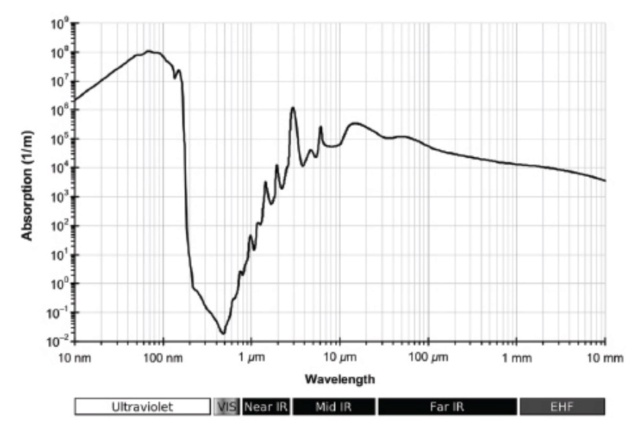
Water is fine-tuned for life
https://reasonandscience.catsboard.com/t1532-water-is-fine-tuned-for-life
Water is finely-tuned for life
An example of cosmic fine-tuning is with respect to the physics of water and ice. Normally a solid for some particular substance will tend to sink in the liquid state of that same substance. In other words, the solid-state is denser than the liquid state. However, there is one important exception to the rule. Just based on our knowledge that solids sink in their liquid equivalents that ice cubes should sink in a glass of water. Of course, we know, that ice floats on water. If that weren’t the case, life would/could not exist, and we probably wouldn’t be here to talk about it. Why? If ice sank in water, all the bodies of water would freeze solid from the bottom up, leaving a thin film of liquid water on the surface. That scenario would play havoc with the origin of life as we know it. There would be a near-permanent ‘Snowball Earth’. Considering the biological importance and relevance of ice floating on water relative to the other way around, we should thank this particular exception to the rule. The question is, why is it so?
https://www.closertotruth.com/series/why-cosmic-fine-tuning-demands-explanation
Lisa Grossman Water's quantum weirdness makes life possible 19 October 2011
WATER’S life-giving properties exist on a knife-edge. It turns out that life as we know it relies on a fortuitous, but incredibly delicate, the balance of quantum forces. Water is one of the planet’s weirdest liquids, and many of its most bizarre features make it life-giving. For example, its higher density as a liquid than as a solid means ice floats on water, allowing fish to survive under partially frozen rivers and lakes. And unlike many liquids, it takes a lot of heat to warm water up even a little, a quality that allows mammals to regulate their body temperature. But computer simulations show that quantum mechanics nearly robbed water of these life-giving features. Most of them arise due to weak hydrogen bonds that hold H2O molecules together in a networked structure. For example, it is hydrogen bonds that hold ice molecules in a more open structure than in liquid water, leading to a lower density. By contrast, without hydrogen bonds, liquid molecules move freely and take up more space than in rigid solid structures.
Yet in simulations that include quantum effects, hydrogen bond lengths keep changing thanks to the Heisenberg uncertainty principle, which says no molecule can have a definite position with respect to the others. This destabilizes the network, removing many of water’s special properties. How water continues to exist as a network of hydrogen bonds, in the face of these destabilizing quantum effects, was a mystery. In 2009, theorist Thomas Markland, now at Stanford University, suggested a reason why water’s fragile structure does not break down completely. They calculated that the uncertainty principle should also affect the bond lengths within each water molecule, and proposed that it does so in such a way as to strengthen the attraction between molecules and maintain the hydrogen-bond network. “Water fortuitously has two quantum effects which cancel each other out.
Until recently, though, there was no way to discover whether there is any variation in bond length within the water molecule. Now, Salmon’s team has done this. Their trick was to use so-called heavy water, in which the molecule’s two hydrogen atoms are replaced with deuterium. This isotope of hydrogen contains a neutron as well as a proton. The extra bulk makes it less vulnerable to quantum uncertainties. It’s like turning the quantum mechanics half off.
