Nucleosynthesis - evidence of design
https://reasonandscience.catsboard.com/t3141-nucleosynthesis-evidence-of-design
Elisabeth Vangioni Cosmic origin of the chemical elements rarety in nuclear astrophysics 23 November 2017
There are only three nucleosynthetic astrophysical sites:
(i) big bang nucleosynthesis, where hydrogen and helium are produced;
(ii) stars, where all elements from carbon to uranium are synthesized and
(iii) interstellar medium in galaxies where lithium (a part of), beryllium and boron are made by non-thermal collisions between cosmic rays and interstellar matter.
The origin of the atoms is now well understood. It is one of the greatest astrophysical discovery in the twentieth century.
Big bang nucleosynthesis
There are a few atoms per unit volume in the Universe, only a few 10−31 g/cm3 on average. Moreover, there are only five atoms of lithium-7 synthesized for 10^10 atoms of hydrogen. In the standard big bang cosmology, none of the chemical elements existed from the very beginning. Three were synthesized during the first minutes by nuclear reactions. About one second after the big bang, the Universe had expanded and cooled to the point where nuclear physics plays its role. The individual protons and neutrons in the primordial soup started sticking together to make heavier, more complex nuclei. Before that moment it was just too hot, with a temperature that exceeded 10 billion Kelvin, about a million times hotter than the surface of the Sun today. In a short period called the ‘Era of Nucleosynthesis’, the Universe became a thermonuclear reactor where nuclei of the lightest elements did form. All present hydrogen and deuterium, almost all present helium and a very few lithium (but a significant fraction of the present-day ones) were created. The remnants of these light elements that are detectable today in the cosmos give us an important information about those early conditions and prove that our Universe indeed began in a very hot, condensed state. They can also be used to make a good estimate of the average density of normal matter today. Just before the Era of Nucleosynthesis and after the baryogenesis phase, the Universe was a hot soup of mostly electrons and positrons, photons and neutrinos. However, for every proton or neutron, there were at least a billion photons. The conditions of the Universe, say the kinetic energies of particles, during this epoch, were not too different from those studied here on Earth by nuclear physicists, so its primordial evolution is well understood. We have the greatest confidence that we can make valid statements about the cosmology of this epoch based on our knowledge of nuclear physics. Let us give a closer look.
A key to understand the physics of this period is the concept of thermal equilibrium. This phenomenon represents a state of balance between opposing nuclear reactions. When a system is in thermal equilibrium, its temperature alone determines the relative quantities of the different interacting species, here nuclei, that are present. When the Universe was in thermal equilibrium its temperature governed the ratio of neutrons to protons, n/p. Before one second, the temperature was above ten billions kelvin, the number of neutrons and protons were roughly equal, because these particles of about the same mass were easily converted into each other. After ten seconds, the temperature had fallen to three billions kelvin, and the reactions producing neutrons from protons were slowing down and the ratio n/p had fallen to 1/6. As the Universe kept expanding and cooling, n/p continued to drop because of neutron decay. So after about two hundred seconds, it was only 1/7. This is when the temperature of the Universe had dropped to the point where proton and neutron merged together to form a deuterium nucleus D, which is the heavy isotope of hydrogen, the starting point of further nucleosynthesis. Then, two neutrons plus a proton made a nucleus of tritium, two protons plus a neutron made 3He or two protons and two neutrons made 4He, the most stable of nucleus of all the light element is 4He. Figure 7 presents the nuclear network of BBN.
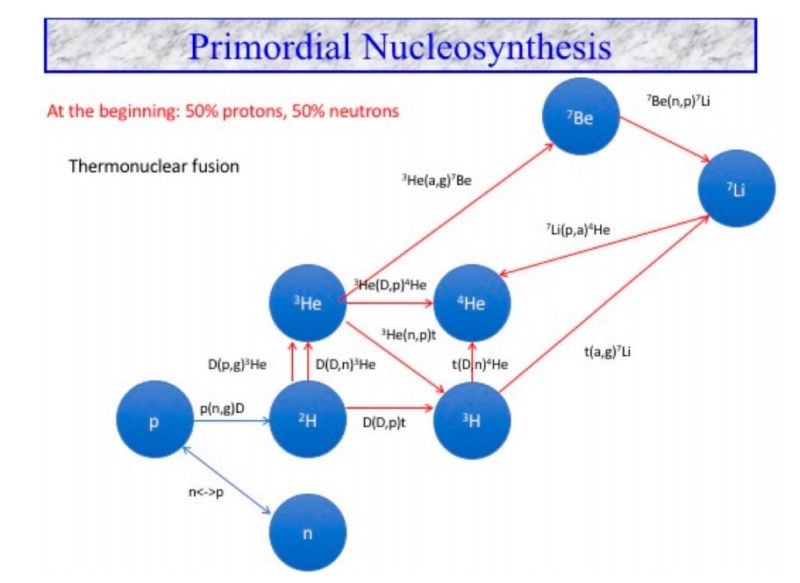
Network of nuclear reactions involved in BBN. There are 12 nuclear reactions responsible for the production of hydrogen, helium and lithium.
There are 12 nuclear reactions that are responsible for the production of these elements via thermonuclear fusion. At the end of BBN, essentially all the baryons in the Universe existed either freely as single protons or were trapped inside of 4He nuclei. Some tiny residues of deuterium D, tritium and 3He remained, along with a trace of 7Li, which has three protons and four neutrons in its nucleus. One sees that the abundances are stabilized after 10,000 seconds or below 108 Kelvin. On the observational side, to derive the most primitive abundances of these elements, one has to extract them from observations of astrophysical sites which are thought to be nonevolved and primitive. Deuterium is a very fragile isotope (since it is an odd–odd nucleus), easily destroyed in stars. Its most primitive abundance is determined from the observation of clouds present on the line of sight of distant quasars (very distant primeval galaxies). Deuterium is only produced during the BBN. Consequently, it is a very good cosmological tracer. 4 He is produced in BBN and by stars. Its primitive abundance is deduced from observations in ionized hydrogen regions of compact blue galaxies, rather primitive. 4He mass fraction is obtained from the extrapolation to zero metallicity (in astrophysics, metallicity represents all elements heavier than helium). The primitive lithium abundance is deduced from observations of low metallicity stars in the halo of our Galaxy where the lithium abundance is almost independent of metallicity. Thanks to this BBN calculation we determined the total quantity of atoms in the Universe: only 4.9% of the total cosmological substance. Presently, primordial nucleosynthesis remains an invaluable tool for probing the physics of the early Universe.
https://www.tandfonline.com/doi/pdf/10.1080/21553769.2017.1411838?needAccess=true
John D. Barrow The Anthropic Cosmological Principle 1986 page 295
The existence of deuterium and the non-existence of the diproton therefore hinge precariously on the precise strength of the nuclear force. If the strong interaction were a little stronger the diproton would be a stable bound state with catastrophic consequences—all the hydrogen in the Universe would have been burnt to He 2 during the early stages of the Big Bang and no hydrogen compounds or long-lived stable stars would exist today. If the di-proton existed we would not! Also, if the nuclear force was a little weaker the deuteron would be unbound with other adverse consequences for the nucleosynthesis of biological elements because a key link in the chain of nucleosynthesis would be removed
https://3lib.net/book/1131892/9a639b
LUKE A. BARNES: The Cosmic Revolutionary’s Handbook (Or: How to Beat the Big Bang) 2020
An important principle is at work here: if you want to make something in the universe, whether a proton or a lead nucleus, you’ll need to wait until things cool off enough for it to survive the ambient temperature. Our focus here is on the elements, so we ask: when is the universe cool enough for nuclei to survive? For most of the periodic table, this would be between 1 and 10 seconds after the big bang. But we’ve got a problem: most of the periodic table doesn’t exist yet. We have to make small nuclei before we can make big ones. And the smallest stable nucleus is that of our old friend deuterium: one proton and one neutron. Why is this a problem? Because deuterium is more fragile than most other elements: it takes about four times less energy to break a deuterium nucleus than any other. So, we have to wait a whole minute before the universe is cool enough that appreciable quantities of deuterium can build up. This delay is known as the deuterium bottleneck. But now the universe can finally make some progress! Protons join with neutrons to form deuterium. Because there are more protons than neutrons, they don’t all pair off in this way – there are left-over protons. After this, and very quickly, deuterium and more protons combine to form helium-3 (two protons and a neutron) and then these combine into ordinary helium (known as helium-4, two protons and two neutrons). It seems that the universe is on its way to making a whole host of elements, including the carbon and oxygen essential to you and me.
