The Endoplasmic Reticulum
https://reasonandscience.catsboard.com/t2197-the-endoplasmic-reticulum
All eukaryotic cells have an endoplasmic reticulum (ER). Its membrane typically constitutes more than half of the total membrane of an average animal cell. The ER is organized into a netlike labyrinth of branching tubules and flattened sacs that extends throughout the cytosol. The tubules and sacs interconnect, and their membrane is continuous with the outer nuclear membrane; the compartment that they enclose therefore is also continuous with the space between the inner and outer nuclear membranes. Thus, the ER and nuclear membranes form a continuous sheet enclosing a single internal space, called the ER lumen or the ER cisternal space, which often occupies more than 10% of the total cell volume. The ER has a central role in both lipid and protein biosynthesis, and it also serves as an intracellular Ca2+ store that is used in many cell signaling responses. The ER membrane is the site of production of all the transmembrane proteins and lipids for most of the cell’s organelles, including the ER itself,
That raises the frequent catch22 question : How did ER lipids emerge, how were they synthesized the first time, if the ER is required to make the lipids of the ER itself ? Had the ER not have to arise all at once, fully functional ?
the Golgi apparatus, lysosomes, endosomes, secretory vesicles, and the plasma membrane. The ER membrane is also the site at which most of the lipids for mitochondrial and peroxisomal membranes are made.
How could the endosymbiotic theory be true, if it takes the ER to make the lipids of mitochondria ?
In addition, almost all of the proteins that will be secreted to the cell exterior—plus those destined for the lumen of the ER, Golgi apparatus, or lysosomes—are initially delivered to the ER lumen.
The ER Is Structurally and Functionally Diverse
While the various functions of the ER are essential to every cell, their relative importance varies greatly between individual cell types. To meet different functional demands, distinct regions of the ER become highly specialized. We observe such functional specialization as dramatic changes in ER structure, and different cell types can therefore possess characteristically different types of ER membrane. One of the most remarkable ER specializations is the rough ER. Mammalian cells begin to import most proteins into the ER before complete synthesis of the polypeptide chain—that is, import is a co-translational process (Figure A).

In contrast, the import of proteins into mitochondria, chloroplasts, nuclei, and peroxisomes is a post-translational process (Figure B). In co-translational transport, the ribosome that is synthesizing the protein is attached
directly to the ER membrane, enabling one end of the protein to be translocated into the ER while the rest of the polypeptide chain is being synthesized. These membrane-bound ribosomes coat the surface of the ER, creating regions termed rough endoplasmic reticulum, or rough ER; regions of ER that lack bound ribosomes are called smooth endoplasmic reticulum, or smooth ER

Most cells have scanty regions of smooth ER, and the ER is often partly smooth and partly rough. Areas of smooth ER from which transport vesicles carrying newly synthesized proteins and lipids bud off for transport to the Golgi apparatus are called transitional ER. In certain specialized cells, the smooth ER is abundant and has additional functions. It is prominent, for example, in cells that specialize in lipid metabolism, such as cells that synthesize steroid hormones from cholesterol; the expanded smooth ER accommodates the enzymes that make cholesterol and modify it to form the hormones (see Figure B). The main cell type in the liver, the hepatocyte, also has a substantial amount of smooth ER. It is the principal site of production of lipoprotein particles, which carry lipids via the bloodstream to other parts of the body. The enzymes that synthesize the lipid components of the particles are located in the membrane of the smooth ER, which also contains enzymes that catalyze a series of reactions to detoxify both lipid-soluble drugs and various harmful compounds produced by metabolism. The most extensively studied of these detoxification reactions are carried out by the cytochrome P450 family of enzymes, which catalyze a series of reactions in which water-insoluble drugs or metabolites that would otherwise accumulate to toxic levels in cell membranes are rendered sufficiently water-soluble to leave the cell and be excreted in the urine. Because the rough ER alone cannot house enough of these and other necessary enzymes, a substantial portion