Science Advances: Deep-Dipping, Grooved Tongue Helps Bats Feast on Nectar Researchers have captured on video what appears to be a new tongue mechanism some bats use to feast on nectar. The researchappears in the 25 September issue of the journal Science Advances.Nectar is a primary food source for many animals but a few, including hummingbirds, honey eaters and sun birds and bats, possess mouthparts specifically designed to slurp up the sweet liquid found in flowers. Specialized nectar-feeding bats typically siphon nectar from flowers using extremely long, protruding tongues sprinkled with hair-like papillae. But some bats sport another type of grooved tongue — one that scientists haven't studied in depth. 1
Marco Tschapka at the University of Ulm and colleagues show that grooved-tongued bats in the group of New World leaf-nosed bats known as the Lonchophyllinae display a unique feeding behavior. Using high-speed cameras focused on bats trained to obtain nectar from artificial "test tube" flowers containing honey water in the lab, the researchers' video captured bats hovering in short flights (rarely lasting a second) over the feeders.Unlike bats with hairy papillae, which moved their tongues in short lapping movements resembling a cat, the grooved-tongued bats lowered their tongues into the test tubes and did not move them during the entire visit. The bats' grooved tongues appear to never separate from the liquid nectar, acting a bit like a conveyor belt to transport the food up the tongue and straight into the animals' mouths.The researchers aren't exactly sure how the bats are able to do this, but suspect it occurs by some combination of tongue deformation and the ability of fluids like nectar to flow without external force in certain narrow spaces, a phenomenon known as capillary action.
THE GROOVE-TONGUED LONCHOPHYLLA ROBUSTA BAT VISITS A BROMELIAD FLOWER. | M. TSCHAPKA/ UNIVERSITY OF ULMBoth methods for obtaining nectar were effective, but the researchers think that the grooved-tongue bats may have an advantage in acquiring nectar from flowers of certain shapes that hold the sweet liquid differently.For example, some flowers have diffusely distributed nectar, while others offer one small pool of nectar. Different nectar extraction mechanisms might be useful depending on the flower. The pumping mechanism of Lonchophyllinae bats might work more efficiently in flowers that allow a more complete submersion of the tongue in the nectar pool, the authors suggest.Another important factor might be nectar viscosity. Nectar from bat-visited flowers is generally dilute, but the sugar concentration in nectar varies between flowers. Nectar with a low sugar concentration that is less viscous and more free-flowing might be more easily harvested by the pumping mechanism compared to nectar of high sugar concentration and viscosity.Nectar uptake in bats using a pumping-tongue mechanism 2Abstract
Many insects use nectar as their principal diet and have mouthparts specialized in nectarivory, whereas most nectar-feeding vertebrates are opportunistic users of floral resources and only a few species show distinct morphological specializations. Specialized nectar-feeding bats extract nectar from flowers using elongated tongues that correspond to two vastly different morphologies: Most species have tongues with hair-like papillae, whereas one group has almost hairless tongues that show distinct lateral grooves. Recent molecular data indicate a convergent evolution of groove- and hair-tongued bat clades into the nectar-feeding niche. Using high-speed video recordings on experimental feeders, we show distinctly divergent nectar-feeding behavior in clades. Grooved tongues are held in contact with nectar for the entire duration of visit as nectar is pumped into the mouths of hovering bats, whereas hairy tongues are used in conventional sinusoidal lapping movements. Bats with grooved tongues use a specific fluid uptake mechanism not known from any other mammal. Nectar rises in semiopen lateral grooves, probably driven by a combination of tongue deformation and capillary action. Extraction efficiency declined for both tongue types with a similar slope toward deeper nectar levels. Our results highlight a novel drinking mechanism in mammals and raise further questions on fluid mechanics and ecological niche partitioning.INTRODUCTION
Nectar is an easily attainable resource because it is openly provided and advertised by flowers in return for pollination services from floral visitors. Its predominant components are various sugars that are used by the visitors as an energy source (
1,
2). Nectar is therefore a highly sought-after food item, primarily by invertebrates, but is also regularly consumed by vertebrates, including a few reptiles, birds, and mammals (
3,
4). Nectarivorous insects regularly consume floral nectar as their principal diet, whereas most nectar-feeding vertebrates are opportunistic users of floral resources. Accordingly and in contrast to many insects that have mouthparts specialized in nectarivory, very few obligate nectar-feeding birds (hummingbirds, honey eaters, and sunbirds) and mammals (the honey possum
Tarsipes rostratus and various species of bats) show corresponding morphological specializations, mainly of the tongue (
4,
5).