But the forces of nature are full of surprises. The next reaction has protons and helium-4 to work with. The options are:
• Proton + proton makes deuterium. This won’t work, because it requires a very slow weak force interaction.
• Helium-4 + proton makes lithium-5 (3 protons, 2 neutrons). This won’t work, because lithium-5 is unstable. It will fall apart before any further reactions can use it.
• Helium + helium makes beryllium-8 (4 protons, 4 neutrons). This won’t work either, because beryllium-8 is also unstable.
Inside stars, the next reaction that makes something stable is, in fact, helium + helium + helium makes carbon. But that reaction, because it involves three nuclei coming together, requires high heat and density. Stars can always collapse a bit more and heat up, but the universe, having had to wait a minute for deuterium to bind, is now too cool. There was a time when carbon could be formed, but it has passed, and it’s not coming back. The universe just keeps cooling, and carbon has missed its chance. After only a few minutes of existence, the universe is too cool for nuclear reactions. The repulsive push of nuclei due to their positive electric charge keeps them apart, and big bang nucleosynthesis is over!
The fundamental machinery
The Standard Model of particle physics lists the particles that we believe are fundamental, out of which everything else is made. Let’s meet the whole gang. As hard as we have tried, we have not succeeded in splitting an electron into smaller pieces. The electron appears to be fundamental, an ultimate building block of the Universe. However, with powerful atom-smashers (which do exactly what it says on the tin, and are more commonly known as particle accelerators) we have been able to poke the innards of protons and neutrons.
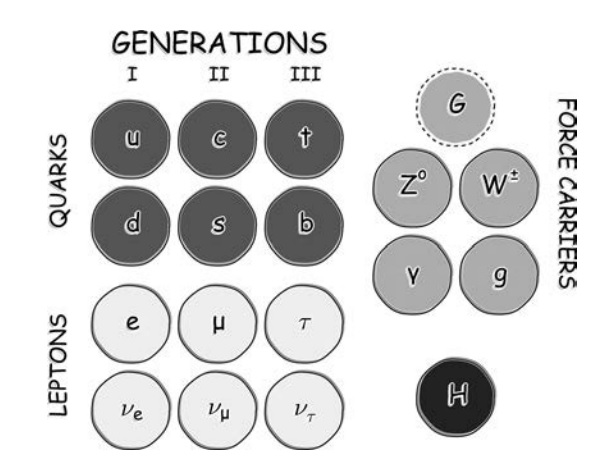
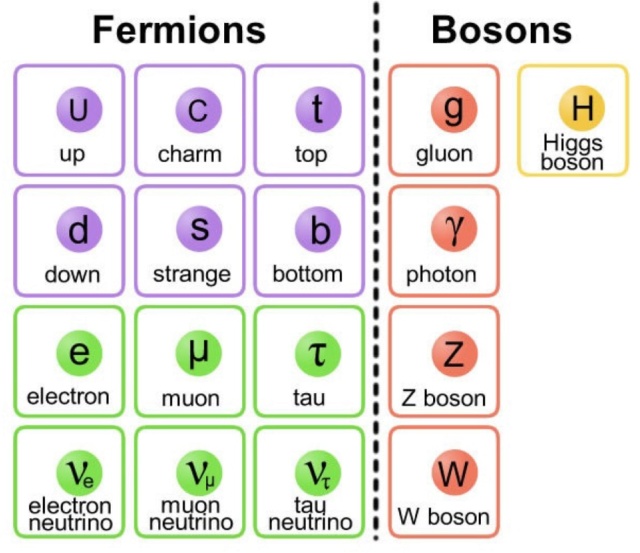
In the Standard Model, the leptons and the quarks are the building blocks of all matter, although virtually everything we see in the Universe is composed of up and down quarks, and electrons. These are coupled with the force carriers and the Higgs boson, which is responsible for mass.
Inside, we have found pieces known as quarks. As with the electron, we have not been able to smash quarks into smaller pieces and we think that they too are the fundamental building blocks of matter. Figure above shows all the pieces. Inside the proton and neutron we have found the up (u) and the down (d) quark. The electron (e) can be found on the left. The electron has −1 units of electric charge, the up quark has +⅔ units, and the down quark −⅓ units. Together, these three particles make up all the matter with which you are familiar. To make a proton, you combine two up quarks with a down quark, to give a total charge of +1, and to make a neutron, you add one up quark to two down quarks, with a resulting charge of zero.
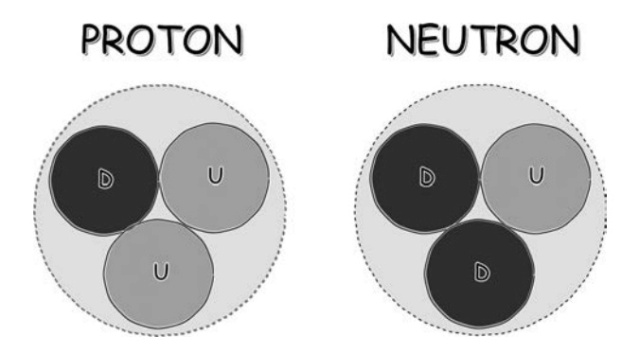
The quark constituents of the proton and neutron. While there are six different quarks available, these key particles, which are the bits of the nucleus of every atom in the Universe, are built from only the two lightest quarks, the up and the down.
The proton and neutron are examples of particles known as baryons, made from three quarks bound together. Once you have protons and neutrons, all you need is to join them to get atomic nuclei, add electrons to get atoms, and onwards to molecules, cells, and human beings. The astute reader may wonder, ‘there are a couple of ways we can combine the up quark and the down quark, so could we join three ups together, or three downs?’ Congratulations, you are now a (novice) particle physicist. Yes, these additional combinations exist. Combining three up quarks gives the attractively named Delta-plus-plus particle (symbolically, Δ++) that has a positive charge of twice that of the proton. The other, the mixture of three down quarks, is the Delta-minus particle (Δ− ), a negatively charged particle with the same charge as the electron. Why don’t we see these other combinations of quarks in everyday life? Because these combinations are unstable and rapidly (and we mean rapidly, in about 0.000000000000001 seconds) decay into the normal matter we see around us. So what of the other particles in the Standard Model? Where did we find those? A little over half a century ago, particle physics was a mess. While perhaps not as messy as cell biology, new experiments were finding huge numbers of different particles. The Standard Model is the final product of decades of intense labor from physicists, searching for underlying simplicity. They discovered that more than the up quark and down quark is required to explain all of the properties of the particles we see in particle accelerators.
Specifically, we need four more quarks, two more with a charge of +⅔, the charm quark and the top quark, as well as two more with a charge of −⅓, the strange quark, and the bottom quark. The up and the down quark are the lightest of the quarks, and as we move along the quarks get more and more massive. These three layers are referred to as the quark generations. Why there are three generations when the Universe seems to only use one is an as-yet unexplained mystery of the cosmos. Why the goofy quark names? Because we had to call them something. It’s not easy naming something you cannot see, and which in our theories is basically a point with a few numbers attached. Not to be left out, the electron has heavier siblings, namely the muon (μ) and tauon (τ), which make up what is known as the lepton generations. The fact that there are three generations of both the leptons and the quarks is probably telling us something pretty deep about the Universe. We just don’t know what. To round off this picture, each member of the lepton family is accompanied by a ghostly, almost massless counterpart, known as the neutrino (ν). While produced in copious amounts in nuclear reactions, these ‘little neutral ones’ (from the Italian) rarely interact and thus stream through matter as if it is not there. In fact, in every second, trillions of neutrinos produced in the heart of the Sun pass harmlessly through your body. Each particle also has a twin: its antiparticle. For every particle that we have observed in a particle accelerator, we have also observed a particle with exactly the same mass but opposite charge. For historical reasons, the electron’s antiparticle is called the positron; the others just have ‘anti’ added to the front of their names. Some particles, like the photon (particle of light), are their own antiparticles. When a particle meets its antiparticle, it can annihilate into two photons. Those twelve little pieces (and their antiparticle twins) are the ultimate building blocks in the Universe. We have arrived at simplicity. All of the complexity we see is built out of a handful of fundamental particles, arranged, like Lego blocks, in a myriad of ways, held together with an even smaller number of fundamental forces. The whole Universe (and any other life form out there) is built the same way.
blocks, in a myriad of ways, held together with an even smaller number of fundamental forces. The whole Universe (and any other life form out there) is built the same way.
