connectivity. These pathways transmit information that guides the formation, strengthening, and elimination of synapses.
Synaptic Activity and Plasticity Genes: Certain genes are associated with synaptic plasticity—the ability of synapses to change their strength in response to activity. These genes, such as those involved in the regulation of neurotransmitter receptors and synaptic structure, contribute to the dynamic nature of synaptogenesis and pruning.
Epigenetic Modifications: Epigenetic modifications, which influence gene expression without altering the underlying DNA sequence, also play a role in neuronal development. These modifications can be influenced by experiences and environmental factors, contributing to the adaptive nature of the nervous system.
Cell-Cell Interactions: Cell adhesion molecules and guidance cues are essential for establishing and refining neural connections. These molecules, guided by genetic information, help neurons find their appropriate partners and form synapses in specific patterns.
The genetic information necessary for neuronal pruning and synaptogenesis involves the coordination of existing genes, signaling pathways, and molecular mechanisms. Rather than requiring entirely new genetic elements, these processes rely on the careful regulation and interaction of existing genetic information to sculpt the intricate neural circuits and adaptability observed in the nervous system.
What manufacturing codes and languages would have had to emerge and be employed for the processes of neuronal pruning and synaptogenesis?
The processes of neuronal pruning and synaptogenesis involve intricate cellular and molecular interactions rather than literal manufacturing codes and languages like those used in human-made technologies. Nevertheless, we can draw an analogy to the concept of "codes" and "languages" in biological terms to describe the molecular instructions and interactions that guide these processes. Here are some analogies to help understand the concept:
Molecular Signaling Pathways: In a metaphorical sense, molecular signaling pathways can be seen as analogous to a "language" that cells use to communicate with each other. Various molecules, such as neurotransmitters, growth factors, and receptors, act as "words" in this cellular communication. Cells "read" these signals to initiate processes like neuronal pruning and synaptogenesis.
Genetic Information and Expression: The genetic code present in an organism's DNA can be likened to a "manufacturing code." Genes contain the instructions for producing proteins and molecules needed for neuronal development and plasticity. The process of gene expression, where DNA is transcribed into RNA and then translated into proteins, can be seen as the "manufacturing" process based on these codes.
Epigenetic Marks and Modifications: Epigenetic modifications, which can influence gene expression without changing the underlying DNA sequence, could be considered as a form of regulatory "coding." These modifications act like switches that turn genes on or off, impacting the course of neuronal development and the dynamics of synaptogenesis.
Cell-Cell Communication: Cell-adhesion molecules and guidance cues can be thought of as a type of "communication language" that cells use to establish proper connections. These molecules guide neurons to their appropriate partners during synaptogenesis and contribute to the spatial organization of neural circuits.
While there aren't literal manufacturing codes and languages involved in neuronal pruning and synaptogenesis, the analogy helps us grasp the complexity of molecular interactions and instructions that guide these processes. The language of molecular signaling, genetic information, epigenetic regulation, and cell-cell communication collectively orchestrates the intricate development and refinement of neural connections in the nervous system.
Which epigenetic regulatory mechanisms are critical for directing neuronal pruning and synaptogenesis?
Epigenetic regulatory mechanisms play a vital role in shaping the processes of neuronal pruning and synaptogenesis by modulating gene expression and influencing the formation and elimination of synapses. Here are some of the critical epigenetic mechanisms involved:
DNA Methylation: DNA methylation involves the addition of methyl groups to specific regions of DNA, typically cytosine residues in CpG dinucleotides. In neuronal development, DNA methylation can influence the expression of genes involved in synaptic plasticity, axon guidance, and cell adhesion. Changes in DNA methylation patterns can lead to lasting alterations in synaptic connectivity.
Histone Modifications: Histones are proteins around which DNA is wound, forming chromatin. Modifications to histone proteins, such as acetylation, methylation, phosphorylation, and more, can influence how tightly DNA is packaged and thus affect gene accessibility. Specific histone modifications are associated with active or repressed gene expression, impacting processes like neuronal pruning and synaptogenesis.
Non-coding RNAs: Non-coding RNAs, such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), can regulate gene expression by binding to target messenger RNAs (mRNAs) and influencing their stability or translation. These RNA molecules can have profound effects on neuronal development, including synapse formation and elimination.
Activity-Dependent Epigenetic Changes: Neuronal activity, such as synaptic stimulation, can trigger epigenetic modifications that influence gene expression. For example, neuronal activity can lead to changes in DNA methylation and histone modifications, allowing the cell to respond to environmental stimuli and modulate synaptic plasticity.
Epigenetic Regulation of Synaptic Genes: Many genes involved in synapse formation, function, and elimination are under the control of epigenetic regulation. For instance, genes encoding cell adhesion molecules, neurotransmitter receptors, and other synaptic proteins can be epigenetically modulated to fine-tune the establishment and maintenance of synapses.
Epigenetic regulatory mechanisms are critical for directing neuronal pruning and synaptogenesis by modulating gene expression and influencing the molecular processes underlying neural connectivity. These mechanisms allow the nervous system to adapt to experiences, shape neural circuits, and optimize synaptic connections for proper functionality.
Signaling pathways that are indispensable for the orchestration of neuronal pruning and synaptogenesis
Several signaling pathways are crucial for the proper orchestration of neuronal pruning and synaptogenesis. These pathways transmit molecular signals that guide the formation, strengthening, and elimination of synapses, as well as the refinement of neural connections. Here are some of the indispensable signaling pathways involved:
Brain-Derived Neurotrophic Factor (BDNF) Pathway: BDNF, a member of the neurotrophin family, is critical for promoting neuronal survival, differentiation, and synaptic plasticity. BDNF signaling through its receptor, TrkB, plays a pivotal role in synaptogenesis and synaptic refinement by enhancing the growth and maintenance of synapses.