Nectar-feeding bats constitute the largest number of specialized nectarivorous mammals and are found in two families: the Old World fruit bats (Pteropodidae) and the New World leaf-nosed bats (Phyllostomidae) in a number of genera traditionally placed in the subfamily Glossophaginae (
6,
7). Floral nectar is generally extracted from flowers by protrusible tongues that may even exceed the body length of bats and are covered with long hair-like papillae (
8,
9). However, some nectar-feeding bat genera present a strikingly different tongue morphology. Here, elongated papillae are almost absent, whereas deep longitudinal grooves run laterally along the entire length of the tongue (fig. S1). On the basis of these “markedly different adaptations for nectarivory,” Griffiths (
10) first proposed a taxonomic separation of these species but offered no explanation as to the function of these morphological structures. After some debate on the validity of this proposal (
11–
13), the recent molecular consensus is that groove-tongued bats form the subfamily Lonchophyllinae, which is a sister group to several predominantly frugivorous subfamilies, and that the subfamily Glossophaginae sensu strictu is the sister group to all of these subfamilies (
14,
15). Considering these phylogenetic relations and the divergent lingual morphology (tongues with hair-like papillae versus grooves), we hypothesized that independently evolved nectarivorous habits should result in distinct differences in nectar-feeding behavior between the two taxa. We predicted that the differences in tongue morphology between glossophagine and lonchophylline bats would translate into drastic differences in tongue movement patterns. Behavioral differences could in turn lead to different nectar uptake and extraction efficiency in the two groups of nectar-feeding phyllostomids. Using a high-speed camera, we compared nectar uptake behavior between species representing both clades (the groove-tongued
Lonchophylla robusta and
Glossophaga soricina, which show a tongue with hair-like papillae) and measured extraction efficiency at different nectar levels.
Tongue movements
All bats visited the feeders in short hovering flights rarely lasting longer than a second. High-speed video recordings revealed distinct differences in tongue movement patterns between the two species. Upon inserting its snout into the feeder opening,
G. soricina initiated repeated sinusoidal movements of the tongue, which alternated between dipping into the nectar and retracting into the mouth (
Fig. 1A and video S1). The amplitude of tongue-tip movements was ca. 25 mm at a distance of 20 mm between the fluid level and the rim of the feeder. These discrete lapping movements were rather stereotypic and were repeated four to seven times per visit.
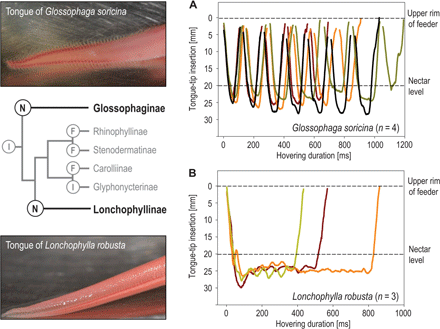 Fig. 1Tongue movement.
Fig. 1Tongue movement.(Left) Extended tongues of drinking: (top) G. soricina (Glossophaginae) and (bottom) L. robusta (Lonchophyllinae). Although the tongue of G. soricina is covered by long filiform papillae, the tongue of L. robusta shows a distinct lateral canal. (Right) Movement patterns of the tongue tips of G. soricina (A) and L. robusta (B) drinking at a feeder offering honey water at 20 mm below the opening. The tongue of L. robusta submerges in the fluid at the beginning of the visit and stays there with only small movements, whereas the tongue of G. soricina extends and retracts repeatedly in stereotypic lapping movements. Interruptions of Glossophaga curves near the upper rim of the feeder represent the total retraction of the tongue into the mouth. (Left, center) Phylogenetic relations between the two groups [modified from (14, 27)] (figs. S1 and S2 and videos S1 to S4).
