13. Cytokinesis
Cytokinesis is the final stage of cell division, following mitosis or meiosis, where a single eukaryotic cell divides into two daughter cells. It ensures the distribution of cellular contents, including organelles and genetic material, into the daughter cells. Cytokinesis involves a series of coordinated processes that lead to the physical separation of the two daughter cells, each containing a nucleus and necessary organelles.
Process of Cytokinesis
Initiation: Cytokinesis begins during late stages of mitosis or meiosis when the cellular components are distributed within two distinct nuclei within the same cell.
Contractile Ring Formation: In animal cells, a contractile ring composed of actin and myosin filaments forms just beneath the cell membrane at the equatorial plane. This ring contracts, causing the membrane to pinch inwards.
Cleavage Furrow Formation: The contracting ring creates a cleavage furrow, which deepens as the contractile ring contracts further.
Cell Membrane Ingrowth: As the cleavage furrow deepens, the cell membrane is progressively drawn inwards, dividing the cytoplasm into two separate compartments.
Daughter Cell Separation: Once the cleavage furrow reaches its maximum depth, the two daughter cells are physically separated, each with its own nucleus and cellular contents.
Importance in Biological Systems
Cytokinesis is crucial for maintaining organismal health and growth. It ensures the even distribution of cellular components, including genetic material and organelles, between the two daughter cells. Accurate cytokinesis is essential to prevent aneuploidy (imbalanced chromosome number) and maintain genetic stability in the organism's tissues.
Developmental Processes Shaping Organismal Form and Function
Cytokinesis plays a pivotal role in the development of multicellular organisms by influencing various aspects of their form and function:
Tissue Formation: During development, cells undergo numerous rounds of division and cytokinesis, which collectively contribute to the formation of tissues and organs with specific shapes and functions.
Organ Growth: Cytokinesis allows tissues to grow in size and complexity. Controlled cell division and cytokinesis are essential for generating and maintaining the appropriate size and proportion of organs.
Cell Differentiation: Differentiation of cells into specialized cell types is influenced by cytokinesis. It ensures that the right number of specialized cells is generated in the right place and at the right time during development.
Pattern Formation: Proper cytokinesis is necessary for generating intricate patterns and structures during embryonic development. It helps shape the organism's body plan and coordinates the positioning of different cell types.
Regeneration and Repair: In tissues that undergo constant renewal, such as the skin and intestinal lining, accurate cytokinesis is essential for proper regeneration and repair after injury.
What are the mechanisms that ensure proper cytokinesis and cell division?
Proper cytokinesis and cell division are ensured through a combination of regulatory mechanisms that coordinate various cellular processes. These mechanisms work together to accurately distribute cellular contents, maintain genetic stability, and prevent errors. Some key mechanisms include:
Checkpoint Control: Checkpoint mechanisms monitor the progress of cell division to ensure accurate DNA replication and chromosome segregation before cytokinesis proceeds. The G1/S, intra-S, and G2/M checkpoints regulate the cell cycle's progression, preventing cell division if errors are detected.
Spindle Assembly Checkpoint (SAC): This checkpoint ensures proper attachment and alignment of chromosomes on the mitotic spindle before cell division proceeds. If chromosomes are not properly positioned, the SAC halts the cell cycle until corrections are made.
Mitotic Spindle Formation: The mitotic spindle, composed of microtubules and associated proteins, is responsible for segregating chromosomes into daughter cells. Accurate spindle formation and function are essential for proper chromosome segregation during cytokinesis.
Cytokinesis Regulatory Proteins: Various proteins are involved in cytokinesis regulation, including those that control contractile ring formation, cell membrane ingrowth, and furrow stabilization. For example, the Rho family of GTPases and associated effectors play a crucial role in actin filament assembly for contractile ring formation.
Cytokinesis Checkpoint: A checkpoint mechanism ensures that cytokinesis is delayed until mitosis is completed. This prevents premature cell division before chromosome segregation is finished.
Anaphase-Promoting Complex (APC/C): APC/C is a protein complex that controls the degradation of specific cell cycle regulators, allowing for the timely progression through different phases of cell division.
Cyclin-Dependent Kinases (CDKs): CDKs are protein kinases that regulate cell cycle progression by phosphorylating target proteins. CDK activity is tightly controlled by cyclins, which activate CDKs at specific points in the cell cycle.
Checkpoint Kinases: Checkpoint kinases, such as ATM and ATR, detect DNA damage or replication stress and transmit signals to halt the cell cycle, providing time for repair processes before division proceeds.
DNA Damage Response: If DNA damage is detected, the cell cycle can be arrested to allow for DNA repair before cell division. If the damage is irreparable, apoptosis (programmed cell death) may be initiated to prevent the propagation of damaged DNA.
Centrosome Duplication and Function: Centrosomes organize microtubules and play a role in spindle formation. Proper centrosome duplication and function are essential for accurate chromosome segregation.
Chromosome Segregation Mechanisms: Protein complexes like the cohesin complex hold sister chromatids together until anaphase. Separase enzyme cleaves cohesin during anaphase, allowing chromatids to separate.
Kinetochore-Microtubule Attachment: Kinetochore proteins attach to microtubules, ensuring correct chromosome alignment on the spindle and facilitating proper chromosome segregation.
These mechanisms collectively contribute to the fidelity of cell division, ensuring that genetic material is accurately distributed to daughter cells and maintaining genomic stability. Dysregulation of these mechanisms can lead to various cellular abnormalities, including aneuploidy, which is associated with several diseases, including cancer.
How do cells coordinate the separation of cytoplasm and organelles during cytokinesis?
Cells coordinate the separation of cytoplasm and organelles during cytokinesis through a combination of cytoskeletal elements, membrane trafficking, and regulatory proteins. The process varies between different types of cells, such as animal cells and plant cells, due to differences in cell structure and mechanisms. Here's an overview of how this coordination is achieved:
Animal Cells
Contractile Ring Formation: In animal cells, a contractile ring composed of actin and myosin filaments forms just beneath the cell membrane at the site of cleavage. The assembly of this contractile ring is a key step in cytokinesis.
Actin-Myosin Contraction: The contractile ring contracts, leading to the constriction of the cell's equator. Myosin motor proteins move along actin filaments, causing them to slide past each other, reducing the diameter of the cell.
Membrane Ingrowth: As the contractile ring contracts, the cell membrane is pulled inward along with the ring. This process creates a cleavage furrow that divides the cytoplasm into two separate portions.
Vesicle Fusion: Membrane-bound vesicles, derived from the Golgi apparatus, fuse with the forming cleavage furrow. These vesicles contribute additional membrane material, which is necessary to accommodate the membrane ingrowth.
Cytokinesis Regulators: Various regulatory proteins, including those from the Rho family of GTPases (such as RhoA), play a role in coordinating actin-myosin contraction and vesicle trafficking during cytokinesis.
Plant Cells
Cell Plate Formation: Plant cells have rigid cell walls that prevent the use of contractile rings. Instead, during cytokinesis, a structure called the cell plate forms at the center of the cell.
Golgi-Derived Vesicles: Vesicles derived from the Golgi apparatus carry cell wall components, such as cellulose and other polysaccharides, to the center of the cell.
Fusion of Vesicles: These vesicles fuse together at the center of the cell, forming the cell plate. The vesicles contribute membrane material and cell wall components to the growing structure.
Cell Wall Synthesis: Enzymes present in the vesicles catalyze the synthesis of new cell wall material, causing the cell plate to expand outward and fuse with the existing cell wall.
Phragmoplast: During this process, a structure called the phragmoplast guides the vesicles to the center of the cell and ensures their proper fusion.
Both in animal and plant cells, the coordination of cytoplasm and organelle separation involves the precise orchestration of cytoskeletal elements, vesicle trafficking, and regulatory proteins. The cell's architecture and specific requirements determine the mechanisms employed. Regardless of the differences, the ultimate goal of cytokinesis is to ensure the formation of two distinct daughter cells, each equipped with the necessary cellular components for independent function.
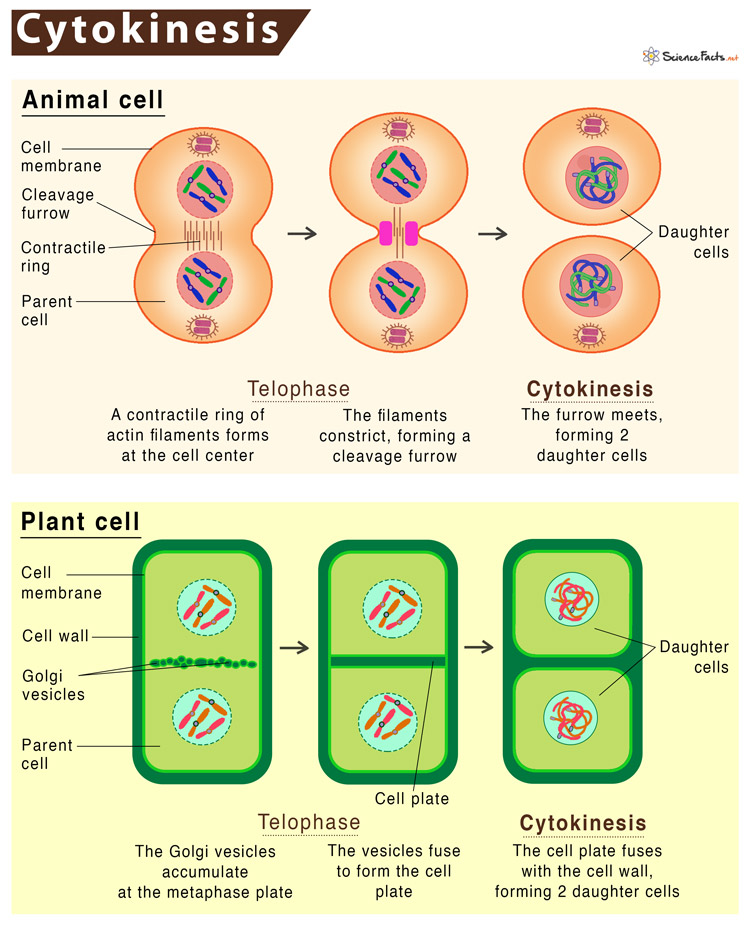
Cytokinesis in Animal Cells
In animal cells, cytokinesis takes place without the presence of cell walls and occurs from anaphase through telophase. This process unfolds through the following stages:
A contractile ring composed of actin filaments forms beneath the cell membrane, positioning itself around the cell's center.
