Photoreceptor cells point to intelligent design
https://reasonandscience.catsboard.com/t1696-photoreceptor-cells-point-to-intelligent-design
http://en.wikipedia.org/wiki/Photoreceptor_cell
A photoreceptor cell is a specialized type of neuron found in the retina that is capable of phototransduction. The great biological importance of photoreceptors is that they convert light (visible electromagnetic radiation) into signals that can stimulate biological processes. To be more specific, photoreceptor proteins in the cell absorb photons, triggering a change in the cell's membrane potential.
http://www.evolutionnews.org/2012/08/eye_of_the_fly063111.html
3. Visual pigments and visual transduction.
Vertebrate photoreceptors can respond to light by virtue of their containing a visual pigment embedded in the bilipid membranous discs that make up the outer segment. The visual pigment consists of a protein called opsin and a chromophore derived from vitamin A known as retinal. The vitamin A is manufactured from beta-carotene in the food we eat, and the protein is manufactured in the photoreceptor cell (see above). The opsin and the chromophore are bound together and lie buried in the membranes of the outer segment discs

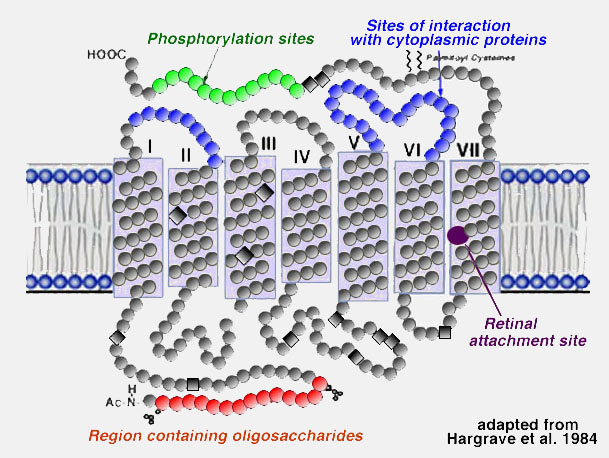
About 50% of the opsin is within the bilipid membrane connected by short protein loops outside. Each molecule of rhodopsin consists of seven of these transmembrane portions surrounding the chromophore (11-cis retinal) in the lipid bilayer. The chromophore apparently lies horizontally in the membrane and is bound at a lysine residue to helix seven . Each outer segment disc, of course, contains many (thousands) visual pigment molecules. Upon absorption of a photon of light, the retinal isomerizes from the 11-cis form to an all-trans form which starts conformational changes in the molecule resulting in bleaching. Several intermediaries are formed in bleaching among them metarhodopsin II which activates the G-protein transducin and a further cascade of events summarized below (see review by Hargrave and McDowell (1992) and by Archer, 1995)and chapter by Yingbin Fu (webvision).
Light transduces the visual pigment via the following enzyme cascade: photons – rhodopsin – activated rhodopsin (metarhodopsin II) – a GTP binding protein (transducin) – an enzyme hydrolyzing cGMP (cGMP-phosphodiesterase) – closes a membrane bound cGMP-gated cation channel.
In the dark a steady current flows into the open channels, carried mainly by Na ions, constituting a “dark current” that partially depolarizes the photoreceptor cell (Fig. 10). Thus, the depolarized photoreceptor releases neurotransmitter (the amino acid glutamate) from its synaptic terminals upon second-order neurons in the dark. On light stimulation the rhodopsin molecules are isomerized to the active form, the above cascade ensues, leading to closure of the cation channels of the photoreceptor membrane, stopping the dark current and causing the photoreceptor cell membrane to hyperpolarize and cease neurotransmitter release to second-order neurons (Fig. 10) (see Stryer, 1991; Yau, 1994, and Kawamura, 1995, and Fu (webvision) for reviews).


Neuron :
Signal transduction pathway
https://www.youtube.com/watch?v=qOVkedxDqQo
The absorption of light leads to a isomeric change in the retinal molecule.
