An animal or plant starts its life as a single cell—a fertilized egg, or zygote. During development, this cell divides repeatedly to produce many different kinds of cells, arranged in a final pattern of spectacular complexity and precision. The goal of developmental cell biology is to understand the cellular and molecular mechanisms that direct this amazing transformation. Plants and animals have very different ways of life, and they use different
developmental strategies; in this chapter, we focus mainly on animals. Four processes are fundamental to animal development:
(1) cell proliferation, which produces many cells from one;
(2) cell–cell interactions, which coordinate the behavior of each cell with that of its neighbors;
(3) cell specialization, or differentiation, which creates cells with different characteristics at different positions; and
(4) cell movement, which rearranges the cells to form structured tissues and organs

It is on the fourth point that plant development differs radically: plant cells are unable to migrate or move independently through the embryo because each one is contained within a cell wall, through which it is cemented to its neighbors. In a developing animal embryo, the four fundamental processes are happening in a kaleidoscopic variety of ways, as they give rise to different parts of the organism. Like the members of an orchestra, the cells in the embryo have to play their individual parts in a highly coordinated manner. In the embryo, however, there is no conductor—no central authority—to direct the performance. Instead, development is a self-assembly process in which the cells, as they grow and proliferate, organize themselves into increasingly complex structures. Each of the millions of cells has to choose for itself how to behave, selectively utilizing the geneticinstructions in its chromosomes. At each stage in its development, the cell is presented with a limited set of options, so that its developmental pathway branches repeatedly, reflecting a large set of sequential choices. Like the decisions we make in our own lives, the choices made by the cell are based on its internal state—which largely reflects its history—and on current influences from other cells, especially its close neighbors. To understand development, we need to know how each choice is controlled and how it depends on previous choices. Beyond that, we need to understand how the choices, once made, influence the cell’s chemistry and behavior, and how cell behaviors act synergistically to determine the structure and function of the body. As cells become specialized they change not only their chemistry but also their shape and their attachments to other cells and to the extracellular matrix. They move and rearrange themselves to create the complex architecture of the body, with all its tissues and organs, each structured precisely and defined in size. To understand this process of form generation, or morphogenesis, we will need to take account of the mechanical, as well as the biochemical, interactions between the cells. At first glance, one would no more expect the worm, the flea, the eagle, and the giant squid all to be generated by the same developmental mechanisms than one would suppose that the same methods were used to make a shoe and an airplane. Remarkably, however, research in the past 30 years has revealed that much of the basic machinery of development is essentially the same in all animals—not just in all vertebrates, but in all the major phyla of invertebrates too. Recognizably similar, molecules define the specialized animal cell types, mark the differences between body regions, and help create the animal body pattern. Homologous proteins are often functionally interchangeable between very different species. Thus, a human protein produced artificially in a fly, for example, can perform the same function as the fly’s own version of that protein

Thanks to an underlying unity of mechanisms, developmental biologists have been making great strides toward a coherent understanding of animal development. We begin this chapter with an overview of some of the basic mechanisms that operate in animal development. We then discuss, in sequence, how cells in the embryo diversify to form patterns in space, how the timing of developmental events is controlled, how cell movements contribute to morphogenesis, and how the size of an animal is regulated. We end by considering the most challenging aspect of development—the mechanisms that enable a highly complex nervous system to form.
OVERVIEW OF DEVELOPMENT
Animals live by eating other organisms. Thus, despite their remarkable diversity, animals as different as worms, mollusks, insects, and vertebrates share anatomical features that are fundamental to this way of life. Epidermal cells form a protective outer layer; gut cells absorb nutrients from ingested food; muscle cells allow movement; and neurons and sensory cells control behavior. These diverse cell types are organized into tissues and organs, forming a sheet of skin covering the exterior, a mouth for feeding, and an internal gut tube to digest food—with muscles, nerves, and other tissues arranged in the space between the skin and the gut tube. Many animals have clearly defined axes—an anteroposterior axis, with mouth and brain anterior and anus posterior; a dorsoventral axis, with back dorsal and belly ventral; and a left-right axis. In this section, we discuss some fundamental mechanisms underlying animal development, beginning with how the basic animal body plan is established.
Conserved Mechanisms Establish the Basic Animal Body Plan
The shared anatomical features of animals develop through conserved mechanisms. After fertilization, the zygote usually divides rapidly, or cleaves, to form many smaller cells; during this cleavage, the embryo, which cannot yet feed, does not grow. This phase of development is initially driven and controlled entirely by the material deposited in the egg by the mother. The embryonic genome remains inactive until a point is reached when maternal mRNAs and proteins rather abruptly begin to be degraded. The embryo’s genome is activated, and the cells cohere to form a blastula—typically a solid or a hollow fluid-filled ball of cells. Complex cell rearrangements called gastrulation (from the Greek “gaster,” meaning “belly”) then transform the blastula into a multilayered structure containing a rudimentary internal gut