Actin filaments contract, causing the cleavage furrow to deepen progressively from the cell's periphery towards its center.
The contractile ring contracts further, ultimately resulting in the separation of the two daughter cells. This separation occurs at the midbody, a narrowed region of cytoplasm connecting the two new cells.
As the cleavage furrow meets at the center, the cell membrane becomes completely pinched off, giving rise to two distinct daughter cells enclosed within their individual cell membranes.
This outward-to-inward separation process is referred to as centripetal cytokinesis due to the progression starting outside and moving toward the cell's center.
Cytokinesis in Plant Cells
Plant cells, possessing cell walls, initiate cytokinesis earlier, during interphase, and continue through telophase. The process unfolds in the following manner:
The Golgi apparatus gathers enzymes, structural proteins, and glucose, which later break down into vesicles that disperse throughout the cell.
During telophase, Golgi vesicles migrate towards the metaphase plate, assembling into a structure known as the phragmoplast.
These vesicles then fuse, initiating the formation of a structure termed the cell plate.
The cell plate gradually extends outward, ultimately merging with the existing cell wall. This division process results in the creation of two new daughter cells, each enclosed within its own cell membrane.
The formation of the cell plate and subsequent cell wall is essential in dividing the plant cell.
These distinctive processes highlight the differences between cytokinesis in animal and plant cells. The presence or absence of a cell wall influences the timing and progression of cytokinesis, ultimately leading to the successful division of cells in both types of organisms.
Appearance of cytokinesis in the evolutionary timeline
The evolutionary timeline of cytokinesis is not fully elucidated due to the lack of direct evidence from the distant past. However, scientists have proposed hypotheses based on comparative studies, molecular analysis, and observations of modern organisms. Here's a simplified overview of the hypothesized appearance of cytokinesis in the evolutionary timeline:
Early Single-Celled Organisms: In the earliest stages of life on Earth, simple single-celled organisms, such as bacteria and archaea, would have undergone a form of binary fission to reproduce. While not cytokinesis in the eukaryotic sense, this basic process involved the division of a cell into two daughter cells through growth and splitting.
Emergence of Eukaryotes: With the advent of eukaryotic cells, which are more complex and compartmentalized than prokaryotic cells, the need for a more sophisticated form of cell division arose. The ancestral mechanisms for cytokinesis in eukaryotes are uncertain, but it is supposed that it has involved rudimentary processes like membrane pinching and septum formation.
Evolving Cytoskeletal Elements: Over time, the evolution of cytoskeletal elements such as actin and microtubules would have allowed for more precise cell division in eukaryotic cells. These structures would have been adapted for generating contractile forces and guiding vesicle trafficking.
Formation of Contractile Rings: The development of actin-based contractile rings in animal cells and similar structures in other eukaryotes would have enhanced the accuracy and efficiency of cytokinesis. The emergence of regulatory proteins, like those from the Rho family of GTPases, would have contributed to the coordination of these processes.
Plant Cell Innovations: Plant cells, with their rigid cell walls, would have evolved a distinct mechanism for cytokinesis involving the formation of a cell plate. This innovation allowed for the construction of new cell walls between daughter cells.
Fine-Tuning and Diversification: As multicellularity emerged and organisms became more complex, the mechanisms of cytokinesis would have undergone further refinement and diversification. The evolution of regulatory networks and signaling pathways would have played a role in coordinating cytokinesis within different cell types and tissues.
It's important to note that the evolutionary timeline of cytokinesis is still a subject of ongoing research, and our understanding is based on hypotheses and comparative studies. While some general trends and innovations are proposed, the specific details of how cytokinesis evolved remain a topic of exploration and debate within the scientific community.
De Novo Genetic Information necessary to instantiate cytokinesis
The hypothetical process of generating and introducing new genetic information to create the mechanisms of cytokinesis, starting from scratch, would involve the de novo creation of various components necessary for cell division:
Formation of Cytoskeletal Elements: The genetic information required for the creation of cytoskeletal elements such as actin and microtubules would need to originate. These structural proteins play a vital role in generating contractile forces and guiding vesicle trafficking during cytokinesis.
Regulatory Proteins: New genetic information would have to emerge to encode regulatory proteins that coordinate the intricate processes of cytokinesis. These proteins would be responsible for initiating the formation of contractile rings or equivalent structures, and for controlling their activation and contraction.
Membrane Trafficking Machinery: Genetic instructions for the assembly of membrane-bound vesicles, Golgi-derived vesicles, and other components of the cellular trafficking machinery would need to be introduced. These vesicles play a role in contributing membrane material to the cleavage furrow or cell plate during cytokinesis.
Coordination Mechanisms: Information would have to originate to encode mechanisms for precise coordination between various components involved in cytokinesis. This would include signaling pathways and checkpoints that ensure proper progression through each step of cell division.
Cell Wall Components (For Plant Cells): If considering plant cells, new genetic information would be required to generate cell wall components such as cellulose and other polysaccharides. These components contribute to the formation and growth of the cell plate during cytokinesis in plant cells.
Anaphase-Promoting Complex (APC/C): The genetic code for the anaphase-promoting complex, which controls the degradation of specific cell cycle regulators, would need to originate. This complex is crucial for the timely progression through different phases of cell division.
Checkpoint Control Systems: Genetic information for checkpoint control systems that monitor the progress of cell division would have to be introduced. These systems ensure accurate DNA replication and chromosome segregation before cytokinesis proceeds.
In this hypothetical scenario, the new genetic information would need to be introduced in a coordinated and precise sequence to ensure the proper assembly and functioning of the components involved in cytokinesis. The complexity of this process underscores the challenge of generating the intricate mechanisms of cell division de novo, and it emphasizes the interdependence of various genetic codes and regulatory networks for the successful execution of cytokinesis.
Manufacturing codes and languages that would have to emerge and be employed to instantiate cytokinesis
The transition from an organism without cytokinesis to one with a fully developed cytokinesis would require the creation and instantiation of a complex array of manufacturing codes and languages beyond genetic information. These mechanisms would ensure the assembly, coordination, and operation of cellular components essential for cytokinesis:
Cytoskeletal Protein Production: Manufacturing codes and pathways for producing cytoskeletal proteins like actin and microtubules would be essential. These proteins contribute to the formation of contractile rings, spindle fibers, and other structural elements involved in cytokinesis.
Vesicle Formation and Trafficking: A coherent system for the generation of vesicles from cellular organelles, particularly the Golgi apparatus, would need to be instantiated. These vesicles play a vital role in providing membrane material required for cleavage furrow or cell plate formation.
Membrane Fusion Proteins: Codes for membrane fusion proteins and their associated regulatory factors would be required. These proteins facilitate the fusion of vesicles with the plasma membrane or cell plate, enabling the incorporation of new membrane material during cytokinesis.
Actin-Myosin Interactions: Elaborate instructions for the assembly and interactions of actin and myosin filaments would need to be established. These interactions generate the contractile forces responsible for cell membrane ingrowth during cytokinesis.
Regulatory Proteins and Signaling Pathways: Codes for regulatory proteins such as the Rho family of GTPases and other signaling molecules would be essential. These proteins coordinate various steps of cytokinesis, ensuring proper timing and localization of key events.
Spindle Formation and Centrosome Organization: Codes for proteins involved in spindle formation, centrosome duplication, and centrosome positioning would have to be created. These proteins help orchestrate the alignment and separation of chromosomes during cytokinesis.
Cell Plate Formation (For Plant Cells): For plant cells, manufacturing codes for enzymes and components responsible for synthesizing cell wall materials like cellulose would be required. These materials contribute to the construction and expansion of the cell plate.
Motor Protein Assembly: Codes for motor proteins like myosins and kinesins would be necessary. These proteins facilitate the movement of organelles, vesicles, and other cellular components required for cytokinesis.
In this scenario, the creation and proper coordination of manufacturing codes and languages beyond genetic information are indispensable. These codes would govern the production, transport, assembly, and interaction of various cellular components, ensuring the successful execution of cytokinesis. The complexity of orchestrating these processes from scratch underscores the intricate interplay of multiple systems required for the development of a fully functional cytokinesis mechanism.
Epigenetic Regulatory Mechanisms necessary to be instantiated for cytokinesis
Epigenetic regulation plays a crucial role in the development of various cellular processes, including cytokinesis. Cytokinesis is the process by which a single eukaryotic cell divides into two daughter cells.
