The electromagnetic spectrum, fine-tuned for life
https://reasonandscience.catsboard.com/t2033-the-electromagnetic-spectrum-fine-tuned-for-life
In 1801, the English doctor Thomas Young made a famous double-slit experiment, which clearly showed that light diffracted, and therefore must travel in the form of a wave. 9 James Clerk Maxwell developed in a series of papers in 1861 and 1862 a single theory of electricity and magnetism that was able to explain all of the experimental work of Faraday, Ampère and others. But Maxwell’s crowning glory came in 1864 when he published a paper that is undoubtedly one of the greatest achievements in the history of science. Albert Einstein later described Maxwell’s 1860s papers as ‘the most profound and the most fruitful that physics has experienced since the time of Newton.’ Maxwell discovered that by unifying electrical and magnetic phenomena together into a single mathematical theory, a startling prediction emerges. Electricity and magnetism can be unified by introducing two new concepts: electric and magnetic fields.
The idea of a field is central to modern physics; a simple example of something that can be represented by a field is the temperature in a room. If you could measure the temperature at each point in the room and note it down, eventually you would have a vast array of numbers that described how the temperature changes from the door to the windows and from the floor to the ceiling. This array of numbers is called the temperature field. In a similar way, you could introduce the concept of a magnetic field by holding a compass at places around a wire carrying an electric current and noting down how much the needle deflects, and in what direction. The numbers and directions are the magnetic fields. This might seem rather abstract and not much of a simplification, but Maxwell found that by introducing the electric and magnetic fields and placing them centre stage, he was able to write down a single set of equations that described all the known electrical and magnetic phenomena.
At this point, you may be wondering what all this has to do with the story of light. Well, here is something profound that provides a glimpse into the true power and beauty of modern physics. In writing down his laws of electricity and magnetism using fields, Maxwell noticed that by using a bit of simple mathematics, he could rearrange his equations into a more compact and magically revealing form. His new equations took the form of what are known as wave equations. In other words, they had exactly the same form as the equations that describe how soundwaves move through air or how water waves move through the ocean. But waves of what? The waves Maxwell discovered were waves in the electric and magnetic fields themselves. His equations showed that as an electric field changes, it creates a changing magnetic field. But in turn as the magnetic field changes, it creates a changing electric field, which creates a changing magnetic field, and so on. In other words, once you’ve wiggled a few electric charges around to create a changing electric and magnetic field, you can take the charges away and the fields will continue sloshing around – as one falls, the other will rise. And this will continue to happen forever, as long as you do nothing to them. This is profound in itself, but there is an extra, more profound conclusion. Maxwell’s equations also predict exactly how fast these waves must fly away from the electric charges that create them. The speed of the waves is the ratio of the strengths of the electric and magnetic fields – quantities that had been measured by Faraday, Ampère, and others and were well known to Maxwell. When Maxwell did the sums, he must have fallen off his chair. He found that his equations predicted that the waves in the electric and magnetic fields traveled at the speed of light! In other words, Maxwell had discovered that light is nothing more than oscillating electric and magnetic fields, sloshing back and forth and propelling each other through space as they do so. How beautiful that the work of Faraday, Ampère, and others with coils of wire and pieces of magnets could lead to such a profound conclusion through the use of a bit of mathematics and a sprinkling of Scottish genius! In modern language, we would say that light is an electromagnetic wave. 9
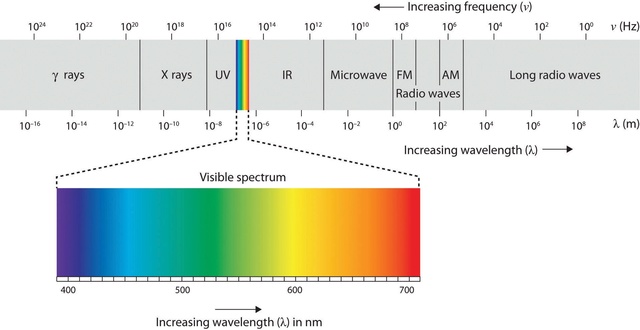
Gamma rays have the shortest wavelengths, < 0.001 nm (about the size of an atomic nucleus). This is the highest frequency and most energetic region of the electromagnetic spectrum. Gamma rays can result from nuclear reactions taking place in objects such as pulsars, quasars, and black holes.
X-rays range in wavelength from 0.001 - 10 nm (about the size of an atom). They are generated, for example, by superheated gas from exploding stars and quasars, where temperatures are near a million to ten million degrees.
Ultraviolet radiation has wavelengths of 10 - 400 nm (about the size of a virus). Young, hot stars produce a lot of ultraviolet light and bathe interstellar space with this energetic light.
Visible light covers the range of wavelengths from 400 - 700 nm (from the size of a molecule to a protozoan). Our sun emits the most of its radiation in the visible range, which our eyes perceive as the colors of the rainbow. Our eyes are sensitive only to this small portion of the electromagnetic spectrum.
Infrared wavelengths span from 700 nm - 1 mm (from the width of a pinpoint to the size of small plant seeds). At a temperature of 37 degrees C, our bodies radiate with a peak intensity near 900 nm.
Radio waves are longer than 1 mm. Since these are the longest waves, they have the lowest energy and are associated with the lowest temperatures. Radio wavelengths are found everywhere: in the background radiation of the universe, in interstellar clouds, and in the cool remnants supernova explosions, to name a few. Radio stations use radio wavelengths of electromagnetic radiation to send signals that our radios then translate into sound. These wavelengths are typically a few feet long in the FM band and up to 300 yards or more in the AM band. Radio stations transmit electromagnetic radiation, not sound. The radio station encodes a pattern on the electromagnetic radiation it transmits, and then our radios receive the electromagnetic radiation, decode the pattern and translate the pattern into sound. 11
The wavelengths of radio waves can vary from about one millimeter to about 100 kilometers, so they cover quite a wide range. 12
electromagnetic waves have been observed with incredibly long wavelengths -- these waves are known as ultra-low frequency (ULF) waves, or micropulsations. 13 the name "ultra-low frequency" is equivalent to "ultra long wavelength" -- although nobody refers to them as such. The range of wavelengths which refer to ULF waves is disputable, and different sources cite different ranges. The consensus seems to be that the wavelength of the longest electromagnetic wave is in the range from 10^6 to 10^11 M.
The frequency distribution of electromagnetic radiation produced by the sun is also critical, as it needs to be tuned to the energies of chemical bonds on earth. If the photons of radiation are too energetic (too much ultraviolet radiation), then chemical bonds are destroyed and molecules are unstable; if the photons are too weak (too much-infrared radiation), then chemical reactions will be too sluggish. The radiation produced is dependent on a careful balancing of the electromagnetic force (alpha-E) and the gravity force (alpha-G), with the mathematical relationship including (alpha-E)12, making the specification for the electromagnetic force particularly critical. On the other hand, the chemical bonding energy comes from quantum mechanical calculations that include the electromagnetic force, the mass of the electron, and Planck's constant. Thus, all of these constants have to be sized relative to each other to give a universe in which radiation is tuned to the necessary chemical reactions that are essential for life.
The Energy in Photons
Photons do not all possess the same amount of energy (figure 10.4). Instead, the energy content of a photon is inversely proportional to the wavelength of the light: short wavelength light contains photons of higher energy than long-wavelength light. X rays, which contain a great deal of energy, have very short wavelengths—much shorter than visible light, making them ideal for high-resolution microscopes. Hertz had noted that the strength of the photoelectric effect depends on the wavelength of light; short wavelengths are much more effective than long ones in producing the photoelectric effect. Einstein’s theory of the photoelectric effect provides an explanation: sunlight contains photons of many different energy levels, only some of which our eyes perceive as visible light. The highest energy photons, at the short-wavelength end of the electromagnetic spectrum (see figure 10.4), are gamma rays, with wavelengths of less than 1 nanometer; the lowest energy photons, with wavelengths of up to thousands of meters, are radio waves. Within the visible portion of the spectrum, violet light has the shortest wavelength and the most energetic photons, and red light has the longest wavelength and the least energetic photons. 15
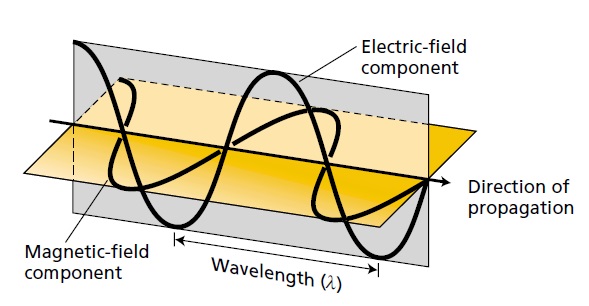
Light is a transverse electromagnetic wave, consisting of oscillating electric and magnetic fields that are perpendicular to each other and to the direction of propagation of the light. Light moves at a speed of 3 × 108 m s–1. The wavelength (l) is the distance between successive crests of the wave.