Wnt Signaling Pathway: The Wnt pathway is involved in a variety of developmental processes, including neuronal connectivity. Wnt signaling influences axon guidance, dendrite development, and synapse formation by regulating the cytoskeleton and intracellular pathways within neurons.
Notch Signaling Pathway: The Notch pathway is essential for cell-cell communication and has roles in neural development. Notch signaling influences the balance between neuronal differentiation and maintenance of precursor cells. Disruption of Notch signaling can impact synaptic connectivity.
Ephrin Receptor Pathway: Ephrin receptors and their ligands, ephrins, are involved in axon guidance and synaptic organization. The interaction between ephrins on one neuron and their corresponding receptors on another plays a role in shaping synaptic connections and neural circuits.
Neuregulin-ErbB Pathway: Neuregulins, ligands that activate ErbB receptor tyrosine kinases, are involved in the development of glial cells and synapses. This pathway plays a role in coordinating the formation of pre- and postsynaptic elements during synaptogenesis.
Calcium Signaling: Intracellular calcium plays a critical role in neuronal activity and synaptic plasticity. Calcium signaling is involved in synaptic vesicle release, postsynaptic response, and the activation of various signaling cascades that influence synaptogenesis.
Activity-Dependent Pathways: Neuronal activity itself, often initiated by synaptic transmission, triggers signaling pathways that contribute to synaptic plasticity and connectivity refinement. NMDA receptor-dependent calcium influx is a key player in activity-dependent processes.
Specific signaling pathways are indispensable for directing neuronal pruning and synaptogenesis. These pathways orchestrate various aspects of neural development and connectivity, ensuring the precise formation, strengthening, and elimination of synapses that are essential for the functional wiring of the nervous system.
What regulatory codes maintain and oversee the operation of neuronal pruning and synaptogenesis?
The "regulatory codes" that maintain and oversee the operation of neuronal pruning and synaptogenesis involve a complex interplay of molecular mechanisms, gene expression, and cellular signaling. These codes ensure the precise execution of these processes while adapting to developmental needs and environmental cues. Here are some of the key regulatory elements that govern neuronal pruning and synaptogenesis:
Activity-Dependent Regulation: Neuronal activity, driven by synaptic transmission and sensory experiences, acts as a regulatory code. It guides the strengthening of active synapses and the elimination of less active ones, contributing to the refinement of neural circuits.
Transcriptional Regulation: Transcription factors and other regulatory molecules control gene expression patterns during neuronal development. These factors determine which genes are turned on or off, influencing synaptogenesis, dendritic branching, and other processes.
Epigenetic Modification Patterns: Epigenetic marks, such as DNA methylation and histone modifications, form regulatory codes that impact gene expression. These marks can be dynamically altered in response to neural activity, experience, and environmental factors.
Molecular Signaling Networks: Signaling pathways, such as BDNF-TrkB, Wnt, and Notch, form interconnected networks that convey instructions for synaptogenesis and pruning. These pathways regulate cellular responses to molecular cues.
Neurotrophins and Growth Factors: Neurotrophic factors, like BDNF, NGF, and others, play crucial roles in regulating neuronal survival, differentiation, and synaptic plasticity. They ensure that proper connections are established and maintained.
Guidance Molecules and Receptors: Guidance cues and their receptors direct axon pathfinding and dendritic arborization. These molecules ensure that neurons connect to their correct targets during development.
Cell Adhesion Molecules: Cell adhesion molecules ensure that synapses are formed between appropriate pre- and postsynaptic partners. They contribute to the precise wiring of neural circuits.
MicroRNAs and Non-Coding RNAs: MicroRNAs and other non-coding RNAs regulate gene expression post-transcriptionally. They fine-tune the levels of specific proteins involved in synaptogenesis and pruning.
The regulatory codes governing neuronal pruning and synaptogenesis encompass a diverse array of mechanisms that interact to ensure the proper development and refinement of neural connections. These codes integrate genetic information, molecular signaling, cellular responses, and environmental inputs to sculpt the intricate connectivity of the nervous system.
Is there scientific evidence that supports the notion that neuronal pruning and synaptogenesis were brought about by the process of evolution?
The intricate processes of neuronal pruning and synaptogenesis, fundamental to the development and functionality of the nervous system, present significant challenges for an evolutionary explanation. The idea that these processes could have emerged gradually, through a stepwise evolutionary progression, faces substantial hurdles given the interdependence of various codes, languages, signaling networks, and proteins that must be operational from the beginning.
Complexity and Functional Requirements: Neuronal pruning and synaptogenesis involve a remarkable level of complexity, requiring precise coordination of multiple molecular interactions and genetic regulations. The establishment of synaptic connections necessitates intricate guidance cues, molecular signaling, and precise cellular interactions. A stepwise evolutionary approach would demand the gradual development of each of these components, without the guarantee of functionality at intermediate stages. It is difficult to conceive how partially formed systems with no immediate function could have been selected for, as they would provide no selective advantage to an organism.
Interdependence and Instantiation: What makes the evolutionary pathway even more implausible is the interdependence of the various components. Signaling pathways, gene expression networks, and molecular codes are not independent entities; they rely on each other for their functionality. A fully operational system is required for neuronal pruning and synaptogenesis to occur. The language of molecular signaling pathways needs a coherent molecular vocabulary that includes proteins, receptors, and other elements. Without all these components functioning together, no functional outcome would be achieved, rendering any intermediate stages non-adaptive and non-selectable.