For example, the up and down quarks are 4.5 and 9.4 times heavier than the electron.10 These aren’t nice, neat numbers. And yet, they are fundamental to the Standard Model of particle physics. Frustratingly, we can measure them, but we can’t explain them in terms of anything else. The other paraphernalia of the Standard Model aren’t any better. The other four quarks are 190, 2495, 8180 and 338,960 times heavier than the electron, while the muon and tau are 206.768 284 and 3477.15 times heavier. Is there anything special about the particular values they have? What happens in a universe in which the electron and quark masses are slightly different? One might think that, since life is so hardy and robust, you’d just get a different form of life. It might not look like us, but since life in our Universe can make use of the hodge-podge of chemical reactions on offer, any old universe would do something. Right?
Masses of the fundamental zoo
In fact, it is rather easy to arrange for a universe to have no chemistry at all. Grab a hold of the particle mass dials and let’s create a few universes. For simplicity, we will change the mass of only the up and down quarks, the quarks that are the basic constituents of protons and neutrons. You might think that this would simply make heavier protons and neutrons, and hence slightly heavier things in general. However, the picture is a little more complicated than that. Remember why, despite there being so many types of quarks and so many ways of putting them together, we only see matter built from protons (up-up-down) and neutrons (up-down-down). When we use particle accelerators to make heavier particles, such as the Δ++ (Delta++plus-plus, up-up-up), Σ+ (Sigma-plus, up-up-strange) or even the muon, they decay into lighter particles. Why can heavier particles decay into lighter particles? The answer is in Einstein’s famous E = mc2 . By multiplying the particle’s mass (m) by the speed of light (c) squared, we can calculate how much energy (E) can be released. That energy can be used to purchase new particles, so long as the old particle can afford it. Consider the example in Figure below.

The Δ++ is more massive than the sum of the masses of the proton and the pion (π+ ). This means that it is energetically possible for the Δ++ to decay into a proton and a pion
The Δ++ particle has a mass of 1232 in suitable units (these are megaelectronvolts, or MeVs, the particle physicist’s energy unit of choice), while the proton and the pion (a meson, that is, a combination of a quark and an antiquark) have energies associated with their masses of 938 and 140 MeV, respectively. The Δ++ particle has more than enough energy to settle the tab, so the decay into a proton and a pion are allowed. The leftover energy is given to the proton and pion as a little extra kinetic energy, the energy of motion. They fly quickly from the site of the transaction. We’ll come to conservation laws in more detail when we take a deeper look at symmetries in the Universe, but having enough energy is not enough to ensure that the transaction goes through. The Δ++ decay also conserves what is known as baryon number: the total number of quarks minus the number of antiquarks is the same before and after the decay. The meson, being a quark/antiquark pair, has a baryon number of zero and so does not contribute to the tally. This conservation of baryon number means that, even if it had enough mass, and hence energy, the Δ++ could not decay into two protons; baryon number would not be conserved. So the proton holds a very important title: it is the lightest particle made from three quarks. The proton is stable because there is no lighter baryon to decay into. The buck stops here. The neutron, by contrast, decays. If left on its own, it has about 15 minutes before it becomes a proton, an electron and an antineutrino. In the early Universe, where there was nothing but a hot, dense soup of particles and radiation, violent collisions were continuously producing and destroying particles of all masses and types. As the Universe cooled, the more massive baryons decayed into protons and neutrons. The Universe managed to lock some neutrons away inside nuclei in the first few minutes before they decayed. By messing with the up-and-down quark masses, we can change a lot of this story. We can strip the proton of its ‘most stable’ title, and even affect neutrons inside nuclei. Let’s see what happens.
The Delta-Plus-Plus Universe:
Let’s start by increasing the mass of the down quark by a factor of about 70. Down quarks would readily transform into up quarks (and other stuff), even inside protons and neutrons. Thus, they would rapidly decay into the new ‘most stable’ title-holder, our old friend the Δ++ particle. We would find ourselves in the ‘Delta-plus-plus universe’. As we’ve seen, the Δ++ particle is a baryon containing three up quarks. Unlike the proton and neutron, however, the extra charge, and hence electromagnetic repulsion, on the Δ++ particles makes them much harder to bind together. Individual Δ++ particles can capture two electrons to make a helium-like element. And this will be the only element in the universe. Farewell, periodic table! The online PubChem database in our Universe lists 60, 770, 909 chemical compounds (and counting); in the Δ++ universe it would list just one. And, being like helium, it would undergo zero chemical reactions.
The Delta-Minus Universe:
Beginning with our Universe again, let’s instead increase the mass of the up quark by a factor of 130. Again, the proton and neutron will be replaced by one kind of stable particle made of three down quarks, known as the Δ− . Within this Δ− universe, with no neutrons to help dilute the repulsive force of their negative charge, there again will be just one type of atom, and, in a dramatic improvement on the Δ++ universe, one chemical reaction! Two Δ− particles can form a molecule, assuming that we replace all electrons with their positively charged alter-ego, the positron.
The Hydrogen Universe:
To create a hydrogen-only universe, we increase the mass of the down quark by at least a factor of 3. Here, no neutron is safe. Even inside nuclei, neutrons decay. Once again, kiss your chemistry textbook goodbye, as we’d be left with one type of atom and one chemical reaction.
The Neutron Universe:
If you think the hydrogen universe is rather featureless, let’s instead increase the mass of the up quark by a factor of 6. The result is that the proton falls apart. In a reversal of what we see in our Universe, the proton, including protons buried in the apparent safety of the atomic nucleus, decay into neutrons, positrons and neutrinos. This is by far the worst universe we’ve so far encountered: no atoms, no chemical reactions. Just endless, featureless space filled with inert, boring neutrons. There is more than one way to create a neutron universe. Decrease the mass of the down quark by just 8 percent and protons in atoms will capture the electrons in orbit around them, forming neutrons. Atoms would dissolve into clouds of featureless, chemical-free neutrons. What about the other particle of everyday stuff, the electron? Since the electron (and its antiparticle, the positron) is involved in the decay of neutron and proton, it too can sterilize a universe. For example, increase its mass by a factor of 2.5, and we’re in the neutron universe again. The situation is summarized in Figure below.

This summarizes the effect of changing the mass of the electron and the difference in the down and up quark masses. The region is sliced up into universes with uninteresting chemistry, shaded in grey, while the small white region allows chemistry for life. The black dot is our Universe.
An important part of this plot to keep a firm eye on is the limits of the axes. We’ve plotted the quark difference from 0 to 7 MeV, and the electron mass from 0 to 4 MeV. This region is the interesting bit of the plot; the rest is boring and grey. If we wanted, instead, to get an impression of the smallness of the white, life-permitting region, we could extend the axes up to the mass of the largest quark we’ve observed: the top quark. To see this, add 4 kilometres of grey paper to the right, top and bottom of the plot. (If you make this 10,000 acre plot, please send us a photo.) If we extend to the limits of our experimental data, we’d mark 6.5 million MeV at the ends a plot that could cover most of Tasmania, or all of Switzerland, or half of South Korea, or most of West Virginia, or two Death Stars,13 depending on which continent/universe you inhabit. The maximum mass that our theories can handle is called the Planck mass. At this mass, a particle is predicted to become its own black hole. We don’t currently have a theory of quantum gravity – that is, a theory that knows what to do with quantum things (like particles) in the grip of their own gravity. So the Planck mass is a firm limit to our physical theories. And if you can imagine extending the plot above by a few tens of thousands of light-years on each side, then you’re doing better than us. As you can imagine, this is the first step in a long journey, with much more to come. And we have not finished with the masses of the fundamental particles. Stars, the source of the energy that powers our existence, are driven by nuclear reactions. As we’ll see in the next chapter, messing with the properties of quarks and electrons can dramatically affect the stability and lifetime of the energy source of life as we know it.