Light Has Characteristics of Both a Particle and a Wave
In Berlin at the beginning of the twentieth century Max Planck and Albert Einstein, two Nobel Prize winners, carried out the epoch-making studies proving that light has a dual nature. It can be regarded as an electromagnetic wave as well as an emission of particles, which are termed light quanta or photons.
A triumph of physics in the early twentieth century was the realization that light has properties of both particles and waves. A wave is characterized by a wavelength, both electric and magnetic fields oscillate perpendicularly
to the direction of propagation of the wave and at 90° with respect to each other. Light is also a particle, which we call a photon. Each photon contains an amount of energy that is called a quantum (plural quanta). The energy content of light is not continuous but rather is delivered in these discrete packets, the quanta. The energy (E) of a photon depends on the frequency of the light according to a relation known as Planck’s law: E = hn Sunlight is like a rain of photons of different frequencies. Our eyes are sensitive to only a small range of frequencies— the visible-light region of the electromagnetic spectrum.
Another interesting fine tuning coincidence is that the emission spectrum of the sun not only peaks at an energy level which is idea to facilitate chemical reaction but it also peaks in the optical window for water. Water is 107 more opaque to ultraviolet and infrared radiation than it is to radiation in the visible spectra (or what we call light). Since living tissue in general and eyes, in particular, are composed mainly of water, communication by sight would be impossible were it not for this unique window of light transmission by water being ideally matched to the radiation from the sun. Yet this matching requires carefully prescribing the values of the gravity and electromagnetic force constants as well as the Planck's constant and the mass of the electron. 4
The electromagnetic radiation of the sun is restricted to a tiny region of the total electromagnetic spectrum, equivalent to one card in a deck of 10^25, and that the very same infinitely minute region is precisely that required for life. In addition, both the atmospheric gases and water are opaque to all regions of the spectrum except this same tiny region. Denton concludes: "it is as if a card player had drawn precisely the same card on four occasions from a deck of 10^25" 1
That the radiation from the Sun (and from many sequence stars) should be concentrated into a minuscule band of the electromagnetic spectrum which provides precisely the radiation required to maintain life on earth is very remarkable. 2
Nearly all of the Sun's radiation is restricted to a narrow band of wavelengths ranging from 0.3 to 1.50 microns. This band encompasses near ultraviolet, visible, and infrared light. 3
In the whole electromagnetic spectrum, there is just one little band that has the energy to cross this threshold exactly. Its wavelengths range between 0.70 microns and 0.40 microns and if you'd like to see it, you can: just raise your head and look around–it's called "visible light". This radiation causes chemical reactions to take place in your eyes and that is why you are able to see.
Electromagnetic radiation and the light spectrum also depend on the relative strengths of the gravity and electromagnetic forces and their associated constants. Furthermore, the frequency distribution of electromagnetic radiation produced by the sun must be precisely tuned to the energies of the various chemical bonds on Earth. Excessively energetic photons of radiation (i.e., the ultraviolet radiation emitted from a blue giant star) destroy chemical bonds and destabilize organic molecules. Insufficiently energetic photons (e.g., infrared and longer wavelength radiation from a red dwarf star) would result in chemical reactions that are either too sluggish or would not occur at all. All life on Earth depends upon fine-tuned solar radiation, which requires, in turn, a very precise balancing of the electromagnetic and gravitational forces. 5
Leaf color is fine-tuned on the solar spectra to avoid strand direct solar radiation 6
Terrestrial green plants are fine-tuned to spectral dynamics of incident solar radiation and PAR absorption is increased in various structural hierarchies.
The Right Wavelength 3
Recent research indicates that sunlight has magnificent features that inspire amazement. Both light and heat are different manifestations of electromagnetic radiation. In all its manifestations, electromagnetic radiation moves through space in waves similar to those created when a stone is thrown into a lake. And just as the ripples created by the stone may have different heights and the distances between them may vary, electromagnetic radiation also has different wavelengths. The stars and other sources of light in the universe do not all give out the same kind of radiation. Instead, they radiate energy with a broad range of wavelengths. Gamma rays, which have the shortest wavelengths, are just 1 of 10^25 the length of the longest radio waves. Strangely enough, nearly all of the radiation emitted by the Sun falls into a single band that is also 1 of10^25 of the whole spectrum. The reason is that the only kinds of radiation that are necessary and fit for life fall in this narrow band. Some wavelengths are several kilometers long while others are shorter than a billionth of a centimeter and the other wavelengths are to be found in a smooth, unbroken spectrum everywhere in between. To make things easier, scientists divide this spectrum up according to wavelength and they assign different names to different parts of it. The radiation with the shortest wavelength (one-trillionth of a centimeter) for example is called "gamma rays": these rays pack tremendous amounts of energy. The longest wavelengths are called "radio waves": they can be several kilometers long but carry very little energy. (One result of this is that radio waves are quite harmless to us while exposure to gamma rays can be fatal.) Light is a form of electromagnetic radiation that lies between these two extremes. The first thing to be noticed about the electromagnetic spectrum is how broad it is: the longest wavelength is 10^25 times the size of the shortest one. A number that big is pretty meaningless by itself. Let's make a few comparisons.
For example, in 4 billion years (the estimated age of the Earth) there are about 10^17 seconds. If you wanted to count from 1 to 10^25 and did so at the rate of one number a second nonstop, day and night, it would take you 100 million times longer than the age of the earth! If we were to build a pile of 10^25 playing cards, we would end up with a stack stretching halfway across the observable universe.
This is the vast spectrum over which the different wavelengths of the universe's electromagnetic energy extend. Now the curious thing about this is that the electromagnetic energy radiated by our Sun is restricted to a very, very narrow section of this spectrum. 70% of the Sun's radiation has wavelengths between 0.3 and 1.50 microns and within that narrow band, there are three types of light: visible light, near-infrared light, and ultraviolet light. Three kinds of light might seem quite enough but all three combined make up an almost insignificant section of the total spectrum. Remember our 10^25 playing cards extending halfway across the universe? Compared with the total, the width of the band of light radiated by the Sun corresponds to just one of those cards!


Figure 3. The visible portion of the electromagnetic spectrum (~1 micron) is the most intense radiation from the sun (Figure 3.1); has the greatest biological utility (Figure 3.2); and passes through atmosphere of Earth (Figure 3.3) and water (Figure 3.4) with almost no absorption. It is uniquely this same wavelength of radiation that is idea to foster the chemistry of life. This is either a truly amazing series of coincidences or else the result of careful design. 15
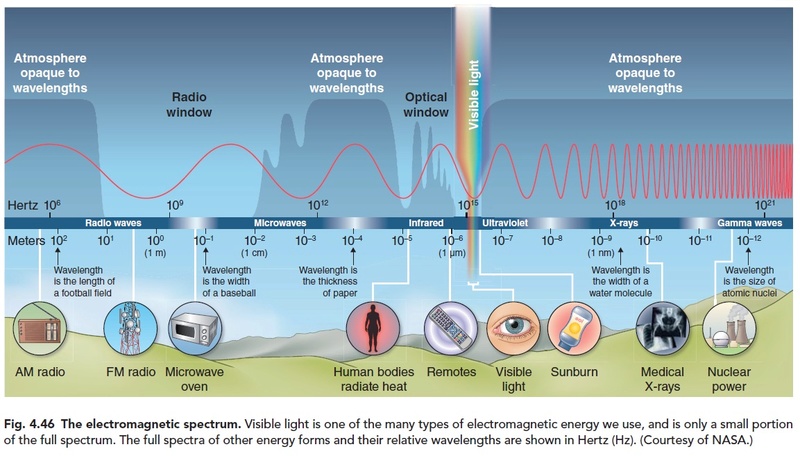
Happily, our star (the sun) emits radiation (light) that is finely tuned to drive the chemical reactions necessary for life. But there is still a critical potential problem: getting that radiation from the sun to the place where the chemical reactions occur. Passing through the near vacuum of space is no problem. However, absorption of light by either Earth's atmosphere or by water where the necessary chemical reactions occur could render life on Earth impossible. It is remarkable that both the Earth's atmosphere and water have "optical windows" that allow visible light (just the radiation necessary for life) to pass through with very little absorption, whereas shorter wavelength (destructive ultraviolet radiation) and longer wavelength (infrared) radiation are both highly absorbed, as seen in Figure 3.{23} This allows solar energy in the form of light to reach the reacting chemicals in the universal solvent, which is water. The Encyclopedia Britannica{24} observes in this regard:
Considering the importance of visible sunlight for all aspects of terrestrial life, one cannot help being awed by the dramatically narrow window in the atmospheric absorption...and in the absorption spectrum of water.