Epigenetic Regulation
DNA Methylation: Epigenetic marks involving the addition of methyl groups to DNA molecules can influence gene expression during cytokinesis. Methylation patterns can affect the accessibility of genes required for cell division.
Histone Modifications: Post-translational modifications of histone proteins, such as acetylation, methylation, and phosphorylation, impact chromatin structure and gene expression during cytokinesis. Histone modifications can alter the compaction of DNA, making certain genes more or less accessible.
Non-Coding RNAs: Small non-coding RNAs, like microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), are involved in post-transcriptional regulation of gene expression during cytokinesis. They can fine-tune the levels of specific mRNAs and proteins required for the process.
Systems Employed to Instantiate Regulation
DNA Methylation Machinery: Enzymes like DNA methyltransferases are responsible for adding methyl groups to specific cytosine residues in DNA, affecting gene expression patterns during cytokinesis.
Histone Modification Complexes: Complexes involving histone acetyltransferases (HATs), histone methyltransferases (HMTs), and histone deacetylases (HDACs) modify histone proteins, influencing chromatin structure and accessibility of genes required for cytokinesis.
RNA Interference Machinery: Enzymes like Dicer and Argonaute are involved in processing and incorporating non-coding RNAs, such as miRNAs, into the RNA-induced silencing complex (RISC), which then targets specific mRNA molecules for degradation or translational repression.
Collaborative Systems for Balanced Operation
Cell Cycle Control: The cell cycle machinery, involving cyclins and cyclin-dependent kinases (CDKs), ensures the orderly progression of cells through different phases of the cell cycle, including cytokinesis. Proper regulation of the cell cycle is essential for balanced cell division.
DNA Repair Mechanisms: DNA damage response pathways monitor and repair DNA lesions that might arise during the intense DNA replication and chromosomal segregation required for cytokinesis. Maintaining genome integrity is critical for successful cell division.
Chromatin Remodeling Complexes: These complexes ensure that DNA remains accessible for transcription by regulating chromatin compaction. They play a role in ensuring that genes involved in cytokinesis are appropriately expressed.
Cell Signaling Pathways: Signaling pathways such as the mitogen-activated protein kinase (MAPK) pathway and the phosphoinositide 3-kinase (PI3K) pathway communicate extracellular signals to the nucleus, affecting gene expression and cellular processes, including cytokinesis.
These systems, in a joint venture, collaborate to maintain the balance and proper operation of cytokinesis. Epigenetic regulation and the associated machinery ensure that genes required for cytokinesis are appropriately expressed, while collaborating systems maintain cell cycle progression, DNA integrity, and chromatin structure. This intricate coordination is essential for successful cell division and overall cellular health.
Signaling Pathways necessary to create, and maintain cytokinesis
The emergence of cytokinesis involves the orchestration of various signaling pathways that are interconnected, interdependent, and often crosstalk with each other and with other biological systems. Here are some key signaling pathways involved in the process of cytokinesis:
Mitogen-Activated Protein Kinase (MAPK) Pathway: The MAPK pathway is a crucial signaling cascade that responds to extracellular signals, such as growth factors. It regulates cell growth, proliferation, and differentiation. During cytokinesis, MAPK pathway components can influence the expression of genes required for cell division and coordinate cell cycle progression.
Phosphoinositide 3-Kinase (PI3K)/Akt Pathway: This pathway responds to growth factors and promotes cell survival, growth, and metabolism. It intersects with the MAPK pathway to influence gene expression and cell cycle regulation during cytokinesis.
Cyclin-Dependent Kinase (CDK) Signaling: CDKs, in collaboration with their regulatory cyclin partners, govern cell cycle progression. CDKs are central to the transition from one cell cycle phase to another, including cytokinesis. The activation of specific CDK-cyclin complexes drives the cell cycle forward.
Wnt/β-Catenin Pathway: The Wnt pathway plays a role in embryonic development and cell polarity. It can influence cytokinesis by affecting cytoskeletal rearrangements and cell shape changes.
Notch Signaling: Notch signaling is involved in cell fate determination and tissue patterning. It can affect cytokinesis indirectly by influencing cell differentiation and proliferation.
Hedgehog (Hh) Pathway: The Hh pathway regulates tissue development and patterning. It can affect cell division by influencing gene expression and cellular responses to growth signals.
JAK-STAT Pathway: The JAK-STAT pathway transmits signals from cytokines and growth factors. It regulates immune responses and cell growth. In the context of cytokinesis, this pathway might influence cell cycle progression and cell differentiation.
Interconnections, Interdependence, and Crosstalk
These signaling pathways are interconnected and often share components, such as kinases and transcription factors. Crosstalk between pathways enables cells to integrate diverse signals for precise control of cytokinesis and other cellular processes. For instance, the MAPK pathway can activate transcription factors that regulate the expression of genes involved in cell division. These factors can crosstalk with those activated by the PI3K/Akt pathway, influencing gene expression patterns during cytokinesis. CDK-cyclin complexes, essential for cell cycle progression and cytokinesis, can be influenced by signals from various pathways, including MAPK and PI3K/Akt. CDKs also regulate transcription factors that impact gene expression during cytokinesis. The interplay between these pathways and other cellular systems, such as DNA repair mechanisms, chromatin remodeling, and cytoskeletal dynamics, ensures proper coordination of processes required for successful cytokinesis. Crosstalk between signaling pathways extends beyond cytokinesis itself, affecting broader cellular functions, such as cell proliferation, differentiation, and survival. For instance, the Wnt pathway's impact on cell polarity can indirectly influence cytokinesis by shaping cell division orientation.
Regulatory codes necessary for maintenance and operation of cytokinesis
The maintenance and operation of cytokinesis involve intricate regulatory codes and languages that enable precise coordination and execution of this cellular process. These regulatory elements communicate information and instructions within the cell. Here are some key regulatory codes and languages involved:
DNA Sequence and Genetic Code: The genetic code encoded in DNA provides the fundamental instructions for synthesizing proteins required for cytokinesis. Regulatory sequences, such as promoters and enhancers, dictate when and where specific genes are transcribed, ensuring proper expression of cytokinesis-related genes.
Epigenetic Marks: Epigenetic modifications, such as DNA methylation and histone modifications, act as a regulatory code that influences gene expression during cytokinesis. These marks dictate whether genes are accessible for transcription, fine-tuning their expression levels.
Transcription Factors: Transcription factors are proteins that bind to specific DNA sequences and act as molecular switches that turn genes on or off. They form a regulatory language that determines which genes are activated or repressed during cytokinesis.
Non-Coding RNAs: Non-coding RNAs, including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), serve as regulators of gene expression. MiRNAs can target messenger RNAs (mRNAs) for degradation or translational repression, while lncRNAs can interact with chromatin-modifying complexes, affecting gene accessibility.
Protein Signaling Pathways: Signaling pathways involve the transmission of information through protein-protein interactions and post-translational modifications. Ligands, receptors, kinases, and downstream effectors communicate messages that influence gene expression, cell cycle progression, and cytokinesis.
Post-Translational Modifications: Proteins involved in cytokinesis undergo post-translational modifications, such as phosphorylation, acetylation, and ubiquitination. These modifications act as a regulatory language that affects protein activity, stability, localization, and interactions.
Cell Cycle Checkpoints: Checkpoints are control points within the cell cycle that ensure proper progression. Regulatory mechanisms monitor DNA integrity, chromosome segregation, and other factors critical for cytokinesis. These checkpoints communicate whether the cell is ready to proceed or needs to halt division.
Cytoskeletal Dynamics and Mechanics: The cytoskeleton communicates mechanical signals that influence cytokinesis. Actin filaments and microtubules generate forces necessary for cell division. Mechanosensitive proteins translate mechanical cues into biochemical responses, contributing to proper cytokinesis.
Feedback Loops: Regulatory networks often involve feedback loops where the output of a process influences the input. Positive feedback amplifies signals, while negative feedback regulates and maintains equilibrium. These loops contribute to the precision of cytokinesis regulation.
Chromatin Remodeling Complexes: These complexes modify chromatin structure to regulate gene accessibility. They communicate instructions for opening or compacting chromatin domains, affecting the expression of cytokinesis-related genes.
Together, these regulatory codes and languages form a complex communication network that ensures proper maintenance and operation of cytokinesis. They enable cells to respond to external cues, internal signals, and developmental contexts to orchestrate the precise execution of cell division.
How did the machinery for cytokinesis supposedly evolve to support cell proliferation and tissue growth?
The supposed evolution of the machinery for cytokinesis would have been a complex process that likely would have involved a series of incremental changes over millions of years. Cytokinesis is crucial for cell proliferation and tissue growth, and its machinery would have evolved to ensure accurate and efficient division of cells. While the exact details of its evolution remain speculative, here is a general overview of how the machinery for cytokinesis would have evolved:
Ancestral Mechanisms: The simplest forms of cytokinesis would have involved mechanical processes such as pinching, cleaving, or budding to physically separate daughter cells. These mechanisms would have been driven by the contraction of filaments, actin-myosin interactions, or other basic cellular structures.
Emergence of Cytoskeletal Elements: As eukaryotic cells supposedly evolved, the emergence of more complex cytoskeletal elements, including actin filaments and microtubules, would have provided the basis for more sophisticated mechanisms of cytokinesis. These elements would have allowed cells to generate the forces needed to drive the separation of daughter cells.
Divisome Formation: In more advanced organisms, the evolution of specific protein complexes, known as divisomes, would have emerged to coordinate cytokinesis. Divisomes are composed of proteins that interact with cytoskeletal elements and help guide the process of cell division.