of the membrane in a hepatocyte normally consists of smooth ER. Another crucially important function of the ER in most eukaryotic cells is to sequester Ca2+ from the cytosol. The release of Ca2+ into the cytosol from the ER, and its subsequent reuptake, occurs in many rapid responses to extracellular signals. A Ca2+ pump transports Ca2+ from the cytosol into the ER lumen. A high concentration of Ca2+-binding proteins in the ER facilitates Ca2+ storage. In some cell types, and perhaps in most, specific regions of the ER are specialized for Ca2+ storage. Muscle cells have an abundant, modified smooth ER called the sarcoplasmic reticulum. The release and reuptake of Ca2+ by the sarcoplasmic reticulum trigger myofibril contraction and relaxation, respectively, during each round of muscle contraction. To study the functions and biochemistry of the ER, it is necessary to isolate it. This may seem to be a hopeless task because the ER is intricately interleaved with other components of the cytoplasm. Fortunately, when tissues or cells are disrupted by homogenization, the ER breaks into fragments, which reseal to form small (~100–200 nm in diameter) closed vesicles called microsomes. Microsomes are relatively easy to purify. To the biochemist, microsomes represent small authentic versions of the ER, still capable of protein translocation, protein glycosylation, Ca2+ uptake and release, and lipid synthesis. Microsomes derived from rough ER are studded with ribosomes and are called rough microsomes. The ribosomes are always found on the outside surface, so the interior of the microsome is biochemically equivalent to the lumen of the ER. Many vesicles similar in size to rough microsomes, but lacking attached ribosomes, are also found in cell homogenates. Such smooth microsomes are derived in part from smooth portions of the ER and in part from vesiculated fragments of the plasma membrane, Golgi apparatus, endosomes, and mitochondria (the ratio depending on the tissue). Thus, whereas rough microsomes are clearly derived from rough portions of ER, it is not easy to separate smooth microsomes derived from different organelles. The smooth microsomes prepared from liver or muscle cells are an exception. Because of the unusually large quantities of smooth ER or sarcoplasmic reticulum, respectively, most of the smooth microsomes in homogenates of these tissues are derived from the smooth ER or sarcoplasmic reticulum. The ribosomes attached to rough microsomes make them more dense than smooth microsomes. As a result, we can use equilibrium centrifugation to separate the rough and smooth microsomes. Microsomes have been invaluable in elucidating the molecular aspects of ER function.
Signal Sequences Were First Discovered in Proteins Imported into the Rough ER
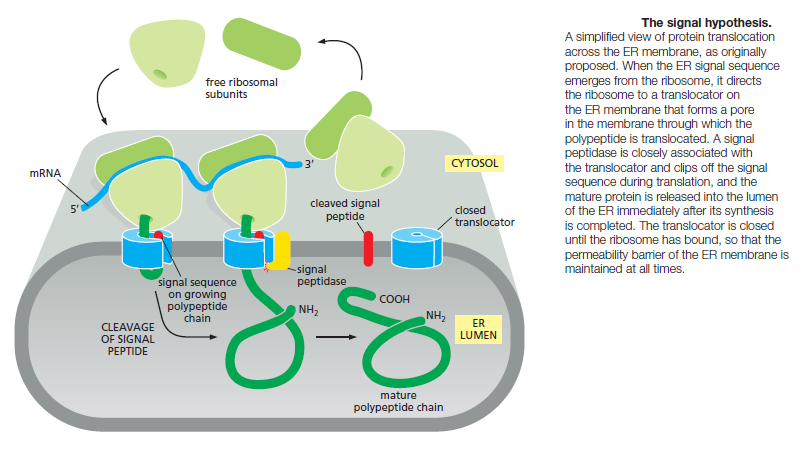
The ER captures selected proteins from the cytosol as they are being synthesized. These proteins are of two types: transmembrane proteins, which are only partly translocated across the ER membrane and become embedded in it, and water-soluble proteins, which are fully translocated across the ER membrane and are released into the ER lumen. Some of the transmembrane proteins function in the ER, but many are destined to reside in the plasma membrane or the membrane of another organelle. The water-soluble proteins are destined either for secretion or for residence in the lumen of the ER or of another organelle. All of these proteins, regardless of their subsequent fate, are directed to the ER membrane by an ER signal sequence, which initiates their translocation by a common mechanism. Signal sequences (and the signal sequence strategy of protein sorting) were first discovered in the early 1970s in secreted proteins that are translocated across the ER membrane as a first step toward their eventual discharge from the cell. In the key experiment, the mRNA encoding a secreted protein was translated by ribosomes in vitro. When microsomes were omitted from this cell-free system, the protein synthesized was slightly larger than the normal secreted protein. In the presence of microsomes derived from the rough ER, however, a protein of the correct size was produced. According to the signal hypothesis, the size difference reflects the initial presence of a signal sequence that directs the secreted protein to the ER membrane and is then cleaved off by a signal peptidase in the ER membrane before the polypeptide chain has been completed


Cell-free systems in which proteins are imported into microsomes have provided powerful procedures for identifying, purifying, and studying the various components of the molecular machinery responsible for the ER import process.