It is remarkable that the optical properties of water and our atmosphere, the chemical bonding energies of the chemicals of life, and the radiation from the sun are all precisely harmonized to allow living systems to utilize the energy from the sun, without which life could not exist. It is quite analogous to your car, which can only run using gasoline as a fuel. Happily, but not accidentally, the service station has an ample supply of exactly the right fuel for your automobile. But someone had to drill for and produce the oil, someone had to refine it into liquid fuel (gasoline) that has been carefully optimized for your internal combustion engine, and others had to truck it to your service station. The production and transportation of the right energy from the sun for the metabolic motors of plants and animals is much more remarkable, and hardly accidental.
Why should sunlight be limited to such a narrow range?
The answer to that question is crucial because the only radiation that is capable of supporting life on earth is the kind that has wavelengths falling within this narrow range.
In Energy and the Atmosphere, the British physicist Ian Campbell addresses this question and says "That the radiation from the Sun (and from many sequence stars) should be concentrated into a minuscule band of the electromagnetic spectrum which provides precisely the radiation required to maintain life on earth is very remarkable." According to Campbell, this situation is "staggering".
Let us now examine these "staggering features of light" more closely.
In the whole electromagnetic spectrum, there is just one little band that has the energy to cross this threshold exactly. Its wavelengths range between 0.70 microns and 0.40 microns and if you'd like to see it, you can: just raise your head and look around–it's called "visible light". This radiation causes chemical reactions to take place in your eyes and that is why you are able to see. "The radiation is known as "visible light" makes up 41% of sunlight even though it occupies less than 1/10^25 of the whole electromagnetic spectrum. In his famous article "Life and Light", which appeared in Scientific American, the renowned physicist George Wald considered this matter and wrote:
"the radiation that is useful in promoting orderly chemical reactions comprises the great bulk of that of our sun." That the Sun should radiate light so exactly right for life is indeed an important example of design.
Is the rest of the light the Sun radiates good for anything?
When we look at this part of the light we see that a large part of solar radiation falling outside the range of visible light is in the section of the spectrum called "near infrared". This begins where visible light ends and again occupies a very small part of the total spectrum–less than 1/10^25 Is infrared light good for anything? Yes, but this time it's no use to look around because you can't see it with the naked eye. However, you can easily feel it: the warmth you feel on your face when you look up on a bright sunny summer or spring day is caused by infrared radiation coming from the Sun. The Sun's infrared radiation is what carries the thermal energy that keeps Earth warm. It too is as essential for life as visible light is. And the fascinating thing is that our Sun was apparently created just to serve for these two purposes because these two kinds of light make up the greatest part of sunlight.
And the third part of sunlight? Is that of any benefit?
You can bet on it. This is "near ultraviolet light" and it makes up the smallest fraction of sunlight. Like all ultraviolet light, it is highly energized and it can cause damage to living cells. The Sun's ultraviolet light, however, is the "least harmful" kind since it is closest to visible light. Although overexposure to solar ultraviolet light has been shown to cause cancer and cellular mutations, it has one vital benefit: the ultraviolet light concentrated in such a minuscule band is needed for the synthesis of vitamin D in humans and other vertebrates. (Vitamin D is necessary for the formation and nourishment of bone: without it, bones become soft or malformed, a disease called rickets that occurs in people deprived of sunlight for great lengths of time.) In other words, all the radiation emitted by the Sun is essential to life: none of it is wasted. The amazing thing is that all this radiation is limited to a 1/10^25 interval of the whole electromagnetic spectrum yet it is sufficient to keep us warm, see, and allow all the chemical reactions necessary for life to take place. Even if all the other conditions necessary for life and mentioned elsewhere in this book existed, if the light radiated by the Sun fell into any other part of the electromagnetic spectrum, there could be no life on Earth. It is certainly impossible to explain the fulfillment of this condition having a probability of 1 in 10^25 with a logic of coincidence. And if all this were not enough, light does something else: it keeps us fed, too!
The energy provided by the Sun has to come in the right amount, shape, and form to be useful to Life on Earth. Life cannot use X-rays or radio waves as an energy source. Visible light is just right the plants use it to make plant matter by photosynthesis, we and many other organisms use it to see by. Likewise, the amount of energy delivered by the Sun to our planet is just right for the hydrologic cycle to work, with water and water vapor changing back and forth, and some minor amount of ice (2 percent of the total water) collecting near the poles. Thus, the climate is between cold and warm, dry and wet just about right.
The electromagnetic spectrum.
The Sun sends light and heat rays (called infrared, IR) and some ultraviolet light (UV). The UV is dangerous to living organisms; it damages eyes, human skin and tree leaves, among other things. Fortunately only a few percent of the energy arrives as UV, the rest is half visible light, half (invisible) IR. In addition, the atmosphere takes out most of the UV before it reaches the ground. The ozone layer in the lower stratosphere (just above the highest clouds) is especially important in protecting living things from UV exposure. 7
Visible light is also incredibly fine-tuned for life to exist. Though visible light is only a tiny fraction of the total electromagnetic spectrum coming from the sun, it happens to be the “most permitted” portion of the sun’s spectrum allowed to filter through our atmosphere. All the other bands of electromagnetic radiation, directly surrounding visible light, happen to be harmful to organic molecules and are almost completely absorbed by the atmosphere. The tiny amount of harmful UV radiation, which is not visible light, allowed to filter through the atmosphere is needed to keep various populations of single cell bacteria from overpopulating the world (Ross; reasons.org). The size of light’s wavelengths and the constraints on the size allowable for the protein molecules of organic life, also seem to be tailor-made for each other. This “tailor-made fit” allows photosynthesis, the miracle of sight, and many other things that are necessary for human life. These specific frequencies of light (that enable plants to manufacture food and astronomers to observe the cosmos) represent less than 1 trillionth of a trillionth (10^-24) of the universe’s entire range of electromagnetic emissions. Like water, visible light also appears to be of optimal biological utility (Denton; Nature’s Destiny).
Transparency of the atmosphere 8
About 60% of the sun’s radiation is in the visible range, only about one trillionth of one trillionth of the entire electromagnetic spectrum. Amazingly, this same range is what the air, the atmosphere, is transparent to. Of course, you may think, air is transparent! Obviously, everyone knows that! But, the amazing thing that everyone does not know, is that air is not transparent to wavelengths on either side of the visible range. A plot of atmospheric opacity versus wavelength has a notch in just the visible range of the spectrum. Opacity is the lack of transparency: the more opaque, the less transparent and vice-versa. This transparency of the atmosphere allows the light to penetrate to the earth’s surface, where photosynthesis can then occur. Also, the water of the earth’s oceans is also transparent— in the same “notch”—in exactly the same range of the electromagnetic spectrum as is the atmosphere. This allows photosynthesis to occur in the oceans, underneath the water where plankton reside. But ultraviolet (UV) light is blocked by a thin layer of water, protecting aquatic life from damage. The UV light from the sun can be damaging. The amount of light of this wavelength reaching earth’s surface is limited by the ozone layer and also by water vapor in the atmosphere. Also, the sun itself does not put out as much UV light as it does visible light. But some UV light does get through, and this is good! UV light is used to produce vitamin D, which is essential for life. Also, the visible range of light provides just the right amount of energy required to stimulate most biochemical reactions (15–65 kilocalories per mole). Wavelengths with less energy would fail to stimulate reactions needed by life, and more energy would damage life.