The early stages of development, as exemplified by a frog.
(A) A fertilized egg divides to produce a blastula—a sheet of epithelial cells often surrounding a cavity. During gastrulation, some of the cells tuck into the interior to form the mesoderm (green) and endoderm (yellow). Ectodermal cells (blue) remain on the outside. (B) A cross section through the trunk of an amphibian embryo shows the basic animal body plan, with a sheet of ectoderm on the outside, a tube of endoderm on the inside, and mesoderm sandwiched between them. The endoderm forms the epithelial lining of the gut, from the mouth to the anus. It gives rise not only to the pharynx, esophagus, stomach, and intestines, but also to many associated
structures. The salivary glands, liver, pancreas, trachea, and lungs, for example, all develop from the wall of the digestive tract and grow to become systems of branching tubes that open into the gut or pharynx. The endoderm forms only the epithelial components of these structures— the lining of the gut and the secretory cells of the pancreas, for example. The supporting muscular and fibrous elements arise from the mesoderm.
The mesoderm gives rise to the connective tissues—at first, to the loose mesh of cells in the embryo known as mesenchyme, and ultimately to cartilage, bone, and fibrous tissue, including the dermis (the inner layer of the skin). The mesoderm also forms the muscles, the entire vascular system—including the heart, blood vessels, and blood cells—and the tubules, ducts, and supporting tissues of the kidneys and gonads. The notochord
forms from the mesoderm and serves as the core of the future backbone and the source of signals that coordinate the development of surrounding tissues. The ectoderm will form the epidermis (the outer, epithelial layer of the skin) and epidermal appendages such as hair, sweat glands, and mammary glands. It will also give rise to the whole of the nervous system, central and peripheral, including not only neurons and glia but also the
sensory cells of the nose, the ear, the eye, and other sense organs.
Some cells of the blastula remain external, constituting the ectoderm, which will give rise to the epidermis and the nervous system; other cells invaginate, forming the endoderm, which will give rise to the gut tube and its appendages, such as lung, pancreas, and liver. Another group of cells moves into the space between ectoderm and endoderm and forms the mesoderm, which will give rise to muscles, connective tissues, blood, kidney, and
various other components. Further cell movements and accompanying cell differentiations create and refine the embryo’s architecture. The ectoderm, mesoderm, and endoderm formed during gastrulation constitute
the three germ layers of the early embryo. Many later developmental transformations will produce the elaborately structured organs. But the basic body plan and axes set up in miniature during gastrulation are preserved into adult life, when the organism may be billions of times larger
The Developmental Potential of Cells Becomes Progressively Restricted
Concomitant with the refinement of the body plan, the individual cells become more and more restricted in their developmental potential. During the blastula stages, cells are often totipotent or pluripotent—they have the potential to give rise to all or almost all of the cell types of the adult body. The pluripotency is lost as gastrulation proceeds: a cell located in the endodermal germ layer, for example, can give rise to the cell types that will line the gut or form gut-derived organs such as the liver or pancreas, but it no longer has the potential to form mesoderm- derived structures such as skeleton, heart, or kidney. Such a cell is said to be determined for an endodermal fate. Thus, cell determination starts early and progressively narrows the options as the cell steps through a programmed series of intermediate states—guided at each step by its genome, its history, and
its interactions with neighbors. The process reaches its limit when a cell undergoes terminal differentiation to form one of the highly specialized cell types of the adult body. Although there are cell types in the adult that retain some degree of pluripotency, their range of options is generally narrow