Salmon and colleagues shot beams of neutrons at different versions of water, and studied the way they bounced off the atoms – a precise way to measure bond lengths. They also substituted heavier oxygen atoms into both heavy and normal water, which allowed them to determine which bonds they were measuring. They found that the hydrogen-oxygen bonds were slightly longer than the deuterium-oxygen ones, which is what you would expect if quantum uncertainty was affecting water’s structure “No one has ever really measured that before,” says Benmore. “Water fortuitously has two quantum uncertainty effects which cancel each other out” We are used to the idea that the cosmos’s physical constants are fine-tuned for life. Now it seems water’s quantum forces can be added to this “just right” list.
https://www.newscientist.com/article/mg21228354-900-waters-quantum-weirdness-makes-life-possible/
Anita Zeidler Oxygen as a Site Specific Probe of the Structure of Water and Oxide Materials 30 SEPTEMBER 2011
https://sci-hub.ren/10.1103/physrevlett.107.145501
Michael Denton The Miracle of the Cell page 103, 2019
All the evidence suggests that the range of viscosities and diffusion rates of water must be very close to what it is, within a range of about 0.5 mP-s to 3 mP-s. at the fitness of the viscosity of water must fall within such a narrow range highlights just how fine-tuned is the natural order for life. The viscosity of common substances varies greatly. 21 Measured in millipascals-seconds, the the viscosity of air is 0.017, water 1.0, olive oil 84, glycerin 1420 and honey 10,000. 22 The total range of viscosities of substances on our planet is more than twenty-seven orders of magnitude, from the viscosity of air to the viscosity of crustal rocks. 23 Thus, the range of life-friendly viscosities is a tiny, vital band within the inconceivably vast range of viscosities in nature.
https://3lib.net/book/6147845/318a4f
Anders Nilsson The structural origin of anomalous properties of liquid water 08 December 2015
Water is the most important liquid for our existence and plays an essential role in physics, chemistry, biology and geoscience. What makes water unique is not only its importance but also the anomalous behaviour of many of its macroscopic properties.… If water would not behave in this unusual way it is most questionable if life could have developed on planet Earth.
https://www.nature.com/articles/ncomms9998
The expansion of water upon freezing is vital to life on Earth. It is what causes ice to be less dense as a solid than as a liquid. This means that ice floats in liquid water. Hydrogen bonding is once again the source of this rare property. Water freezes when its molecules are no longer moving around enough to break their hydrogen bonds. When water freezes it becomes locked into a crystalline lattice, and each water molecule bonds to four neighboring molecules. The hydrogen bonds allow for the molecules to be kept far enough apart so that ice is less dense than liquid water at 4 degrees Celsius.This may not seem like a very important property, but the fitness of the environment would drastically change if water was less dense than ice. All of bodies of water would eventually freeze over if ice did sink, essentially making life on Earth impossible.
https://apbiologychapter3.weebly.com/expansion-upon-freezing.html#:~:text=The%20expansion%20of%20water%20upon,ice%20floats%20in%20liquid%20water.&text=The%20hydrogen%20bonds%20allow%20for,water%20at%204%20degrees%20Celsius.
Jonathan Sarfati The wonders of water December 1997
Insight into ice
A vital and very unusual property of water is that it expands as it freezes, unlike most other substances. That is why icebergs float. In fact, water contracts normally as it is cooled, until it reaches 4°C (39.2°F), when it starts to expand again. This means that icy-cold water is less dense, so tends to move upwards. This is very important. Most liquids exposed to cold air would cool, and the cold liquid would sink, forcing more liquid to rise and be cooled by the air. Eventually all the liquid would lose heat to the air and freeze, from the bottom up, till completely frozen. But with water, the cold regions, being less dense, stay on top, allowing the warmer regions to stay below and avoid losing heat to the air. This means that the surface may be frozen, but fish can still live in the water below. But if water were like other substances, large bodies of water, such as North America’s Great Lakes, would be frozen solid, with dire effects on life on earth as a whole.