Cell Plate Formation (Plant Cells): In plant cells, the evolution of the cell plate mechanism would have allowed for cytokinesis. During this process, vesicles carrying cell wall components would have accumulated at the cell equator and fuse, creating a new cell wall between daughter cells. This mechanism would have been adapted to support the rigid cell walls of plants.
Actomyosin Contraction (Animal Cells): In animal cells, actomyosin contraction would have become a central mechanism for cytokinesis. The assembly of an actin ring, combined with the action of myosin motor proteins, constricts the cell membrane, leading to the formation of a cleavage furrow and eventual separation of daughter cells.
Microtubule-Based Mechanisms (Fungi and Animal Cells): In some organisms, microtubules play a more prominent role in cytokinesis. For instance, some fungi use a process known as "centrally located spindle pole bodies" to segregate chromosomes and guide cytokinesis.
Evolution of Regulatory Pathways: Over time, the machinery for cytokinesis would have become integrated into broader regulatory networks that govern the cell cycle, DNA replication, and cell growth. This integration would have allowed cells to coordinate cytokinesis with other cellular processes, ensuring proper cell division and tissue growth.
Increased Complexity and Specialization: As multicellularity would have evolved, the machinery for cytokinesis would have adapted to support the growth of complex tissues and organs. Specialized cell types and tissue structures would have developed, requiring precise control of cell division to maintain tissue integrity and function.
Fine-Tuning and Optimization: Throughout evolution, the machinery for cytokinesis would have undergone numerous refinements and optimizations to ensure accurate and efficient division. Genetic mutations and natural selection would have had to play roles in shaping the machinery to fit specific cellular and organismal requirements.
It's important to note that our understanding of the evolutionary history of cytokinesis is based on current scientific knowledge and hypotheses. The exact sequence of events and the mechanisms involved are subject to ongoing research and investigation.
Is there scientific evidence supporting the idea that cytokinesis was brought about by the process of evolution?
The step-by-step evolution of the complex machinery for cytokinesis faces significant challenges due to the intricate interdependence and functional requirements of its various components. The complexity of the processes involved, the need for precise coordination of multiple systems, and the essential nature of each component suggest that an evolutionary progression is extremely unlikely. Here's why:
Functional Interdependence: Cytokinesis requires the precise orchestration of various components, including cytoskeletal elements, regulatory codes, signaling pathways, and protein machinery. These components are interdependent, meaning that one cannot function without the other. For instance, the actin-myosin contractile ring depends on regulatory signals from upstream pathways, as well as the structural support provided by microtubules and other cytoskeletal elements. In an evolutionary scenario, the absence of any one of these components would render the system non-functional, making it implausible that they could have evolved gradually.
Irreducible Complexity: Cytokinesis is often considered an example of irreducible complexity, where the removal of any essential part leads to a loss of function. This concept suggests that the entire system must have been created all at once, fully operational, in order to be functional. Intermediate stages with partially developed components would not confer any advantage and would not be subject to natural selection, thus hindering their evolution.
Simultaneous Instantiation: The intricate regulatory codes, languages, and signaling pathways required for cytokinesis need to be operational from the outset. The genetic information, epigenetic marks, and protein interactions must exist in a coordinated manner for cytokinesis to function. A stepwise evolution would involve stages where these elements would have no functional purpose, and therefore, would not be selected for. This challenges the notion that these complex systems could evolve incrementally over time.
Cellular Integrity and Survival: Incomplete or malfunctioning cytokinesis can have dire consequences for the cell's survival and overall health. Evolutionary pressures would not favor the development of intermediate stages that compromise essential processes, as such cells would likely face reduced fitness or even death.
Lack of Fossil Evidence: The fossil record provides no evidence of gradual transitions between non-cytokinetic cells and fully functioning cytokinetic cells. This absence of intermediate forms challenges the hypothesis of stepwise evolution.
Considering the interdependent nature of the components required for cytokinesis, the functional requirements of the process, and the absence of evidence for gradual transitions, the emergence of cytokinesis is best explained by the idea that it was intentionally designed and instantiated with all its intricate features fully operational right from the beginning. The complex machinery and interrelated systems required for cytokinesis reflect the work of an intelligent creator rather than a result of gradual evolutionary processes.
Irreducibility and Interdependence of the systems to instantiate and operate cytokinesis
From the perspective of a proponent of intelligent design, the complexity of creating, developing, and operating cytokinesis is apparent in the interdependence and irreducibility of its manufacturing, signaling, and regulatory codes and languages. These intricate systems are essential for proper cellular function and are deeply intertwined, making an evolutionary stepwise progression highly implausible.
Irreducible Complexity and Interdependence
Manufacturing Codes and Languages: The genetic code encodes the instructions for building the proteins essential for cytokinesis. This code is interdependent with the transcription and translation machinery, without which protein synthesis cannot occur. The genetic information itself relies on epigenetic marks, such as DNA methylation and histone modifications, to control gene expression. These epigenetic marks are regulated by specific enzymes and signaling pathways.
Signaling Pathways and Regulatory Codes: Signaling pathways like the MAPK and PI3K/Akt pathways communicate external cues to the nucleus, influencing gene expression and cell cycle progression. These pathways crosstalk with each other, with feedback loops that fine-tune their activation. Regulatory codes involve transcription factors that bind to DNA, initiating or inhibiting gene expression. These factors are often regulated by post-translational modifications, such as phosphorylation, which are controlled by other signaling pathways.
Crosstalk and Communication: The communication systems within the cell are essential for proper function. Signaling pathways crosstalk to integrate diverse signals and coordinate cellular responses. Regulatory codes and transcription factors communicate with one another to orchestrate gene expression. Epigenetic marks affect chromatin structure and accessibility, influencing the binding of transcription factors. This intricate interplay ensures that the cell can respond to its environment and maintain homeostasis.
Interdependence Evolution Challenge
The interdependence of these systems presents a significant challenge to the gradual evolution of cytokinesis. In an evolutionary scenario, each component would need to develop incrementally, waiting for the others to catch up in a stepwise fashion. However, many of these components would not have any selective advantage without the presence and functionality of others.
For instance: Transcription factors would have no function without the genetic code and functional signaling pathways that activate them.
Epigenetic marks would be meaningless without the machinery to interpret and respond to these marks.
Signaling pathways would be ineffective without genes to regulate, and transcription factors to activate.
Additionally, intermediate stages of these systems would likely be non-functional and possibly even detrimental to cell survival, as they would disrupt critical processes. This challenges the idea of natural selection favoring gradual evolutionary transitions.
Given the irreducibility and interdependence of these codes, languages, and systems, proponents of intelligent design argue that the complexity of cytokinesis is best explained by the notion that these systems were intentionally designed and instantiated all at once, fully operational, from scratch. This viewpoint suggests that an intelligent creator orchestrated the intricate interplay of these elements to ensure the proper functioning of cytokinesis and cellular life as a whole.
Once is instantiated and operational, what other intra and extracellular systems is it interdependent with?
Once cytokinesis is instantiated and operational, it becomes interdependent with a multitude of intra and extracellular systems. These interdependencies ensure proper cell division, growth, and overall cellular health. Here are some of the key systems with which cytokinesis is interdependent:
Cell Cycle Control: Cytokinesis is tightly coordinated with the cell cycle. The progression of cell cycle phases, including DNA replication and mitosis, is interdependent with cytokinesis. The cell cycle machinery, including cyclins, cyclin-dependent kinases (CDKs), and checkpoints, ensures that cytokinesis occurs at the appropriate time and in the correct sequence.
DNA Replication and Repair: DNA replication must be completed before cytokinesis, as dividing cells need accurate copies of their genomes. Additionally, DNA repair mechanisms ensure the integrity of genetic material, and their interplay with cytokinesis prevents the propagation of damaged DNA to daughter cells.
Cell Signaling Networks: Cell signaling pathways, including those involved in growth factors, stress responses, and developmental cues, influence cytokinesis. Signals from the extracellular environment impact intracellular processes, affecting the timing and efficiency of cytokinesis.
Metabolism and Energy Production: Proper metabolism is essential for providing the energy and resources necessary for cell division, including cytokinesis. Metabolic pathways, such as glycolysis and oxidative phosphorylation, supply the ATP required for cytoskeletal rearrangements and membrane dynamics during cytokinesis.
Cytoskeletal Dynamics: The cytoskeleton, composed of microtubules and actin filaments, plays a critical role in cytokinesis. The dynamic rearrangements of these filaments during cytokinesis are essential for generating forces that drive cell division.
Cell Adhesion and ECM Interaction: Interaction with the extracellular matrix (ECM) and neighboring cells influences cytokinesis. Cell adhesion molecules and integrins play a role in anchoring cells during division and coordinating cellular responses.
Cell Polarity and Morphogenesis: Proper cell polarity and morphogenesis are essential for guiding cytokinesis. Signaling pathways and cellular structures that control cell shape and polarity influence where and how cytokinesis occurs.
Immune and Inflammatory Responses: Immune responses and inflammation can impact cytokinesis. Cytokines released during immune responses can affect cell cycle progression and cytokinesis in neighboring cells.
Nutrient Availability and Growth Factors: Adequate nutrient availability and growth factor signaling support cell division and cytokinesis. Nutrient deficiencies or growth factor imbalances can hinder proper cell division.
Cell Differentiation and Development: In multicellular organisms, the differentiation and development of specialized cell types can impact cytokinesis. Differentiated cells might have altered cytokinesis patterns or requirements.