Coordinated Emergence of Multiple Mechanisms: The coordinated emergence of gene expression regulations, epigenetic modifications, molecular signaling, and cellular interactions is a substantial challenge for stepwise evolution. The likelihood of these mechanisms independently evolving, and then coincidentally aligning to support neuronal pruning and synaptogenesis, stretches the bounds of probability. This complex coordination is best explained by the concept of intelligent design, where all necessary components are instantiated simultaneously to achieve a functional outcome.
Irreducible Complexity and Intelligent Design: The concept of irreducible complexity arises when a system relies on multiple interacting components, none of which can be removed without disrupting function. Neuronal pruning and synaptogenesis could be seen as irreducibly complex systems. These systems were most likely designed and implemented all at once with all their intricate interdependencies in place, rather than evolving in a piecemeal fashion.
The simultaneous emergence of neuronal pruning and synaptogenesis as fully operational systems seems more plausible than the stepwise evolution of their various components. The interdependence of codes, languages, signaling networks, and proteins required for their function, along with the complexity and functional demands of these processes, presents a compelling case for intelligent design as the best explanation for the origins of these intricate mechanisms.
How might the systems and structures involved in neuronal pruning and synaptogenesis be considered irreducibly complex or interdependent?
Neuronal pruning and synaptogenesis represent intricate processes that exhibit features of irreducible complexity and interdependence, reinforcing the notion that they are the result of intelligent design rather than gradual evolution.
Molecular Signaling and Receptors: The language of molecular signaling involves complex interactions between signaling molecules and their receptors. This signaling is indispensable for guiding axons, dendrites, and synaptic connections. The absence of any key signaling component would lead to an incomplete and non-functional process.
Guidance Cues and Cell Adhesion Molecules: The guidance cues that direct the growth of axons and dendrites are interdependent with cell adhesion molecules that enable synaptic connections. Without proper guidance cues, neurons might not reach their targets, and without functional adhesion molecules, synapses would not form properly.
Gene Expression and Transcription Factors: The genetic code, transcription factors, and gene expression are intricately involved in shaping neuronal connectivity. The absence of specific genes or regulatory elements would disrupt the orchestration of synaptogenesis and pruning.
Synaptic Activity and Plasticity: The plasticity of synapses, allowing them to strengthen or weaken based on activity, is intertwined with the overall process of pruning. The absence of synaptic activity would hinder both the refinement of synapses and the elimination of excess connections.
Molecular Codes and Signaling Pathways: The "codes" for molecular signaling pathways must be present alongside functional receptors, ligands, and downstream effectors. The absence of any of these components would result in disrupted communication and misdirection of neural growth.
Epigenetic Regulation and Genetic Expression: Epigenetic modifications, such as DNA methylation and histone modifications, regulate gene expression critical for proper development. The intricate interplay between epigenetic marks, gene expression, and neural connectivity is essential for the successful operation of these processes.
Once neuronal pruning and synaptogenesis are fully instantiated and operational, with which other intra and extracellular systems do they closely interact or rely?
Neuronal pruning and synaptogenesis, once fully instantiated and operational, closely interact with various intra and extracellular systems to ensure the proper development and function of the nervous system. These interactions contribute to the establishment of functional neural circuits and the precise wiring of the brain.
Intracellular Interactions
Intracellular Signaling Pathways: Neuronal pruning and synaptogenesis rely on intricate intracellular signaling pathways that regulate processes such as gene expression, cytoskeletal dynamics, and organelle transport. These pathways help neurons respond to extracellular cues and adapt their connectivity.
Cytoskeletal Dynamics: The cytoskeleton, comprising microtubules, microfilaments, and intermediate filaments, plays a vital role in axon and dendrite growth, guidance, and synaptic plasticity. Cytoskeletal elements are crucial for maintaining neuronal structure and connectivity.
Intracellular Transport Systems: Molecular motors and transport mechanisms facilitate the movement of organelles, vesicles, and other cellular components within neurons. Proper intracellular transport is necessary for the delivery of essential materials to growing axons and dendrites.
Extracellular Interactions:
Synaptic Activity and Neurotransmission: Neuronal pruning and synaptogenesis closely interact with synaptic activity and neurotransmission. Active synapses strengthen through activity-dependent mechanisms, while less active synapses are eliminated through pruning. This interaction fine-tunes neural connectivity.
Cell-Cell Communication: Interactions between neurons and other cell types, such as glia, are crucial for guiding axon growth, providing trophic support, and modulating synaptic connections. These interactions help create a conducive environment for proper neuronal development.
Neurotrophic Factors and Growth Factors: Neurotrophic factors play a key role in promoting neuronal survival, differentiation, and synaptic plasticity. They interact with neuronal pruning and synaptogenesis by influencing cell survival, axon guidance, and synapse formation.
Extracellular Matrix (ECM): The ECM provides physical and molecular cues that guide axon and dendrite growth, influence synaptic maturation, and help establish proper neural circuits. The interactions between neurons and the ECM play a role in shaping neural connectivity.
Epigenetic Regulations and Feedback Loops
Epigenetic Mechanisms: Epigenetic regulations, including DNA methylation and histone modifications, influence gene expression patterns that impact neuronal connectivity. These mechanisms are influenced by neuronal activity, shaping the interactions between synaptic activity and gene expression.
Activity-Dependent Feedback Loops: Synaptic activity influences epigenetic modifications, which in turn affect gene expression. This creates feedback loops that allow the nervous system to adapt to experiences and optimize neural circuitry.