The lineage from blastomere to differentiated cell type.
As development proceeds, cells become more and more specialized. Blastomeres have the potential to give rise to most or all cell types. Under the influence of signaling molecules and gene regulatory factors, cells acquire more restricted fates until they differentiate into highly specialized cell types, such as the pancreatic β-islet cells that secrete the hormone insulin.
Cell Memory Underlies Cell Decision-Making Underlying the richness and astonishingly complex outcomes of development is cell memory. Both the genes a cell expresses and the way it behaves depend on the cell’s past, as well as on its present circumstances. The cells of our body—the muscle cells, the neurons, the skin cells, the gut cells, and so on—maintain their specialized characters largely because they retain a record of the extracellular signals their ancestors received during development, rather than because they continually receive such instructions from their surroundings. Despite their radically different phenotypes, they retain the same complete genome that was present in the zygote; their differences arise instead from differential gene expression. We have discussed the molecular mechanisms of gene regulation, cell memory, cell division, cell signaling, and cell movement in previous chapters. In this chapter, we shall see how these basic processes are collectively deployed to create an animal.
Several Model Organisms Have Been Crucial for Understanding Development
The anatomical features that animals share have undergone many extreme modifications in the course of evolution. As a result, the differences between species are usually more striking to our human eye than the similarities. But at the level of the underlying molecular mechanisms and the macromolecules that mediate them, the reverse is true: the similarities among all animals are profound and extensive. Through more than half a billion years of evolutionary divergence, all animals have retained unmistakably similar sets of genes and proteins that are responsible for generating their body plans and for forming their specialized cells and organs. This astonishing degree of conservation was discovered not by broad surveys of animal diversity, but through intensive study of a small number of representative species. For animal developmental biology, the most important have been the fly Drosophila melanogaster, the frog Xenopus laevis, the roundworm Caenorhabditis elegans, the mouse Mus musculus, and the zebrafish Danio rerio. In discussing the mechanisms of development, we shall draw our examples mainly from these few species.
Genes Involved in Cell–Cell Communication and Transcriptional Control Are Especially Important for Animal Development
What are the genes that animals share with one another but not with other kingdoms of life? These would be expected to include genes required specifically for animal development but not needed for unicellular existence. Comparison of animal genomes with the genome of budding yeast—a unicellular eukaryote— suggests that three classes of genes are especially important for multicellular organization. The first class includes genes that encode proteins used for cell–cell adhesion and cell signaling; hundreds of human genes encode signal proteins, cell-surface receptors, cell adhesion proteins, or ion channels that are either not present in yeast or present in much smaller numbers. The second class includes genes encoding proteins that regulate transcription and chromatin structure: more than 1000 human genes encode transcription regulators, but only about 250 yeast genes do so. As we shall see, the development of animals is dominated by cell–cell interactions and by differential gene expression. The third class of noncoding RNAs has a more uncertain status: it includes genes that encode microRNAs (miRNAs); there are at least 500 of these in humans. Along with the regulatory proteins, they play a significant part in controlling gene expression during animal development, but the full extent of their importance is still unclear. The loss of individual miRNA genes in C. elegans, where their functions have been well studied, rarely leads to obvious phenotypes, suggesting that the roles of miRNAs during animal development are often subtle, serving to fine-tune the developmental machinery rather than to form its core structures.
Regulatory DNA Seems Largely Responsible for the Differences Between Animal Species
As discussed in Chapter 7, each gene in a multicellular organism is associated with many thousands of nucleotides of noncoding DNA that contains regulatory elements. These regulatory elements determine when, where, and how strongly the gene is to be expressed, according to the transcription regulators and chromatin structures that are present in the particular cell