A Signal-Recognition Particle (SRP) Directs the ER Signal Sequence to a Specific Receptor in the Rough ER Membrane
The ER signal sequence is guided to the ER membrane by at least two components: a signal-recognition particle (SRP), which cycles between the ER membrane and the cytosol and binds to the signal sequence, and an SRP receptor in the ER membrane. The SRP is a large complex; in animal cells, it consists of six different polypeptide chains bound to a single small RNA molecule. While the SRP and SRP receptor have fewer subunits in bacteria, homologs are present in all cells.
1. Information is more than the physical coding used to represent it. The sender and receiver must agree in advance on conventions to represent whatever is to be communicated in the future.
2. Information exchange requires that the frame of reference or context be agreed to in advance.
3. Random processes cannot generate coded information; rather, they only reflect the underlying mechanistic and probabilistic properties of the components which created that physical arrangement.
4. Information efficiency may be denser than implied by Shannon’s log2(n) equation, since a common basis of understanding exists between sender and receiver, often allowing implications with various degrees of certainty to be assumed by both parties, in addition to the raw data of the message.
5. In addition to the data encoded in the physical message the intention of the original sender must be considered. An encoding system can be devised to ensure transmission accuracy or to avoid understanding by an unwanted party.
6. A message allows information to survive over time. Assuming that the physical medium is not destroyed, there is some flexibility as to when the receiver can interpret the information.
7. The underlying meaning of coded information is external to the mere nature and properties of the sender.
8. The physical medium upon which a message is encoded is subject to physical laws such as a natural trend towards increased entropy in the long run (and thereby loss of ncoded information which is dependent on a physical medium).
9. Information content of messages is more easily quantified in a comparative than absolute sense.
ER signal sequences vary greatly in amino acid sequence, but each has eight or more nonpolar amino acids at its center. How can the SRP bind specifically to so many different sequences? The answer has come from the crystal structure of the SRP protein, which shows that the signal-sequence-binding site is a large hydrophobic pocket lined by methionines. Because methionines have unbranched, flexible side chains, the pocket is sufficiently plastic to accommodate hydrophobic signal sequences of different sequences, sizes, and shapes. The SRP is a rodlike structure, which wraps around the large ribosomal subunit, with one end binding to the ER signal sequence as it emerges from the ribosome as part of the newly made polypeptide chain; the other end blocks the elongation factor binding site at the interface between the large and small ribosomal subunits



https://reasonandscience.catsboard.com/t2197-the-endoplasmic-reticulum
All eukaryotic cells have an endoplasmic reticulum (ER). Its membrane typically constitutes more than half of the total membrane of an average animal cell. The ER is organized into a netlike labyrinth of branching tubules and flattened sacs that extends throughout the cytosol. The tubules and sacs interconnect, and their membrane is continuous with the outer nuclear membrane; the compartment that they enclose therefore is also continuous with the space between the inner and outer nuclear membranes. Thus, the ER and nuclear membranes form a continuous sheet enclosing a single internal space, called the ER lumen or the ER cisternal space, which often occupies more than 10% of the total cell volume. The ER has a central role in both lipid and protein biosynthesis, and it also serves as an intracellular Ca2+ store that is used in many cell signaling responses. The ER membrane is the site of production of all the transmembrane proteins and lipids for most of the cell’s organelles, including the ER itself,
That raises the frequent catch22 question : How did ER lipids emerge, how were they synthesized the first time, if the ER is required to make the lipids of the ER itself ? Had the ER not have to arise all at once, fully functional ?
the Golgi apparatus, lysosomes, endosomes, secretory vesicles, and the plasma membrane. The ER membrane is also the site at which most of the lipids for mitochondrial and peroxisomal membranes are made.
How could the endosymbiotic theory be true, if it takes the ER to make the lipids of mitochondria ?
In addition, almost all of the proteins that will be secreted to the cell exterior—plus those destined for the lumen of the ER, Golgi apparatus, or lysosomes—are initially delivered to the ER lumen.