Neuronal pruning and synaptogenesis closely interact with a network of intra and extracellular systems to ensure the proper development, refinement, and maintenance of neural connectivity. These interactions highlight the intricate coordination required for the formation of functional neural circuits and emphasize the complexity of the mechanisms involved in shaping the brain's intricate wiring.
1. Neuronal pruning and synaptogenesis rely on intricate semiotic codes and languages for proper communication and coordination between cells and molecules.
2. The systems involved in neuronal pruning and synaptogenesis are highly interdependent, requiring the simultaneous presence and precise coordination of multiple components for functionality.
3. Interlocking codes and interdependence suggest a carefully designed setup rather than an unguided, stepwise evolutionary process.
1. Wikipedia: Synapse pruning
How do neuronal pruning and synaptogenesis regulate neural circuits during development and in response to experience?
Neuronal pruning and synaptogenesis are critical processes that regulate neural circuits during development and in response to experience. These processes shape the intricate network of connections within the brain, allowing it to efficiently process information and adapt to changing environments. Here's how neuronal pruning and synaptogenesis contribute to the regulation of neural circuits:
Neuronal Pruning
Overproduction of Neurons and Connections: During early brain development, there is an overproduction of neurons and synapses. This abundance of connections is important to ensure that the brain has the potential to establish a wide range of circuits.
Competition for Resources: Neurons and synapses compete for limited resources, such as nutrients and trophic factors. This competition leads to the selective survival of the fittest neurons and synapses while eliminating weaker ones.
Synaptic Elimination: Neuronal pruning involves the selective elimination of excess synapses. This process is often guided by neural activity; synapses that are less active are more likely to be eliminated. This activity-dependent pruning helps refine and strengthen the most relevant connections.
Role of Apoptosis: In some cases, the elimination of excess neurons occurs through programmed cell death, or apoptosis. This controlled cell death is a natural part of neural development and helps sculpt the brain's architecture.
Synaptogenesis
Formation of New Synapses: Synaptogenesis is the process by which new synapses are formed between neurons. This process begins early in development and continues throughout life, allowing the brain to adapt to new experiences and learn new information.
Activity-Dependent Wiring: Neural activity plays a crucial role in synaptogenesis. Neurons that fire together establish connections, leading to the strengthening of synapses and the creation of functional circuits. This process is a basis for learning and memory.
Structural and Functional Plasticity: Synaptogenesis contributes to the brain's plasticity – its ability to reorganize itself in response to experience. New synapses can form in response to learning, environmental changes, or sensory input.
Critical Periods: During certain developmental stages, such as critical periods, the brain is particularly sensitive to experience, and synaptogenesis is highly active. These periods are essential for the proper wiring of sensory systems and the development of complex skills.
Neuronal pruning and synaptogenesis work in concert to refine neural circuits by eliminating unnecessary connections and strengthening relevant ones. This dynamic interplay between elimination and formation of synapses is crucial for the development, plasticity, and adaptability of the brain's neural circuits in both early development and throughout life.
How do these processes contribute to the overall functionality and plasticity of the nervous system?
Neuronal pruning and synaptogenesis play pivotal roles in shaping the functionality and plasticity of the nervous system. These processes collectively contribute to the refinement, efficiency, and adaptability of neural circuits, allowing the brain to process information, learn, and respond to experiences in a dynamic manner.
Overall Functionality:
Elimination of Redundant Connections: Neuronal pruning ensures that only the most relevant and effective connections are retained in the neural network. By eliminating redundant or weaker connections, the brain optimizes the transmission of signals and reduces noise, leading to more efficient information processing.
Circuit Specialization: Pruning and synaptogenesis help neural circuits become specialized for specific functions. As connections are refined, distinct circuits dedicated to sensory processing, motor control, memory, and other cognitive functions emerge. This specialization enhances the overall functionality of the nervous system.
Network Balance: Neuronal pruning prevents circuits from becoming overly complex and unwieldy. This maintains a balance between different neuronal populations, preventing an excessive number of connections that could impede efficient information flow.
Plasticity and Adaptability:
Experience-Dependent Changes: Synaptogenesis allows the nervous system to adapt to changing environments and experiences. New synapses can form as a response to learning or exposure to novel stimuli, enabling the brain to incorporate new information into existing networks.
Learning and Memory: The ability of synapses to strengthen or weaken in response to activity, known as synaptic plasticity, underlies learning and memory processes. Long-term potentiation (LTP) and long-term depression (LTD) are forms of synaptic plasticity that contribute to the encoding and retention of information.
Sensory Development: During critical periods in development, synaptogenesis is particularly active, allowing sensory systems to wire themselves in response to specific experiences. This is crucial for the proper development of sensory perception.
Recovery from Injury: Neuronal pruning and synaptogenesis also play roles in recovery after neural injury. The brain can rewire itself to some extent by forming new connections around damaged areas, aiding in functional recovery.
Neurodevelopmental Disorders and Plasticity:
Imbalance and Disorders: Disruptions in neuronal pruning and synaptogenesis can lead to neurodevelopmental disorders. For instance, conditions like autism spectrum disorder (ASD) are associated with altered synapse formation and connectivity.
Therapeutic Potential: Understanding these processes is essential for developing therapies for neurodevelopmental disorders. Promoting adaptive synaptogenesis and modifying pruning patterns could potentially help treat certain conditions.
Neuronal pruning and synaptogenesis are crucial mechanisms that not only refine neural circuits for optimal functionality but also provide the nervous system with the capacity to adapt, learn, and respond to experiences throughout life. These processes are foundational to the brain's remarkable ability to process information, form memories, and continuously reshape itself in response to the world around it.