These interdependencies illustrate the interconnectedness of cellular processes and systems. Cytokinesis relies on the proper functioning of multiple pathways and mechanisms to ensure successful cell division and maintain cellular health. The complexity of these interrelationships raises questions about how such intricate coordination could have emerged through gradual stepwise evolution, leading proponents of intelligent design to argue for an intentionally designed and integrated cellular system.
Premise 1: Cytokinesis involves an intricate interplay of various intra and extracellular systems, including manufacturing codes, signaling networks, and regulatory languages.
Premise 2: The functionality of these systems is deeply interdependent, such that each component relies on the presence and proper function of others to achieve successful cell division.
Premise 3: The components involved in cytokinesis, including genetic codes, epigenetic marks, transcription factors, and signaling pathways, are semiotic in nature, representing information and communication.
Conclusion: The simultaneous emergence and interlocking of these complex, interdependent systems, each with its own specific language and code, suggests a designed setup. The intricate coordination required for cytokinesis to function seamlessly points to the need for an intentional and integrated design from the outset, rather than a gradual stepwise evolution, since isolated components without their interdependent counterparts would bear no function.
Cytokinesis is the final stage of cell division, following mitosis or meiosis, where a single eukaryotic cell divides into two daughter cells. It ensures the distribution of cellular contents, including organelles and genetic material, into the daughter cells. Cytokinesis involves a series of coordinated processes that lead to the physical separation of the two daughter cells, each containing a nucleus and necessary organelles.
Process of Cytokinesis
Initiation: Cytokinesis begins during late stages of mitosis or meiosis when the cellular components are distributed within two distinct nuclei within the same cell.
Contractile Ring Formation: In animal cells, a contractile ring composed of actin and myosin filaments forms just beneath the cell membrane at the equatorial plane. This ring contracts, causing the membrane to pinch inwards.
Cleavage Furrow Formation: The contracting ring creates a cleavage furrow, which deepens as the contractile ring contracts further.
Cell Membrane Ingrowth: As the cleavage furrow deepens, the cell membrane is progressively drawn inwards, dividing the cytoplasm into two separate compartments.
Daughter Cell Separation: Once the cleavage furrow reaches its maximum depth, the two daughter cells are physically separated, each with its own nucleus and cellular contents.
Importance in Biological Systems
Cytokinesis is crucial for maintaining organismal health and growth. It ensures the even distribution of cellular components, including genetic material and organelles, between the two daughter cells. Accurate cytokinesis is essential to prevent aneuploidy (imbalanced chromosome number) and maintain genetic stability in the organism's tissues.
Developmental Processes Shaping Organismal Form and Function
Cytokinesis plays a pivotal role in the development of multicellular organisms by influencing various aspects of their form and function:
Tissue Formation: During development, cells undergo numerous rounds of division and cytokinesis, which collectively contribute to the formation of tissues and organs with specific shapes and functions.
Organ Growth: Cytokinesis allows tissues to grow in size and complexity. Controlled cell division and cytokinesis are essential for generating and maintaining the appropriate size and proportion of organs.
Cell Differentiation: Differentiation of cells into specialized cell types is influenced by cytokinesis. It ensures that the right number of specialized cells is generated in the right place and at the right time during development.
Pattern Formation: Proper cytokinesis is necessary for generating intricate patterns and structures during embryonic development. It helps shape the organism's body plan and coordinates the positioning of different cell types.
Regeneration and Repair: In tissues that undergo constant renewal, such as the skin and intestinal lining, accurate cytokinesis is essential for proper regeneration and repair after injury.
What are the mechanisms that ensure proper cytokinesis and cell division?
Proper cytokinesis and cell division are ensured through a combination of regulatory mechanisms that coordinate various cellular processes. These mechanisms work together to accurately distribute cellular contents, maintain genetic stability, and prevent errors. Some key mechanisms include:
Checkpoint Control: Checkpoint mechanisms monitor the progress of cell division to ensure accurate DNA replication and chromosome segregation before cytokinesis proceeds. The G1/S, intra-S, and G2/M checkpoints regulate the cell cycle's progression, preventing cell division if errors are detected.
Spindle Assembly Checkpoint (SAC): This checkpoint ensures proper attachment and alignment of chromosomes on the mitotic spindle before cell division proceeds. If chromosomes are not properly positioned, the SAC halts the cell cycle until corrections are made.
Mitotic Spindle Formation: The mitotic spindle, composed of microtubules and associated proteins, is responsible for segregating chromosomes into daughter cells. Accurate spindle formation and function are essential for proper chromosome segregation during cytokinesis.
Cytokinesis Regulatory Proteins: Various proteins are involved in cytokinesis regulation, including those that control contractile ring formation, cell membrane ingrowth, and furrow stabilization. For example, the Rho family of GTPases and associated effectors play a crucial role in actin filament assembly for contractile ring formation.
Cytokinesis Checkpoint: A checkpoint mechanism ensures that cytokinesis is delayed until mitosis is completed. This prevents premature cell division before chromosome segregation is finished.
Anaphase-Promoting Complex (APC/C): APC/C is a protein complex that controls the degradation of specific cell cycle regulators, allowing for the timely progression through different phases of cell division.
Cyclin-Dependent Kinases (CDKs): CDKs are protein kinases that regulate cell cycle progression by phosphorylating target proteins. CDK activity is tightly controlled by cyclins, which activate CDKs at specific points in the cell cycle.
Checkpoint Kinases: Checkpoint kinases, such as ATM and ATR, detect DNA damage or replication stress and transmit signals to halt the cell cycle, providing time for repair processes before division proceeds.
DNA Damage Response: If DNA damage is detected, the cell cycle can be arrested to allow for DNA repair before cell division. If the damage is irreparable, apoptosis (programmed cell death) may be initiated to prevent the propagation of damaged DNA.
Centrosome Duplication and Function: Centrosomes organize microtubules and play a role in spindle formation. Proper centrosome duplication and function are essential for accurate chromosome segregation.
Chromosome Segregation Mechanisms: Protein complexes like the cohesin complex hold sister chromatids together until anaphase. Separase enzyme cleaves cohesin during anaphase, allowing chromatids to separate.
Kinetochore-Microtubule Attachment: Kinetochore proteins attach to microtubules, ensuring correct chromosome alignment on the spindle and facilitating proper chromosome segregation.
These mechanisms collectively contribute to the fidelity of cell division, ensuring that genetic material is accurately distributed to daughter cells and maintaining genomic stability. Dysregulation of these mechanisms can lead to various cellular abnormalities, including aneuploidy, which is associated with several diseases, including cancer.
How do cells coordinate the separation of cytoplasm and organelles during cytokinesis?
Cells coordinate the separation of cytoplasm and organelles during cytokinesis through a combination of cytoskeletal elements, membrane trafficking, and regulatory proteins. The process varies between different types of cells, such as animal cells and plant cells, due to differences in cell structure and mechanisms. Here's an overview of how this coordination is achieved:
Animal Cells
Contractile Ring Formation: In animal cells, a contractile ring composed of actin and myosin filaments forms just beneath the cell membrane at the site of cleavage. The assembly of this contractile ring is a key step in cytokinesis.
Actin-Myosin Contraction: The contractile ring contracts, leading to the constriction of the cell's equator. Myosin motor proteins move along actin filaments, causing them to slide past each other, reducing the diameter of the cell.
Membrane Ingrowth: As the contractile ring contracts, the cell membrane is pulled inward along with the ring. This process creates a cleavage furrow that divides the cytoplasm into two separate portions.
Vesicle Fusion: Membrane-bound vesicles, derived from the Golgi apparatus, fuse with the forming cleavage furrow. These vesicles contribute additional membrane material, which is necessary to accommodate the membrane ingrowth.
Cytokinesis Regulators: Various regulatory proteins, including those from the Rho family of GTPases (such as RhoA), play a role in coordinating actin-myosin contraction and vesicle trafficking during cytokinesis.
Plant Cells
Cell Plate Formation: Plant cells have rigid cell walls that prevent the use of contractile rings. Instead, during cytokinesis, a structure called the cell plate forms at the center of the cell.
Golgi-Derived Vesicles: Vesicles derived from the Golgi apparatus carry cell wall components, such as cellulose and other polysaccharides, to the center of the cell.
Fusion of Vesicles: These vesicles fuse together at the center of the cell, forming the cell plate. The vesicles contribute membrane material and cell wall components to the growing structure.
Cell Wall Synthesis: Enzymes present in the vesicles catalyze the synthesis of new cell wall material, causing the cell plate to expand outward and fuse with the existing cell wall.
Phragmoplast: During this process, a structure called the phragmoplast guides the vesicles to the center of the cell and ensures their proper fusion.
Both in animal and plant cells, the coordination of cytoplasm and organelle separation involves the precise orchestration of cytoskeletal elements, vesicle trafficking, and regulatory proteins. The cell's architecture and specific requirements determine the mechanisms employed. Regardless of the differences, the ultimate goal of cytokinesis is to ensure the formation of two distinct daughter cells, each equipped with the necessary cellular components for independent function.

Cytokinesis in Animal Cells
In animal cells, cytokinesis takes place without the presence of cell walls and occurs from anaphase through telophase. This process unfolds through the following stages:
A contractile ring composed of actin filaments forms beneath the cell membrane, positioning itself around the cell's center.
Actin filaments contract, causing the cleavage furrow to deepen progressively from the cell's periphery towards its center.