The ER Is Structurally and Functionally Diverse
While the various functions of the ER are essential to every cell, their relative importance varies greatly between individual cell types. To meet different functional demands, distinct regions of the ER become highly specialized. We observe such functional specialization as dramatic changes in ER structure, and different cell types can therefore possess characteristically different types of ER membrane. One of the most remarkable ER specializations is the rough ER. Mammalian cells begin to import most proteins into the ER before complete synthesis of the polypeptide chain—that is, import is a co-translational process (Figure A).

In contrast, the import of proteins into mitochondria, chloroplasts, nuclei, and peroxisomes is a post-translational process (Figure B). In co-translational transport, the ribosome that is synthesizing the protein is attached
directly to the ER membrane, enabling one end of the protein to be translocated into the ER while the rest of the polypeptide chain is being synthesized. These membrane-bound ribosomes coat the surface of the ER, creating regions termed rough endoplasmic reticulum, or rough ER; regions of ER that lack bound ribosomes are called smooth endoplasmic reticulum, or smooth ER

Most cells have scanty regions of smooth ER, and the ER is often partly smooth and partly rough. Areas of smooth ER from which transport vesicles carrying newly synthesized proteins and lipids bud off for transport to the Golgi apparatus are called transitional ER. In certain specialized cells, the smooth ER is abundant and has additional functions. It is prominent, for example, in cells that specialize in lipid metabolism, such as cells that synthesize steroid hormones from cholesterol; the expanded smooth ER accommodates the enzymes that make cholesterol and modify it to form the hormones (see Figure B). The main cell type in the liver, the hepatocyte, also has a substantial amount of smooth ER. It is the principal site of production of lipoprotein particles, which carry lipids via the bloodstream to other parts of the body. The enzymes that synthesize the lipid components of the particles are located in the membrane of the smooth ER, which also contains enzymes that catalyze a series of reactions to detoxify both lipid-soluble drugs and various harmful compounds produced by metabolism. The most extensively studied of these detoxification reactions are carried out by the cytochrome P450 family of enzymes, which catalyze a series of reactions in which water-insoluble drugs or metabolites that would otherwise accumulate to toxic levels in cell membranes are rendered sufficiently water-soluble to leave the cell and be excreted in the urine. Because the rough ER alone cannot house enough of these and other necessary enzymes, a substantial portion of the membrane in a hepatocyte normally consists of smooth ER. Another crucially important function of the ER in most eukaryotic cells is to sequester Ca2+ from the cytosol. The release of Ca2+ into the cytosol from the ER, and its subsequent reuptake, occurs in many rapid responses to extracellular signals. A Ca2+ pump transports Ca2+ from the cytosol into the ER lumen. A high concentration of Ca2+-binding proteins in the ER facilitates Ca2+ storage. In some cell types, and perhaps in most, specific regions of the ER are specialized for Ca2+ storage. Muscle cells have an abundant, modified smooth ER called the sarcoplasmic reticulum. The release and reuptake of Ca2+ by the sarcoplasmic reticulum trigger myofibril contraction and relaxation, respectively, during each round of muscle contraction. To study the functions and biochemistry of the ER, it is necessary to isolate it. This may seem to be a hopeless task because the ER is intricately interleaved with other components of the cytoplasm. Fortunately, when tissues or cells are disrupted by homogenization, the ER breaks into fragments, which reseal to form small (~100–200 nm in diameter) closed vesicles called microsomes. Microsomes are relatively easy to purify. To the biochemist, microsomes represent small authentic versions of the ER, still capable of protein translocation, protein glycosylation, Ca2+ uptake and release, and lipid synthesis. Microsomes derived from rough ER are studded with ribosomes and are called rough microsomes. The ribosomes are always found on the outside surface, so the interior of the microsome is biochemically equivalent to the lumen of the ER. Many vesicles similar in size to rough microsomes, but lacking attached ribosomes, are also found in cell homogenates. Such smooth microsomes are derived in part from smooth portions of the ER and in part from vesiculated fragments of the plasma membrane, Golgi apparatus, endosomes, and mitochondria (the ratio depending on the tissue). Thus, whereas rough microsomes are clearly derived from rough portions of ER, it is not easy to separate smooth microsomes derived from different organelles. The smooth microsomes prepared from liver or muscle cells are an exception. Because of the unusually large quantities of smooth ER or sarcoplasmic reticulum, respectively, most of the smooth microsomes in homogenates of these tissues are derived from the smooth ER or sarcoplasmic reticulum. The ribosomes attached to rough microsomes make them more dense than smooth microsomes. As a result, we can use equilibrium centrifugation to separate the rough and smooth microsomes. Microsomes have been invaluable in elucidating the molecular aspects of ER function.