Vision
As already noted, both water and air are transparent to the same wavelengths, visible light, which is amazingly well-suited for vision. If a shorter wavelength were used for vision, photons would carry additional energy, which would be destructive to biological molecules. Also, this radiation would be much more difficult to focus with a lens because higher-energy waves tend to not be bent (or blocked) by many materials. (This is why X-rays pass through flesh.) On the other hand, if longer waves were used for vision, then we would loose resolution or detail in what we see. Longer wavelengths would be more difficult to detect since they would carry less energy. The energy of a single photon must be amplified many times by the photoreceptors in the eye in order for the weak signal to be useful. This is also evident in man-made devices, such as radios, which make use of longer wavelengths. The signal must be amplified. If any of you have ever used a crystal radio set, you know that there is no amplification, and the sound is very weak. Yet even this weak signal consists of not one, but many, photons. The eye, remember, can detect a single photon, which is a much weaker signal. In order to amplify this weaker signal there would need to be a larger amplification apparatus, which would increase the physical size of the photoreceptor unit. This would change the “pixel size” of an image to a larger size and would reduce the resolution, which means less detail. As it is, the wavelengths of visible light are just right—not too long and not too short!Remember, also, that we have a coincidence of several necessary factors for vision: the transparency of both air and water—the eye contains water—to the same visible light range and the fitness of this visible range for vision. Vision is limited by spherical and chromatic aberration, which increase with the size of the opening receiving light in the eye. To keep this opening small and limit these distortions, we have another problem—diffraction limiting resolution. The larger the wavelength, the larger the diffraction and the less the resolution. Increased resolution, as microscopes and telescopes provide, comes at the cost of a decreased field of view—obviously not desirable for living things. To optimally balance all these factors requires a design like that actually found in the eye of man. The minimum size formed on the retina will be about 2 microns, which cannot be made smaller due to the limitations discussed above. Amazingly, this is actually the size of light detectors found in the eye of man. The size of an eye is limited by the fact that the supply of nutrients to the internal parts of the eye depends upon diffusion, and the supply by diffusion becomes more limited as the distance nutrients must travel increases. On the other hand, an eye too small would suffer from too few photoreceptors and consequent insufficient resolution. Also, due to the physics of light waves, even if photoreceptors could be made smaller than 2 microns, there would be no improvement in resolution. As Denton states: “This is a remarkable coincidence. The two optical limits to the resolving power of the eye—the one set by the waveguide optics…the other set by the laws of classical optics…have the same value: 2 microns.” Another limit on the physical size of photoreceptors to about 2 microns is that photoreceptors require many millions of rhodopsin molecules per cell to increase the probability that a single photon will encounter a rhodopsin molecule and thus be detected—important for low light or night vision. Reducing the cell size to gain more resolution means lowering the number of rhodopsin molecules and thereby reducing the probability of detecting single photons. It seems that the size of photoreceptor cells is just right for vision and that the properties of visible light are just right for that size!
CLEAR VISION 10
Consider the atmosphere’s transparency, which is actually just part of the story. Our atmosphere participates in one of the most extraordinary coincidences known to science: an eerie harmony among the range of wavelengths of light emitted by the Sun, transmitted by Earth’s atmosphere, converted by plants into chemical energy, and detected by the human eye. The human eye perceives light of different wavelengths as different colors, ranging from violet blue (the shortest wavelengths in the visible spectrum) to red (the longest). Looking at a diagram of the electromagnetic spectrum, we see the visible light emerging gracefully from the ultraviolet, differentiating into the familiar colors of the rainbow, and disappearing seamlessly into the warm, invisible infrared. The visible wavelengths rise and fall at the nano-scale, the distances from their peaks measured in ten-billionths of a meter, called Ångstroms (Å). We see blue at approximately 4,800 Å, green at 5,200 Å, yellow at 5,800 Å, and red at 6,600 Å. Earth’s atmosphere is transparent to radiation between 3,100 to 9,500 Å and to the much longer radio wavelengths. The radiation we see is near the middle of that range, between 4,000 and 7,000 Å, the range in which the Sun emits 40 percent of its energy. Its spectrum peaks smack in the middle of this visible spectrum, at 5,500 Å. This is but a tiny sample of the entire range. The near-ultraviolet, visible, and near-infrared spectra— the light most useful to life and sight—are a razor-thin sliver of the universe’s natural, electromagnetic emissions: about one part in 10^25. That is much smaller than one star out of all the stars in the entire visible universe: about 10^22. As it happens, our atmosphere strikes a nearly perfect balance, transmitting most of the radiation that is useful for life while blocking most of the lethal energy. Water vapor in the atmosphere is likewise accommodating, a fact that even the fifteenth edition of the staid Encyclopaedia Britannica picks up on: “Considering the importance of visible sunlight for all aspects of terrestrial life, one cannot help being awed by the dramatically narrow window in the atmospheric absorption . . . and in the absorption spectrum of water.” The oceans transmit an even narrower window of the spectrum, mainly the blues and greens, while halting the other wavelengths near the surface, where they nourish the marine life that figures prominently in Earth’s biosphere. It seems plausible that this is merely an artifact of our eyes having evolved through natural selection to decipher just the spectrum of light that happens to get through the atmosphere. But this fact isn’t so easily dismissed. As George Greenstein notes:
One might think that a certain adaptation has been at work here: the adaptation of plant life to the properties of sunlight. After all, if the Sun were a different temperature could not some other molecule, tuned to absorb light of a different color, take the place of chlorophyll? Remarkably enough the answer is no, for within broad limits all molecules absorb light of similar colors. The absorption of light is accomplished by the excitation of electrons in molecules to higher energy states, and the general scale of energy required to do this is the same no matter what molecule you are discussing. Furthermore, light is composed of photons, packets of energy, and photons of the wrong energy simply cannot be absorbed.
In other words, because of the basic properties of matter, the typical energy involved in chemical reactions corresponds to the typical energy of optical light photons. Otherwise, photosynthetic life wouldn’t be possible. Photons with too much energy would tear molecules apart, while those with too little energy could not trigger chemical reactions. Similar arguments hold for the wavelength range over which the atmosphere is transparent. So stars that don’t emit the right sort of radiation in the right amounts won’t qualify as useful energy sources for life. The radiation a star emits and its peak emission depend on its temperature. Temperatures of normal stars range over a factor of about twenty. The Sun is a little below the mid-range, while exotic stars emit most of their energy on the far ends of the electromagnetic spectrum, as gamma rays, X-rays, or radio waves. A star like our Sun, which produces mostly photons that can energize chemical reactions on Earth, will shine light that comes from just that region in its photosphere where atoms can combine to form stable molecules. So we can’t explain away one part of this coincidence in terms of the others. Life can’t use just any type of light from any type of star. Our Sun, it turns out, is near the optimum for any plausible kind of chemical life. To see the stars, though, a translucent atmosphere that merely allows light to reach the surface won’t cut it. Like a clean, clear pane of glass as compared to a frosted one, we need an atmosphere, like Earth’s, that is transparent in order to see the stars and the wider cosmos. Of course, we enjoy these views because we have the good fortune of living on land. The view would not be nearly as good if we lived underwater.
But even dry land and a transparent atmosphere aren’t enough, since they wouldn’t be much use without the dark nights. Although we take them for granted, dark nights depend on several astronomical variables, some local and some not. A dark sky requires that our planet regularly rotate away from the intense direct light of the Sun. If our day were the same length as our year, Earth would always keep the same face pointed toward the Sun, much as the Moon does Earth. The resulting large temperature difference between the day and night sides would be hostile to complex life. Any complex life, such as there could be, would stay on the day side. Unless the planet enjoyed total solar eclipses, like Lagash, it would never see dark skies. We would suffer similar but less severe problems if we had several moons staring unblinkingly in the night sky like headlights on a busy highway. Our single moon does interfere with astronomers’ ability to observe distant faint objects, but only when it’s out. Moreover, visibility is better now than it was in the distant past, when the Moon was nearly three times closer to Earth, and thus nearly nine times brighter. Of course, a translucent atmosphere might work for merely converting sunlight into chemical energy or hunting prey and avoiding predators. But because we are big-brained, mobile, surface-dwelling creatures, we need a certain type of atmosphere. It just so happens that that very atmosphere— predominantly nitrogen and oxygen with some carbon dioxide and water vapor—is mostly transparent to optical radiation. All four of these chemical constituents are essential for our biosphere. Water does partially hinder our view of the distant universe, but the valuable trade-offs it provides more than compensate for this hindrance.
For example, one particularly impressive consequence of atmospheric water is the rainbow. Rainbows, like total solar eclipses, seem equal parts whimsy and mystery, summoning the artist’s creativity and the naturalist’s curiosity. The early attempts to explain their formation started the first of our many tutorials on the nature of light. The scientists who followed those clues eventually learned how to unweave the white light of the Sun. René Descartes and, later, Isaac Newton, who performed experiments with sunlight and prisms in 1666, groped toward the modern explanation for the rainbow and the modern science of spectroscopy. A rainbow is in effect a natural spectroscope as big as the sky. Once scientists learned how to use a prism to replicate a rainbow, it was only a matter of time before someone scrutinized the solar spectrum. But rainbows won’t appear on just any planet. A good rainbow needs a partially cloudy atmosphere, the golden mean between the uniformly cloudy and uniformly dry. Earth’s water results in skies that, on average, are about 68 percent cloudy. Clouds help balance the global energy by contributing to the global albedo, that fraction of sunlight reflected back into space. Earth currently reflects about 30 percent of the sunlight that strikes it. Four major types of reflecting surfaces contribute to the global albedo: land, ice, oceans, and clouds, each with its own particular reflective properties.