The contractile ring contracts further, ultimately resulting in the separation of the two daughter cells. This separation occurs at the midbody, a narrowed region of cytoplasm connecting the two new cells.
As the cleavage furrow meets at the center, the cell membrane becomes completely pinched off, giving rise to two distinct daughter cells enclosed within their individual cell membranes.
This outward-to-inward separation process is referred to as centripetal cytokinesis due to the progression starting outside and moving toward the cell's center.
Cytokinesis in Plant Cells
Plant cells, possessing cell walls, initiate cytokinesis earlier, during interphase, and continue through telophase. The process unfolds in the following manner:
The Golgi apparatus gathers enzymes, structural proteins, and glucose, which later break down into vesicles that disperse throughout the cell.
During telophase, Golgi vesicles migrate towards the metaphase plate, assembling into a structure known as the phragmoplast.
These vesicles then fuse, initiating the formation of a structure termed the cell plate.
The cell plate gradually extends outward, ultimately merging with the existing cell wall. This division process results in the creation of two new daughter cells, each enclosed within its own cell membrane.
The formation of the cell plate and subsequent cell wall is essential in dividing the plant cell.
These distinctive processes highlight the differences between cytokinesis in animal and plant cells. The presence or absence of a cell wall influences the timing and progression of cytokinesis, ultimately leading to the successful division of cells in both types of organisms.
Appearance of cytokinesis in the evolutionary timeline
The evolutionary timeline of cytokinesis is not fully elucidated due to the lack of direct evidence from the distant past. However, scientists have proposed hypotheses based on comparative studies, molecular analysis, and observations of modern organisms. Here's a simplified overview of the hypothesized appearance of cytokinesis in the evolutionary timeline:
Early Single-Celled Organisms: In the earliest stages of life on Earth, simple single-celled organisms, such as bacteria and archaea, would have undergone a form of binary fission to reproduce. While not cytokinesis in the eukaryotic sense, this basic process involved the division of a cell into two daughter cells through growth and splitting.
Emergence of Eukaryotes: With the advent of eukaryotic cells, which are more complex and compartmentalized than prokaryotic cells, the need for a more sophisticated form of cell division arose. The ancestral mechanisms for cytokinesis in eukaryotes are uncertain, but it is supposed that it has involved rudimentary processes like membrane pinching and septum formation.
Evolving Cytoskeletal Elements: Over time, the evolution of cytoskeletal elements such as actin and microtubules would have allowed for more precise cell division in eukaryotic cells. These structures would have been adapted for generating contractile forces and guiding vesicle trafficking.
Formation of Contractile Rings: The development of actin-based contractile rings in animal cells and similar structures in other eukaryotes would have enhanced the accuracy and efficiency of cytokinesis. The emergence of regulatory proteins, like those from the Rho family of GTPases, would have contributed to the coordination of these processes.
Plant Cell Innovations: Plant cells, with their rigid cell walls, would have evolved a distinct mechanism for cytokinesis involving the formation of a cell plate. This innovation allowed for the construction of new cell walls between daughter cells.
Fine-Tuning and Diversification: As multicellularity emerged and organisms became more complex, the mechanisms of cytokinesis would have undergone further refinement and diversification. The evolution of regulatory networks and signaling pathways would have played a role in coordinating cytokinesis within different cell types and tissues.
It's important to note that the evolutionary timeline of cytokinesis is still a subject of ongoing research, and our understanding is based on hypotheses and comparative studies. While some general trends and innovations are proposed, the specific details of how cytokinesis evolved remain a topic of exploration and debate within the scientific community.
De Novo Genetic Information necessary to instantiate cytokinesis
The hypothetical process of generating and introducing new genetic information to create the mechanisms of cytokinesis, starting from scratch, would involve the de novo creation of various components necessary for cell division:
Formation of Cytoskeletal Elements: The genetic information required for the creation of cytoskeletal elements such as actin and microtubules would need to originate. These structural proteins play a vital role in generating contractile forces and guiding vesicle trafficking during cytokinesis.
Regulatory Proteins: New genetic information would have to emerge to encode regulatory proteins that coordinate the intricate processes of cytokinesis. These proteins would be responsible for initiating the formation of contractile rings or equivalent structures, and for controlling their activation and contraction.
Membrane Trafficking Machinery: Genetic instructions for the assembly of membrane-bound vesicles, Golgi-derived vesicles, and other components of the cellular trafficking machinery would need to be introduced. These vesicles play a role in contributing membrane material to the cleavage furrow or cell plate during cytokinesis.
Coordination Mechanisms: Information would have to originate to encode mechanisms for precise coordination between various components involved in cytokinesis. This would include signaling pathways and checkpoints that ensure proper progression through each step of cell division.
Cell Wall Components (For Plant Cells): If considering plant cells, new genetic information would be required to generate cell wall components such as cellulose and other polysaccharides. These components contribute to the formation and growth of the cell plate during cytokinesis in plant cells.
Anaphase-Promoting Complex (APC/C): The genetic code for the anaphase-promoting complex, which controls the degradation of specific cell cycle regulators, would need to originate. This complex is crucial for the timely progression through different phases of cell division.
Checkpoint Control Systems: Genetic information for checkpoint control systems that monitor the progress of cell division would have to be introduced. These systems ensure accurate DNA replication and chromosome segregation before cytokinesis proceeds.
In this hypothetical scenario, the new genetic information would need to be introduced in a coordinated and precise sequence to ensure the proper assembly and functioning of the components involved in cytokinesis. The complexity of this process underscores the challenge of generating the intricate mechanisms of cell division de novo, and it emphasizes the interdependence of various genetic codes and regulatory networks for the successful execution of cytokinesis.
Manufacturing codes and languages that would have to emerge and be employed to instantiate cytokinesis
The transition from an organism without cytokinesis to one with a fully developed cytokinesis would require the creation and instantiation of a complex array of manufacturing codes and languages beyond genetic information. These mechanisms would ensure the assembly, coordination, and operation of cellular components essential for cytokinesis:
Cytoskeletal Protein Production: Manufacturing codes and pathways for producing cytoskeletal proteins like actin and microtubules would be essential. These proteins contribute to the formation of contractile rings, spindle fibers, and other structural elements involved in cytokinesis.
Vesicle Formation and Trafficking: A coherent system for the generation of vesicles from cellular organelles, particularly the Golgi apparatus, would need to be instantiated. These vesicles play a vital role in providing membrane material required for cleavage furrow or cell plate formation.
Membrane Fusion Proteins: Codes for membrane fusion proteins and their associated regulatory factors would be required. These proteins facilitate the fusion of vesicles with the plasma membrane or cell plate, enabling the incorporation of new membrane material during cytokinesis.
Actin-Myosin Interactions: Elaborate instructions for the assembly and interactions of actin and myosin filaments would need to be established. These interactions generate the contractile forces responsible for cell membrane ingrowth during cytokinesis.
Regulatory Proteins and Signaling Pathways: Codes for regulatory proteins such as the Rho family of GTPases and other signaling molecules would be essential. These proteins coordinate various steps of cytokinesis, ensuring proper timing and localization of key events.
Spindle Formation and Centrosome Organization: Codes for proteins involved in spindle formation, centrosome duplication, and centrosome positioning would have to be created. These proteins help orchestrate the alignment and separation of chromosomes during cytokinesis.
Cell Plate Formation (For Plant Cells): For plant cells, manufacturing codes for enzymes and components responsible for synthesizing cell wall materials like cellulose would be required. These materials contribute to the construction and expansion of the cell plate.
Motor Protein Assembly: Codes for motor proteins like myosins and kinesins would be necessary. These proteins facilitate the movement of organelles, vesicles, and other cellular components required for cytokinesis.
In this scenario, the creation and proper coordination of manufacturing codes and languages beyond genetic information are indispensable. These codes would govern the production, transport, assembly, and interaction of various cellular components, ensuring the successful execution of cytokinesis. The complexity of orchestrating these processes from scratch underscores the intricate interplay of multiple systems required for the development of a fully functional cytokinesis mechanism.
Epigenetic Regulatory Mechanisms necessary to be instantiated for cytokinesis
Epigenetic regulation plays a crucial role in the development of various cellular processes, including cytokinesis. Cytokinesis is the process by which a single eukaryotic cell divides into two daughter cells.
Epigenetic Regulation
DNA Methylation: Epigenetic marks involving the addition of methyl groups to DNA molecules can influence gene expression during cytokinesis. Methylation patterns can affect the accessibility of genes required for cell division.
Histone Modifications: Post-translational modifications of histone proteins, such as acetylation, methylation, and phosphorylation, impact chromatin structure and gene expression during cytokinesis. Histone modifications can alter the compaction of DNA, making certain genes more or less accessible.
Non-Coding RNAs: Small non-coding RNAs, like microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), are involved in post-transcriptional regulation of gene expression during cytokinesis. They can fine-tune the levels of specific mRNAs and proteins required for the process.
Systems Employed to Instantiate Regulation
DNA Methylation Machinery: Enzymes like DNA methyltransferases are responsible for adding methyl groups to specific cytosine residues in DNA, affecting gene expression patterns during cytokinesis.
Histone Modification Complexes: Complexes involving histone acetyltransferases (HATs), histone methyltransferases (HMTs), and histone deacetylases (HDACs) modify histone proteins, influencing chromatin structure and accessibility of genes required for cytokinesis.
RNA Interference Machinery: Enzymes like Dicer and Argonaute are involved in processing and incorporating non-coding RNAs, such as miRNAs, into the RNA-induced silencing complex (RISC), which then targets specific mRNA molecules for degradation or translational repression.