In contrast, L. robusta lowered its tongue into the fluid at the beginning of a feeding visit and maintained this position during the entire visit without any intermittent retraction into the mouth (Fig. 1B and video S2). The amplitude of the tongue tip was generally small (<5 mm) and was also partly related to the movements of the bat hovering above the feeder. Close-up recordings with the high-speed camera showed that the tongue tip assumed an almost horizontal position just below the nectar surface, with lateral grooves widely open. Although frontal views showed no distinct tongue deformation during drinking, lateral recordings showed peristaltic movements along the edges of the groove and fluid moving along the canal (video S3). The edges of the canal (up to 2 mm deep) are unconnected over the entire length of the tongue and therefore do not form a hermetically closed tube. However, we suggest that muscular action largely closes the edges, supported by a row of elongated triangular papillae that spring from the ventral edge and loosely cover the canal (fig. S2). Feeding was possible once the very tip of the tongue had been submerged in the nectar (video S4). Although the edges of the canal (in proximity to the bat’s mouth) remained in tight contact with each other, it was possible to see fluid rising up into the mouth shortly after the initiation of drinking.
In a quantitative comparison (see Statistical Analysis of Tongue Movement), the species showed significant differences in tongue movement patterns (Mann-Whitney U test: Z = −2.121,N1 = 4, N2 = 3, P = 0.034). Correspondingly, G. soricina individuals retracted their tongues significantly more often than did L. robusta individuals [Mann-Whitney U test: Z = −2.223, N1 = 4, N2 = 3, P = 0.026; median, 6.5 (G. soricina) and 1 (L. robusta)].
Nectar extraction efficiency
Artificial flowers were presented on a precision balance and the amount of nectar extracted after each visit was recorded. Visit duration was timed using light traps connected to a computer. Nectar extraction efficiency was defined as grams of nectar per second of hovering and was standardized on the basis of daily energy expenditure to account for differences in body size. We recorded 354 visits from 8
G. soricina individuals at nectar levels from 10 to 40 mm and 516 visits from 10
L. robusta at nectar levels between 10 and 50 mm.
At all nectar levels, the larger
L. robusta individuals extracted more nectar than did the small
G. soricina individuals (mean ± SE;
L. robusta: 0.11 ± 0.01 g;
G. soricina: 0.06 ± 0.01 g). Uptake steadily decreased in both species when bats had to extend the tongue farther down to lower nectar levels into the feeder (
Fig. 2A). Both species showed a tendency to hover and feed longer at decreasing nectar levels (
Fig. 2B). However, upon reaching the limit of their tongue extension capability, the bats tended to abort an unproductive feeding attempt, and hovering duration decreased. The standardized extraction efficiency declined significantly with decreasing nectar levels [general linear mixed modeling (GLMM);
F4,75.014 = 50.009,
P < 0.0001, Akaike information criterion = −676.452]. Even after correction for size difference,
L. robusta was significantly more efficient than
G. soricina (GLMM;
F1,77.216 = 41.395,
P < 0.0001); however, the observed decline in efficiency toward deeper nectar levels progressed similarly in both species (linear regression;
L. robusta:
y = 0.006 − 0.001
x;
G. soricina:
y = 0.005 − 0.001
x) (
Fig. 2C). The interaction between nectar level and species had no significant influence on extraction efficiency (GLMM;
F3,74.632 = 1.264,
P = 0.293).