Signal Sequences Were First Discovered in Proteins Imported into the Rough ER
The ER captures selected proteins from the cytosol as they are being synthesized. These proteins are of two types: transmembrane proteins, which are only partly translocated across the ER membrane and become embedded in it, and water-soluble proteins, which are fully translocated across the ER membrane and are released into the ER lumen. Some of the transmembrane proteins function in the ER, but many are destined to reside in the plasma membrane or the membrane of another organelle. The water-soluble proteins are destined either for secretion or for residence in the lumen of the ER or of another organelle. All of these proteins, regardless of their subsequent fate, are directed to the ER membrane by an ER signal sequence, which initiates their translocation by a common mechanism. Signal sequences (and the signal sequence strategy of protein sorting) were first discovered in the early 1970s in secreted proteins that are translocated across the ER membrane as a first step toward their eventual discharge from the cell. In the key experiment, the mRNA encoding a secreted protein was translated by ribosomes in vitro. When microsomes were omitted from this cell-free system, the protein synthesized was slightly larger than the normal secreted protein. In the presence of microsomes derived from the rough ER, however, a protein of the correct size was produced. According to the signal hypothesis, the size difference reflects the initial presence of a signal sequence that directs the secreted protein to the ER membrane and is then cleaved off by a signal peptidase in the ER membrane before the polypeptide chain has been completed



Cell-free systems in which proteins are imported into microsomes have provided powerful procedures for identifying, purifying, and studying the various components of the molecular machinery responsible for the ER import process.
A Signal-Recognition Particle (SRP) Directs the ER Signal Sequence to a Specific Receptor in the Rough ER Membrane
The ER signal sequence is guided to the ER membrane by at least two components: a signal-recognition particle (SRP), which cycles between the ER membrane and the cytosol and binds to the signal sequence, and an SRP receptor in the ER membrane. The SRP is a large complex; in animal cells, it consists of six different polypeptide chains bound to a single small RNA molecule. While the SRP and SRP receptor have fewer subunits in bacteria, homologs are present in all cells.
1. Information is more than the physical coding used to represent it. The sender and receiver must agree in advance on conventions to represent whatever is to be communicated in the future.
2. Information exchange requires that the frame of reference or context be agreed to in advance.
3. Random processes cannot generate coded information; rather, they only reflect the underlying mechanistic and probabilistic properties of the components which created that physical arrangement.
4. Information efficiency may be denser than implied by Shannon’s log2(n) equation, since a common basis of understanding exists between sender and receiver, often allowing implications with various degrees of certainty to be assumed by both parties, in addition to the raw data of the message.
5. In addition to the data encoded in the physical message the intention of the original sender must be considered. An encoding system can be devised to ensure transmission accuracy or to avoid understanding by an unwanted party.
6. A message allows information to survive over time. Assuming that the physical medium is not destroyed, there is some flexibility as to when the receiver can interpret the information.
7. The underlying meaning of coded information is external to the mere nature and properties of the sender.
8. The physical medium upon which a message is encoded is subject to physical laws such as a natural trend towards increased entropy in the long run (and thereby loss of ncoded information which is dependent on a physical medium).
9. Information content of messages is more easily quantified in a comparative than absolute sense.
ER signal sequences vary greatly in amino acid sequence, but each has eight or more nonpolar amino acids at its center. How can the SRP bind specifically to so many different sequences? The answer has come from the crystal structure of the SRP protein, which shows that the signal-sequence-binding site is a large hydrophobic pocket lined by methionines. Because methionines have unbranched, flexible side chains, the pocket is sufficiently plastic to accommodate hydrophobic signal sequences of different sequences, sizes, and shapes. The SRP is a rodlike structure, which wraps around the large ribosomal subunit, with one end binding to the ER signal sequence as it emerges from the ribosome as part of the newly made polypeptide chain; the other end blocks the elongation factor binding site at the interface between the large and small ribosomal subunits