How do neuronal pruning and synaptogenesis contribute to the overall functionality and plasticity of the nervous system?
Neuronal pruning and synaptogenesis are fundamental processes that play crucial roles in shaping the functionality and plasticity of the nervous system. These processes involve the refinement of neural connections, leading to more efficient neural circuits and adaptive responses. Here's how neuronal pruning and synaptogenesis contribute to the overall functionality and plasticity of the nervous system:
Neuronal Pruning
Exuberant Connection Formation: During early development, neurons form an excessive number of connections, resulting in a dense network of synapses. This exuberant connectivity allows the nervous system to establish a wide range of potential pathways and interactions.
Competition and Refinement: Neuronal activity plays a crucial role in determining which synapses are strengthened and which are weakened. Synapses that are frequently activated are reinforced, while those that are less active are eliminated through a process called synaptic pruning. This competitive process refines neural connections, eliminating unnecessary or weak synapses and enhancing the efficiency of information transmission.
Sculpting Circuitry: Neuronal pruning is responsible for sculpting neural circuits into more precise and functional configurations. This fine-tuning of connections enhances the specificity of neural pathways, allowing for more accurate and efficient signal processing.
Synaptogenesis
Formation of New Synapses: Synaptogenesis involves the formation of new synapses between neurons. This process occurs throughout life, not just during development, and it contributes to learning, memory, and adaptive responses to environmental changes.
Experience-Dependent Plasticity: Synaptogenesis is influenced by experiences and environmental factors. Learning new skills or adapting to new situations often involves the creation of new synapses or the strengthening of existing ones. This experience-dependent plasticity allows the nervous system to adapt and learn from its surroundings.
Neuroplasticity and Recovery: Following injuries or changes in sensory input, synaptogenesis can contribute to the brain's ability to rewire itself and recover lost function. Neurons can establish new connections or alter existing ones to compensate for damage or changes in input.
Neuronal pruning and synaptogenesis are essential processes that optimize the structure and function of the nervous system. Neuronal pruning refines neural connections, while synaptogenesis allows for the formation of new synapses, enabling learning, memory, and adaptive responses. These processes together contribute to the remarkable plasticity and adaptability of the nervous system throughout life.

A model view of the synapse 1
At what point in the evolutionary timeline did neuronal pruning and synaptogenesis first appear?
Neuronal pruning and synaptogenesis are complex processes that are intimately linked to the development and functionality of the nervous system. While the exact point in the evolutionary timeline when these processes first appeared is not definitively known, it's supposed that they emerged gradually as nervous systems became more sophisticated.
The evolution of nervous systems would have been a gradual process that spans millions of years, making it challenging to pinpoint precise stages in which specific mechanisms like neuronal pruning and synaptogenesis emerged.
Early Nervous System Evolution: In the earliest multicellular organisms, nerve cells (neurons) would have started to form basic networks, allowing for simple sensory and motor responses. These early networks would have lacked the complex pruning and refinement mechanisms seen in more advanced nervous systems.
Emergence of Synaptic Connections: As nervous systems would have become more complex, the formation of synaptic connections would have became more important. Synapses, the junctions between neurons, would have allowed for communication and signal transmission between nerve cells. Over time, mechanisms that promoted the strengthening or weakening of synapses would have emerged to enhance the efficiency of signal transmission.
Refinement and Pruning: As nervous systems would have continued to evolve, mechanisms of neuronal pruning probably would have developed as a way to fine-tune neural connections. This would have been driven by the need for more efficient neural circuits, as well as the optimization of limited resources in the developing organisms.
Adaptation and Plasticity: The ability to form new synapses and adapt existing ones, which is a hallmark of synaptogenesis, would have provided significant evolutionary advantages. Organisms with the ability to adjust their neural circuits based on experiences and environmental changes would have been better equipped to survive and thrive in changing conditions.
What de novo genetic information is thought to have been necessary to instantiate neuronal pruning and synaptogenesis?
The mechanisms underlying neuronal pruning and synaptogenesis involve intricate genetic and molecular processes that regulate the formation, refinement, and elimination of neural connections. While it's not necessarily the case that entirely new genetic information was required to instantiate these processes, the proper orchestration of existing genetic information would have been crucial. Here are some key aspects of genetic information and molecular mechanisms thought to be involved:
Gene Expression and Regulation: Existing genes in an organism's genome are responsible for producing the proteins and molecules necessary for neuronal development and plasticity. The activation or repression of specific genes during different developmental stages is critical for initiating and guiding processes like synaptogenesis and neuronal pruning.
Signaling Pathways: Various signaling pathways, involving proteins and molecules such as growth factors, neurotransmitters, and their receptors, play essential roles in regulating neuronal development and connectivity. These pathways transmit information that guides the formation, strengthening, and elimination of synapses.
Synaptic Activity and Plasticity Genes: Certain genes are associated with synaptic plasticity—the ability of synapses to change their strength in response to activity. These genes, such as those involved in the regulation of neurotransmitter receptors and synaptic structure, contribute to the dynamic nature of synaptogenesis and pruning.
Epigenetic Modifications: Epigenetic modifications, which influence gene expression without altering the underlying DNA sequence, also play a role in neuronal development. These modifications can be influenced by experiences and environmental factors, contributing to the adaptive nature of the nervous system.
Cell-Cell Interactions: Cell adhesion molecules and guidance cues are essential for establishing and refining neural connections. These molecules, guided by genetic information, help neurons find their appropriate partners and form synapses in specific patterns.