Consequently, a change in the regulatory DNA, even without any change in the coding DNA, can alter the logic of the gene-regulatory network and change the outcome of development. when we compare the genomes of different animal species, we find that evolution has altered the coding and regulatory DNA to different extents. The coding DNA, for the most part, has been highly conserved, the noncoding regulatory DNA much less so. It seems that changes in regulatory DNA are largely responsible for the dramatic differences between one class of animals and another. We can view the protein products of the coding sequences as a conserved kit of common molecular parts, and the regulatory DNA as instructions for assembly: with different instructions, the same kit of parts can be used to make a whole variety of different body structures. We will return to this important concept later.
Small Numbers of Conserved Cell–Cell Signaling Pathways Coordinate Spatial Patterning
Spatial patterning of a developing animal requires that cells become different according to their positions in the embryo, which means that cells must respond to extracellular signals produced by other cells, especially their neighbors. In what is probably the commonest mode of spatial patterning, a group of cells starts out with the same developmental potential, and a signal from cells outside the group then induces one or more members of the group to change their character. This process is called inductive signaling. Generally, the inductive signal is limited in time and space so that only a subset of the cells capable of responding—the cells close to the source of the signal—take on the induced character

Some inductive signals depend on cell–cell contact; others act over a longer range and are mediated by molecules that diffuse through the extracellular medium or are transported in the bloodstream. Most of the known inductive events in animal development are governed by a small number of highly conserved signaling pathways, including transforming growth factor-β (TGFβ), Wnt, Hedgehog, Notch, and receptor tyrosine kinase (RTK) pathways. The discovery of the limited vocabulary that developing cells use for intercellular communication has emerged over the past 25 years as one of the great simplifying features of developmental biology.
Through Combinatorial Control and Cell Memory, Simple Signals Can Generate Complex Patterns
But how can this small number of signaling pathways generate the huge diversity of cells and patterns? Three kinds of mechanisms are responsible. First, through gene duplication, the basic components of a pathway often come to be encoded by small families of closely related homologous genes. This allows for diversity in the operation of the pathway, according to which family member is employed in a given situation. Notch signaling, for example, may be mediated by Notch1 in one tissue, but by its homolog Notch4 in another. Second, the response of a cell to a given signal protein depends on the other signals that the cell is receiving concurrently ( Figure A below )

Two mechanisms for generating different responses to the same inductive signal. (A) In combinatorial signaling, the effect of a signal depends on the presence of other signals received at the same time. (B) Through cell memory, previous signals (or other events) can leave a lasting trace that alters the response to the current signal. The memory trace is represented here in the coloring of the cell nucleus.
As a result, different combinations of signals can generate a large variety of different responses. Third, and most fundamental, the effect of activating a signaling pathway depends on the previous experiences of the responding cell: past influences leave a lasting mark, registered in the state of the cell’s chromatin and the selection of transcription regulatory proteins and RNA molecules that the cell contains. This cell memory enables cells with different histories to respond to the same signals differently (Figure B). Thus, the same few signaling pathways can be used repeatedly at different times and places with different outcomes, so as to generate patterns of unlimited complexity.
Morphogens Are Long-Range Inductive Signals That Exert Graded Effects
Signal molecules often govern simple yes–no choices—one outcome when their concentration is high, another when it is low. In many cases, however, the responses are more finely graded: a high concentration of a signal molecule may, for example, direct cells into one developmental pathway, an intermediate concentration into another, and a low concentration into yet another. One common way to generate such different concentrations of a signal molecule is for the molecule to diffuse out from a localized signaling source, creating a concentration gradient. Cells at different distances from the source are driven to behave in a variety of different ways, according to the signal concentration that they experience