https://creation.com/the-wonders-of-water
Guillermo Gonzalez, Jay W. Richards: The Privileged Planet: How Our Place in the Cosmos Is Designed for Discovery 2004 page 33
Life also needs a solvent, which provides a medium for chemical reactions. The best possible solvent should dissolve many types of molecules, transporting them to reaction sites while preserving their integrity. It should be in the liquid state since the solid state doesn’t allow for mobility and the gaseous one doesn’t allow for sufficiently frequent reactions. Further, the solvent should be liquid over the same range of temperatures where the basic molecules of life remain largely intact and in the liquid or gaseous state. Water, the most abundant chemical compound in the universe, exquisitely meets these requirements. In fact, water far exceeds these basic requirements for life chemistry. First, water is virtually unique in being denser as a liquid than as a solid (the element bismuth is another substance with this property). As a result, ice floats on water, insulating the water underneath from further loss of heat. This simple fact also prevents lakes and oceans from freezing from the bottom up. It’s very difficult, if not impossible, to alter such a situation once attained. If ice were to sink to the bottom, it would remain there, unable to melt, separated from the Sun’s warmth. Surface ice also helps to regulate the climate by altering Earth’s ability to absorb or reflect sunlight. Second, water has very high latent heats when changing from a solid to a liquid to a gas. So more heat is needed to vaporize one gram of water than the same amount of any other known substance at ambient surface temperature (and higher than most others at any temperature). This means that it takes an unusually large amount of heat to convert liquid water to vapor. Similarly, vapor releases the same amount of heat when it condenses back to liquid water. As a result, water helps moderate Earth’s climate and helps larger organisms regulate their body temperatures. This characteristic also permits smallish bodies of water to exist on land; otherwise, ponds and lakes would evaporate more easily. In all three cases, if a gram of water evaporated with less heat, it would remove less heat from a surface. It’s probably no coincidence that water is found in all three states at Earth’s surface, and that the mean surface temperature is near the triple point of water—a unique combination of pressure and temperature where all three states can coexist. Not only does this provide a diverse set of surfaces, but it also best exploits water’s anomalous properties for regulating the temperature. Third, liquid water’s surface tension, which is higher than that of almost all other liquids, gives it better capillary action in soils, trees, and circulatory systems, a greater ability to form discrete structures with membranes, and the power to speed up chemical reactions at its surface. Finally, water is probably essential for starting and maintaining Earth’s plate tectonics, an important part of the climate regulation system. Frank H. Stillinger, an expert on water, observed, “It is striking that so many eccentricities should occur together in one substance.” While water has more properties that are valuable for life than nearly all other elements or compounds, each property also interacts with the others to yield a biologically useful end. Michael Denton describes one of these ends, the weathering of rock:
Take, for example, the weathering of rocks and its end result, the distribution of vital minerals upon which life depends via rivers to the oceans and ultimately throughout the hydrosphere. It is the high surface tension of water which draws it into the crevices of the rock; it is its highly anomalous expansion on freezing which cracks the rock, producing additional crevices for further weathering and increasing the surface area available for the solvation action of water in leaching out the elements. On top of all this, ice possesses the appropriate viscosity and strength to form hard, grinding rivers or glaciers which reduce the rocks broken and fractured by repeated cycles of freezing and thawing to tiny particles of glacial silt. The low viscosity of water confers on it the ability to flow rapidly in rivers and mountain streams and to carry at high speed those tiny particles of rock and glacial silt which contribute further to the weathering process and the breaking down of the mountains. The chemical reactivity of water and its great solvation power also contribute to the weathering process, dissolving out the minerals and elements from the rocks and eventually distributing them throughout the hydrosphere. This chemical and mechanical distribution of vital elements is an important part of chemical weathering, which is also an important part of Earth’s climate regulation system
https://3lib.net/book/5102561/45e43d
The Chemical Revolution of Antoine-Laurent Lavoisier