The genetic information necessary for neuronal pruning and synaptogenesis involves the coordination of existing genes, signaling pathways, and molecular mechanisms. Rather than requiring entirely new genetic elements, these processes rely on the careful regulation and interaction of existing genetic information to sculpt the intricate neural circuits and adaptability observed in the nervous system.
What manufacturing codes and languages would have had to emerge and be employed for the processes of neuronal pruning and synaptogenesis?
The processes of neuronal pruning and synaptogenesis involve intricate cellular and molecular interactions rather than literal manufacturing codes and languages like those used in human-made technologies. Nevertheless, we can draw an analogy to the concept of "codes" and "languages" in biological terms to describe the molecular instructions and interactions that guide these processes. Here are some analogies to help understand the concept:
Molecular Signaling Pathways: In a metaphorical sense, molecular signaling pathways can be seen as analogous to a "language" that cells use to communicate with each other. Various molecules, such as neurotransmitters, growth factors, and receptors, act as "words" in this cellular communication. Cells "read" these signals to initiate processes like neuronal pruning and synaptogenesis.
Genetic Information and Expression: The genetic code present in an organism's DNA can be likened to a "manufacturing code." Genes contain the instructions for producing proteins and molecules needed for neuronal development and plasticity. The process of gene expression, where DNA is transcribed into RNA and then translated into proteins, can be seen as the "manufacturing" process based on these codes.
Epigenetic Marks and Modifications: Epigenetic modifications, which can influence gene expression without changing the underlying DNA sequence, could be considered as a form of regulatory "coding." These modifications act like switches that turn genes on or off, impacting the course of neuronal development and the dynamics of synaptogenesis.
Cell-Cell Communication: Cell-adhesion molecules and guidance cues can be thought of as a type of "communication language" that cells use to establish proper connections. These molecules guide neurons to their appropriate partners during synaptogenesis and contribute to the spatial organization of neural circuits.
While there aren't literal manufacturing codes and languages involved in neuronal pruning and synaptogenesis, the analogy helps us grasp the complexity of molecular interactions and instructions that guide these processes. The language of molecular signaling, genetic information, epigenetic regulation, and cell-cell communication collectively orchestrates the intricate development and refinement of neural connections in the nervous system.
Which epigenetic regulatory mechanisms are critical for directing neuronal pruning and synaptogenesis?
Epigenetic regulatory mechanisms play a vital role in shaping the processes of neuronal pruning and synaptogenesis by modulating gene expression and influencing the formation and elimination of synapses. Here are some of the critical epigenetic mechanisms involved:
DNA Methylation: DNA methylation involves the addition of methyl groups to specific regions of DNA, typically cytosine residues in CpG dinucleotides. In neuronal development, DNA methylation can influence the expression of genes involved in synaptic plasticity, axon guidance, and cell adhesion. Changes in DNA methylation patterns can lead to lasting alterations in synaptic connectivity.
Histone Modifications: Histones are proteins around which DNA is wound, forming chromatin. Modifications to histone proteins, such as acetylation, methylation, phosphorylation, and more, can influence how tightly DNA is packaged and thus affect gene accessibility. Specific histone modifications are associated with active or repressed gene expression, impacting processes like neuronal pruning and synaptogenesis.
Non-coding RNAs: Non-coding RNAs, such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), can regulate gene expression by binding to target messenger RNAs (mRNAs) and influencing their stability or translation. These RNA molecules can have profound effects on neuronal development, including synapse formation and elimination.
Activity-Dependent Epigenetic Changes: Neuronal activity, such as synaptic stimulation, can trigger epigenetic modifications that influence gene expression. For example, neuronal activity can lead to changes in DNA methylation and histone modifications, allowing the cell to respond to environmental stimuli and modulate synaptic plasticity.
Epigenetic Regulation of Synaptic Genes: Many genes involved in synapse formation, function, and elimination are under the control of epigenetic regulation. For instance, genes encoding cell adhesion molecules, neurotransmitter receptors, and other synaptic proteins can be epigenetically modulated to fine-tune the establishment and maintenance of synapses.
Epigenetic regulatory mechanisms are critical for directing neuronal pruning and synaptogenesis by modulating gene expression and influencing the molecular processes underlying neural connectivity. These mechanisms allow the nervous system to adapt to experiences, shape neural circuits, and optimize synaptic connections for proper functionality.
Signaling pathways that are indispensable for the orchestration of neuronal pruning and synaptogenesis
Several signaling pathways are crucial for the proper orchestration of neuronal pruning and synaptogenesis. These pathways transmit molecular signals that guide the formation, strengthening, and elimination of synapses, as well as the refinement of neural connections. Here are some of the indispensable signaling pathways involved:
Brain-Derived Neurotrophic Factor (BDNF) Pathway: BDNF, a member of the neurotrophin family, is critical for promoting neuronal survival, differentiation, and synaptic plasticity. BDNF signaling through its receptor, TrkB, plays a pivotal role in synaptogenesis and synaptic refinement by enhancing the growth and maintenance of synapses.
Wnt Signaling Pathway: The Wnt pathway is involved in a variety of developmental processes, including neuronal connectivity. Wnt signaling influences axon guidance, dendrite development, and synapse formation by regulating the cytoskeleton and intracellular pathways within neurons.
Notch Signaling Pathway: The Notch pathway is essential for cell-cell communication and has roles in neural development. Notch signaling influences the balance between neuronal differentiation and maintenance of precursor cells. Disruption of Notch signaling can impact synaptic connectivity.