The signal transduction pathway is the mechanism by which the energy of a photon signals a mechanism in the cell that leads to its electrical polarization. This polarization ultimately leads to either the transmittance or inhibition of a neural signal that will be fed to the brain via the optic nerve. The steps, or signal transduction pathway, in the vertebrate eye's rod and cone photoreceptors are then:
http://www.harunyahya.com/en/Books/592/darwinism-refuted/chapter/51
Thus, a rod or cone photoreceptor actually releases less neurotransmitter when stimulated by light. Less neurotransmitter could either stimulate (depolarize) or inhibit (hyperpolarize) the bi-polar cell it synapses with, dependent on the nature of the receptor on the bipolar cell. This ability is integral to the center on/off mapping of visual units.[citation needed]
ATP provided by the inner segment powers the sodium-potassium pump. This pump is necessary to reset the initial state of the outer segment by taking the sodium ions that are entering the cell and pumping them back out.
Although photoreceptors are neurons, they do not conduct action potentials with the exception of the photosensitive ganglion cell – which are involved mainly in the regulation of circadian rhythms, melatonin, and pupil dilation.
Advantages
Phototransduction in rods and cones is unique in that the stimulus (in this case, light) actually reduces the cell's response or firing rate, which is unusual for a sensory system where the stimulus usually increases the cell's response or firing rate. However, this system offers several key advantages.
First, the classic (rod or cone) photoreceptor is depolarized in the dark, which means many sodium ions are flowing into the cell. Thus, the random opening or closing of sodium channels will not affect the membrane potential of the cell; only the closing of a large number of channels, through absorption of a photon, will affect it and signal that light is in the visual field. Hence, the system is noiseless.
Second, there is a lot of amplification in two stages of classic phototransduction: one pigment will activate many molecules of transducin, and one PDE will cleave many cGMPs. This amplification means that even the absorption of one photon will affect membrane potential and signal to the brain that light is in the visual field. This is the main feature that differentiates rod photoreceptors from cone photoreceptors. Rods are extremely sensitive and have the capacity of registering a single photon of light, unlike cones. On the other hand, cones are known to have very fast kinetics in terms of rate of amplification of phototransduction, unlike rods.
http://www.detectingdesign.com/humaneye.html
Arguments against IC of the signal transduction pathway
http://www.asa3.org/evolution/irred_compl.html
https://reasonandscience.catsboard.com/t1696-photoreceptor-cells-point-to-intelligent-design
http://en.wikipedia.org/wiki/Photoreceptor_cell
A photoreceptor cell is a specialized type of neuron found in the retina that is capable of phototransduction. The great biological importance of photoreceptors is that they convert light (visible electromagnetic radiation) into signals that can stimulate biological processes. To be more specific, photoreceptor proteins in the cell absorb photons, triggering a change in the cell's membrane potential.
http://www.evolutionnews.org/2012/08/eye_of_the_fly063111.html
3. Visual pigments and visual transduction.
Vertebrate photoreceptors can respond to light by virtue of their containing a visual pigment embedded in the bilipid membranous discs that make up the outer segment. The visual pigment consists of a protein called opsin and a chromophore derived from vitamin A known as retinal. The vitamin A is manufactured from beta-carotene in the food we eat, and the protein is manufactured in the photoreceptor cell (see above). The opsin and the chromophore are bound together and lie buried in the membranes of the outer segment discs


About 50% of the opsin is within the bilipid membrane connected by short protein loops outside. Each molecule of rhodopsin consists of seven of these transmembrane portions surrounding the chromophore (11-cis retinal) in the lipid bilayer. The chromophore apparently lies horizontally in the membrane and is bound at a lysine residue to helix seven . Each outer segment disc, of course, contains many (thousands) visual pigment molecules. Upon absorption of a photon of light, the retinal isomerizes from the 11-cis form to an all-trans form which starts conformational changes in the molecule resulting in bleaching. Several intermediaries are formed in bleaching among them metarhodopsin II which activates the G-protein transducin and a further cascade of events summarized below (see review by Hargrave and McDowell (1992) and by Archer, 1995)and chapter by Yingbin Fu (webvision).
Light transduces the visual pigment via the following enzyme cascade: photons – rhodopsin – activated rhodopsin (metarhodopsin II) – a GTP binding protein (transducin) – an enzyme hydrolyzing cGMP (cGMP-phosphodiesterase) – closes a membrane bound cGMP-gated cation channel.