Giving atoms personality
As well as mass, particles such as electrons and quarks carry other properties that dictate their interactions with other particles. One of the most important, and strangest, is particle spin.
Spin
Spin angular momentum is an intrinsic form of angular momentum carried by elementary particles, composite particles (hadrons), and atomic nuclei. Spin is one of two types of angular momentum in quantum mechanics, the other being orbital angular momentum.
https://en.wikipedia.org/wiki/Spin_(physics)
Spin angular momentum is an intrinsic, or internal, property of elementary particles such as electrons and photons, and has no classical counterpart. The significance of angular momentum in classical physics is that it is one of the fundamental constants of motion (together with linear momentum and energy) of an isolated system. The counterpart of this statement also holds for isolated quantum mechanical systems.
As its name suggests, a particle’s spin is like the spin of the Earth, or like a child’s spinning top. There are several key differences, however, which significantly influence the way the matter in the universe behaves. Firstly, spin is quantized, which means a particle may only have a whole number of indivisible chunks of spin. While some particles lack spin, and so are said to be spin-zero particles, all electrons have exactly the same amount of spin, namely one-half (again, in appropriate units). Other particles have one unit of spin, others again with spins of three halves, with increments of half a unit of spin. This might sound like mere bookkeeping; what does it have to do with the workings of the Universe?
Well, physicists divide particles into two families:
those with an integer value of spin (0, 1, 2, ...) which are called bosons, and
those with half-integer spins (½, 1½, 2½, ...) which are known as the fermions.
Of course, the most famous boson in the world is the Higgs boson, but there are others, as we will see shortly. Why the two families? It’s to do with the way particles pack together. Bosons are flexible little things: in a small box, we can continually pack in more and more bosons and there is nothing to stop us. The most familiar boson in our everyday life is the photon, the particle of light, which has a spin of 1. If you’re trying to pack a box with photons, each new addition doesn’t much mind if there are already photons inside. Fermions, however, do not play by the same rules. Fermions don’t like other similar fermions, and once they have established their space, they don’t let anyone else join them. If you try and pack fermions into a box, eventually the box will be full and you will be unable to squeeze any more in.
The most common fermion to our everyday life is the electron, and this packing limitation of electrons has a profound influence on our world, effectively defining the subject of chemistry. This is the famous Pauli exclusion principle in action. Remembering your atomic physics, electrons live in certain energy levels about atoms, with two in the lowest level of an atom, known as the ground state. Why two? Electrons possess a quantized spin of ½, which can point either upwards or downwards, and so the lowest energy state can be occupied by one electron with its spin pointing upwards, as well as a second electron with its spin pointing downwards. (Classically, we define the direction of a spin with the right-hand-rule: wrap your right hand around the spinning object, with your fingers pointing with the spin. Give a thumbs-up; this defines the direction of the spin).

Spin-½
In quantum mechanics, spin is an intrinsic property of all elementary particles. All known fermions, the particles that constitute ordinary matter, have a spin of ½.The spin number describes how many symmetrical facets a particle has in one full rotation; a spin of ½ means that the particle must be fully rotated twice (through 720°) before it has the same configuration as when it started. Particles having net spin ½ include the proton, neutron, electron, neutrino, and quarks. The dynamics of spin-½ objects cannot be accurately described using classical physics; they are among the simplest systems which require quantum mechanics to describe them. As such, the study of the behavior of spin-½ systems forms a central part of quantum mechanics.
https://en.wikipedia.org/wiki/Spin-½
In terms of packing electrons, this lowest level of the atom is full, and any additional electrons must go into higher levels. And as these upper levels fill, incoming electrons settle into higher and higher energy levels. What does this have to do with chemistry? Well, chemistry is about how atoms interact, and this is completely defined by the locations of the electrons in the outermost energy levels; these are the most loosely bound electrons, and it is these that can be swapped between atoms to allow them to bond and form complex molecules. It is these electrons, and the properties of their spin and orbits, that give atoms their personality. The same is true of atomic nuclei. Protons and neutrons, themselves fermions, can only be found on distinct energy levels with the nucleus, packed in accordance with the Pauli exclusion principle. This explains why, while isolated neutrons rapidly decay, a neutron inside an atomic nucleus is effectively stable. It cannot decay because no lower energy positions are available for the resultant proton.
What if electrons were bosons rather than fermions?
The result would be disastrous as there would be nothing to prevent all the electrons occupying the lowest level in an atom, like stuffing as many photons in the box as you can. Once again, wave chemistry – and the chemical complexity and flexibility needed by life – goodbye. These bosonic electrons would be very tightly bound to their nuclei, with little inclination to be shared with other atoms. This universe would be a sea of individual atoms floating through the cosmos, minding their own business and not getting involved in this messy work of forming molecules. However, the situation could be even more complicated! As well as the electrons, the quarks are also fermions, also possessing a spin of one-half. And this spin is imprinted (in a rather complex fashion) onto the particle they form. And both the proton and the neutron, even though they are composite objects, have a total spin of one-half, also making them fermions, and ensuring they obey the fermion rules of packing. This means that the protons and neutrons of an atom are arranged in orbits very similar to the orbits of the much more distant electrons. If quarks, and so also the protons and neutrons, had an integer spin, just like the electrons, there would be nothing to prevent all of them collapsing down and occupying the lowest energy level. Collapsing all of the protons and neutrons in your atoms is not necessarily a catastrophe; there are more important things to worry about.
Melting the solid-state
There is another way that electrons can bring about detrimental changes to our Universe. When two atoms or molecules come together and shuffle electrons, a chemical reaction has occurred. Sometimes, the electron is not pilfered ( steal (typically things of relatively little value)). but shared: as the two atoms or molecules wrestle for possession of the electron, a chemical bond is formed. In solids, atoms are held together by chemical bonds in a fixed lattice. Imagine a three-dimensional grid of balls held together by springs, something like the one in Figure below.
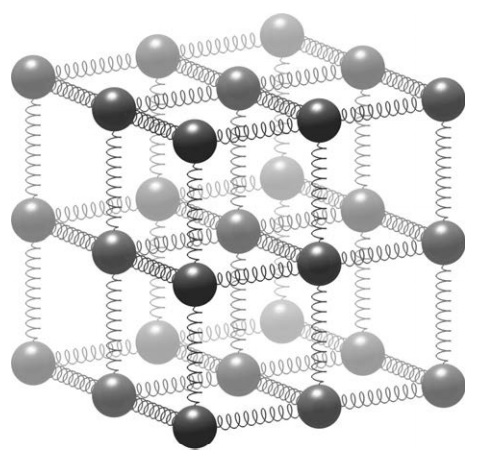
A simple ball and spring model for a molecular lattice. Atoms are held in a repeating crystal structure and vibrate as the bonds between them stretch and compress. Heating the solid causes the atoms to vibrate faster and further, straining the bonds. If enough heat is added, the bonds break and the substance melts. The melting point of the lattice depends upon the masses of the fundamental particles and the strength of the fundamental forces. Changing these can make the springs break more easily, or the atoms vibrate more violently, melting solids into liquids
We can break the lattice by shaking it vigorously. Similarly, we can melt a solid by heating it up. Heat consists of microscopic random motions, and too much jiggling of the lattice will break the chemical bonds between the atoms. The atoms move too quickly for any particular bond to hold for very long, and the material loses its rigidity, turning into a liquid or a gas. There is a natural, unavoidable jiggling in all objects, which comes from quantum mechanics. Unlike heating, this jiggling is a fundamental property of the quantum world, preventing us from ever cooling something to absolute zero as this would require the atoms in our lattice to be completely still. In our Universe, this jiggling is relatively gentle and won’t melt objects so long as electrons are much lighter than protons. The quantum jiggling will cause electrons to buzz around the nuclei, but they’re too light (1836.15 times lighter than the proton) to knock nuclei out of the lattice. And so, solid materials stay solid as long as the temperature is sufficiently low. However, if the electron mass were within a factor of a hundred of the proton mass, the quantum jiggling of electrons would destroy the lattice. In short: no solids. No solid planets, no stable DNA molecules, no bones, no semi-permeable living cell walls, no organs. A complete mess, and almost certainly no life!