- Download high-res image
- Open in new tab
- Download Powerpoint
Fig. 2 Feeding behavior.(A) The amount of nectar extracted after each visit decreases steadily toward deeper levels. (B) Hovering duration in G. soricina and L. robusta increases when bats have to reach deeper into the feeder. The final decline occurs when bats abort their visit upon reaching the limit of their tongue extension capability. (C) Standardized extraction efficiency in both species decreases at a very similar slope. All figures are presented as mean ±1 SE. (D) L. robustavisiting a bromeliad flower (Werauhia sp.). Photo was taken at the Bocas del Toro Field Station of the Smithsonian Tropical Research Institute on March 2009.DISCUSSION
Animals extract nectar from flowers principally using one of three mechanisms: active suction, capillary suction, and viscous dipping (16). The drinking behavior of G. soricina has been investigated previously and consists of stereotypic lapping movements of an elongated tongue, assisted by long papillae (17) that are hemodynamically actively erected and help in effectively mopping up nectar out of a flower (18). This behavior has been classified as viscous dipping, and variations have been found in ants and bees (16). In contrast, the specific and so far undescribed nectar uptake technique of L. robusta transports nectar in deep lateral canals inside the tongue (fig. S1) (10). No lapping movements are observed. Instead, the tongue tip enters the fluid and remains submerged during the entire visit, and nectar is actively pumped into the bat’s mouth. All nectar-feeding mammals studied so far use variations of the brush-tongue lapping technique, whereas grooved tongues seem to be specific for Lonchophylla and some closely related genera (Lionycteris, Platalina, Xeronycteris, and Hsunycteris). Future investigations will probably reveal that all species in this clade share the use of a pumping-tongue drinking mechanism. The pumping tongue’s highly dynamic lingual canal system is not tightly sealed when active, which becomes obvious when the fluid rises and the opening of the canal becomes visibly moist. Feeding through active suction along the entire length of the canal (as found, for example, in moths and butterflies) (16) is therefore not possible because the semiopen canal cannot support the buildup of a necessary pressure difference. Nectar is probably extracted through a combination of active pumping movements of the canals realized through complex bundles of skeletal muscles (10) and capillary forces in small canals. By loosely connecting both sides of the tongue canal, triangular papillae (fig. S2) might additionally help to minimize leakage. As in G. soricina and other glossophagine species (19), the amount of food extracted by L. robusta decreased with lower nectar levels, which was partly compensated for by an increased duration of foraging. Given the fundamental differences between nectar uptake mechanisms, it is remarkable that standardized feeding efficiency decreased in both species at almost the same rate because they could be affected by different parameters. Loss of efficiency in brush-tongued bats at deeper flowers might be mainly attributable to shorter tongue-nectar contact per licking cycle and leakage during tongue retraction. In contrast, the complex muscular arrangement of a grooved tongue at near-maximum extension might progressively lose its degree of mobility, resulting in a decrease in pumping efficiency.
The drastic differences in nectar extraction techniques between L. robusta and G. soricina are striking given their rather close phylogenetic relationship. Apparently, nectarivory has independently evolved twice in a relatively small group of bat species, realizing two totally different methods that functionally only share the enormous elongation of the tongue in common. Because bat-pollinated flowers seem to be at least basically accessible to both convergently developed nectar extraction mechanisms, selection from one bat clade might have indirectly increased the number of floral partners and resource availability in the other clade, thus initially stabilizing the dichotomy.
Species from both clades co-occur in most regions of the neotropics from southern Mexico to Peru, Bolivia, and Brazil (20, 21). This coexistence suggests that nature offers fitting niche options for both. The nectar volume of bat-pollinated flowers ranges from less than 0.05 ml to more than 10 ml in one night (22–24). Some flowers have diffusely distributed nectar, whereas others present one small pool of nectar. The different nectar extraction mechanisms of the two nectar-feeding bat clades might correlate with this variability in nectar volume and distribution in flowers. It is feasible that small and distributed nectar quantities are more efficiently mopped up by the long hair-like papillae of the glossophagine tongue. In contrast, the pumping mechanism of Lonchophyllinae could work more efficiently in flowers that allow a more complete submersion of the tongue in the nectar pool. In fact, the significantly higher standardized extraction efficiency observed in Lonchophylla could be partly attributable to our experimental setup, with copiously available nectar that might have favored the pumping mechanism. Another important parameter, which is hardly studied in bat flowers, might be nectar viscosity. Nectar from bat-visited flowers is generally rather dilute, but sugar concentrations range between 4% and nearly 30% (23). Although this is far from the extremes found in nature, nectar of low sugar concentration and viscosity might be more easily harvested by the pumping mechanism than nectar of high sugar concentration and viscosity (16). The different extraction mechanisms described here might match the nectar presentation of some flowers better than others and thus could ultimately provide possibilities for resource partitioning and coexistence of species (Fig. 2D).