Ephrin Receptor Pathway: Ephrin receptors and their ligands, ephrins, are involved in axon guidance and synaptic organization. The interaction between ephrins on one neuron and their corresponding receptors on another plays a role in shaping synaptic connections and neural circuits.
Neuregulin-ErbB Pathway: Neuregulins, ligands that activate ErbB receptor tyrosine kinases, are involved in the development of glial cells and synapses. This pathway plays a role in coordinating the formation of pre- and postsynaptic elements during synaptogenesis.
Calcium Signaling: Intracellular calcium plays a critical role in neuronal activity and synaptic plasticity. Calcium signaling is involved in synaptic vesicle release, postsynaptic response, and the activation of various signaling cascades that influence synaptogenesis.
Activity-Dependent Pathways: Neuronal activity itself, often initiated by synaptic transmission, triggers signaling pathways that contribute to synaptic plasticity and connectivity refinement. NMDA receptor-dependent calcium influx is a key player in activity-dependent processes.
Specific signaling pathways are indispensable for directing neuronal pruning and synaptogenesis. These pathways orchestrate various aspects of neural development and connectivity, ensuring the precise formation, strengthening, and elimination of synapses that are essential for the functional wiring of the nervous system.
What regulatory codes maintain and oversee the operation of neuronal pruning and synaptogenesis?
The "regulatory codes" that maintain and oversee the operation of neuronal pruning and synaptogenesis involve a complex interplay of molecular mechanisms, gene expression, and cellular signaling. These codes ensure the precise execution of these processes while adapting to developmental needs and environmental cues. Here are some of the key regulatory elements that govern neuronal pruning and synaptogenesis:
Activity-Dependent Regulation: Neuronal activity, driven by synaptic transmission and sensory experiences, acts as a regulatory code. It guides the strengthening of active synapses and the elimination of less active ones, contributing to the refinement of neural circuits.
Transcriptional Regulation: Transcription factors and other regulatory molecules control gene expression patterns during neuronal development. These factors determine which genes are turned on or off, influencing synaptogenesis, dendritic branching, and other processes.
Epigenetic Modification Patterns: Epigenetic marks, such as DNA methylation and histone modifications, form regulatory codes that impact gene expression. These marks can be dynamically altered in response to neural activity, experience, and environmental factors.
Molecular Signaling Networks: Signaling pathways, such as BDNF-TrkB, Wnt, and Notch, form interconnected networks that convey instructions for synaptogenesis and pruning. These pathways regulate cellular responses to molecular cues.
Neurotrophins and Growth Factors: Neurotrophic factors, like BDNF, NGF, and others, play crucial roles in regulating neuronal survival, differentiation, and synaptic plasticity. They ensure that proper connections are established and maintained.
Guidance Molecules and Receptors: Guidance cues and their receptors direct axon pathfinding and dendritic arborization. These molecules ensure that neurons connect to their correct targets during development.
Cell Adhesion Molecules: Cell adhesion molecules ensure that synapses are formed between appropriate pre- and postsynaptic partners. They contribute to the precise wiring of neural circuits.
MicroRNAs and Non-Coding RNAs: MicroRNAs and other non-coding RNAs regulate gene expression post-transcriptionally. They fine-tune the levels of specific proteins involved in synaptogenesis and pruning.
The regulatory codes governing neuronal pruning and synaptogenesis encompass a diverse array of mechanisms that interact to ensure the proper development and refinement of neural connections. These codes integrate genetic information, molecular signaling, cellular responses, and environmental inputs to sculpt the intricate connectivity of the nervous system.
Is there scientific evidence that supports the notion that neuronal pruning and synaptogenesis were brought about by the process of evolution?
The intricate processes of neuronal pruning and synaptogenesis, fundamental to the development and functionality of the nervous system, present significant challenges for an evolutionary explanation. The idea that these processes could have emerged gradually, through a stepwise evolutionary progression, faces substantial hurdles given the interdependence of various codes, languages, signaling networks, and proteins that must be operational from the beginning.
Complexity and Functional Requirements: Neuronal pruning and synaptogenesis involve a remarkable level of complexity, requiring precise coordination of multiple molecular interactions and genetic regulations. The establishment of synaptic connections necessitates intricate guidance cues, molecular signaling, and precise cellular interactions. A stepwise evolutionary approach would demand the gradual development of each of these components, without the guarantee of functionality at intermediate stages. It is difficult to conceive how partially formed systems with no immediate function could have been selected for, as they would provide no selective advantage to an organism.
Interdependence and Instantiation: What makes the evolutionary pathway even more implausible is the interdependence of the various components. Signaling pathways, gene expression networks, and molecular codes are not independent entities; they rely on each other for their functionality. A fully operational system is required for neuronal pruning and synaptogenesis to occur. The language of molecular signaling pathways needs a coherent molecular vocabulary that includes proteins, receptors, and other elements. Without all these components functioning together, no functional outcome would be achieved, rendering any intermediate stages non-adaptive and non-selectable.
Coordinated Emergence of Multiple Mechanisms: The coordinated emergence of gene expression regulations, epigenetic modifications, molecular signaling, and cellular interactions is a substantial challenge for stepwise evolution. The likelihood of these mechanisms independently evolving, and then coincidentally aligning to support neuronal pruning and synaptogenesis, stretches the bounds of probability. This complex coordination is best explained by the concept of intelligent design, where all necessary components are instantiated simultaneously to achieve a functional outcome.
Irreducible Complexity and Intelligent Design: The concept of irreducible complexity arises when a system relies on multiple interacting components, none of which can be removed without disrupting function. Neuronal pruning and synaptogenesis could be seen as irreducibly complex systems. These systems were most likely designed and implemented all at once with all their intricate interdependencies in place, rather than evolving in a piecemeal fashion.