Lars Bergström Cosmology and particle astrophysics 2006
Baryonic Matter
Ordinary matter, made of protons and neutrons, is generically called baryonic matter. The particle physics definition of baryonic matter also includes other shortlived particles (see Chapter 6) but those are not stable over cosmological time scales. In normal matter, electrons are of course also present in equal numbers as protons. However, being almost 2000 times lighter, they do not contribute much to the present mass density of the Universe. Baryons are found in a variety of forms: in gaseous clouds, either of neutral atoms or molecules, or in ionized plasma, in frozen condensations in comets and in dense, hot, environments such as planets, stars and stellar remnants such as white dwarfs, neutron stars and presumably in black holes. Except for planets and stellar remnants, baryonic condensations consist mainly of hydrogen and helium.
https://3lib.net/book/451734/719a46
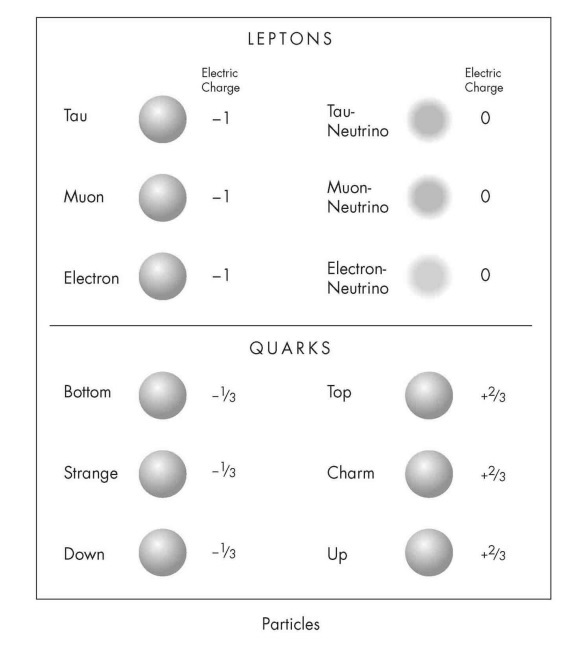
The twelve particles that make up all known matter. They all have spin ½. Each has a corresponding antiparticle. The quarks are not found in isolation but exist either in combinations of three or in unstable quark-antiquark pairs. Electric charge is given here in units of the charge on the proton. The particles in Families 2 and 3 decay into Family 1 particles.
B.D. Fields Big Bang Nucleosynthesis 2020
Big-Bang nucleosynthesis (BBN) offers the deepest reliable probe of the early Universe, being based on well-understood Standard Model physics [1]. Predictions of the abundances of the light elements, D, 3He, 4He, and 7Li, synthesized at the end of the first three minutes, are in good overall agreement with the primordial abundances inferred from observational data, thus validating the standard hot Big-Bang cosmology (see [2–5] for reviews). This is particularly impressive given that these abundances span nine orders of magnitude – from 4He/H∼ 0.08 down to 7Li/H∼ 10−10 (ratios by number). Thus BBN provides powerful constraints on possible deviations from the standard cosmology, and on new physics beyond the Standard Model
https://pdg.lbl.gov/2020/reviews/rpp2020-rev-bbang-nucleosynthesis.pdf
https://reasonandscience.catsboard.com/t3141-nucleosynthesis-evidence-of-design
Elisabeth Vangioni Cosmic origin of the chemical elements rarety in nuclear astrophysics 23 November 2017
There are only three nucleosynthetic astrophysical sites:
(i) big bang nucleosynthesis, where hydrogen and helium are produced;
(ii) stars, where all elements from carbon to uranium are synthesized and
(iii) interstellar medium in galaxies where lithium (a part of), beryllium and boron are made by non-thermal collisions between cosmic rays and interstellar matter.
The origin of the atoms is now well understood. It is one of the greatest astrophysical discovery in the twentieth century.
Big bang nucleosynthesis
There are a few atoms per unit volume in the Universe, only a few 10−31 g/cm3 on average. Moreover, there are only five atoms of lithium-7 synthesized for 10^10 atoms of hydrogen. In the standard big bang cosmology, none of the chemical elements existed from the very beginning. Three were synthesized during the first minutes by nuclear reactions. About one second after the big bang, the Universe had expanded and cooled to the point where nuclear physics plays its role. The individual protons and neutrons in the primordial soup started sticking together to make heavier, more complex nuclei. Before that moment it was just too hot, with a temperature that exceeded 10 billion Kelvin, about a million times hotter than the surface of the Sun today. In a short period called the ‘Era of Nucleosynthesis’, the Universe became a thermonuclear reactor where nuclei of the lightest elements did form. All present hydrogen and deuterium, almost all present helium and a very few lithium (but a significant fraction of the present-day ones) were created. The remnants of these light elements that are detectable today in the cosmos give us an important information about those early conditions and prove that our Universe indeed began in a very hot, condensed state. They can also be used to make a good estimate of the average density of normal matter today. Just before the Era of Nucleosynthesis and after the baryogenesis phase, the Universe was a hot soup of mostly electrons and positrons, photons and neutrinos. However, for every proton or neutron, there were at least a billion photons. The conditions of the Universe, say the kinetic energies of particles, during this epoch, were not too different from those studied here on Earth by nuclear physicists, so its primordial evolution is well understood. We have the greatest confidence that we can make valid statements about the cosmology of this epoch based on our knowledge of nuclear physics. Let us give a closer look.
A key to understand the physics of this period is the concept of thermal equilibrium. This phenomenon represents a state of balance between opposing nuclear reactions. When a system is in thermal equilibrium, its temperature alone determines the relative quantities of the different interacting species, here nuclei, that are present. When the Universe was in thermal equilibrium its temperature governed the ratio of neutrons to protons, n/p. Before one second, the temperature was above ten billions kelvin, the number of neutrons and protons were roughly equal, because these particles of about the same mass were easily converted into each other. After ten seconds, the temperature had fallen to three billions kelvin, and the reactions producing neutrons from protons were slowing down and the ratio n/p had fallen to 1/6. As the Universe kept expanding and cooling, n/p continued to drop because of neutron decay. So after about two hundred seconds, it was only 1/7. This is when the temperature of the Universe had dropped to the point where proton and neutron merged together to form a deuterium nucleus D, which is the heavy isotope of hydrogen, the starting point of further nucleosynthesis. Then, two neutrons plus a proton made a nucleus of tritium, two protons plus a neutron made 3He or two protons and two neutrons made 4He, the most stable of nucleus of all the light element is 4He. Figure 7 presents the nuclear network of BBN.

Network of nuclear reactions involved in BBN. There are 12 nuclear reactions responsible for the production of hydrogen, helium and lithium.
There are 12 nuclear reactions that are responsible for the production of these elements via thermonuclear fusion. At the end of BBN, essentially all the baryons in the Universe existed either freely as single protons or were trapped inside of 4He nuclei. Some tiny residues of deuterium D, tritium and 3He remained, along with a trace of 7Li, which has three protons and four neutrons in its nucleus. One sees that the abundances are stabilized after 10,000 seconds or below 108 Kelvin. On the observational side, to derive the most primitive abundances of these elements, one has to extract them from observations of astrophysical sites which are thought to be nonevolved and primitive. Deuterium is a very fragile isotope (since it is an odd–odd nucleus), easily destroyed in stars. Its most primitive abundance is determined from the observation of clouds present on the line of sight of distant quasars (very distant primeval galaxies). Deuterium is only produced during the BBN. Consequently, it is a very good cosmological tracer. 4 He is produced in BBN and by stars. Its primitive abundance is deduced from observations in ionized hydrogen regions of compact blue galaxies, rather primitive. 4He mass fraction is obtained from the extrapolation to zero metallicity (in astrophysics, metallicity represents all elements heavier than helium). The primitive lithium abundance is deduced from observations of low metallicity stars in the halo of our Galaxy where the lithium abundance is almost independent of metallicity. Thanks to this BBN calculation we determined the total quantity of atoms in the Universe: only 4.9% of the total cosmological substance. Presently, primordial nucleosynthesis remains an invaluable tool for probing the physics of the early Universe.