In the dark a steady current flows into the open channels, carried mainly by Na ions, constituting a “dark current” that partially depolarizes the photoreceptor cell (Fig. 10). Thus, the depolarized photoreceptor releases neurotransmitter (the amino acid glutamate) from its synaptic terminals upon second-order neurons in the dark. On light stimulation the rhodopsin molecules are isomerized to the active form, the above cascade ensues, leading to closure of the cation channels of the photoreceptor membrane, stopping the dark current and causing the photoreceptor cell membrane to hyperpolarize and cease neurotransmitter release to second-order neurons (Fig. 10) (see Stryer, 1991; Yau, 1994, and Kawamura, 1995, and Fu (webvision) for reviews).


Neuron :
Signal transduction pathway
https://www.youtube.com/watch?v=qOVkedxDqQo
The absorption of light leads to a isomeric change in the retinal molecule.
The signal transduction pathway is the mechanism by which the energy of a photon signals a mechanism in the cell that leads to its electrical polarization. This polarization ultimately leads to either the transmittance or inhibition of a neural signal that will be fed to the brain via the optic nerve. The steps, or signal transduction pathway, in the vertebrate eye's rod and cone photoreceptors are then:
1.The rhodopsin or iodopsin in the disc membrane of the outer segment absorbs a photon, changing the configuration of a retinal Schiff base cofactor inside the protein from the cis-form to the trans-form, causing the retinal to change shape.
2.This results in a series of unstable intermediates, the last of which binds stronger to the G protein in the membrane and activates transducin, a protein inside the cell. This is the first amplification step – each photoactivated rhodopsin triggers activation of about 100 transducins. (The shape change in the opsin activates a G protein called transducin.)
3.Each transducin then activates the enzyme cGMP-specific phosphodiesterase (PDE).
4.PDE then catalyzes the hydrolysis of cGMP to 5' GMP. This is the second amplification step, where a single PDE hydrolyses about 1000 cGMP molecules.
5.The net concentration of intracellular cGMP is reduced (due to its conversion to 5' GMP via PDE), resulting in the closure of cyclic nucleotide-gated Na+ ion channels located in the photoreceptor outer segment membrane.
6.As a result, sodium ions can no longer enter the cell, and the photoreceptor outer segment membrane becomes hyperpolarized, due to the charge inside the membrane becoming more negative.
7.This change in the cell's membrane potential causes voltage-gated calcium channels to close. This leads to a decrease in the influx of calcium ions into the cell and thus the intracellular calcium ion concentration falls.
8.A decrease in the intracellular calcium concentration means that less glutamate is released via calcium-induced exocytosis to the bipolar cell (see below). (The decreased calcium level slows the release of the neurotransmitter glutamate, which can either excite or inhibit the postsynaptic bipolar cells.)
9.Reduction in the release of glutamate means one population of bipolar cells will be depolarized and a separate population of bipolar cells will be hyperpolarized, depending on the nature of receptors (ionotropic or metabotropic) in the postsynaptic terminal (see receptive field).
http://www.harunyahya.com/en/Books/592/darwinism-refuted/chapter/51
So, how does this system, which Darwin glossed over as a simple structure, actually work? How do the cells in the eye's retinal layer perceive the light rays that fall on them?
The answer to that question is rather complicated. When photons hit the cells of the retina they activate a chain action, rather like a domino effect. The first of these domino pieces is a molecule called "11-cis-retinal" that is sensitive to photons. When struck by a photon, this molecule changes shape, which in turn changes the shape of a protein called "rhodopsin" to which it is tightly bound. Rhodopsin then takes a form that enables it to stick to another resident protein in the cell called "transducin."
Prior to reacting with rhodopsin, transducin is bound to another molecule called GDP. When it connects with rhodopsin, transducin releases the GDP molecule and is linked to a new molecule called GTP. That is why the new complex consisting of the two proteins (rhodopsin and transducin) and a smaller molecule (GTP) is called "GTP-transducin-rhodopsin."
But the process has only just begun. The new GTP-transducin-rhodopsin complex can now very quickly bind to another protein resident in the cell called "phosphodiesterase." This enables the phosphodiesterase protein to cut yet another molecule resident in the cell, called cGMP. Since this process takes place in the millions of proteins in the cell, the cGMP concentration is suddenly decreased.