Collaborative Systems for Balanced Operation
Cell Cycle Control: The cell cycle machinery, involving cyclins and cyclin-dependent kinases (CDKs), ensures the orderly progression of cells through different phases of the cell cycle, including cytokinesis. Proper regulation of the cell cycle is essential for balanced cell division.
DNA Repair Mechanisms: DNA damage response pathways monitor and repair DNA lesions that might arise during the intense DNA replication and chromosomal segregation required for cytokinesis. Maintaining genome integrity is critical for successful cell division.
Chromatin Remodeling Complexes: These complexes ensure that DNA remains accessible for transcription by regulating chromatin compaction. They play a role in ensuring that genes involved in cytokinesis are appropriately expressed.
Cell Signaling Pathways: Signaling pathways such as the mitogen-activated protein kinase (MAPK) pathway and the phosphoinositide 3-kinase (PI3K) pathway communicate extracellular signals to the nucleus, affecting gene expression and cellular processes, including cytokinesis.
These systems, in a joint venture, collaborate to maintain the balance and proper operation of cytokinesis. Epigenetic regulation and the associated machinery ensure that genes required for cytokinesis are appropriately expressed, while collaborating systems maintain cell cycle progression, DNA integrity, and chromatin structure. This intricate coordination is essential for successful cell division and overall cellular health.
Signaling Pathways necessary to create, and maintain cytokinesis
The emergence of cytokinesis involves the orchestration of various signaling pathways that are interconnected, interdependent, and often crosstalk with each other and with other biological systems. Here are some key signaling pathways involved in the process of cytokinesis:
Mitogen-Activated Protein Kinase (MAPK) Pathway: The MAPK pathway is a crucial signaling cascade that responds to extracellular signals, such as growth factors. It regulates cell growth, proliferation, and differentiation. During cytokinesis, MAPK pathway components can influence the expression of genes required for cell division and coordinate cell cycle progression.
Phosphoinositide 3-Kinase (PI3K)/Akt Pathway: This pathway responds to growth factors and promotes cell survival, growth, and metabolism. It intersects with the MAPK pathway to influence gene expression and cell cycle regulation during cytokinesis.
Cyclin-Dependent Kinase (CDK) Signaling: CDKs, in collaboration with their regulatory cyclin partners, govern cell cycle progression. CDKs are central to the transition from one cell cycle phase to another, including cytokinesis. The activation of specific CDK-cyclin complexes drives the cell cycle forward.
Wnt/β-Catenin Pathway: The Wnt pathway plays a role in embryonic development and cell polarity. It can influence cytokinesis by affecting cytoskeletal rearrangements and cell shape changes.
Notch Signaling: Notch signaling is involved in cell fate determination and tissue patterning. It can affect cytokinesis indirectly by influencing cell differentiation and proliferation.
Hedgehog (Hh) Pathway: The Hh pathway regulates tissue development and patterning. It can affect cell division by influencing gene expression and cellular responses to growth signals.
JAK-STAT Pathway: The JAK-STAT pathway transmits signals from cytokines and growth factors. It regulates immune responses and cell growth. In the context of cytokinesis, this pathway might influence cell cycle progression and cell differentiation.
Interconnections, Interdependence, and Crosstalk
These signaling pathways are interconnected and often share components, such as kinases and transcription factors. Crosstalk between pathways enables cells to integrate diverse signals for precise control of cytokinesis and other cellular processes. For instance, the MAPK pathway can activate transcription factors that regulate the expression of genes involved in cell division. These factors can crosstalk with those activated by the PI3K/Akt pathway, influencing gene expression patterns during cytokinesis. CDK-cyclin complexes, essential for cell cycle progression and cytokinesis, can be influenced by signals from various pathways, including MAPK and PI3K/Akt. CDKs also regulate transcription factors that impact gene expression during cytokinesis. The interplay between these pathways and other cellular systems, such as DNA repair mechanisms, chromatin remodeling, and cytoskeletal dynamics, ensures proper coordination of processes required for successful cytokinesis. Crosstalk between signaling pathways extends beyond cytokinesis itself, affecting broader cellular functions, such as cell proliferation, differentiation, and survival. For instance, the Wnt pathway's impact on cell polarity can indirectly influence cytokinesis by shaping cell division orientation.
Regulatory codes necessary for maintenance and operation of cytokinesis
The maintenance and operation of cytokinesis involve intricate regulatory codes and languages that enable precise coordination and execution of this cellular process. These regulatory elements communicate information and instructions within the cell. Here are some key regulatory codes and languages involved:
DNA Sequence and Genetic Code: The genetic code encoded in DNA provides the fundamental instructions for synthesizing proteins required for cytokinesis. Regulatory sequences, such as promoters and enhancers, dictate when and where specific genes are transcribed, ensuring proper expression of cytokinesis-related genes.
Epigenetic Marks: Epigenetic modifications, such as DNA methylation and histone modifications, act as a regulatory code that influences gene expression during cytokinesis. These marks dictate whether genes are accessible for transcription, fine-tuning their expression levels.
Transcription Factors: Transcription factors are proteins that bind to specific DNA sequences and act as molecular switches that turn genes on or off. They form a regulatory language that determines which genes are activated or repressed during cytokinesis.
Non-Coding RNAs: Non-coding RNAs, including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), serve as regulators of gene expression. MiRNAs can target messenger RNAs (mRNAs) for degradation or translational repression, while lncRNAs can interact with chromatin-modifying complexes, affecting gene accessibility.
Protein Signaling Pathways: Signaling pathways involve the transmission of information through protein-protein interactions and post-translational modifications. Ligands, receptors, kinases, and downstream effectors communicate messages that influence gene expression, cell cycle progression, and cytokinesis.
Post-Translational Modifications: Proteins involved in cytokinesis undergo post-translational modifications, such as phosphorylation, acetylation, and ubiquitination. These modifications act as a regulatory language that affects protein activity, stability, localization, and interactions.
Cell Cycle Checkpoints: Checkpoints are control points within the cell cycle that ensure proper progression. Regulatory mechanisms monitor DNA integrity, chromosome segregation, and other factors critical for cytokinesis. These checkpoints communicate whether the cell is ready to proceed or needs to halt division.
Cytoskeletal Dynamics and Mechanics: The cytoskeleton communicates mechanical signals that influence cytokinesis. Actin filaments and microtubules generate forces necessary for cell division. Mechanosensitive proteins translate mechanical cues into biochemical responses, contributing to proper cytokinesis.
Feedback Loops: Regulatory networks often involve feedback loops where the output of a process influences the input. Positive feedback amplifies signals, while negative feedback regulates and maintains equilibrium. These loops contribute to the precision of cytokinesis regulation.
Chromatin Remodeling Complexes: These complexes modify chromatin structure to regulate gene accessibility. They communicate instructions for opening or compacting chromatin domains, affecting the expression of cytokinesis-related genes.
Together, these regulatory codes and languages form a complex communication network that ensures proper maintenance and operation of cytokinesis. They enable cells to respond to external cues, internal signals, and developmental contexts to orchestrate the precise execution of cell division.
How did the machinery for cytokinesis supposedly evolve to support cell proliferation and tissue growth?
The supposed evolution of the machinery for cytokinesis would have been a complex process that likely would have involved a series of incremental changes over millions of years. Cytokinesis is crucial for cell proliferation and tissue growth, and its machinery would have evolved to ensure accurate and efficient division of cells. While the exact details of its evolution remain speculative, here is a general overview of how the machinery for cytokinesis would have evolved:
Ancestral Mechanisms: The simplest forms of cytokinesis would have involved mechanical processes such as pinching, cleaving, or budding to physically separate daughter cells. These mechanisms would have been driven by the contraction of filaments, actin-myosin interactions, or other basic cellular structures.
Emergence of Cytoskeletal Elements: As eukaryotic cells supposedly evolved, the emergence of more complex cytoskeletal elements, including actin filaments and microtubules, would have provided the basis for more sophisticated mechanisms of cytokinesis. These elements would have allowed cells to generate the forces needed to drive the separation of daughter cells.
Divisome Formation: In more advanced organisms, the evolution of specific protein complexes, known as divisomes, would have emerged to coordinate cytokinesis. Divisomes are composed of proteins that interact with cytoskeletal elements and help guide the process of cell division.
Cell Plate Formation (Plant Cells): In plant cells, the evolution of the cell plate mechanism would have allowed for cytokinesis. During this process, vesicles carrying cell wall components would have accumulated at the cell equator and fuse, creating a new cell wall between daughter cells. This mechanism would have been adapted to support the rigid cell walls of plants.
Actomyosin Contraction (Animal Cells): In animal cells, actomyosin contraction would have become a central mechanism for cytokinesis. The assembly of an actin ring, combined with the action of myosin motor proteins, constricts the cell membrane, leading to the formation of a cleavage furrow and eventual separation of daughter cells.
Microtubule-Based Mechanisms (Fungi and Animal Cells): In some organisms, microtubules play a more prominent role in cytokinesis. For instance, some fungi use a process known as "centrally located spindle pole bodies" to segregate chromosomes and guide cytokinesis.
Evolution of Regulatory Pathways: Over time, the machinery for cytokinesis would have become integrated into broader regulatory networks that govern the cell cycle, DNA replication, and cell growth. This integration would have allowed cells to coordinate cytokinesis with other cellular processes, ensuring proper cell division and tissue growth.