https://www.tandfonline.com/doi/pdf/10.1080/21553769.2017.1411838?needAccess=true
John D. Barrow The Anthropic Cosmological Principle 1986 page 295
The existence of deuterium and the non-existence of the diproton therefore hinge precariously on the precise strength of the nuclear force. If the strong interaction were a little stronger the diproton would be a stable bound state with catastrophic consequences—all the hydrogen in the Universe would have been burnt to He 2 during the early stages of the Big Bang and no hydrogen compounds or long-lived stable stars would exist today. If the di-proton existed we would not! Also, if the nuclear force was a little weaker the deuteron would be unbound with other adverse consequences for the nucleosynthesis of biological elements because a key link in the chain of nucleosynthesis would be removed
https://3lib.net/book/1131892/9a639b
LUKE A. BARNES: The Cosmic Revolutionary’s Handbook (Or: How to Beat the Big Bang) 2020
An important principle is at work here: if you want to make something in the universe, whether a proton or a lead nucleus, you’ll need to wait until things cool off enough for it to survive the ambient temperature. Our focus here is on the elements, so we ask: when is the universe cool enough for nuclei to survive? For most of the periodic table, this would be between 1 and 10 seconds after the big bang. But we’ve got a problem: most of the periodic table doesn’t exist yet. We have to make small nuclei before we can make big ones. And the smallest stable nucleus is that of our old friend deuterium: one proton and one neutron. Why is this a problem? Because deuterium is more fragile than most other elements: it takes about four times less energy to break a deuterium nucleus than any other. So, we have to wait a whole minute before the universe is cool enough that appreciable quantities of deuterium can build up. This delay is known as the deuterium bottleneck. But now the universe can finally make some progress! Protons join with neutrons to form deuterium. Because there are more protons than neutrons, they don’t all pair off in this way – there are left-over protons. After this, and very quickly, deuterium and more protons combine to form helium-3 (two protons and a neutron) and then these combine into ordinary helium (known as helium-4, two protons and two neutrons). It seems that the universe is on its way to making a whole host of elements, including the carbon and oxygen essential to you and me.
But the forces of nature are full of surprises. The next reaction has protons and helium-4 to work with. The options are:
• Proton + proton makes deuterium. This won’t work, because it requires a very slow weak force interaction.
• Helium-4 + proton makes lithium-5 (3 protons, 2 neutrons). This won’t work, because lithium-5 is unstable. It will fall apart before any further reactions can use it.
• Helium + helium makes beryllium-8 (4 protons, 4 neutrons). This won’t work either, because beryllium-8 is also unstable.
Inside stars, the next reaction that makes something stable is, in fact, helium + helium + helium makes carbon. But that reaction, because it involves three nuclei coming together, requires high heat and density. Stars can always collapse a bit more and heat up, but the universe, having had to wait a minute for deuterium to bind, is now too cool. There was a time when carbon could be formed, but it has passed, and it’s not coming back. The universe just keeps cooling, and carbon has missed its chance. After only a few minutes of existence, the universe is too cool for nuclear reactions. The repulsive push of nuclei due to their positive electric charge keeps them apart, and big bang nucleosynthesis is over!
The fundamental machinery
The Standard Model of particle physics lists the particles that we believe are fundamental, out of which everything else is made. Let’s meet the whole gang. As hard as we have tried, we have not succeeded in splitting an electron into smaller pieces. The electron appears to be fundamental, an ultimate building block of the Universe. However, with powerful atom-smashers (which do exactly what it says on the tin, and are more commonly known as particle accelerators) we have been able to poke the innards of protons and neutrons.

In the Standard Model, the leptons and the quarks are the building blocks of all matter, although virtually everything we see in the Universe is composed of up and down quarks, and electrons. These are coupled with the force carriers and the Higgs boson, which is responsible for mass.
Inside, we have found pieces known as quarks. As with the electron, we have not been able to smash quarks into smaller pieces and we think that they too are the fundamental building blocks of matter. Figure above shows all the pieces. Inside the proton and neutron we have found the up (u) and the down (d) quark. The electron (e) can be found on the left. The electron has −1 units of electric charge, the up quark has +⅔ units, and the down quark −⅓ units. Together, these three particles make up all the matter with which you are familiar. To make a proton, you combine two up quarks with a down quark, to give a total charge of +1, and to make a neutron, you add one up quark to two down quarks, with a resulting charge of zero.

The quark constituents of the proton and neutron. While there are six different quarks available, these key particles, which are the bits of the nucleus of every atom in the Universe, are built from only the two lightest quarks, the up and the down.
The proton and neutron are examples of particles known as baryons, made from three quarks bound together. Once you have protons and neutrons, all you need is to join them to get atomic nuclei, add electrons to get atoms, and onwards to molecules, cells, and human beings. The astute reader may wonder, ‘there are a couple of ways we can combine the up quark and the down quark, so could we join three ups together, or three downs?’ Congratulations, you are now a (novice) particle physicist. Yes, these additional combinations exist. Combining three up quarks gives the attractively named Delta-plus-plus particle (symbolically, Δ++) that has a positive charge of twice that of the proton. The other, the mixture of three down quarks, is the Delta-minus particle (Δ− ), a negatively charged particle with the same charge as the electron. Why don’t we see these other combinations of quarks in everyday life? Because these combinations are unstable and rapidly (and we mean rapidly, in about 0.000000000000001 seconds) decay into the normal matter we see around us. So what of the other particles in the Standard Model? Where did we find those? A little over half a century ago, particle physics was a mess. While perhaps not as messy as cell biology, new experiments were finding huge numbers of different particles. The Standard Model is the final product of decades of intense labor from physicists, searching for underlying simplicity. They discovered that more than the up quark and down quark is required to explain all of the properties of the particles we see in particle accelerators.
Specifically, we need four more quarks, two more with a charge of +⅔, the charm quark and the top quark, as well as two more with a charge of −⅓, the strange quark, and the bottom quark. The up and the down quark are the lightest of the quarks, and as we move along the quarks get more and more massive. These three layers are referred to as the quark generations. Why there are three generations when the Universe seems to only use one is an as-yet unexplained mystery of the cosmos. Why the goofy quark names? Because we had to call them something. It’s not easy naming something you cannot see, and which in our theories is basically a point with a few numbers attached. Not to be left out, the electron has heavier siblings, namely the muon (μ) and tauon (τ), which make up what is known as the lepton generations. The fact that there are three generations of both the leptons and the quarks is probably telling us something pretty deep about the Universe. We just don’t know what. To round off this picture, each member of the lepton family is accompanied by a ghostly, almost massless counterpart, known as the neutrino (ν). While produced in copious amounts in nuclear reactions, these ‘little neutral ones’ (from the Italian) rarely interact and thus stream through matter as if it is not there. In fact, in every second, trillions of neutrinos produced in the heart of the Sun pass harmlessly through your body. Each particle also has a twin: its antiparticle. For every particle that we have observed in a particle accelerator, we have also observed a particle with exactly the same mass but opposite charge. For historical reasons, the electron’s antiparticle is called the positron; the others just have ‘anti’ added to the front of their names. Some particles, like the photon (particle of light), are their own antiparticles. When a particle meets its antiparticle, it can annihilate into two photons. Those twelve little pieces (and their antiparticle twins) are the ultimate building blocks in the Universe. We have arrived at simplicity. All of the complexity we see is built out of a handful of fundamental particles, arranged, like Lego
For example, the up and down quarks are 4.5 and 9.4 times heavier than the electron.10 These aren’t nice, neat numbers. And yet, they are fundamental to the Standard Model of particle physics. Frustratingly, we can measure them, but we can’t explain them in terms of anything else. The other paraphernalia of the Standard Model aren’t any better. The other four quarks are 190, 2495, 8180 and 338,960 times heavier than the electron, while the muon and tau are 206.768 284 and 3477.15 times heavier. Is there anything special about the particular values they have? What happens in a universe in which the electron and quark masses are slightly different? One might think that, since life is so hardy and robust, you’d just get a different form of life. It might not look like us, but since life in our Universe can make use of the hodge-podge of chemical reactions on offer, any old universe would do something. Right?