The simultaneous emergence of neuronal pruning and synaptogenesis as fully operational systems seems more plausible than the stepwise evolution of their various components. The interdependence of codes, languages, signaling networks, and proteins required for their function, along with the complexity and functional demands of these processes, presents a compelling case for intelligent design as the best explanation for the origins of these intricate mechanisms.
How might the systems and structures involved in neuronal pruning and synaptogenesis be considered irreducibly complex or interdependent?
Neuronal pruning and synaptogenesis represent intricate processes that exhibit features of irreducible complexity and interdependence, reinforcing the notion that they are the result of intelligent design rather than gradual evolution.
Molecular Signaling and Receptors: The language of molecular signaling involves complex interactions between signaling molecules and their receptors. This signaling is indispensable for guiding axons, dendrites, and synaptic connections. The absence of any key signaling component would lead to an incomplete and non-functional process.
Guidance Cues and Cell Adhesion Molecules: The guidance cues that direct the growth of axons and dendrites are interdependent with cell adhesion molecules that enable synaptic connections. Without proper guidance cues, neurons might not reach their targets, and without functional adhesion molecules, synapses would not form properly.
Gene Expression and Transcription Factors: The genetic code, transcription factors, and gene expression are intricately involved in shaping neuronal connectivity. The absence of specific genes or regulatory elements would disrupt the orchestration of synaptogenesis and pruning.
Synaptic Activity and Plasticity: The plasticity of synapses, allowing them to strengthen or weaken based on activity, is intertwined with the overall process of pruning. The absence of synaptic activity would hinder both the refinement of synapses and the elimination of excess connections.
Molecular Codes and Signaling Pathways: The "codes" for molecular signaling pathways must be present alongside functional receptors, ligands, and downstream effectors. The absence of any of these components would result in disrupted communication and misdirection of neural growth.
Epigenetic Regulation and Genetic Expression: Epigenetic modifications, such as DNA methylation and histone modifications, regulate gene expression critical for proper development. The intricate interplay between epigenetic marks, gene expression, and neural connectivity is essential for the successful operation of these processes.
Once neuronal pruning and synaptogenesis are fully instantiated and operational, with which other intra and extracellular systems do they closely interact or rely?
Neuronal pruning and synaptogenesis, once fully instantiated and operational, closely interact with various intra and extracellular systems to ensure the proper development and function of the nervous system. These interactions contribute to the establishment of functional neural circuits and the precise wiring of the brain.
Intracellular Interactions
Intracellular Signaling Pathways: Neuronal pruning and synaptogenesis rely on intricate intracellular signaling pathways that regulate processes such as gene expression, cytoskeletal dynamics, and organelle transport. These pathways help neurons respond to extracellular cues and adapt their connectivity.
Cytoskeletal Dynamics: The cytoskeleton, comprising microtubules, microfilaments, and intermediate filaments, plays a vital role in axon and dendrite growth, guidance, and synaptic plasticity. Cytoskeletal elements are crucial for maintaining neuronal structure and connectivity.
Intracellular Transport Systems: Molecular motors and transport mechanisms facilitate the movement of organelles, vesicles, and other cellular components within neurons. Proper intracellular transport is necessary for the delivery of essential materials to growing axons and dendrites.
Extracellular Interactions:
Synaptic Activity and Neurotransmission: Neuronal pruning and synaptogenesis closely interact with synaptic activity and neurotransmission. Active synapses strengthen through activity-dependent mechanisms, while less active synapses are eliminated through pruning. This interaction fine-tunes neural connectivity.
Cell-Cell Communication: Interactions between neurons and other cell types, such as glia, are crucial for guiding axon growth, providing trophic support, and modulating synaptic connections. These interactions help create a conducive environment for proper neuronal development.
Neurotrophic Factors and Growth Factors: Neurotrophic factors play a key role in promoting neuronal survival, differentiation, and synaptic plasticity. They interact with neuronal pruning and synaptogenesis by influencing cell survival, axon guidance, and synapse formation.
Extracellular Matrix (ECM): The ECM provides physical and molecular cues that guide axon and dendrite growth, influence synaptic maturation, and help establish proper neural circuits. The interactions between neurons and the ECM play a role in shaping neural connectivity.
Epigenetic Regulations and Feedback Loops
Epigenetic Mechanisms: Epigenetic regulations, including DNA methylation and histone modifications, influence gene expression patterns that impact neuronal connectivity. These mechanisms are influenced by neuronal activity, shaping the interactions between synaptic activity and gene expression.
Activity-Dependent Feedback Loops: Synaptic activity influences epigenetic modifications, which in turn affect gene expression. This creates feedback loops that allow the nervous system to adapt to experiences and optimize neural circuitry.
Neuronal pruning and synaptogenesis closely interact with a network of intra and extracellular systems to ensure the proper development, refinement, and maintenance of neural connectivity. These interactions highlight the intricate coordination required for the formation of functional neural circuits and emphasize the complexity of the mechanisms involved in shaping the brain's intricate wiring.
1. Neuronal pruning and synaptogenesis rely on intricate semiotic codes and languages for proper communication and coordination between cells and molecules.
2. The systems involved in neuronal pruning and synaptogenesis are highly interdependent, requiring the simultaneous presence and precise coordination of multiple components for functionality.
3. Interlocking codes and interdependence suggest a carefully designed setup rather than an unguided, stepwise evolutionary process.
1. Wikipedia: Synapse pruning