Increased Complexity and Specialization: As multicellularity would have evolved, the machinery for cytokinesis would have adapted to support the growth of complex tissues and organs. Specialized cell types and tissue structures would have developed, requiring precise control of cell division to maintain tissue integrity and function.
Fine-Tuning and Optimization: Throughout evolution, the machinery for cytokinesis would have undergone numerous refinements and optimizations to ensure accurate and efficient division. Genetic mutations and natural selection would have had to play roles in shaping the machinery to fit specific cellular and organismal requirements.
It's important to note that our understanding of the evolutionary history of cytokinesis is based on current scientific knowledge and hypotheses. The exact sequence of events and the mechanisms involved are subject to ongoing research and investigation.
Is there scientific evidence supporting the idea that cytokinesis was brought about by the process of evolution?
The step-by-step evolution of the complex machinery for cytokinesis faces significant challenges due to the intricate interdependence and functional requirements of its various components. The complexity of the processes involved, the need for precise coordination of multiple systems, and the essential nature of each component suggest that an evolutionary progression is extremely unlikely. Here's why:
Functional Interdependence: Cytokinesis requires the precise orchestration of various components, including cytoskeletal elements, regulatory codes, signaling pathways, and protein machinery. These components are interdependent, meaning that one cannot function without the other. For instance, the actin-myosin contractile ring depends on regulatory signals from upstream pathways, as well as the structural support provided by microtubules and other cytoskeletal elements. In an evolutionary scenario, the absence of any one of these components would render the system non-functional, making it implausible that they could have evolved gradually.
Irreducible Complexity: Cytokinesis is often considered an example of irreducible complexity, where the removal of any essential part leads to a loss of function. This concept suggests that the entire system must have been created all at once, fully operational, in order to be functional. Intermediate stages with partially developed components would not confer any advantage and would not be subject to natural selection, thus hindering their evolution.
Simultaneous Instantiation: The intricate regulatory codes, languages, and signaling pathways required for cytokinesis need to be operational from the outset. The genetic information, epigenetic marks, and protein interactions must exist in a coordinated manner for cytokinesis to function. A stepwise evolution would involve stages where these elements would have no functional purpose, and therefore, would not be selected for. This challenges the notion that these complex systems could evolve incrementally over time.
Cellular Integrity and Survival: Incomplete or malfunctioning cytokinesis can have dire consequences for the cell's survival and overall health. Evolutionary pressures would not favor the development of intermediate stages that compromise essential processes, as such cells would likely face reduced fitness or even death.
Lack of Fossil Evidence: The fossil record provides no evidence of gradual transitions between non-cytokinetic cells and fully functioning cytokinetic cells. This absence of intermediate forms challenges the hypothesis of stepwise evolution.
Considering the interdependent nature of the components required for cytokinesis, the functional requirements of the process, and the absence of evidence for gradual transitions, the emergence of cytokinesis is best explained by the idea that it was intentionally designed and instantiated with all its intricate features fully operational right from the beginning. The complex machinery and interrelated systems required for cytokinesis reflect the work of an intelligent creator rather than a result of gradual evolutionary processes.
Irreducibility and Interdependence of the systems to instantiate and operate cytokinesis
From the perspective of a proponent of intelligent design, the complexity of creating, developing, and operating cytokinesis is apparent in the interdependence and irreducibility of its manufacturing, signaling, and regulatory codes and languages. These intricate systems are essential for proper cellular function and are deeply intertwined, making an evolutionary stepwise progression highly implausible.
Irreducible Complexity and Interdependence
Manufacturing Codes and Languages: The genetic code encodes the instructions for building the proteins essential for cytokinesis. This code is interdependent with the transcription and translation machinery, without which protein synthesis cannot occur. The genetic information itself relies on epigenetic marks, such as DNA methylation and histone modifications, to control gene expression. These epigenetic marks are regulated by specific enzymes and signaling pathways.
Signaling Pathways and Regulatory Codes: Signaling pathways like the MAPK and PI3K/Akt pathways communicate external cues to the nucleus, influencing gene expression and cell cycle progression. These pathways crosstalk with each other, with feedback loops that fine-tune their activation. Regulatory codes involve transcription factors that bind to DNA, initiating or inhibiting gene expression. These factors are often regulated by post-translational modifications, such as phosphorylation, which are controlled by other signaling pathways.
Crosstalk and Communication: The communication systems within the cell are essential for proper function. Signaling pathways crosstalk to integrate diverse signals and coordinate cellular responses. Regulatory codes and transcription factors communicate with one another to orchestrate gene expression. Epigenetic marks affect chromatin structure and accessibility, influencing the binding of transcription factors. This intricate interplay ensures that the cell can respond to its environment and maintain homeostasis.
Interdependence Evolution Challenge
The interdependence of these systems presents a significant challenge to the gradual evolution of cytokinesis. In an evolutionary scenario, each component would need to develop incrementally, waiting for the others to catch up in a stepwise fashion. However, many of these components would not have any selective advantage without the presence and functionality of others.
For instance: Transcription factors would have no function without the genetic code and functional signaling pathways that activate them.
Epigenetic marks would be meaningless without the machinery to interpret and respond to these marks.
Signaling pathways would be ineffective without genes to regulate, and transcription factors to activate.
Additionally, intermediate stages of these systems would likely be non-functional and possibly even detrimental to cell survival, as they would disrupt critical processes. This challenges the idea of natural selection favoring gradual evolutionary transitions.
Given the irreducibility and interdependence of these codes, languages, and systems, proponents of intelligent design argue that the complexity of cytokinesis is best explained by the notion that these systems were intentionally designed and instantiated all at once, fully operational, from scratch. This viewpoint suggests that an intelligent creator orchestrated the intricate interplay of these elements to ensure the proper functioning of cytokinesis and cellular life as a whole.
Once is instantiated and operational, what other intra and extracellular systems is it interdependent with?
Once cytokinesis is instantiated and operational, it becomes interdependent with a multitude of intra and extracellular systems. These interdependencies ensure proper cell division, growth, and overall cellular health. Here are some of the key systems with which cytokinesis is interdependent:
Cell Cycle Control: Cytokinesis is tightly coordinated with the cell cycle. The progression of cell cycle phases, including DNA replication and mitosis, is interdependent with cytokinesis. The cell cycle machinery, including cyclins, cyclin-dependent kinases (CDKs), and checkpoints, ensures that cytokinesis occurs at the appropriate time and in the correct sequence.
DNA Replication and Repair: DNA replication must be completed before cytokinesis, as dividing cells need accurate copies of their genomes. Additionally, DNA repair mechanisms ensure the integrity of genetic material, and their interplay with cytokinesis prevents the propagation of damaged DNA to daughter cells.
Cell Signaling Networks: Cell signaling pathways, including those involved in growth factors, stress responses, and developmental cues, influence cytokinesis. Signals from the extracellular environment impact intracellular processes, affecting the timing and efficiency of cytokinesis.
Metabolism and Energy Production: Proper metabolism is essential for providing the energy and resources necessary for cell division, including cytokinesis. Metabolic pathways, such as glycolysis and oxidative phosphorylation, supply the ATP required for cytoskeletal rearrangements and membrane dynamics during cytokinesis.
Cytoskeletal Dynamics: The cytoskeleton, composed of microtubules and actin filaments, plays a critical role in cytokinesis. The dynamic rearrangements of these filaments during cytokinesis are essential for generating forces that drive cell division.
Cell Adhesion and ECM Interaction: Interaction with the extracellular matrix (ECM) and neighboring cells influences cytokinesis. Cell adhesion molecules and integrins play a role in anchoring cells during division and coordinating cellular responses.
Cell Polarity and Morphogenesis: Proper cell polarity and morphogenesis are essential for guiding cytokinesis. Signaling pathways and cellular structures that control cell shape and polarity influence where and how cytokinesis occurs.
Immune and Inflammatory Responses: Immune responses and inflammation can impact cytokinesis. Cytokines released during immune responses can affect cell cycle progression and cytokinesis in neighboring cells.
Nutrient Availability and Growth Factors: Adequate nutrient availability and growth factor signaling support cell division and cytokinesis. Nutrient deficiencies or growth factor imbalances can hinder proper cell division.
Cell Differentiation and Development: In multicellular organisms, the differentiation and development of specialized cell types can impact cytokinesis. Differentiated cells might have altered cytokinesis patterns or requirements.
These interdependencies illustrate the interconnectedness of cellular processes and systems. Cytokinesis relies on the proper functioning of multiple pathways and mechanisms to ensure successful cell division and maintain cellular health. The complexity of these interrelationships raises questions about how such intricate coordination could have emerged through gradual stepwise evolution, leading proponents of intelligent design to argue for an intentionally designed and integrated cellular system.
Premise 1: Cytokinesis involves an intricate interplay of various intra and extracellular systems, including manufacturing codes, signaling networks, and regulatory languages.
Premise 2: The functionality of these systems is deeply interdependent, such that each component relies on the presence and proper function of others to achieve successful cell division.
Premise 3: The components involved in cytokinesis, including genetic codes, epigenetic marks, transcription factors, and signaling pathways, are semiotic in nature, representing information and communication.
Conclusion: The simultaneous emergence and interlocking of these complex, interdependent systems, each with its own specific language and code, suggests a designed setup. The intricate coordination required for cytokinesis to function seamlessly points to the need for an intentional and integrated design from the outset, rather than a gradual stepwise evolution, since isolated components without their interdependent counterparts would bear no function.