We owe the modern names for the elemental building blocks of water – hydrogen and oxygen – to Antoine Lavoisier, one of the greatest of the pioneering eighteenth-century chemists. Great though he undoubtedly was, however, he made a fundamental error in naming these two elements that persists to this day. He named hydrogen, entirely appropriately, from the Greek ‘hydro’ (meaning water) and ‘genes’ (meaning creator). Oxygen, however, with its Greek root of ‘oxys’ (meaning acid), incorrectly suggests that oxygen is a component of all acids. It would have been more accurate to call hydrogen ‘oxygen’, in that the majority of common acid-base chemical reactions involve the transfer of protons, which are the nuclei of hydrogen. But Lavoisier’s names have stayed with us, so oxygen will forever be ‘the acid giver’, which it isn’t. By 1804, the final elemental description of water was given in a paper by the French chemist Joseph Louis Gay-Lussac and the German naturalist Alexander von Humboldt. Together, they demonstrated that water consisted of two volumes of hydrogen to one of oxygen, and thus gave the world the most widely known of all chemical formulae: H2O. If Lavoisier had got it right, we’d call water O2H rather than H2O. Such is history. 2
This shouldn’t be surprising when you consider that hydrogen and oxygen are two of the most abundant atoms in the Universe. Hydrogen forms 74 per cent of all the elemental mass. The second lightest element, helium, comprises 24 percent. These two elements dominate because they were formed in the first few minutes after the Big Bang. Oxygen is the third most abundant element in the cosmos, at around 1 per cent by mass. Most of the rest is carbon; all the other elements are present in much smaller quantities. All of the oxygen and carbon atoms in the Universe today, including all of those in your body, were produced in the cores of stars by nuclear fusion and scattered out into space as the stars died. Apart from helium, which is satisfied with its full inner shell of two electrons, these atoms have an affinity for each other because of their desire to pair up their solitary electrons. As a result, they tend to form molecules. After the hydrogen molecule (H2) and carbon monoxide (CO), water is the third most common molecule in the Universe.
https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/lavoisier.html
http://www.sciencedirect.com/science/article/pii/S1364032116000502
A.E. Kabeel, Z.M. Omara, F.A. Essa and A.S. Abdullah
Renewable and Sustainable Energy Reviews, 2016, vol. 59, issue C, pages 839-857

1. A privileged planet, Gonzalez , page 33
2. WONDERS OF LIFE, Brian Cox, page 37
https://reasonandscience.catsboard.com/t1532-water-is-fine-tuned-for-life
Water is finely-tuned for life
An example of cosmic fine-tuning is with respect to the physics of water and ice. Normally a solid for some particular substance will tend to sink in the liquid state of that same substance. In other words, the solid-state is denser than the liquid state. However, there is one important exception to the rule. Just based on our knowledge that solids sink in their liquid equivalents that ice cubes should sink in a glass of water. Of course, we know, that ice floats on water. If that weren’t the case, life would/could not exist, and we probably wouldn’t be here to talk about it. Why? If ice sank in water, all the bodies of water would freeze solid from the bottom up, leaving a thin film of liquid water on the surface. That scenario would play havoc with the origin of life as we know it. There would be a near-permanent ‘Snowball Earth’. Considering the biological importance and relevance of ice floating on water relative to the other way around, we should thank this particular exception to the rule. The question is, why is it so?
https://www.closertotruth.com/series/why-cosmic-fine-tuning-demands-explanation
Lisa Grossman Water's quantum weirdness makes life possible 19 October 2011
WATER’S life-giving properties exist on a knife-edge. It turns out that life as we know it relies on a fortuitous, but incredibly delicate, the balance of quantum forces. Water is one of the planet’s weirdest liquids, and many of its most bizarre features make it life-giving. For example, its higher density as a liquid than as a solid means ice floats on water, allowing fish to survive under partially frozen rivers and lakes. And unlike many liquids, it takes a lot of heat to warm water up even a little, a quality that allows mammals to regulate their body temperature. But computer simulations show that quantum mechanics nearly robbed water of these life-giving features. Most of them arise due to weak hydrogen bonds that hold H2O molecules together in a networked structure. For example, it is hydrogen bonds that hold ice molecules in a more open structure than in liquid water, leading to a lower density. By contrast, without hydrogen bonds, liquid molecules move freely and take up more space than in rigid solid structures.