A signal molecule that imposes a pattern on a whole field of cells in this way is called a morphogen. In the simplest case, a specialized group of cells produces a morphogen at a steady rate, and the morphogen is then degraded as it diffuses away from this source. The speed of diffusion and the half-life of the morphogen will together determine the range and steepness of its resulting gradient
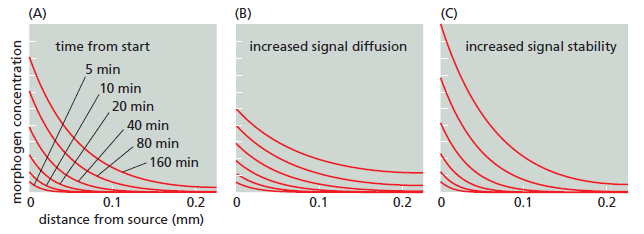
Setting up a signal gradient by diffusion.
(A–C) Each graph shows six successive stages in the buildup of the concentration of a signal molecule that is produced at a steady rate at the origin, with production starting at time 0. In all cases, the molecule undergoes degradation as it diffuses away from the source, and the graphs are calculated on the assumption that diffusion is occurring along two axes in space (for example, radially from a source in an epithelial sheet). (A) The pattern of the morphogen assuming that the molecule has a half-life of 170 minutes, and that it diffuses with an effective diffusion constant of D = 1 μm2 sec–1, typical of a small protein molecule in extracellular tissues. Note that the gradient is already close to its steady-state form within an hour and that the concentration at steady state falls off exponentially with distance. (B) A threefold increase in the diffusion constant of the morphogen extends its range but lowers its concentration next to the source, whereas (C) a threefold increase in morphogen halflife increases its concentration throughout the tissue. Effects of the morphogen will depend not just on its concentration at some critical moment, but also on how each target cell integrates its response over time.
This simple mechanism can be modified in various ways. Receptors on the surface of cells along the way, for example, may trap the diffusing morphogen and cause it to be endocytosed and degraded, shortening its effective half-life. Alternatively, the morphogen may bind to molecules in the extracellular matrix such as heparan sulfate proteoglycan , thereby greatly reducing its diffusion rate.
Lateral Inhibition Can Generate Patterns of Different Cell Types
Morphogen gradients, and other kinds of inductive signal, exploit an existing asymmetry in the embryo to create further asymmetries and differences between cells: already, at the outset, some cells are specialized to produce the morphogen and thereby impose a pattern on another class of cells that are sensitive to it. But what if there is no clear initial asymmetry? Can a regular pattern arise spontaneously within a set of cells that are initially all alike? The answer is yes. The fundamental principle underlying such de novo pattern formation is positive feedback: cells can exchange signals in such a way that any small initial discrepancy between cells at different sites becomes self-amplifying, driving the cells toward different fates. This is most clearly illustrated in the phenomenon of lateral inhibition, a form of cell–cell interaction that forces close neighbors to become different and thereby generates fine-grained patterns of different cell types. Consider a pair of adjacent cells that start off in a similar state. Each of these cells can both produce and respond to a certain signal molecule X, with the added rule that the stronger the signal a cell receives, the weaker the signal it generates

Genesis of asymmetry through lateral inhibition and cell 1 cell 2 positive feedback. In this example, two cells interact, each producing a substance X that acts on the other cell to inhibit its production of X, an effect known as lateral inhibition. An increase of X in one of the cells leads to a positive feedback that tends to increase X in that cell still further, while decreasing X in its neighbor. This can create a runaway instability, making the two cells become radically different. Ultimately, the system comes to rest in one or the other of two opposite stable states. The final choice of state represents a form of memory: the small influence that initially directed the choice is no longer required to maintain it.
If one cell produces more X, the other is forced to produce less. This gives rise to a positive feedback loop that tends to amplify any initial difference between the two adjacent cells. Such a difference may arise from a bias imposed by some present or past external factor, or it may simply originate from spontaneous random fluctuations, or “noise”—an inevitable feature of the genetic control circuitry in cells (discussed in Chapter 7). In either case, lateral inhibition means that if cell 1 makes a little more of X, it will thereby cause cell 2 to make less; and because cell 2 makes less X, it delivers less inhibition to cell 1 and so allows the production of X in cell 1 to rise higher still; and so on, until a steady state is reached where cell 1 produces a lot of X and cell 2 produces very little. In the standard case, the signal molecule X acts in the receiving cell by regulating gene transcription, and the result is that the two cells are driven along different pathways of differentiation. In almost all tissues, a balanced mixture of different cell types is required. Lateral inhibition provides a common way to generate the mixture. As we shall see, lateral inhibition is very often mediated by exchange of signals at cell–cell contacts via the Notch signaling pathway, driving cell diversification by enabling individual cells that express one set of genes to direct their immediate neighbors to express a different set, in exactly the way we have described
Short-Range Activation and Long-Range Inhibition Can Generate Complex Cellular Patterns
Lateral inhibition mediated by the Notch pathway is not the only example of pattern generation through positive feedback: there are other ways in which, through the same basic principle, a system that starts off homogeneous and symmetrical can pattern itself spontaneously, even in the absence of an external morphogen. Positive feedback processes mediated by diffusible signal molecules can operate over broad arrays of cells to create many types of spatial patterns. Mechanisms of this sort are called reaction-diffusion systems. For example, a substance A (a shortrange activator) may stimulate its own production in the cells that contain it and in their immediate neighbors, while also causing these cells to produce a signal I (a long-range inhibitor) that diffuses widely and inhibits the production of A in cells farther away. If the cells all start the same, but one group gains a slight advantage by making a little more A than the rest, the asymmetry can be self-amplifying Such short-range activation combined with long-range inhibition can account for the formation of clusters of cells within an initially homogeneous tissue that become specialized as localized signaling centers.