How does all this help with sight? The last element of this chain reaction supplies the answer. The fall in the cGMP amount affects the ion channels in the cell. The so-called ion channel is a structure composed of proteins that regulate the number of sodium ions within the cell. Under normal conditions, the ion channel allows sodium ions to flow into the cell while another molecule disposes of the excess ions to maintain a balance. When the number of cGMP molecules falls, so does the number of sodium ions. This leads to an imbalance of charge across the membrane, which stimulates the nerve cells connected to these cells, forming what we refer to as an "electrical impulse." Nerves carry the impulses to the brain and "seeing" happens there. 347
In brief, a single photon hits a single cell, and through a series of chain reactions the cell produces an electrical impulse. This stimulus is modulated by the energy of the photon—that is, the brightness of the light. Another fascinating fact is that all of the processes described so far happen in no more than one thousandth of a second. As soon as this chain reaction is completed, other specialized proteins within the cells convert elements such as 11-cis-retinal, rhodopsin and transducin back to their original states. The eye is under a constant shower of photons, and the chain reactions within the eye's sensitive cells enable it to perceive each one of these.
The process of sight is actually a great deal more complicated than the outline presented here would indicate. However, even this brief overview is sufficient to demonstrate the extraordinary nature of the system. There is such a complex, finely calculated system inside the eye that it is nonsensical to claim that it could have come about by chance. The system possesses a totally irreducibly complex structure. If even one of the many molecular parts that enter into a chain reaction with each other were missing, or did not possess a suitable structure, then the system would not function at all.
It is clear that this system deals a heavy blow to Darwin's explanation of life by "chance." Michael Behe makes this comment on the chemistry of the eye and the theory of evolution:
Now that the black box of vision has been opened, it is no longer enough for an evolutionary explanation of that power to consider only the anatomical structures of whole eyes, as Darwin did in the nineteenth century (and as popularizers of evolution continue to do today). Each of the anatomical steps and structures that Darwin thought were so simple actually involves staggeringly complicated biochemical processes that cannot be papered over with rhetoric.
Thus, a rod or cone photoreceptor actually releases less neurotransmitter when stimulated by light. Less neurotransmitter could either stimulate (depolarize) or inhibit (hyperpolarize) the bi-polar cell it synapses with, dependent on the nature of the receptor on the bipolar cell. This ability is integral to the center on/off mapping of visual units.[citation needed]
ATP provided by the inner segment powers the sodium-potassium pump. This pump is necessary to reset the initial state of the outer segment by taking the sodium ions that are entering the cell and pumping them back out.
Although photoreceptors are neurons, they do not conduct action potentials with the exception of the photosensitive ganglion cell – which are involved mainly in the regulation of circadian rhythms, melatonin, and pupil dilation.
Advantages
Phototransduction in rods and cones is unique in that the stimulus (in this case, light) actually reduces the cell's response or firing rate, which is unusual for a sensory system where the stimulus usually increases the cell's response or firing rate. However, this system offers several key advantages.
First, the classic (rod or cone) photoreceptor is depolarized in the dark, which means many sodium ions are flowing into the cell. Thus, the random opening or closing of sodium channels will not affect the membrane potential of the cell; only the closing of a large number of channels, through absorption of a photon, will affect it and signal that light is in the visual field. Hence, the system is noiseless.
Second, there is a lot of amplification in two stages of classic phototransduction: one pigment will activate many molecules of transducin, and one PDE will cleave many cGMPs. This amplification means that even the absorption of one photon will affect membrane potential and signal to the brain that light is in the visual field. This is the main feature that differentiates rod photoreceptors from cone photoreceptors. Rods are extremely sensitive and have the capacity of registering a single photon of light, unlike cones. On the other hand, cones are known to have very fast kinetics in terms of rate of amplification of phototransduction, unlike rods.