Yet in simulations that include quantum effects, hydrogen bond lengths keep changing thanks to the Heisenberg uncertainty principle, which says no molecule can have a definite position with respect to the others. This destabilizes the network, removing many of water’s special properties. How water continues to exist as a network of hydrogen bonds, in the face of these destabilizing quantum effects, was a mystery. In 2009, theorist Thomas Markland, now at Stanford University, suggested a reason why water’s fragile structure does not break down completely. They calculated that the uncertainty principle should also affect the bond lengths within each water molecule, and proposed that it does so in such a way as to strengthen the attraction between molecules and maintain the hydrogen-bond network. “Water fortuitously has two quantum effects which cancel each other out.
Until recently, though, there was no way to discover whether there is any variation in bond length within the water molecule. Now, Salmon’s team has done this. Their trick was to use so-called heavy water, in which the molecule’s two hydrogen atoms are replaced with deuterium. This isotope of hydrogen contains a neutron as well as a proton. The extra bulk makes it less vulnerable to quantum uncertainties. It’s like turning the quantum mechanics half off.
Salmon and colleagues shot beams of neutrons at different versions of water, and studied the way they bounced off the atoms – a precise way to measure bond lengths. They also substituted heavier oxygen atoms into both heavy and normal water, which allowed them to determine which bonds they were measuring. They found that the hydrogen-oxygen bonds were slightly longer than the deuterium-oxygen ones, which is what you would expect if quantum uncertainty was affecting water’s structure “No one has ever really measured that before,” says Benmore. “Water fortuitously has two quantum uncertainty effects which cancel each other out” We are used to the idea that the cosmos’s physical constants are fine-tuned for life. Now it seems water’s quantum forces can be added to this “just right” list.
https://www.newscientist.com/article/mg21228354-900-waters-quantum-weirdness-makes-life-possible/
Anita Zeidler Oxygen as a Site Specific Probe of the Structure of Water and Oxide Materials 30 SEPTEMBER 2011
https://sci-hub.ren/10.1103/physrevlett.107.145501
Michael Denton The Miracle of the Cell page 103, 2019
All the evidence suggests that the range of viscosities and diffusion rates of water must be very close to what it is, within a range of about 0.5 mP-s to 3 mP-s. at the fitness of the viscosity of water must fall within such a narrow range highlights just how fine-tuned is the natural order for life. The viscosity of common substances varies greatly. 21 Measured in millipascals-seconds, the the viscosity of air is 0.017, water 1.0, olive oil 84, glycerin 1420 and honey 10,000. 22 The total range of viscosities of substances on our planet is more than twenty-seven orders of magnitude, from the viscosity of air to the viscosity of crustal rocks. 23 Thus, the range of life-friendly viscosities is a tiny, vital band within the inconceivably vast range of viscosities in nature.
https://3lib.net/book/6147845/318a4f
Anders Nilsson The structural origin of anomalous properties of liquid water 08 December 2015
Water is the most important liquid for our existence and plays an essential role in physics, chemistry, biology and geoscience. What makes water unique is not only its importance but also the anomalous behaviour of many of its macroscopic properties.… If water would not behave in this unusual way it is most questionable if life could have developed on planet Earth.
https://www.nature.com/articles/ncomms9998
The expansion of water upon freezing is vital to life on Earth. It is what causes ice to be less dense as a solid than as a liquid. This means that ice floats in liquid water. Hydrogen bonding is once again the source of this rare property. Water freezes when its molecules are no longer moving around enough to break their hydrogen bonds. When water freezes it becomes locked into a crystalline lattice, and each water molecule bonds to four neighboring molecules. The hydrogen bonds allow for the molecules to be kept far enough apart so that ice is less dense than liquid water at 4 degrees Celsius.This may not seem like a very important property, but the fitness of the environment would drastically change if water was less dense than ice. All of bodies of water would eventually freeze over if ice did sink, essentially making life on Earth impossible.