1. http://www.unav.es/cryf/georgedenton.html
2. Ian M. Campbell, Energy and the Atmosphere, London: Wiley, 1977, p. 1-2
3. http://m.harunyahya.com/tr/buku/966/The-Creation-of-the-Universe/chapter/3177/Chapter-VI-The-Signs-of-Creation-in-Light
4. http://www.discovery.org/a/3681
5. https://www.christianforums.com/threads/a-fine-tuned-universe-em-radiation-light-spectrum-gravity.2043325/
6. http://link.springer.com.secure.sci-hub.tw/article/10.1007/s10265-016-0809-0
7. http://earthguide.ucsd.edu/virtualmuseum/ita/07_1.shtml
8. http://www.tasc-creationscience.org/content/amazing-coincidences-life
9. Wonders of the Universe, Professor Brian Cox & Andrew Cohen, page 30
10. Privileged Planet, Gonzalez, page 65
11. http://amazingspace.org/resources/explorations/light/star-light-science.html
12. https://www.enotes.com/homework-help/what-longest-shortest-radiowave-wavelength-325147
13. https://hypertextbook.com/facts/2001/RachelShapiro.shtml
14. Plant Physiology, 3rd ed., Lincoln Taiz and Eduardo Zeiger, page 116
15. http://www.leaderu.com/offices/bradley/docs/scievidence.html
More readings:
https://courses.lumenlearning.com/boundless-chemistry/chapter/the-nature-of-light/
https://docs.google.com/document/d/1LuWUW26yc_as4QUHau4Q83-4VBjWMMAdQgGv513_YuU/edit
https://reasonandscience.catsboard.com/t2033-the-electromagnetic-spectrum-fine-tuned-for-life

In 1801, the English doctor Thomas Young made a famous double-slit experiment, which clearly showed that light diffracted, and therefore must travel in the form of a wave. 9 James Clerk Maxwell developed in a series of papers in 1861 and 1862 a single theory of electricity and magnetism that was able to explain all of the experimental work of Faraday, Ampère and others. But Maxwell’s crowning glory came in 1864 when he published a paper that is undoubtedly one of the greatest achievements in the history of science. Albert Einstein later described Maxwell’s 1860s papers as ‘the most profound and the most fruitful that physics has experienced since the time of Newton.’ Maxwell discovered that by unifying electrical and magnetic phenomena together into a single mathematical theory, a startling prediction emerges. Electricity and magnetism can be unified by introducing two new concepts: electric and magnetic fields.
The idea of a field is central to modern physics; a simple example of something that can be represented by a field is the temperature in a room. If you could measure the temperature at each point in the room and note it down, eventually you would have a vast array of numbers that described how the temperature changes from the door to the windows and from the floor to the ceiling. This array of numbers is called the temperature field. In a similar way, you could introduce the concept of a magnetic field by holding a compass at places around a wire carrying an electric current and noting down how much the needle deflects, and in what direction. The numbers and directions are the magnetic fields. This might seem rather abstract and not much of a simplification, but Maxwell found that by introducing the electric and magnetic fields and placing them centre stage, he was able to write down a single set of equations that described all the known electrical and magnetic phenomena.
At this point, you may be wondering what all this has to do with the story of light. Well, here is something profound that provides a glimpse into the true power and beauty of modern physics. In writing down his laws of electricity and magnetism using fields, Maxwell noticed that by using a bit of simple mathematics, he could rearrange his equations into a more compact and magically revealing form. His new equations took the form of what are known as wave equations. In other words, they had exactly the same form as the equations that describe how soundwaves move through air or how water waves move through the ocean. But waves of what? The waves Maxwell discovered were waves in the electric and magnetic fields themselves. His equations showed that as an electric field changes, it creates a changing magnetic field. But in turn as the magnetic field changes, it creates a changing electric field, which creates a changing magnetic field, and so on. In other words, once you’ve wiggled a few electric charges around to create a changing electric and magnetic field, you can take the charges away and the fields will continue sloshing around – as one falls, the other will rise. And this will continue to happen forever, as long as you do nothing to them. This is profound in itself, but there is an extra, more profound conclusion. Maxwell’s equations also predict exactly how fast these waves must fly away from the electric charges that create them. The speed of the waves is the ratio of the strengths of the electric and magnetic fields – quantities that had been measured by Faraday, Ampère, and others and were well known to Maxwell. When Maxwell did the sums, he must have fallen off his chair. He found that his equations predicted that the waves in the electric and magnetic fields traveled at the speed of light! In other words, Maxwell had discovered that light is nothing more than oscillating electric and magnetic fields, sloshing back and forth and propelling each other through space as they do so. How beautiful that the work of Faraday, Ampère, and others with coils of wire and pieces of magnets could lead to such a profound conclusion through the use of a bit of mathematics and a sprinkling of Scottish genius! In modern language, we would say that light is an electromagnetic wave. 9
Gamma rays have the shortest wavelengths, < 0.001 nm (about the size of an atomic nucleus). This is the highest frequency and most energetic region of the electromagnetic spectrum. Gamma rays can result from nuclear reactions taking place in objects such as pulsars, quasars, and black holes.
X-rays range in wavelength from 0.001 - 10 nm (about the size of an atom). They are generated, for example, by superheated gas from exploding stars and quasars, where temperatures are near a million to ten million degrees.
Ultraviolet radiation has wavelengths of 10 - 400 nm (about the size of a virus). Young, hot stars produce a lot of ultraviolet light and bathe interstellar space with this energetic light.
Visible light covers the range of wavelengths from 400 - 700 nm (from the size of a molecule to a protozoan). Our sun emits the most of its radiation in the visible range, which our eyes perceive as the colors of the rainbow. Our eyes are sensitive only to this small portion of the electromagnetic spectrum.
Infrared wavelengths span from 700 nm - 1 mm (from the width of a pinpoint to the size of small plant seeds). At a temperature of 37 degrees C, our bodies radiate with a peak intensity near 900 nm.
Radio waves are longer than 1 mm. Since these are the longest waves, they have the lowest energy and are associated with the lowest temperatures. Radio wavelengths are found everywhere: in the background radiation of the universe, in interstellar clouds, and in the cool remnants supernova explosions, to name a few. Radio stations use radio wavelengths of electromagnetic radiation to send signals that our radios then translate into sound. These wavelengths are typically a few feet long in the FM band and up to 300 yards or more in the AM band. Radio stations transmit electromagnetic radiation, not sound. The radio station encodes a pattern on the electromagnetic radiation it transmits, and then our radios receive the electromagnetic radiation, decode the pattern and translate the pattern into sound. 11
The wavelengths of radio waves can vary from about one millimeter to about 100 kilometers, so they cover quite a wide range. 12
electromagnetic waves have been observed with incredibly long wavelengths -- these waves are known as ultra-low frequency (ULF) waves, or micropulsations. 13 the name "ultra-low frequency" is equivalent to "ultra long wavelength" -- although nobody refers to them as such. The range of wavelengths which refer to ULF waves is disputable, and different sources cite different ranges. The consensus seems to be that the wavelength of the longest electromagnetic wave is in the range from 10^6 to 10^11 M.
The frequency distribution of electromagnetic radiation produced by the sun is also critical, as it needs to be tuned to the energies of chemical bonds on earth. If the photons of radiation are too energetic (too much ultraviolet radiation), then chemical bonds are destroyed and molecules are unstable; if the photons are too weak (too much-infrared radiation), then chemical reactions will be too sluggish. The radiation produced is dependent on a careful balancing of the electromagnetic force (alpha-E) and the gravity force (alpha-G), with the mathematical relationship including (alpha-E)12, making the specification for the electromagnetic force particularly critical. On the other hand, the chemical bonding energy comes from quantum mechanical calculations that include the electromagnetic force, the mass of the electron, and Planck's constant. Thus, all of these constants have to be sized relative to each other to give a universe in which radiation is tuned to the necessary chemical reactions that are essential for life.
The Energy in Photons
Photons do not all possess the same amount of energy (figure 10.4). Instead, the energy content of a photon is inversely proportional to the wavelength of the light: short wavelength light contains photons of higher energy than long-wavelength light. X rays, which contain a great deal of energy, have very short wavelengths—much shorter than visible light, making them ideal for high-resolution microscopes. Hertz had noted that the strength of the photoelectric effect depends on the wavelength of light; short wavelengths are much more effective than long ones in producing the photoelectric effect. Einstein’s theory of the photoelectric effect provides an explanation: sunlight contains photons of many different energy levels, only some of which our eyes perceive as visible light. The highest energy photons, at the short-wavelength end of the electromagnetic spectrum (see figure 10.4), are gamma rays, with wavelengths of less than 1 nanometer; the lowest energy photons, with wavelengths of up to thousands of meters, are radio waves. Within the visible portion of the spectrum, violet light has the shortest wavelength and the most energetic photons, and red light has the longest wavelength and the least energetic photons. 15

Light is a transverse electromagnetic wave, consisting of oscillating electric and magnetic fields that are perpendicular to each other and to the direction of propagation of the light. Light moves at a speed of 3 × 108 m s–1. The wavelength (l) is the distance between successive crests of the wave.