Pattern generation by a reaction-diffusion system. From (A) a uniform field of cells, (B) local positive feedback and (C) long-range inhibition can (D) generate patterns within the initially uniform field. The patterns can be complex, resembling the spots of a leopard (as shown) or the stripes of a zebra; or they can be simple, with creation of a single cluster of specialized cells that can, for example, go on to serve as the source of a morphogen gradient.
Asymmetric Cell Division Can Also Generate Diversity
Cell diversification does not always depend on extracellular signals: in some cases, daughter cells are born different as a result of an asymmetric cell division, in which some important molecule or molecules are distributed unequally between the two daughters. This asymmetric inheritance ensures that the two daughter cells develop differently

Two ways of making sister cells different.
Asymmetric division is a common feature of early development, where the fertilized egg already has an internal pattern and cleavage of this large cell segregates different determinants into separate blastomeres. We shall see that asymmetric division also plays a part in some later developmental processes.
Initial Patterns Are Established in Small Fields of Cells and Refined by Sequential Induction as the Embryo Grows
The signals that organize the spatial pattern of cells in an embryo generally act over short distances and govern relatively simple choices. A morphogen, for example, typically acts over a distance of less than 1 mm—an effective range for diffusion —and directs choices between several developmental options for the cells on which it acts. Yet the organs that eventually develop are much larger and more complex than this. The cell proliferation that follows the initial specification accounts for the size increase, while the refinement of the initial pattern is explained by a series of local inductions plus other interactions that add successive levels of detail on an initially simple sketch. For example, as soon as two types of cells are present in a developing tissue, one of them can produce a signal that induces a subset of the neighboring cells to specialize in a third way. The third cell type can in turn signal back to the other two cell types nearby, generating a fourth and a fifth cell type, and so on

This strategy for generating a progressively more complicated pattern is called sequential induction. It is chiefly through sequential inductions that the body plan of a developing animal, after being first roughed out in miniature, becomes elaborated with finer and finer details as development proceeds.
Different Animals Use Different Mechanisms to Establish Their Primary Axes of Polarization
Surprisingly, the earliest steps of animal development are among the most variable, even within a phylum. A frog, a chicken, and a mammal, for example, even though they develop in similar ways later, make eggs that differ radically in size and structure, and they begin their development with different sequences of cell divisions and cell specializations. Gastrulation occurs in all animal embryos, but the details of its timing, of the associated pattern of cell movements, and of the shape and size of the embryo as gastrulation proceeds are highly variable. Likewise, there is great variation in the time and manner in which the primary axes of the body become marked out. However, this polarization of the embryo usually becomes discernible very early, before gastrulation begins: it is the first step of spatial patterning. Three axes generally have to be established. The animal-vegetal (A-V) axis, in most species, defines which parts are to become internal (through the movements of gastrulation) and which are to remain external. (The bizarre name dates from a century ago and has nothing to do with vegetables.) The anteroposterior (A-P) axis specifies the locations of future head and tail. The dorsoventral (D-V) axis specifies the future back and belly. At one extreme, the egg is spherically symmetrical, and the axes only become defined during embryogenesis. The mouse comes close to being an example, with little obvious sign of polarity in the egg. Correspondingly, the blastomeres produced by the first few cell divisions seem to be all alike and are remarkably adaptable. If the early mouse embryo is split in two, a pair of identical twins can be produced— two complete, normal individuals from a single cell. Similarly, if one of the cells in a two-cell mouse embryo is destroyed by pricking it with a needle and the resulting “half-embryo” is placed in the uterus of a foster mother to develop, in many cases a perfectly normal mouse will emerge. At the opposite extreme, the structure of the egg defines the future axes of the body. This is the case for most species, including insects such as Drosophila, as we shall see shortly. Many other organisms lie between the two extremes. The egg of the frog Xenopus, for example, has a clearly defined A-V axis even before fertilization: the nucleus near the top defines the animal pole, while the mass of yolk (the embryo’s food supply, destined to be incorporated in the gut) toward the bottom defines the vegetal pole. Several types of mRNA molecules are already localized in the vegetal cytoplasm of the egg, where they produce their protein products. After fertilization, these mRNAs and proteins act in and on the cells in the lower and middle part of the embryo, giving the cells there specialized characters, both by direct effects and by stimulating the production of secreted signal proteins. For example, mRNA encoding the transcription regulator VegT is deposited at the vegetal pole during oogenesis. After fertilization, this mRNA is translated, and the resulting VegT protein activates a set of genes that code for signal proteins that induce mesoderm and endoderm, as discussed later. The D-V axis of the Xenopus embryo, by contrast, is defined through the act of fertilization. Following entry of the sperm, the outer cortex of the egg cytoplasm rotates relative to the central core of the egg, so that the animal pole of the cortex becomes slightly shifted to one side