https://apbiologychapter3.weebly.com/expansion-upon-freezing.html#:~:text=The%20expansion%20of%20water%20upon,ice%20floats%20in%20liquid%20water.&text=The%20hydrogen%20bonds%20allow%20for,water%20at%204%20degrees%20Celsius.
Jonathan Sarfati The wonders of water December 1997
Insight into ice
A vital and very unusual property of water is that it expands as it freezes, unlike most other substances. That is why icebergs float. In fact, water contracts normally as it is cooled, until it reaches 4°C (39.2°F), when it starts to expand again. This means that icy-cold water is less dense, so tends to move upwards. This is very important. Most liquids exposed to cold air would cool, and the cold liquid would sink, forcing more liquid to rise and be cooled by the air. Eventually all the liquid would lose heat to the air and freeze, from the bottom up, till completely frozen. But with water, the cold regions, being less dense, stay on top, allowing the warmer regions to stay below and avoid losing heat to the air. This means that the surface may be frozen, but fish can still live in the water below. But if water were like other substances, large bodies of water, such as North America’s Great Lakes, would be frozen solid, with dire effects on life on earth as a whole.
https://creation.com/the-wonders-of-water
Guillermo Gonzalez, Jay W. Richards: The Privileged Planet: How Our Place in the Cosmos Is Designed for Discovery 2004 page 33
Life also needs a solvent, which provides a medium for chemical reactions. The best possible solvent should dissolve many types of molecules, transporting them to reaction sites while preserving their integrity. It should be in the liquid state since the solid state doesn’t allow for mobility and the gaseous one doesn’t allow for sufficiently frequent reactions. Further, the solvent should be liquid over the same range of temperatures where the basic molecules of life remain largely intact and in the liquid or gaseous state. Water, the most abundant chemical compound in the universe, exquisitely meets these requirements. In fact, water far exceeds these basic requirements for life chemistry. First, water is virtually unique in being denser as a liquid than as a solid (the element bismuth is another substance with this property). As a result, ice floats on water, insulating the water underneath from further loss of heat. This simple fact also prevents lakes and oceans from freezing from the bottom up. It’s very difficult, if not impossible, to alter such a situation once attained. If ice were to sink to the bottom, it would remain there, unable to melt, separated from the Sun’s warmth. Surface ice also helps to regulate the climate by altering Earth’s ability to absorb or reflect sunlight. Second, water has very high latent heats when changing from a solid to a liquid to a gas. So more heat is needed to vaporize one gram of water than the same amount of any other known substance at ambient surface temperature (and higher than most others at any temperature). This means that it takes an unusually large amount of heat to convert liquid water to vapor. Similarly, vapor releases the same amount of heat when it condenses back to liquid water. As a result, water helps moderate Earth’s climate and helps larger organisms regulate their body temperatures. This characteristic also permits smallish bodies of water to exist on land; otherwise, ponds and lakes would evaporate more easily. In all three cases, if a gram of water evaporated with less heat, it would remove less heat from a surface. It’s probably no coincidence that water is found in all three states at Earth’s surface, and that the mean surface temperature is near the triple point of water—a unique combination of pressure and temperature where all three states can coexist. Not only does this provide a diverse set of surfaces, but it also best exploits water’s anomalous properties for regulating the temperature. Third, liquid water’s surface tension, which is higher than that of almost all other liquids, gives it better capillary action in soils, trees, and circulatory systems, a greater ability to form discrete structures with membranes, and the power to speed up chemical reactions at its surface. Finally, water is probably essential for starting and maintaining Earth’s plate tectonics, an important part of the climate regulation system. Frank H. Stillinger, an expert on water, observed, “It is striking that so many eccentricities should occur together in one substance.” While water has more properties that are valuable for life than nearly all other elements or compounds, each property also interacts with the others to yield a biologically useful end. Michael Denton describes one of these ends, the weathering of rock:
Take, for example, the weathering of rocks and its end result, the distribution of vital minerals upon which life depends via rivers to the oceans and ultimately throughout the hydrosphere. It is the high surface tension of water which draws it into the crevices of the rock; it is its highly anomalous expansion on freezing which cracks the rock, producing additional crevices for further weathering and increasing the surface area available for the solvation action of water in leaching out the elements. On top of all this, ice possesses the appropriate viscosity and strength to form hard, grinding rivers or glaciers which reduce the rocks broken and fractured by repeated cycles of freezing and thawing to tiny particles of glacial silt. The low viscosity of water confers on it the ability to flow rapidly in rivers and mountain streams and to carry at high speed those tiny particles of rock and glacial silt which contribute further to the weathering process and the breaking down of the mountains. The chemical reactivity of water and its great solvation power also contribute to the weathering process, dissolving out the minerals and elements from the rocks and eventually distributing them throughout the hydrosphere. This chemical and mechanical distribution of vital elements is an important part of chemical weathering, which is also an important part of Earth’s climate regulation system
https://3lib.net/book/5102561/45e43d
The Chemical Revolution of Antoine-Laurent Lavoisier
We owe the modern names for the elemental building blocks of water – hydrogen and oxygen – to Antoine Lavoisier, one of the greatest of the pioneering eighteenth-century chemists. Great though he undoubtedly was, however, he made a fundamental error in naming these two elements that persists to this day. He named hydrogen, entirely appropriately, from the Greek ‘hydro’ (meaning water) and ‘genes’ (meaning creator). Oxygen, however, with its Greek root of ‘oxys’ (meaning acid), incorrectly suggests that oxygen is a component of all acids. It would have been more accurate to call hydrogen ‘oxygen’, in that the majority of common acid-base chemical reactions involve the transfer of protons, which are the nuclei of hydrogen. But Lavoisier’s names have stayed with us, so oxygen will forever be ‘the acid giver’, which it isn’t. By 1804, the final elemental description of water was given in a paper by the French chemist Joseph Louis Gay-Lussac and the German naturalist Alexander von Humboldt. Together, they demonstrated that water consisted of two volumes of hydrogen to one of oxygen, and thus gave the world the most widely known of all chemical formulae: H2O. If Lavoisier had got it right, we’d call water O2H rather than H2O. Such is history. 2
This shouldn’t be surprising when you consider that hydrogen and oxygen are two of the most abundant atoms in the Universe. Hydrogen forms 74 per cent of all the elemental mass. The second lightest element, helium, comprises 24 percent. These two elements dominate because they were formed in the first few minutes after the Big Bang. Oxygen is the third most abundant element in the cosmos, at around 1 per cent by mass. Most of the rest is carbon; all the other elements are present in much smaller quantities. All of the oxygen and carbon atoms in the Universe today, including all of those in your body, were produced in the cores of stars by nuclear fusion and scattered out into space as the stars died. Apart from helium, which is satisfied with its full inner shell of two electrons, these atoms have an affinity for each other because of their desire to pair up their solitary electrons. As a result, they tend to form molecules. After the hydrogen molecule (H2) and carbon monoxide (CO), water is the third most common molecule in the Universe.
https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/lavoisier.html

http://www.sciencedirect.com/science/article/pii/S1364032116000502
A.E. Kabeel, Z.M. Omara, F.A. Essa and A.S. Abdullah
Renewable and Sustainable Energy Reviews, 2016, vol. 59, issue C, pages 839-857

1. A privileged planet, Gonzalez , page 33
2. WONDERS OF LIFE, Brian Cox, page 37
Last edited by Otangelo on Tue May 31, 2022 7:02 am; edited 17 times in total