Light Has Characteristics of Both a Particle and a Wave
In Berlin at the beginning of the twentieth century Max Planck and Albert Einstein, two Nobel Prize winners, carried out the epoch-making studies proving that light has a dual nature. It can be regarded as an electromagnetic wave as well as an emission of particles, which are termed light quanta or photons.
A triumph of physics in the early twentieth century was the realization that light has properties of both particles and waves. A wave is characterized by a wavelength, both electric and magnetic fields oscillate perpendicularly
to the direction of propagation of the wave and at 90° with respect to each other. Light is also a particle, which we call a photon. Each photon contains an amount of energy that is called a quantum (plural quanta). The energy content of light is not continuous but rather is delivered in these discrete packets, the quanta. The energy (E) of a photon depends on the frequency of the light according to a relation known as Planck’s law: E = hn Sunlight is like a rain of photons of different frequencies. Our eyes are sensitive to only a small range of frequencies— the visible-light region of the electromagnetic spectrum.
Another interesting fine tuning coincidence is that the emission spectrum of the sun not only peaks at an energy level which is idea to facilitate chemical reaction but it also peaks in the optical window for water. Water is 107 more opaque to ultraviolet and infrared radiation than it is to radiation in the visible spectra (or what we call light). Since living tissue in general and eyes, in particular, are composed mainly of water, communication by sight would be impossible were it not for this unique window of light transmission by water being ideally matched to the radiation from the sun. Yet this matching requires carefully prescribing the values of the gravity and electromagnetic force constants as well as the Planck's constant and the mass of the electron. 4
The electromagnetic radiation of the sun is restricted to a tiny region of the total electromagnetic spectrum, equivalent to one card in a deck of 10^25, and that the very same infinitely minute region is precisely that required for life. In addition, both the atmospheric gases and water are opaque to all regions of the spectrum except this same tiny region. Denton concludes: "it is as if a card player had drawn precisely the same card on four occasions from a deck of 10^25" 1
That the radiation from the Sun (and from many sequence stars) should be concentrated into a minuscule band of the electromagnetic spectrum which provides precisely the radiation required to maintain life on earth is very remarkable. 2

Nearly all of the Sun's radiation is restricted to a narrow band of wavelengths ranging from 0.3 to 1.50 microns. This band encompasses near ultraviolet, visible, and infrared light. 3
In the whole electromagnetic spectrum, there is just one little band that has the energy to cross this threshold exactly. Its wavelengths range between 0.70 microns and 0.40 microns and if you'd like to see it, you can: just raise your head and look around–it's called "visible light". This radiation causes chemical reactions to take place in your eyes and that is why you are able to see.
Electromagnetic radiation and the light spectrum also depend on the relative strengths of the gravity and electromagnetic forces and their associated constants. Furthermore, the frequency distribution of electromagnetic radiation produced by the sun must be precisely tuned to the energies of the various chemical bonds on Earth. Excessively energetic photons of radiation (i.e., the ultraviolet radiation emitted from a blue giant star) destroy chemical bonds and destabilize organic molecules. Insufficiently energetic photons (e.g., infrared and longer wavelength radiation from a red dwarf star) would result in chemical reactions that are either too sluggish or would not occur at all. All life on Earth depends upon fine-tuned solar radiation, which requires, in turn, a very precise balancing of the electromagnetic and gravitational forces. 5
Leaf color is fine-tuned on the solar spectra to avoid strand direct solar radiation 6
Terrestrial green plants are fine-tuned to spectral dynamics of incident solar radiation and PAR absorption is increased in various structural hierarchies.
The Right Wavelength 3
Recent research indicates that sunlight has magnificent features that inspire amazement. Both light and heat are different manifestations of electromagnetic radiation. In all its manifestations, electromagnetic radiation moves through space in waves similar to those created when a stone is thrown into a lake. And just as the ripples created by the stone may have different heights and the distances between them may vary, electromagnetic radiation also has different wavelengths. The stars and other sources of light in the universe do not all give out the same kind of radiation. Instead, they radiate energy with a broad range of wavelengths. Gamma rays, which have the shortest wavelengths, are just 1 of 10^25 the length of the longest radio waves. Strangely enough, nearly all of the radiation emitted by the Sun falls into a single band that is also 1 of10^25 of the whole spectrum. The reason is that the only kinds of radiation that are necessary and fit for life fall in this narrow band. Some wavelengths are several kilometers long while others are shorter than a billionth of a centimeter and the other wavelengths are to be found in a smooth, unbroken spectrum everywhere in between. To make things easier, scientists divide this spectrum up according to wavelength and they assign different names to different parts of it. The radiation with the shortest wavelength (one-trillionth of a centimeter) for example is called "gamma rays": these rays pack tremendous amounts of energy. The longest wavelengths are called "radio waves": they can be several kilometers long but carry very little energy. (One result of this is that radio waves are quite harmless to us while exposure to gamma rays can be fatal.) Light is a form of electromagnetic radiation that lies between these two extremes. The first thing to be noticed about the electromagnetic spectrum is how broad it is: the longest wavelength is 10^25 times the size of the shortest one. A number that big is pretty meaningless by itself. Let's make a few comparisons.
For example, in 4 billion years (the estimated age of the Earth) there are about 10^17 seconds. If you wanted to count from 1 to 10^25 and did so at the rate of one number a second nonstop, day and night, it would take you 100 million times longer than the age of the earth! If we were to build a pile of 10^25 playing cards, we would end up with a stack stretching halfway across the observable universe.
This is the vast spectrum over which the different wavelengths of the universe's electromagnetic energy extend. Now the curious thing about this is that the electromagnetic energy radiated by our Sun is restricted to a very, very narrow section of this spectrum. 70% of the Sun's radiation has wavelengths between 0.3 and 1.50 microns and within that narrow band, there are three types of light: visible light, near-infrared light, and ultraviolet light. Three kinds of light might seem quite enough but all three combined make up an almost insignificant section of the total spectrum. Remember our 10^25 playing cards extending halfway across the universe? Compared with the total, the width of the band of light radiated by the Sun corresponds to just one of those cards!


Figure 3. The visible portion of the electromagnetic spectrum (~1 micron) is the most intense radiation from the sun (Figure 3.1); has the greatest biological utility (Figure 3.2); and passes through atmosphere of Earth (Figure 3.3) and water (Figure 3.4) with almost no absorption. It is uniquely this same wavelength of radiation that is idea to foster the chemistry of life. This is either a truly amazing series of coincidences or else the result of careful design. 15
Happily, our star (the sun) emits radiation (light) that is finely tuned to drive the chemical reactions necessary for life. But there is still a critical potential problem: getting that radiation from the sun to the place where the chemical reactions occur. Passing through the near vacuum of space is no problem. However, absorption of light by either Earth's atmosphere or by water where the necessary chemical reactions occur could render life on Earth impossible. It is remarkable that both the Earth's atmosphere and water have "optical windows" that allow visible light (just the radiation necessary for life) to pass through with very little absorption, whereas shorter wavelength (destructive ultraviolet radiation) and longer wavelength (infrared) radiation are both highly absorbed, as seen in Figure 3.{23} This allows solar energy in the form of light to reach the reacting chemicals in the universal solvent, which is water. The Encyclopedia Britannica{24} observes in this regard:
Considering the importance of visible sunlight for all aspects of terrestrial life, one cannot help being awed by the dramatically narrow window in the atmospheric absorption...and in the absorption spectrum of water.
It is remarkable that the optical properties of water and our atmosphere, the chemical bonding energies of the chemicals of life, and the radiation from the sun are all precisely harmonized to allow living systems to utilize the energy from the sun, without which life could not exist. It is quite analogous to your car, which can only run using gasoline as a fuel. Happily, but not accidentally, the service station has an ample supply of exactly the right fuel for your automobile. But someone had to drill for and produce the oil, someone had to refine it into liquid fuel (gasoline) that has been carefully optimized for your internal combustion engine, and others had to truck it to your service station. The production and transportation of the right energy from the sun for the metabolic motors of plants and animals is much more remarkable, and hardly accidental.
Why should sunlight be limited to such a narrow range?
The answer to that question is crucial because the only radiation that is capable of supporting life on earth is the kind that has wavelengths falling within this narrow range.
In Energy and the Atmosphere, the British physicist Ian Campbell addresses this question and says "That the radiation from the Sun (and from many sequence stars) should be concentrated into a minuscule band of the electromagnetic spectrum which provides precisely the radiation required to maintain life on earth is very remarkable." According to Campbell, this situation is "staggering".
Let us now examine these "staggering features of light" more closely.