http://www.detectingdesign.com/humaneye.html
the first step in vision is the detection of photons. In order to detect a photon, specialized cells use a molecule called 11-cis-retinal. When a photon of light interacts with this molecule, it changes its shape almost instantly. It is now called trans-retinal. This change in shape causes a change in shape of another molecule called rhodopsin. The new shape of rhodopsin is called metarhodopsin II. Metarhodopsin II now sticks to another protein called transducin forcing it to drop an attached molecule called GDP and pick up another molecule called GTP. The GTP-transducin-metarhodopsin II molecule now attaches to another protein called phosphodiesterase. When this happens, phosphodiesterase cleaves molecules called cGMPs. This cleavage of cGMPs reduces their relative numbers in the cell. This reduction in cGMP is sensed by an ion channel. This ion channel shuts off the ability of the sodium ion to enter the cell. This blockage of sodium entrance into the cell causes an imbalance of charge across the cell's membrane. This imbalance of charge sends an electrical current to the brain. The brain then interprets this signal and the result is called vision.
Many other proteins are now needed to convert the proteins and other molecules just mentioned back to their original forms so that they can detect another photon of light and signal the brain. If any one of these proteins or molecules is missing, even in the simplest eye system, vision will not occur
The question now of course is, how could such a system evolve gradually? All the pieces must be in place simultaneously. For example, what good would it be for an earthworm that has no eyes to suddenly evolve the protein 11-cis-retinal in a small group or "spot" of cells on its head? These cells now have the ability to detect photons, but so what? What benefit is that to the earthworm? Now, lets say that somehow these cells develop all the needed proteins to activate an electrical charge across their membranes in response to a photon of light striking them. So what?! What good is it for them to be able to establish an electrical gradient across their membranes if there is no nervous pathway to the worm's minute brain? Now, what if this pathway did happen to suddenly evolve and such a signal could be sent to the worm's brain. So what?! How is the worm going to know what to do with this signal? It will have to learn what this signal means. Learning and interpretation are very complicated processes involving a great many other proteins in other unique systems. Now the earthworm, in one lifetime, must evolve the ability to pass on this ability to interpret vision to its offspring. If it does not pass on this ability, the offspring must learn as well or vision offers no advantage to them. All of these wonderful processes need regulation. No function is beneficial unless it can be regulated (turned off and on). If the light sensitive cells cannot be turned off once they are turned on, vision does not occur. This regulatory ability is also very complicated involving a great many proteins and other molecules - all of which must be in place initially for vision to be beneficial.
Arguments against IC of the signal transduction pathway
http://www.asa3.org/evolution/irred_compl.html
Second, concerning vision. The argument has been made that the vision system is also an irreducibly complex system. Mike Behe has found no fault in Darwin's lack of concern with the origin of light reception at the detailed cellular and molecular level, but now with our opening of the black box of vision, we have no excuse for not concerning ourselves with those sort of details. Mike claims that upon examination of the open box that we must conclude that evolution of this complex system is impossible. So again we must ask, does the pre-adaptation argument get us anywhere in the discussion of the origin of vision? Again, the answer is an obvious yes. First, if we restrict ourselves to light reception, then I think that it's fair to say that a nerve cell is a pre adaptation to vision. Given a nerve cell, I don't have to explain where all those components come from (at least when explaining vision). Second, transducin, one of the key proteins involved in the light signal transduction from rhodopsin to nerve cell, is a member of the G protein family, a large family involved in all sorts of signal transduction events, including hormone signaling. The main novel feature of transducin is its specificity for rhodopsin. The generic G protein is a pre adaptation for transducin. Finally, rhodopsin, the main light reception protein, is a membrane protein similar in structure to other sensory receptors and to hormone receptors. These other receptors whose physiological effects are also mediated by G proteins may have been pre adaptations for rhodopsin. My answer here may be a form of question-begging, because you can always ask where did these other systems come from, but I think that the functional diversification of similar signal transduction sytems is reminiscent of the hemoglobin tale told above. What's needed is detailed sequence and structure information about all these proteins in a variety of organisms that are representative of the tree of life. Then maybe we can say that we have opened the black box. Until then, I think that given the present data that the evolutionary explanation for complexity is not only plausible but likely.
This answer does not address the key issue of IC, namely that unless the process goes all steps through, no function is achieved.
Last edited by Admin on Fri Apr 13, 2018 7:31 pm; edited 13 times in total