Masses of the fundamental zoo
In fact, it is rather easy to arrange for a universe to have no chemistry at all. Grab a hold of the particle mass dials and let’s create a few universes. For simplicity, we will change the mass of only the up and down quarks, the quarks that are the basic constituents of protons and neutrons. You might think that this would simply make heavier protons and neutrons, and hence slightly heavier things in general. However, the picture is a little more complicated than that. Remember why, despite there being so many types of quarks and so many ways of putting them together, we only see matter built from protons (up-up-down) and neutrons (up-down-down). When we use particle accelerators to make heavier particles, such as the Δ++ (Delta++plus-plus, up-up-up), Σ+ (Sigma-plus, up-up-strange) or even the muon, they decay into lighter particles. Why can heavier particles decay into lighter particles? The answer is in Einstein’s famous E = mc2 . By multiplying the particle’s mass (m) by the speed of light (c) squared, we can calculate how much energy (E) can be released. That energy can be used to purchase new particles, so long as the old particle can afford it. Consider the example in Figure below.

The Δ++ is more massive than the sum of the masses of the proton and the pion (π+ ). This means that it is energetically possible for the Δ++ to decay into a proton and a pion
The Δ++ particle has a mass of 1232 in suitable units (these are megaelectronvolts, or MeVs, the particle physicist’s energy unit of choice), while the proton and the pion (a meson, that is, a combination of a quark and an antiquark) have energies associated with their masses of 938 and 140 MeV, respectively. The Δ++ particle has more than enough energy to settle the tab, so the decay into a proton and a pion are allowed. The leftover energy is given to the proton and pion as a little extra kinetic energy, the energy of motion. They fly quickly from the site of the transaction. We’ll come to conservation laws in more detail when we take a deeper look at symmetries in the Universe, but having enough energy is not enough to ensure that the transaction goes through. The Δ++ decay also conserves what is known as baryon number: the total number of quarks minus the number of antiquarks is the same before and after the decay. The meson, being a quark/antiquark pair, has a baryon number of zero and so does not contribute to the tally. This conservation of baryon number means that, even if it had enough mass, and hence energy, the Δ++ could not decay into two protons; baryon number would not be conserved. So the proton holds a very important title: it is the lightest particle made from three quarks. The proton is stable because there is no lighter baryon to decay into. The buck stops here. The neutron, by contrast, decays. If left on its own, it has about 15 minutes before it becomes a proton, an electron and an antineutrino. In the early Universe, where there was nothing but a hot, dense soup of particles and radiation, violent collisions were continuously producing and destroying particles of all masses and types. As the Universe cooled, the more massive baryons decayed into protons and neutrons. The Universe managed to lock some neutrons away inside nuclei in the first few minutes before they decayed. By messing with the up-and-down quark masses, we can change a lot of this story. We can strip the proton of its ‘most stable’ title, and even affect neutrons inside nuclei. Let’s see what happens.
The Delta-Plus-Plus Universe:
Let’s start by increasing the mass of the down quark by a factor of about 70. Down quarks would readily transform into up quarks (and other stuff), even inside protons and neutrons. Thus, they would rapidly decay into the new ‘most stable’ title-holder, our old friend the Δ++ particle. We would find ourselves in the ‘Delta-plus-plus universe’. As we’ve seen, the Δ++ particle is a baryon containing three up quarks. Unlike the proton and neutron, however, the extra charge, and hence electromagnetic repulsion, on the Δ++ particles makes them much harder to bind together. Individual Δ++ particles can capture two electrons to make a helium-like element. And this will be the only element in the universe. Farewell, periodic table! The online PubChem database in our Universe lists 60, 770, 909 chemical compounds (and counting); in the Δ++ universe it would list just one. And, being like helium, it would undergo zero chemical reactions.
The Delta-Minus Universe:
Beginning with our Universe again, let’s instead increase the mass of the up quark by a factor of 130. Again, the proton and neutron will be replaced by one kind of stable particle made of three down quarks, known as the Δ− . Within this Δ− universe, with no neutrons to help dilute the repulsive force of their negative charge, there again will be just one type of atom, and, in a dramatic improvement on the Δ++ universe, one chemical reaction! Two Δ− particles can form a molecule, assuming that we replace all electrons with their positively charged alter-ego, the positron.
The Hydrogen Universe:
To create a hydrogen-only universe, we increase the mass of the down quark by at least a factor of 3. Here, no neutron is safe. Even inside nuclei, neutrons decay. Once again, kiss your chemistry textbook goodbye, as we’d be left with one type of atom and one chemical reaction.
The Neutron Universe:
If you think the hydrogen universe is rather featureless, let’s instead increase the mass of the up quark by a factor of 6. The result is that the proton falls apart. In a reversal of what we see in our Universe, the proton, including protons buried in the apparent safety of the atomic nucleus, decay into neutrons, positrons and neutrinos. This is by far the worst universe we’ve so far encountered: no atoms, no chemical reactions. Just endless, featureless space filled with inert, boring neutrons. There is more than one way to create a neutron universe. Decrease the mass of the down quark by just 8 percent and protons in atoms will capture the electrons in orbit around them, forming neutrons. Atoms would dissolve into clouds of featureless, chemical-free neutrons. What about the other particle of everyday stuff, the electron? Since the electron (and its antiparticle, the positron) is involved in the decay of neutron and proton, it too can sterilize a universe. For example, increase its mass by a factor of 2.5, and we’re in the neutron universe again. The situation is summarized in Figure below.

This summarizes the effect of changing the mass of the electron and the difference in the down and up quark masses. The region is sliced up into universes with uninteresting chemistry, shaded in grey, while the small white region allows chemistry for life. The black dot is our Universe.
An important part of this plot to keep a firm eye on is the limits of the axes. We’ve plotted the quark difference from 0 to 7 MeV, and the electron mass from 0 to 4 MeV. This region is the interesting bit of the plot; the rest is boring and grey. If we wanted, instead, to get an impression of the smallness of the white, life-permitting region, we could extend the axes up to the mass of the largest quark we’ve observed: the top quark. To see this, add 4 kilometres of grey paper to the right, top and bottom of the plot. (If you make this 10,000 acre plot, please send us a photo.) If we extend to the limits of our experimental data, we’d mark 6.5 million MeV at the ends a plot that could cover most of Tasmania, or all of Switzerland, or half of South Korea, or most of West Virginia, or two Death Stars,13 depending on which continent/universe you inhabit. The maximum mass that our theories can handle is called the Planck mass. At this mass, a particle is predicted to become its own black hole. We don’t currently have a theory of quantum gravity – that is, a theory that knows what to do with quantum things (like particles) in the grip of their own gravity. So the Planck mass is a firm limit to our physical theories. And if you can imagine extending the plot above by a few tens of thousands of light-years on each side, then you’re doing better than us. As you can imagine, this is the first step in a long journey, with much more to come. And we have not finished with the masses of the fundamental particles. Stars, the source of the energy that powers our existence, are driven by nuclear reactions. As we’ll see in the next chapter, messing with the properties of quarks and electrons can dramatically affect the stability and lifetime of the energy source of life as we know it.
Giving atoms personality
As well as mass, particles such as electrons and quarks carry other properties that dictate their interactions with other particles. One of the most important, and strangest, is particle spin.
Spin
Spin angular momentum is an intrinsic form of angular momentum carried by elementary particles, composite particles (hadrons), and atomic nuclei. Spin is one of two types of angular momentum in quantum mechanics, the other being orbital angular momentum.
https://en.wikipedia.org/wiki/Spin_(physics)
Spin angular momentum is an intrinsic, or internal, property of elementary particles such as electrons and photons, and has no classical counterpart. The significance of angular momentum in classical physics is that it is one of the fundamental constants of motion (together with linear momentum and energy) of an isolated system. The counterpart of this statement also holds for isolated quantum mechanical systems.
As its name suggests, a particle’s spin is like the spin of the Earth, or like a child’s spinning top. There are several key differences, however, which significantly influence the way the matter in the universe behaves. Firstly, spin is quantized, which means a particle may only have a whole number of indivisible chunks of spin. While some particles lack spin, and so are said to be spin-zero particles, all electrons have exactly the same amount of spin, namely one-half (again, in appropriate units). Other particles have one unit of spin, others again with spins of three halves, with increments of half a unit of spin. This might sound like mere bookkeeping; what does it have to do with the workings of the Universe?