In the whole electromagnetic spectrum, there is just one little band that has the energy to cross this threshold exactly. Its wavelengths range between 0.70 microns and 0.40 microns and if you'd like to see it, you can: just raise your head and look around–it's called "visible light". This radiation causes chemical reactions to take place in your eyes and that is why you are able to see. "The radiation is known as "visible light" makes up 41% of sunlight even though it occupies less than 1/10^25 of the whole electromagnetic spectrum. In his famous article "Life and Light", which appeared in Scientific American, the renowned physicist George Wald considered this matter and wrote:
"the radiation that is useful in promoting orderly chemical reactions comprises the great bulk of that of our sun." That the Sun should radiate light so exactly right for life is indeed an important example of design.
Is the rest of the light the Sun radiates good for anything?
When we look at this part of the light we see that a large part of solar radiation falling outside the range of visible light is in the section of the spectrum called "near infrared". This begins where visible light ends and again occupies a very small part of the total spectrum–less than 1/10^25 Is infrared light good for anything? Yes, but this time it's no use to look around because you can't see it with the naked eye. However, you can easily feel it: the warmth you feel on your face when you look up on a bright sunny summer or spring day is caused by infrared radiation coming from the Sun. The Sun's infrared radiation is what carries the thermal energy that keeps Earth warm. It too is as essential for life as visible light is. And the fascinating thing is that our Sun was apparently created just to serve for these two purposes because these two kinds of light make up the greatest part of sunlight.
And the third part of sunlight? Is that of any benefit?
You can bet on it. This is "near ultraviolet light" and it makes up the smallest fraction of sunlight. Like all ultraviolet light, it is highly energized and it can cause damage to living cells. The Sun's ultraviolet light, however, is the "least harmful" kind since it is closest to visible light. Although overexposure to solar ultraviolet light has been shown to cause cancer and cellular mutations, it has one vital benefit: the ultraviolet light concentrated in such a minuscule band is needed for the synthesis of vitamin D in humans and other vertebrates. (Vitamin D is necessary for the formation and nourishment of bone: without it, bones become soft or malformed, a disease called rickets that occurs in people deprived of sunlight for great lengths of time.) In other words, all the radiation emitted by the Sun is essential to life: none of it is wasted. The amazing thing is that all this radiation is limited to a 1/10^25 interval of the whole electromagnetic spectrum yet it is sufficient to keep us warm, see, and allow all the chemical reactions necessary for life to take place. Even if all the other conditions necessary for life and mentioned elsewhere in this book existed, if the light radiated by the Sun fell into any other part of the electromagnetic spectrum, there could be no life on Earth. It is certainly impossible to explain the fulfillment of this condition having a probability of 1 in 10^25 with a logic of coincidence. And if all this were not enough, light does something else: it keeps us fed, too!
The energy provided by the Sun has to come in the right amount, shape, and form to be useful to Life on Earth. Life cannot use X-rays or radio waves as an energy source. Visible light is just right the plants use it to make plant matter by photosynthesis, we and many other organisms use it to see by. Likewise, the amount of energy delivered by the Sun to our planet is just right for the hydrologic cycle to work, with water and water vapor changing back and forth, and some minor amount of ice (2 percent of the total water) collecting near the poles. Thus, the climate is between cold and warm, dry and wet just about right.
The electromagnetic spectrum.
The Sun sends light and heat rays (called infrared, IR) and some ultraviolet light (UV). The UV is dangerous to living organisms; it damages eyes, human skin and tree leaves, among other things. Fortunately only a few percent of the energy arrives as UV, the rest is half visible light, half (invisible) IR. In addition, the atmosphere takes out most of the UV before it reaches the ground. The ozone layer in the lower stratosphere (just above the highest clouds) is especially important in protecting living things from UV exposure. 7
Visible light is also incredibly fine-tuned for life to exist. Though visible light is only a tiny fraction of the total electromagnetic spectrum coming from the sun, it happens to be the “most permitted” portion of the sun’s spectrum allowed to filter through our atmosphere. All the other bands of electromagnetic radiation, directly surrounding visible light, happen to be harmful to organic molecules and are almost completely absorbed by the atmosphere. The tiny amount of harmful UV radiation, which is not visible light, allowed to filter through the atmosphere is needed to keep various populations of single cell bacteria from overpopulating the world (Ross; reasons.org). The size of light’s wavelengths and the constraints on the size allowable for the protein molecules of organic life, also seem to be tailor-made for each other. This “tailor-made fit” allows photosynthesis, the miracle of sight, and many other things that are necessary for human life. These specific frequencies of light (that enable plants to manufacture food and astronomers to observe the cosmos) represent less than 1 trillionth of a trillionth (10^-24) of the universe’s entire range of electromagnetic emissions. Like water, visible light also appears to be of optimal biological utility (Denton; Nature’s Destiny).
Transparency of the atmosphere 8
About 60% of the sun’s radiation is in the visible range, only about one trillionth of one trillionth of the entire electromagnetic spectrum. Amazingly, this same range is what the air, the atmosphere, is transparent to. Of course, you may think, air is transparent! Obviously, everyone knows that! But, the amazing thing that everyone does not know, is that air is not transparent to wavelengths on either side of the visible range. A plot of atmospheric opacity versus wavelength has a notch in just the visible range of the spectrum. Opacity is the lack of transparency: the more opaque, the less transparent and vice-versa. This transparency of the atmosphere allows the light to penetrate to the earth’s surface, where photosynthesis can then occur. Also, the water of the earth’s oceans is also transparent— in the same “notch”—in exactly the same range of the electromagnetic spectrum as is the atmosphere. This allows photosynthesis to occur in the oceans, underneath the water where plankton reside. But ultraviolet (UV) light is blocked by a thin layer of water, protecting aquatic life from damage. The UV light from the sun can be damaging. The amount of light of this wavelength reaching earth’s surface is limited by the ozone layer and also by water vapor in the atmosphere. Also, the sun itself does not put out as much UV light as it does visible light. But some UV light does get through, and this is good! UV light is used to produce vitamin D, which is essential for life. Also, the visible range of light provides just the right amount of energy required to stimulate most biochemical reactions (15–65 kilocalories per mole). Wavelengths with less energy would fail to stimulate reactions needed by life, and more energy would damage life.
Vision
As already noted, both water and air are transparent to the same wavelengths, visible light, which is amazingly well-suited for vision. If a shorter wavelength were used for vision, photons would carry additional energy, which would be destructive to biological molecules. Also, this radiation would be much more difficult to focus with a lens because higher-energy waves tend to not be bent (or blocked) by many materials. (This is why X-rays pass through flesh.) On the other hand, if longer waves were used for vision, then we would loose resolution or detail in what we see. Longer wavelengths would be more difficult to detect since they would carry less energy. The energy of a single photon must be amplified many times by the photoreceptors in the eye in order for the weak signal to be useful. This is also evident in man-made devices, such as radios, which make use of longer wavelengths. The signal must be amplified. If any of you have ever used a crystal radio set, you know that there is no amplification, and the sound is very weak. Yet even this weak signal consists of not one, but many, photons. The eye, remember, can detect a single photon, which is a much weaker signal. In order to amplify this weaker signal there would need to be a larger amplification apparatus, which would increase the physical size of the photoreceptor unit. This would change the “pixel size” of an image to a larger size and would reduce the resolution, which means less detail. As it is, the wavelengths of visible light are just right—not too long and not too short!Remember, also, that we have a coincidence of several necessary factors for vision: the transparency of both air and water—the eye contains water—to the same visible light range and the fitness of this visible range for vision. Vision is limited by spherical and chromatic aberration, which increase with the size of the opening receiving light in the eye. To keep this opening small and limit these distortions, we have another problem—diffraction limiting resolution. The larger the wavelength, the larger the diffraction and the less the resolution. Increased resolution, as microscopes and telescopes provide, comes at the cost of a decreased field of view—obviously not desirable for living things. To optimally balance all these factors requires a design like that actually found in the eye of man. The minimum size formed on the retina will be about 2 microns, which cannot be made smaller due to the limitations discussed above. Amazingly, this is actually the size of light detectors found in the eye of man. The size of an eye is limited by the fact that the supply of nutrients to the internal parts of the eye depends upon diffusion, and the supply by diffusion becomes more limited as the distance nutrients must travel increases. On the other hand, an eye too small would suffer from too few photoreceptors and consequent insufficient resolution. Also, due to the physics of light waves, even if photoreceptors could be made smaller than 2 microns, there would be no improvement in resolution. As Denton states: “This is a remarkable coincidence. The two optical limits to the resolving power of the eye—the one set by the waveguide optics…the other set by the laws of classical optics…have the same value: 2 microns.” Another limit on the physical size of photoreceptors to about 2 microns is that photoreceptors require many millions of rhodopsin molecules per cell to increase the probability that a single photon will encounter a rhodopsin molecule and thus be detected—important for low light or night vision. Reducing the cell size to gain more resolution means lowering the number of rhodopsin molecules and thereby reducing the probability of detecting single photons. It seems that the size of photoreceptor cells is just right for vision and that the properties of visible light are just right for that size!