In conclusion, our study reveals a specific and hitherto undescribed nectar pumping system in lonchophylline bats that represents a convergent evolution to the brush-tip tongues of glossophagine bats and provides similar feeding efficiency. This new mammalian drinking mechanism raises both mechanistic and ecological questions. For a full functional understanding of the pumping mechanism, it will be necessary to study fluid dynamics and the interaction between active tongue movements and passive capillary actions. In an evolutionary and ecological context, it might be rewarding to evaluate the nectar presentation patterns of chiropterophilous flowers for suitability for the two different methods of nectar extraction.
MATERIAL AND METHODS
Experimental design for tongue movement
Using high-speed video recordings, we compared the nectar-drinking behavior of the Pallas’ long-tongued bat G. soricina (the most common glossophagine species with a brush-tip tongue) to that of the orange nectar-feeding bat L. robusta (a lonchophylline species with a grooved tongue). Experiments with G. soricina were performed in an experimental chamber (4.8 m by 2.4 m by 2.2 m) between September 2009 and August 2010 using bats from a captive colony at the University of Ulm (Ulm, Germany). L. robusta bats captured temporarily from the wild were tested in a flight tent (4 m by 4 m by 2.5 m) at the Bocas del Toro Field Station of the Smithsonian Tropical Research Institute (Balboa, Panama) in March 2009.
Bats (L. robusta, n = 3; G. soricina, n = 4) were recorded visiting a glass tube (10.3 mm by 5.5 mm by 100 mm) filled with artificial nectar (honey water, 17% w/w sugar concentration) up to 20 mm below the opening. All video recordings were made under infrared light-emitting diode light (Sony HVL-IRM) using a black-and-white high-speed camera (Optronis Camrecord 600x2) with Nikkor 60- or 100-mm macro lenses (Nikon) set to 500 to 750 frames/s for 1/1000 to 1/3003 s of exposure. Tongue-tip insertion into the feeder was tracked on all available video frames using ImageJ software (25).
Statistical analysis of tongue movement
We analyzed tongue movements after full extension during the initial insertion at successive 75-ms intervals, which approximately corresponded to the duration of tongue extraction and retraction of lapping G. soricina. We calculated the slope for each interval using linear regressions and averaged absolute slope values for each individual as a proxy for tongue movement patterns. Subsequently, we performed a Mann-Whitney U test on mean slope values to determine species-specific differences in tongue movements. We additionally counted the number of tongue retractions after the initial insertion for all seven individuals and conducted a Mann-Whitney U test to investigate species-specific differences.
Experimental design for nectar extraction efficiency
G. soricina (8 individuals; mean ± SD body mass, 10.7 ± 0.6 g) and L. robusta (10 individuals; mean ± SD body mass, 15.4 ± 1.7 g) visited test tubes (9 mm in diameter) placed on an analytical balance (precision, 1 mg; Mettler Labstyle 152) and filled with nectar at different distances from the upper rim (10, 20, 30, 40, and 50 mm). Readings before and after a visit provided the mass of consumed nectar. An infrared light beam at the entrance of the test tube allowed us to register bat visits on a personal computer (precision, 10 ms) using a custom-written program (Turbo Pascal 5.0). Time differences between subsequent light beam status changes provided the duration of each hovering visit. We defined nectar extraction efficiency, E(g/s), as the ratio of the benefit of a flower visit to the cost of a flower visit [that is, extracted honey water (g) and hovering duration (s)] following an established protocol (19).
Statistical analysis of nectar extraction efficiency
For a biologically meaningful comparison among differently sized species, we standardized the nectar extraction efficiency
Es by dividing
E by the species-specific daily energy expenditure DEE, which is a function of body mass (
19,
26). We used GLMM (normal distribution, identity link function) to compare the standardized nectar extraction efficiency in both species. Fixed effects included species, honey water level, and species–honey water level; individual bats were included as random effect. All calculations were run in Excel 2007 (Microsoft Corp.). Statistical tests were performed with SPSS version 20.0 (IBM).
1) http://www.aaas.org/news/science-advances-deep-dipping-grooved-tongue-helps-bats-feast-nectar
2) http://advances.sciencemag.org/content/1/8/e1500525.full