Well, physicists divide particles into two families:
those with an integer value of spin (0, 1, 2, ...) which are called bosons, and
those with half-integer spins (½, 1½, 2½, ...) which are known as the fermions.
Of course, the most famous boson in the world is the Higgs boson, but there are others, as we will see shortly. Why the two families? It’s to do with the way particles pack together. Bosons are flexible little things: in a small box, we can continually pack in more and more bosons and there is nothing to stop us. The most familiar boson in our everyday life is the photon, the particle of light, which has a spin of 1. If you’re trying to pack a box with photons, each new addition doesn’t much mind if there are already photons inside. Fermions, however, do not play by the same rules. Fermions don’t like other similar fermions, and once they have established their space, they don’t let anyone else join them. If you try and pack fermions into a box, eventually the box will be full and you will be unable to squeeze any more in.
The most common fermion to our everyday life is the electron, and this packing limitation of electrons has a profound influence on our world, effectively defining the subject of chemistry. This is the famous Pauli exclusion principle in action. Remembering your atomic physics, electrons live in certain energy levels about atoms, with two in the lowest level of an atom, known as the ground state. Why two? Electrons possess a quantized spin of ½, which can point either upwards or downwards, and so the lowest energy state can be occupied by one electron with its spin pointing upwards, as well as a second electron with its spin pointing downwards. (Classically, we define the direction of a spin with the right-hand-rule: wrap your right hand around the spinning object, with your fingers pointing with the spin. Give a thumbs-up; this defines the direction of the spin).

Spin-½
In quantum mechanics, spin is an intrinsic property of all elementary particles. All known fermions, the particles that constitute ordinary matter, have a spin of ½.The spin number describes how many symmetrical facets a particle has in one full rotation; a spin of ½ means that the particle must be fully rotated twice (through 720°) before it has the same configuration as when it started. Particles having net spin ½ include the proton, neutron, electron, neutrino, and quarks. The dynamics of spin-½ objects cannot be accurately described using classical physics; they are among the simplest systems which require quantum mechanics to describe them. As such, the study of the behavior of spin-½ systems forms a central part of quantum mechanics.
https://en.wikipedia.org/wiki/Spin-½
In terms of packing electrons, this lowest level of the atom is full, and any additional electrons must go into higher levels. And as these upper levels fill, incoming electrons settle into higher and higher energy levels. What does this have to do with chemistry? Well, chemistry is about how atoms interact, and this is completely defined by the locations of the electrons in the outermost energy levels; these are the most loosely bound electrons, and it is these that can be swapped between atoms to allow them to bond and form complex molecules. It is these electrons, and the properties of their spin and orbits, that give atoms their personality. The same is true of atomic nuclei. Protons and neutrons, themselves fermions, can only be found on distinct energy levels with the nucleus, packed in accordance with the Pauli exclusion principle. This explains why, while isolated neutrons rapidly decay, a neutron inside an atomic nucleus is effectively stable. It cannot decay because no lower energy positions are available for the resultant proton.
What if electrons were bosons rather than fermions?
The result would be disastrous as there would be nothing to prevent all the electrons occupying the lowest level in an atom, like stuffing as many photons in the box as you can. Once again, wave chemistry – and the chemical complexity and flexibility needed by life – goodbye. These bosonic electrons would be very tightly bound to their nuclei, with little inclination to be shared with other atoms. This universe would be a sea of individual atoms floating through the cosmos, minding their own business and not getting involved in this messy work of forming molecules. However, the situation could be even more complicated! As well as the electrons, the quarks are also fermions, also possessing a spin of one-half. And this spin is imprinted (in a rather complex fashion) onto the particle they form. And both the proton and the neutron, even though they are composite objects, have a total spin of one-half, also making them fermions, and ensuring they obey the fermion rules of packing. This means that the protons and neutrons of an atom are arranged in orbits very similar to the orbits of the much more distant electrons. If quarks, and so also the protons and neutrons, had an integer spin, just like the electrons, there would be nothing to prevent all of them collapsing down and occupying the lowest energy level. Collapsing all of the protons and neutrons in your atoms is not necessarily a catastrophe; there are more important things to worry about.
Melting the solid-state
There is another way that electrons can bring about detrimental changes to our Universe. When two atoms or molecules come together and shuffle electrons, a chemical reaction has occurred. Sometimes, the electron is not pilfered ( steal (typically things of relatively little value)). but shared: as the two atoms or molecules wrestle for possession of the electron, a chemical bond is formed. In solids, atoms are held together by chemical bonds in a fixed lattice. Imagine a three-dimensional grid of balls held together by springs, something like the one in Figure below.

A simple ball and spring model for a molecular lattice. Atoms are held in a repeating crystal structure and vibrate as the bonds between them stretch and compress. Heating the solid causes the atoms to vibrate faster and further, straining the bonds. If enough heat is added, the bonds break and the substance melts. The melting point of the lattice depends upon the masses of the fundamental particles and the strength of the fundamental forces. Changing these can make the springs break more easily, or the atoms vibrate more violently, melting solids into liquids
We can break the lattice by shaking it vigorously. Similarly, we can melt a solid by heating it up. Heat consists of microscopic random motions, and too much jiggling of the lattice will break the chemical bonds between the atoms. The atoms move too quickly for any particular bond to hold for very long, and the material loses its rigidity, turning into a liquid or a gas. There is a natural, unavoidable jiggling in all objects, which comes from quantum mechanics. Unlike heating, this jiggling is a fundamental property of the quantum world, preventing us from ever cooling something to absolute zero as this would require the atoms in our lattice to be completely still. In our Universe, this jiggling is relatively gentle and won’t melt objects so long as electrons are much lighter than protons. The quantum jiggling will cause electrons to buzz around the nuclei, but they’re too light (1836.15 times lighter than the proton) to knock nuclei out of the lattice. And so, solid materials stay solid as long as the temperature is sufficiently low. However, if the electron mass were within a factor of a hundred of the proton mass, the quantum jiggling of electrons would destroy the lattice. In short: no solids. No solid planets, no stable DNA molecules, no bones, no semi-permeable living cell walls, no organs. A complete mess, and almost certainly no life!
Lars Bergström Cosmology and particle astrophysics 2006
Baryonic Matter
Ordinary matter, made of protons and neutrons, is generically called baryonic matter. The particle physics definition of baryonic matter also includes other shortlived particles (see Chapter 6) but those are not stable over cosmological time scales. In normal matter, electrons are of course also present in equal numbers as protons. However, being almost 2000 times lighter, they do not contribute much to the present mass density of the Universe. Baryons are found in a variety of forms: in gaseous clouds, either of neutral atoms or molecules, or in ionized plasma, in frozen condensations in comets and in dense, hot, environments such as planets, stars and stellar remnants such as white dwarfs, neutron stars and presumably in black holes. Except for planets and stellar remnants, baryonic condensations consist mainly of hydrogen and helium.
https://3lib.net/book/451734/719a46

The twelve particles that make up all known matter. They all have spin ½. Each has a corresponding antiparticle. The quarks are not found in isolation but exist either in combinations of three or in unstable quark-antiquark pairs. Electric charge is given here in units of the charge on the proton. The particles in Families 2 and 3 decay into Family 1 particles.
B.D. Fields Big Bang Nucleosynthesis 2020
Big-Bang nucleosynthesis (BBN) offers the deepest reliable probe of the early Universe, being based on well-understood Standard Model physics [1]. Predictions of the abundances of the light elements, D, 3He, 4He, and 7Li, synthesized at the end of the first three minutes, are in good overall agreement with the primordial abundances inferred from observational data, thus validating the standard hot Big-Bang cosmology (see [2–5] for reviews). This is particularly impressive given that these abundances span nine orders of magnitude – from 4He/H∼ 0.08 down to 7Li/H∼ 10−10 (ratios by number). Thus BBN provides powerful constraints on possible deviations from the standard cosmology, and on new physics beyond the Standard Model
https://pdg.lbl.gov/2020/reviews/rpp2020-rev-bbang-nucleosynthesis.pdf
Last edited by Otangelo on Fri Jul 16, 2021 1:08 pm; edited 13 times in total