CLEAR VISION 10
Consider the atmosphere’s transparency, which is actually just part of the story. Our atmosphere participates in one of the most extraordinary coincidences known to science: an eerie harmony among the range of wavelengths of light emitted by the Sun, transmitted by Earth’s atmosphere, converted by plants into chemical energy, and detected by the human eye. The human eye perceives light of different wavelengths as different colors, ranging from violet blue (the shortest wavelengths in the visible spectrum) to red (the longest). Looking at a diagram of the electromagnetic spectrum, we see the visible light emerging gracefully from the ultraviolet, differentiating into the familiar colors of the rainbow, and disappearing seamlessly into the warm, invisible infrared. The visible wavelengths rise and fall at the nano-scale, the distances from their peaks measured in ten-billionths of a meter, called Ångstroms (Å). We see blue at approximately 4,800 Å, green at 5,200 Å, yellow at 5,800 Å, and red at 6,600 Å. Earth’s atmosphere is transparent to radiation between 3,100 to 9,500 Å and to the much longer radio wavelengths. The radiation we see is near the middle of that range, between 4,000 and 7,000 Å, the range in which the Sun emits 40 percent of its energy. Its spectrum peaks smack in the middle of this visible spectrum, at 5,500 Å. This is but a tiny sample of the entire range. The near-ultraviolet, visible, and near-infrared spectra— the light most useful to life and sight—are a razor-thin sliver of the universe’s natural, electromagnetic emissions: about one part in 10^25. That is much smaller than one star out of all the stars in the entire visible universe: about 10^22. As it happens, our atmosphere strikes a nearly perfect balance, transmitting most of the radiation that is useful for life while blocking most of the lethal energy. Water vapor in the atmosphere is likewise accommodating, a fact that even the fifteenth edition of the staid Encyclopaedia Britannica picks up on: “Considering the importance of visible sunlight for all aspects of terrestrial life, one cannot help being awed by the dramatically narrow window in the atmospheric absorption . . . and in the absorption spectrum of water.” The oceans transmit an even narrower window of the spectrum, mainly the blues and greens, while halting the other wavelengths near the surface, where they nourish the marine life that figures prominently in Earth’s biosphere. It seems plausible that this is merely an artifact of our eyes having evolved through natural selection to decipher just the spectrum of light that happens to get through the atmosphere. But this fact isn’t so easily dismissed. As George Greenstein notes:
One might think that a certain adaptation has been at work here: the adaptation of plant life to the properties of sunlight. After all, if the Sun were a different temperature could not some other molecule, tuned to absorb light of a different color, take the place of chlorophyll? Remarkably enough the answer is no, for within broad limits all molecules absorb light of similar colors. The absorption of light is accomplished by the excitation of electrons in molecules to higher energy states, and the general scale of energy required to do this is the same no matter what molecule you are discussing. Furthermore, light is composed of photons, packets of energy, and photons of the wrong energy simply cannot be absorbed.
In other words, because of the basic properties of matter, the typical energy involved in chemical reactions corresponds to the typical energy of optical light photons. Otherwise, photosynthetic life wouldn’t be possible. Photons with too much energy would tear molecules apart, while those with too little energy could not trigger chemical reactions. Similar arguments hold for the wavelength range over which the atmosphere is transparent. So stars that don’t emit the right sort of radiation in the right amounts won’t qualify as useful energy sources for life. The radiation a star emits and its peak emission depend on its temperature. Temperatures of normal stars range over a factor of about twenty. The Sun is a little below the mid-range, while exotic stars emit most of their energy on the far ends of the electromagnetic spectrum, as gamma rays, X-rays, or radio waves. A star like our Sun, which produces mostly photons that can energize chemical reactions on Earth, will shine light that comes from just that region in its photosphere where atoms can combine to form stable molecules. So we can’t explain away one part of this coincidence in terms of the others. Life can’t use just any type of light from any type of star. Our Sun, it turns out, is near the optimum for any plausible kind of chemical life. To see the stars, though, a translucent atmosphere that merely allows light to reach the surface won’t cut it. Like a clean, clear pane of glass as compared to a frosted one, we need an atmosphere, like Earth’s, that is transparent in order to see the stars and the wider cosmos. Of course, we enjoy these views because we have the good fortune of living on land. The view would not be nearly as good if we lived underwater.
But even dry land and a transparent atmosphere aren’t enough, since they wouldn’t be much use without the dark nights. Although we take them for granted, dark nights depend on several astronomical variables, some local and some not. A dark sky requires that our planet regularly rotate away from the intense direct light of the Sun. If our day were the same length as our year, Earth would always keep the same face pointed toward the Sun, much as the Moon does Earth. The resulting large temperature difference between the day and night sides would be hostile to complex life. Any complex life, such as there could be, would stay on the day side. Unless the planet enjoyed total solar eclipses, like Lagash, it would never see dark skies. We would suffer similar but less severe problems if we had several moons staring unblinkingly in the night sky like headlights on a busy highway. Our single moon does interfere with astronomers’ ability to observe distant faint objects, but only when it’s out. Moreover, visibility is better now than it was in the distant past, when the Moon was nearly three times closer to Earth, and thus nearly nine times brighter. Of course, a translucent atmosphere might work for merely converting sunlight into chemical energy or hunting prey and avoiding predators. But because we are big-brained, mobile, surface-dwelling creatures, we need a certain type of atmosphere. It just so happens that that very atmosphere— predominantly nitrogen and oxygen with some carbon dioxide and water vapor—is mostly transparent to optical radiation. All four of these chemical constituents are essential for our biosphere. Water does partially hinder our view of the distant universe, but the valuable trade-offs it provides more than compensate for this hindrance.
For example, one particularly impressive consequence of atmospheric water is the rainbow. Rainbows, like total solar eclipses, seem equal parts whimsy and mystery, summoning the artist’s creativity and the naturalist’s curiosity. The early attempts to explain their formation started the first of our many tutorials on the nature of light. The scientists who followed those clues eventually learned how to unweave the white light of the Sun. René Descartes and, later, Isaac Newton, who performed experiments with sunlight and prisms in 1666, groped toward the modern explanation for the rainbow and the modern science of spectroscopy. A rainbow is in effect a natural spectroscope as big as the sky. Once scientists learned how to use a prism to replicate a rainbow, it was only a matter of time before someone scrutinized the solar spectrum. But rainbows won’t appear on just any planet. A good rainbow needs a partially cloudy atmosphere, the golden mean between the uniformly cloudy and uniformly dry. Earth’s water results in skies that, on average, are about 68 percent cloudy. Clouds help balance the global energy by contributing to the global albedo, that fraction of sunlight reflected back into space. Earth currently reflects about 30 percent of the sunlight that strikes it. Four major types of reflecting surfaces contribute to the global albedo: land, ice, oceans, and clouds, each with its own particular reflective properties.
1. http://www.unav.es/cryf/georgedenton.html
2. Ian M. Campbell, Energy and the Atmosphere, London: Wiley, 1977, p. 1-2
3. http://m.harunyahya.com/tr/buku/966/The-Creation-of-the-Universe/chapter/3177/Chapter-VI-The-Signs-of-Creation-in-Light
4. http://www.discovery.org/a/3681
5. https://www.christianforums.com/threads/a-fine-tuned-universe-em-radiation-light-spectrum-gravity.2043325/
6. http://link.springer.com.secure.sci-hub.tw/article/10.1007/s10265-016-0809-0
7. http://earthguide.ucsd.edu/virtualmuseum/ita/07_1.shtml
8. http://www.tasc-creationscience.org/content/amazing-coincidences-life
9. Wonders of the Universe, Professor Brian Cox & Andrew Cohen, page 30
10. Privileged Planet, Gonzalez, page 65
11. http://amazingspace.org/resources/explorations/light/star-light-science.html
12. https://www.enotes.com/homework-help/what-longest-shortest-radiowave-wavelength-325147
13. https://hypertextbook.com/facts/2001/RachelShapiro.shtml
14. Plant Physiology, 3rd ed., Lincoln Taiz and Eduardo Zeiger, page 116
15. http://www.leaderu.com/offices/bradley/docs/scievidence.html
More readings:
https://courses.lumenlearning.com/boundless-chemistry/chapter/the-nature-of-light/
https://docs.google.com/document/d/1LuWUW26yc_as4QUHau4Q83-4VBjWMMAdQgGv513_YuU/edit
Last edited by Otangelo on Sat Apr 02, 2022 1:54 pm; edited 1 time in total