Treatments that block the rotation allow cleavage to occur normally but produce an embryo with a central gut and no dorsal structures or D-V asymmetry. Thus, this cortical rotation is required to define the D-V axis of the future body by creating the D-V axis of the egg. The site of sperm entry that biases the direction of the cortical rotation in Xenopus, perhaps through the centrosome that the sperm brings into the egg— inasmuch as the rotation is associated with a reorganization of the microtubules nucleated from the centrosome in the egg cytoplasm. The reorganization leads to a microtubule-based transport of several cytoplasmic components, including the mRNA coding for Wnt11, a member of the Wnt family of signal proteins, moving it toward the future dorsal side (see Figure above). This mRNA is soon translated and the Wnt11 protein secreted from cells that form in that region of the embryo activates the Wnt signaling pathway . This activation is crucial for triggering the cascade of subsequent events that will organize the dorsoventral axis of the body. (The A-P axis of the embryo will only become clear later, in the process of gastrulation.) Although different animal species use a variety of different mechanisms to specify their axes, the outcome has been relatively well conserved in evolution: head is distinguished from tail, back from belly, and gut from skin. It seems that it does not much matter what tricks the embryo uses to break the initial symmetry and set up this basic body plan.
Studies in Drosophila Have Revealed the Genetic Control Mechanisms Underlying Development
It is the fly Drosophila, more than any other organism, that has provided the key to our present understanding of how genes govern development. Decades of genetic study culminated in a large-scale genetic screen, focusing especially on the early embryo and searching for mutations that disrupt its pattern. This revealed that the key developmental genes fall into a relatively small set of functional classes defined by their mutant phenotypes. The discovery of these genes and the subsequent analysis of their functions was a famous tour de force and had a revolutionary impact on all of developmental biology, earning its discoverers a Nobel Prize. Some parts of the developmental machinery revealed in this way are conserved between flies and vertebrates, some parts not. But the logic of the experimental approach and the general strategies of genetic control that it revealed have transformed our understanding of multicellular development in general. To understand how the early developmental machinery operates in Drosophila, it is important to note a peculiarity of fly development. Like the eggs of other insects, but unlike most vertebrates, the Drosophila egg—shaped like a cucumber— begins its development with an extraordinarily rapid series of nuclear divisions without cell division, producing multiple nuclei in a common cytoplasm—a syncytium. The nuclei then migrate to the cell cortex, forming a structure called the syncytial blastoderm. After about 6000 nuclei have been produced, the plasma membrane folds inward between them and partitions them into separate cells, converting the syncytial blastoderm into the cellular blastoderm
Last edited by Admin on Tue Dec 15, 2015 2:40 pm; edited 2 times in total

